Abstract
Various transplantation strategies have been designed to improve the poor prognosis of adult (ages 15 to 60 years) acute lymphoblastic leukemia (ALL). The GOELAL02 trial evaluated the impact of early allogeneic bone marrow transplantation (alloBMT) or delayed unpurged autologous stem cell transplantation (ASCT) for patients who had no human leukocyte antigen (HLA)–matched sibling donor or who were older than 50 years. Inclusion criteria included at least one of the following: age older than 35 years; non–T-ALL; leukocytosis greater than 30 × 109/L; t(9;22), t(4;11), or t(1; 19); or failure to achieve complete remission (CR) after one induction course. Among 198 patients, the median age was 33 years. The CR rate was 80% with vincristine, idarubicine, l-asparaginase, and randomized intravenous injection or oral steroids (P = nonsignificant [ns]). AlloBMT was performed after 2 consolidation courses while ASCT was delayed after 1 additional reinduction. Intensified conditioning regimen before transplantation included etoposide, cyclophosphamide, and total body irradiation (TBI). Median follow-up was 5.1 years. The median overall survival (OS) was 29 months, with a 6-year OS of 41%. On an intent-to-treat analysis for patients younger than 50 years, alloBMT significantly improved the 6-year OS (75% versus 40% after ASCT; P = .0027). Randomized interferon-α maintenance had no effect on relapse or survival after ASCT. In conclusion, the outcome of adult ALL is better after early alloBMT than after delayed ASCT.
Introduction
Acute lymphoblastic leukemia (ALL) accounts for approximately 15% to 20% of all adult acute leukemias, and the incidence increases with age. ALL is a heterogeneous disorder, and response to therapy can vary dramatically, depending on the clinical features present at diagnosis. More than 70% of afflicted children achieve long-term disease-free survival (DFS), while only 20% to 38% of adults do so.1-5 These results, obtained with conventional chemotherapy, have led many centers to investigate the use of high-dose therapy (HDT) and stem cell transplantation in adult ALL patients. Yet, despite such intensive therapy, many of these patients eventually experience relapse. Prognostic factors have been identified to select patients with a poor predictable outcome and included age older than 35 years; non–T-cell phenotype; white blood cell (WBC) count exceeding 30 × 109/L; poor-risk cytogenetic abnormalities including Philadelphia chromosome positivity (Ph+), t(4;11), or t(1;19); or the absence of complete remission (CR) on day 35 after the first induction course.5-8 In a large prospective German study, the 5-year DFS for adults with at least one of these adverse factors ranged from 11% to 33%.6 To improve the prognosis of these ALL patients, we prospectively evaluated the therapeutic value of early human leukocyte antigen (HLA)–matched sibling allogeneic bone marrow transplantation (alloBMT) to reduce the transplantation-related mortality. For patients without a sibling donor or older than 50 years, autologous stem cell transplantation (ASCT) was delayed until after sequential conventional therapy and performed after an intensified conditioning regimen in an attempt to further reduce the number of residual leukemia cells.
In addition, the impact of an interferon-α maintenance regimen on relapse was evaluated by randomization after ASCT. At that time, several arguments suggested that maintenance interferon-α might be of benefit in ALL even in patients without Philadelphia chromosome. Indeed after alloBMT, the relapse rate was lowered from 74% to 36% (P = .04) when partially purified human leukocyte interferon was given every 3 days to reduce cytomegalovirus infection or graft-versus-host disease.9 Anecdotal reports suggested some efficacy of high-dose interferon-α against chemotherapy-resistant ALL,10-12 and the interferon gene was reported to be deleted in a third of adult ALL patients,13,14 supporting the potential efficacy of interferon-α.
Patients and methods
Patients
Patients aged from 15 to 59 years were eligible for the GOELAL02 trial if they had untreated non-Burkitt type ALL and at least one of the following criteria: age older than 35; B-ALL; WBC count exceeding 30 × 109/L; poor-risk cytogenetic abnormalities including t(9;22) or BCR-ABL transcript, t(4;11) or t(1;19); or failure to achieve CR after the first induction course. Patients without any of these criteria received a less intensive consolidation regimen than the GOELAL02 and will be reported elsewhere. All patients had adequate cardiac, renal, and hepatic functions. Informed consent was signed by every patient or their parents if they were younger than 18 years. ALL was diagnosed according to the French-American-British (FAB) criteria for cytologic and cytochemical features of blood and bone marrow (BM) smears. Flow cytometry immunophenotyping was obtained for 167 (84%) patients, who were classified after central review according to the European Group for the Immunological Characterization of Leukemias (EGIL) criteria (n = 111; 67%).15 Cytogenetic results were also centrally reviewed by 2 independent investigators. Karyotypes could be interpreted for 174 (90%) patients, 8 of whom had a normal result with only 10 to 19 assessable mitoses. Reverse transcriptase-polymerase chain reaction (RT-PCR) specific for BCR-ABL transcripts was applied to 134 (67%) samples at diagnosis following a common protocol. Molecular analysis of E2A-PBX1 and AF4-MLL was not required.
Treatment
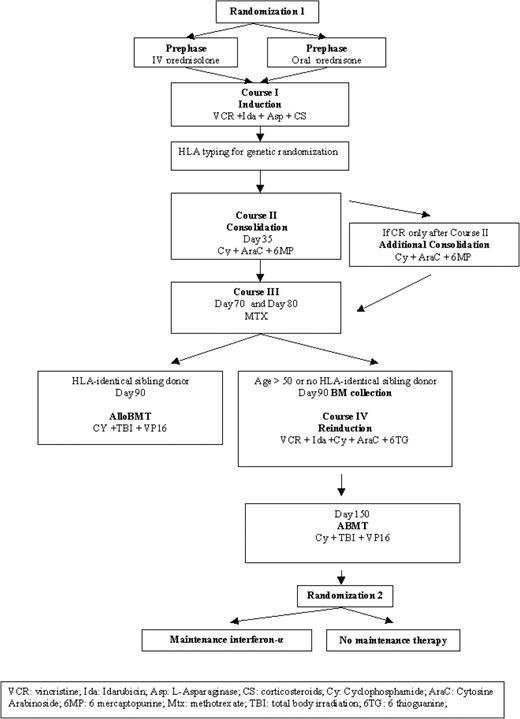
Drugs and doses used for induction, consolidation, reinduction, and intensification phases of treatment are detailed in Figure 1 and Table 1. At diagnosis, a first randomization assigned intravenous injection or oral steroids (40 mg/m2/d) for the induction and served mainly to register every patient. The induction (course I), derived from the Berlin-Frankfurt-Muenster (BFM) regimen,16 consisted of weekly intravenous administration of vincristine and idarubicin for 4 weeks and 6 doses of l-asparaginase in addition to 3 weeks of daily steroids. The fourth vincristine injection could be switched to teniposide (100 mg/m2) in the case of poor neurologic tolerance. Glycemia, blood and urine amylase levels, hepatic function, and hemostasis, including fibrinogen and antithrombin III (ATIII), were evaluated before each l-asparaginase injection. It was recommended that fresh frozen plasma or fibrinogen and ATIII concentrates be transfused to maintain their levels at greater than 1000 mg/L (100 mg/dL) and 60%, respectively. If transfusion was not performed, l-asparaginase induction was delayed for 48 hours. Erwiniase was used in case of anaphylactic reaction. Bone marrow aspiration was re-evaluated on day 35.
GOELAL02 high-risk ALL treatment plan. The trial includes 2 randomizations.
Chemotherapy schedule for adult ALL according to the GOELAL02 trial
. | Route . | Dose . | Days . |
|---|---|---|---|
| Pretreatment, prednisolone | IV | 40 mg/m2 | -3 to -1 |
| Course I, induction | |||
| Prednisone/prednisolone | PO/IV | 40 mg/m2 | 1-21 |
| Vincristine | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Idarubicin | IV | 5 mg/m2 | 1, 8, 15, 22 |
| L-asparaginase | IV | 7500 IU/m2 | 10, 13, 16, 19, 22, 25 |
| Methotrexate | IT | 10 mg/m2 | 3 |
| Methylprednisolone | IT | 40 mg | 3 |
| Course II, consolidation (day 35 after induction)* | |||
| Cyclophosphamide | IV | 650 mg/m2 | 1, 15, 29 |
| Cytarabine | IV | 75 mg/m2 | 3-6, 10-13, 17-20, 24-27 |
| 6-Mercaptopurine | PO | 60 mg/m2 | 1-29 |
| Methotrexate | IT | 10 mg/m2 | 3, 10, 17, 24, 31 |
| Methylprednisolone | IT | 40 mg | 3, 10, 17, 24, 31 |
| Course III (day 70 from induction)† | |||
| Methotrexate | IV 4 hours | 3000 mg/m2 | 1, 10 |
| Folinic acid | IV | 25 mg/m2/6 h | 2-4, 11-13 |
| Course IV, reinduction (day 90 after induction) | |||
| Dexamethasone | PO | 10 mg/m2 | 1-28 |
| Vincristine | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Idarubicin | IV | 5 mg/m2 | 1, 8, 15, 22 |
| Cyclophosphamide | IV | 650 mg/m2 | 29 |
| Cytarabine | IV | 75 mg/m2 | 31-34, 38-41 |
| 6-Thioguanine | PO | 60 mg/m2 | 29-42 |
| Day 150, intensification conditioning before ABMT | |||
| Etoposide | IV 8 hours | 40 mg/kg | -6 |
| Cyclophosphamide | IV | 120 mg/kg | -5, -4 |
| Total body irradiation | Fractionated | 12 Gy | -3, -2, -1 |
| Randomized maintenance therapy for patients after ABMT | |||
| Interferon-α | SC | 3-4.5 MIU | 3/wk for 2 y |
. | Route . | Dose . | Days . |
|---|---|---|---|
| Pretreatment, prednisolone | IV | 40 mg/m2 | -3 to -1 |
| Course I, induction | |||
| Prednisone/prednisolone | PO/IV | 40 mg/m2 | 1-21 |
| Vincristine | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Idarubicin | IV | 5 mg/m2 | 1, 8, 15, 22 |
| L-asparaginase | IV | 7500 IU/m2 | 10, 13, 16, 19, 22, 25 |
| Methotrexate | IT | 10 mg/m2 | 3 |
| Methylprednisolone | IT | 40 mg | 3 |
| Course II, consolidation (day 35 after induction)* | |||
| Cyclophosphamide | IV | 650 mg/m2 | 1, 15, 29 |
| Cytarabine | IV | 75 mg/m2 | 3-6, 10-13, 17-20, 24-27 |
| 6-Mercaptopurine | PO | 60 mg/m2 | 1-29 |
| Methotrexate | IT | 10 mg/m2 | 3, 10, 17, 24, 31 |
| Methylprednisolone | IT | 40 mg | 3, 10, 17, 24, 31 |
| Course III (day 70 from induction)† | |||
| Methotrexate | IV 4 hours | 3000 mg/m2 | 1, 10 |
| Folinic acid | IV | 25 mg/m2/6 h | 2-4, 11-13 |
| Course IV, reinduction (day 90 after induction) | |||
| Dexamethasone | PO | 10 mg/m2 | 1-28 |
| Vincristine | IV | 1.5 mg/m2 | 1, 8, 15, 22 |
| Idarubicin | IV | 5 mg/m2 | 1, 8, 15, 22 |
| Cyclophosphamide | IV | 650 mg/m2 | 29 |
| Cytarabine | IV | 75 mg/m2 | 31-34, 38-41 |
| 6-Thioguanine | PO | 60 mg/m2 | 29-42 |
| Day 150, intensification conditioning before ABMT | |||
| Etoposide | IV 8 hours | 40 mg/kg | -6 |
| Cyclophosphamide | IV | 120 mg/kg | -5, -4 |
| Total body irradiation | Fractionated | 12 Gy | -3, -2, -1 |
| Randomized maintenance therapy for patients after ABMT | |||
| Interferon-α | SC | 3-4.5 MIU | 3/wk for 2 y |
IV indicates intravenous injection; PO, per os; IT, intrathecal; MIU, million international units.
An additional course II was performed for patients in CR after this cycle.
On day 90, alloBMT was performed if there was an HLA-matched sibling donor. Unpurged BM collection was collected from patients older than 50 y or without an HLA-matched sibling donor.
Consolidation (course II), comprising cyclophosphamide, cytarabine, oral 6-mercaptopurine (6MP) and 6 intrathecal (IT) methotrexatemethylprednisolone injections, was initiated as soon as neutrophils reached 1 × 109/L. Folinic-acid (25 mg) rescue was administered orally 6 hours after each IT injection. Four 4-day cytarabine injections were given when neutrophils reached 0.2 × 109/L and platelets 30 × 109/L. Administration of 6MP was interrupted when neutrophils were less than 0.5 × 109/L for a total of 28 doses, and the dose was reduced by 50% when liver enzymes were twice their baseline levels. Patients achieving CR only after this cycle received an additional cyclophosphamide-mercaptopurine-cytarabine course.
Central nervous system (CNS) prophylaxis included 6 IT injections of methotrexate (10 mg/m2) and methylprednisolone (40 mg), and 2 intravenous high doses of methotrexate (course III), without cranial irradiation. Additional folinic acid was infused when methotrexate levels exceeded 10–5, 10–6, and 10–7 μM, respectively, at 24, 48, and 72 hours after completion of methotrexate infusion. Patients with CNS disease at the time of diagnosis received IT doses of 3 drugs (10 mg/m2 methotrexate, 20 mg/m2 cytarabine, and 40 mg methylprednisolone) twice weekly until clearance of CNS disease, followed by 6 additional IT doses of the 3 drugs.
All patients no older than 50 years achieving CR (after courses I or II) were HLA typed. Patients with a sibling donor matched by 6 of 6 HLA antigens underwent an alloBMT procedure performed after 1 consolidation course and 2 high doses of methotrexate.
In patients older than 50 years, or without an HLA-matched sibling, unpurged stem cells were collected after high-dose methotrexate. Stem cells were collected mainly from bone marrow, but a few centers decided to use peripheral blood stem cells. Patients received an ASC transplant around day 150 after 1 reinduction (course IV). AlloBMT and ASCT conditioning regimen included etoposide (40 mg/kg), cyclophosphamide (120 mg/kg), and fractionated TBI (12 Gy in 6 fractions). Because of its instability, etoposide was administered in 4 consecutive infusions of 10 mg/kg lasting 2 hours each.
The use of hematopoietic growth factors and prophylactic antibiotics and the management of febrile episodes and transfusions were left to institutional guidelines.
After hematopoietic recovery from ASCT (neutrophils exceeding 2 × 109/L, platelets exceeding 100 × 109/L), a second randomization assigned patients to receive or not to receive a maintenance regimen of 3 MIU interferon-α (Roche, Paris, France) administered subcutaneously 3 times a week for 2 years. Interferon-α was interrupted when neutrophils were lower than 0.5 × 109/L or platelets were lower than 30 × 109/L.
Response criteria
Patients were considered to be in CR when BM cytology was normal (fewer than 5% blasts and greater than 25% cellularity), neutrophil counts were higher than 1.5 × 109/L, platelet counts were higher than 100 × 109/L, and all extramedullary disease had resolved. To remain enrolled in the study, all patients had to achieve CR by the end of the second chemotherapy course.
Statistical methods
Allocation to study group. In this prospective, randomized trial, patients were randomized at diagnosis by the coordinating center (Angers, France). The second randomization was performed after hematologic recovery following ASCT. Treatment allocation was issued by phone after confirmation of patient eligibility and confirmed by fax or mail.
Sample size. The major objective of this second randomization was to compare relapse rates in the 2 groups. Because 60% of adults in first CR experience relapse after ASCT,17 interferon-α maintenance was given in an attempt to lower this rate to 20%, a rate similar to that observed after alloBMT18 and in agreement with the observed 40% reduction of the relapse rate with human leukocyte interferon after alloBMT.9 To ensure a significance level of 5% and a power of 80% to these assumptions, 28 patients were required in each treatment arm.
Analysis. All primary analyses were conducted with the use of the intention-to-treat rule; that is, the outcome for each patient was counted in the trial group to which the patient was originally assigned (donor versus no donor or age older than 50), regardless of whether the patient later deviated from the protocol.
We compared the prevalence of risk factors and the incidence of end points in groups using a χ2 or a Fisher exact test when necessary. Survival was defined as the time from initial randomization to death or date of last follow-up. DFS was defined as the time from CR to all events including relapse, death in CR, or date of last follow-up. Cumulative incidence of relapse was calculated from the time of CR to relapse or date of last follow-up. Maintenance therapy–related survival rates were calculated from the time of second randomization.
Survival curves were estimated by the Kaplan-Meier method, and differences were analyzed with the log-rank test. All tests were 2-sided, with P < .05 considered statistically significant. Median follow-up time was estimated by reversing the codes for the censoring indicator in a Kaplan-Meier analysis. The last date of follow-up was May 1, 2003.
Logistic regression analysis and Cox proportional hazards regression models were applied to select and retain, respectively, the risk factors significantly affecting the CR rate and time to events. SPSS (Chicago, IL) software version 10.1.3 for Windows was used for these analyses.
Results
Pretreatment characteristics
Between September 1994 and December 1998, 198 consecutive patients from 21 institutions with non-Burkitt ALL were randomized in the GOELAL02 trial. Seventeen additional patients younger than 35, with T-ALL, WBC count below 30 × 109/L, no poor-risk cytogenetic factors, and in CR on day 35 were considered to have good-risk ALL and therefore were not included. The main initial characteristics of the 198 patients are listed in Table 2. The median age was 33 years (range, 15-59 years); 60% of the patients were male; 42% of patients had initial WBC counts exceeding 30 × 109/L; 14% had WBC count exceeding 100 × 109/L. Fifty-six patients (28%) had fever or infection before chemotherapy; 5 of them had proven infections. Three patients had CNS involvement at diagnosis. Mediastinal involvement was present in 11% of the patients, while clinical hepatomegaly and splenomegaly were detected in 23% and 41%, respectively.
CR, OS, and DFS rates as a function of clinical and biologic characteristics
. | No. (%) . | CRI, no. (%) [P] . | Overall CR, no. (%) [P] . | Median OS, mo. [P] . | Median DFS, mo. [P] . |
|---|---|---|---|---|---|
| Patients | 198 | 159 (80) | 170 (86) | 29 | 28 |
| Age | |||||
| Younger than 35 y | 110 (56) | 97 (88)§ | 104 (94)[<10-5] | 50.5 [.0019] | 37.2§ |
| 35 y or older | 88 (44) | 62 (70) | 66 (75) | 15.5 | 14.8 |
| WBC count | |||||
| Less than 30 × 109/L | 115 (58.1) | 92 (80)§ | 101 (88)§ | 51.6§ | 37.4§ |
| At least 30 × 109/L | 83 (41.9) | 67 (81) | 69 (89) | 19.1 | 16.8 |
| Cytogenetic results, no. = 174 | |||||
| Poor risk | 51 (29) | 32 (63) [.0002] | 36 (71) [< 10-4] | 14.9 [< 10-5] | 10.1 [.0021] |
| Others | 123 (71) | 107 (87) | 113 (92) | 71.8 | 70.0 |
| Ph∥ | 37∥ (21) | 20 (54) | 24 (67) | 14.8 | 8.8 |
| t(4;11) | 8 (5) | 6 (75) | 6 (75) | 5.6 | 5.8 |
| t(1;19) | 6 (3) | 6 (100) | 6 (100) | 30.2 | 10.1 |
| Complex | 22 (13) | 18 (82) | 19 (86) | 19 | 12.6 |
| Normal | 44 (25) | 40 (91) | 41 (93) | 71.8 | 70.0 |
| Others | 57 (33) | 49 (86) | 53 (93) | 72.7 | 70.8 |
| Immunophenotype | |||||
| B-ALL, total | 129 (77) | 104 (81)§ | 112 (87)§ | 27.7§ | 17.1§ |
| T-ALL, total | 35 (21) | 29 (83) | 31 (89) | 60.9 | 57.6 |
| B-ALL, subset* | 48 | NA | NA | 72.7 | 70.8 |
| T-ALL, subset† | 10 | NA | NA | 71.8 | 70 |
| No. induction cycles needed for CR | |||||
| 1 | 159 | NA | NA | 71.8 [.03] | 37.4§ |
| 2 | 11 | NA | NA | 23.3 | 13.1 |
| No. poor-risk factors at diagnosis‡ | |||||
| 0 | 1 | 1 (0) | 1 (100) | 25.3 [< 10-5] | 20.9 [< 10-5] |
| 1 | 81 (41) | 73 (90) | 77 (95) | 71.8 | 57.6 |
| 2 | 64 (32) | 53 (83) | 56 (86) | 38.4 | 70.8 |
| 3 | 36 (18) | 22 (61) | 25 (69) | 14.8 | 10.9 |
| 4 | 16 (8) | 11 (69) | 11 (69) | 9.9 | 6.4 |
| No. poor-risk factors after induction | |||||
| 1 | 74 (37) | 73 (99) | 74 (100) | 71.8 [< 10-5] | 70 [< 10-5] |
| 2 | 64 (32) | 53 (83) | 57 (89) | 72.7 | 70.8 |
| 3 | 30 (15) | 21 (70) | 24 (80) | 15.5 | 12.8 |
| 4 | 25 (13) | 11 (44) | 14 (56) | 9.7 | 5.6 |
| 5 | 5 (2) | 0 | 0 | 10.6 | NA |
. | No. (%) . | CRI, no. (%) [P] . | Overall CR, no. (%) [P] . | Median OS, mo. [P] . | Median DFS, mo. [P] . |
|---|---|---|---|---|---|
| Patients | 198 | 159 (80) | 170 (86) | 29 | 28 |
| Age | |||||
| Younger than 35 y | 110 (56) | 97 (88)§ | 104 (94)[<10-5] | 50.5 [.0019] | 37.2§ |
| 35 y or older | 88 (44) | 62 (70) | 66 (75) | 15.5 | 14.8 |
| WBC count | |||||
| Less than 30 × 109/L | 115 (58.1) | 92 (80)§ | 101 (88)§ | 51.6§ | 37.4§ |
| At least 30 × 109/L | 83 (41.9) | 67 (81) | 69 (89) | 19.1 | 16.8 |
| Cytogenetic results, no. = 174 | |||||
| Poor risk | 51 (29) | 32 (63) [.0002] | 36 (71) [< 10-4] | 14.9 [< 10-5] | 10.1 [.0021] |
| Others | 123 (71) | 107 (87) | 113 (92) | 71.8 | 70.0 |
| Ph∥ | 37∥ (21) | 20 (54) | 24 (67) | 14.8 | 8.8 |
| t(4;11) | 8 (5) | 6 (75) | 6 (75) | 5.6 | 5.8 |
| t(1;19) | 6 (3) | 6 (100) | 6 (100) | 30.2 | 10.1 |
| Complex | 22 (13) | 18 (82) | 19 (86) | 19 | 12.6 |
| Normal | 44 (25) | 40 (91) | 41 (93) | 71.8 | 70.0 |
| Others | 57 (33) | 49 (86) | 53 (93) | 72.7 | 70.8 |
| Immunophenotype | |||||
| B-ALL, total | 129 (77) | 104 (81)§ | 112 (87)§ | 27.7§ | 17.1§ |
| T-ALL, total | 35 (21) | 29 (83) | 31 (89) | 60.9 | 57.6 |
| B-ALL, subset* | 48 | NA | NA | 72.7 | 70.8 |
| T-ALL, subset† | 10 | NA | NA | 71.8 | 70 |
| No. induction cycles needed for CR | |||||
| 1 | 159 | NA | NA | 71.8 [.03] | 37.4§ |
| 2 | 11 | NA | NA | 23.3 | 13.1 |
| No. poor-risk factors at diagnosis‡ | |||||
| 0 | 1 | 1 (0) | 1 (100) | 25.3 [< 10-5] | 20.9 [< 10-5] |
| 1 | 81 (41) | 73 (90) | 77 (95) | 71.8 | 57.6 |
| 2 | 64 (32) | 53 (83) | 56 (86) | 38.4 | 70.8 |
| 3 | 36 (18) | 22 (61) | 25 (69) | 14.8 | 10.9 |
| 4 | 16 (8) | 11 (69) | 11 (69) | 9.9 | 6.4 |
| No. poor-risk factors after induction | |||||
| 1 | 74 (37) | 73 (99) | 74 (100) | 71.8 [< 10-5] | 70 [< 10-5] |
| 2 | 64 (32) | 53 (83) | 57 (89) | 72.7 | 70.8 |
| 3 | 30 (15) | 21 (70) | 24 (80) | 15.5 | 12.8 |
| 4 | 25 (13) | 11 (44) | 14 (56) | 9.7 | 5.6 |
| 5 | 5 (2) | 0 | 0 | 10.6 | NA |
CRI indicates complete remission after 1 induction course; CR, complete remission; DFS, disease-free survival; NA, not applicable.
WBC count below 30 × 109/L, no poor-risk cytogenetic factors in CR after the first induction course.
Patient older than 35 years without poor-risk cytogenetic factors in CR after the first induction course.
For this analysis, 1 patient with Philadelphia chromosome, no mediastinal involvement, but no immunophenotype at diagnosis was considered non-T lineage. In addition, patients without cytogenetic results were considered not to have t(9;22), t(1;19), or t(4;11).
P is nonsignificant.
Two patients with BCR-ABL transcripts were included despite failure for one and normal karyotype for the other.
Immunophenotyping was reviewed in 167 cases (M.-C. Béné). The 129 B-lineage ALL cases represented 77% of the cases while the 35 T-lineage ALL cases represented 21% of cases. In 28% of the cases, we noted aberrant expression of myeloid antigens but without scores high enough to establish biphenotypic acute leukemia according to the EGIL criteria. Among 174 karyotypes, 45 had a normal number of chromosomes, while hypodiploidy (fewer than 46 chromosomes), low hyperdiploidy (47-51 chromosomes), high hyperdiploidy (52-60 chromosomes), neartriploidy (61-69 chromosomes), tetraploidy, and pseudodiploidy were observed in 28 (16%), 20 (12%), 8 (5%), 2 (1%), 1 (.5%), and 70 (40%) patients, respectively. Translocations t(9;22), t(1;19), and t(4;11) and complex abnormalities (3 or more structural abnormalities including monosomy 7 and trisomy 8) were observed in 35, 6, 8, and 22 patients, respectively. Molecular BCR gene rearrangement was detected in 30 of 134 (22%) of the evaluated samples (17 were m-BCR; 12, M-BCR; 1, both). Two patients with a BCR-ABL gene rearrangement without cytogenetic abnormalities were evaluated in the Ph+ group for statistical analysis. Overall, 41%, 32%, 18%, and 8% of the patients had 1, 2, 3, or 4 poor-risk factors, respectively, at diagnosis.
Treatment results
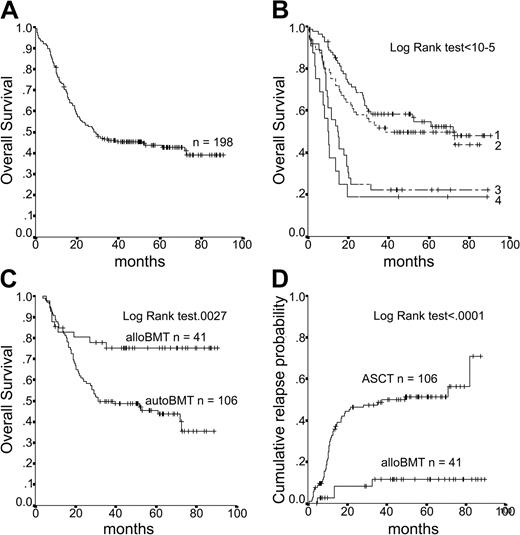
Treatments received. Median follow-up was 5.1 years. Median OS for the entire population was 29 months (Figure 2A). At 6 years, 41% of the patients were alive in CR.
Data on OS. (A) Overall survival of the entire population (n = 198). (B) OS according to the number of prognostic factors at diagnosis. (C) OS according to alloBMT and ASCT in an intent-to-treat analysis. (D) Cumulative incidence of relapse according to alloBMT and ASCT in an intent-to-treat analysis.
Data on OS. (A) Overall survival of the entire population (n = 198). (B) OS according to the number of prognostic factors at diagnosis. (C) OS according to alloBMT and ASCT in an intent-to-treat analysis. (D) Cumulative incidence of relapse according to alloBMT and ASCT in an intent-to-treat analysis.
Induction and consolidation. Of 198 eligible patients, 170 (86%) achieved CR. As expected, no significant differences were observed in pretreatment characteristics, CR rate, or outcome according to the first randomization (intravenous injection or oral steroids). There were 159 patients (80%) who achieved CR after the first induction course; 3 patients died of infection before day-35 BM evaluation, and 36 (18%) patients failed to achieve CR after the first induction course. Of the 23 patients who received an additional induction course, 11 achieved CR. Thirteen patients (7%) in CR did not follow the protocol because of early toxic death (n = 6) or toxicity (n = 7). At the end of induction, 156 (79%) patients were alive, in CR, and able to receive the planned treatment. The predominant cause of induction- and consolidation-associated mortality was infection: 6 septic shock syndromes, 3 pulmonary aspergillosis, 1 liver failure, and 1 cerebral hemorrhage. Most bacterial infections occurred during the induction and consolidation phases in 40 (20%) and 31 (18%) patients, respectively, mainly with staphylococci (42%). In contrast, the rate of bacterial infectious complications was low during courses III and IV (5 of 148 [3%] and 10 of 128 [8%], respectively). Before the intensification phase, 11 patients developed invasive aspergillosis (7 during induction, 3 during consolidation, and 1 under high-dose methotrexate); 3 had pneumocystosis (1 during induction, 1 during consolidation, and 1 during phase 4); and 1 had herpes zoster infection (phase 4).
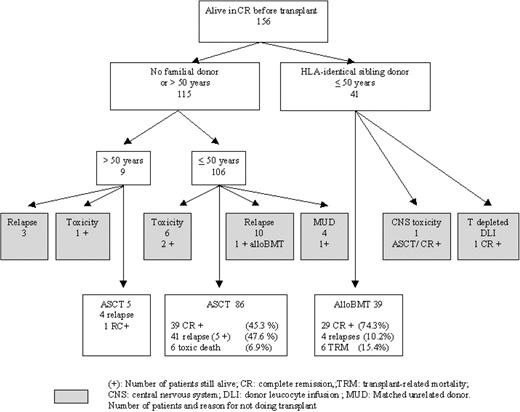
Allogeneic BMT. There were 41 patients who had an HLA-identical sibling donor, but only 39 received an alloBMT according to the protocol (Figure 3). The 2 other patients received T-cell–depleted alloBMT and donor lymphocyte infusion (n = 1) or ASCT because of CNS toxicity after high-dose methotrexate (n = 1). Matched, unrelated alloBMT was performed in 4 patients; in the intent-to-treat analysis, we analyzed these patients in the ASCT group. The median time from CR to AlloBMT was 3.0 months (range, 1-8.5 months). Transplantation-related mortality at 6 months was 15% (1 aspergillosis; 1 diffuse cytomegalovirus disease; 1 septic shock syndrome; 1 cardiac dysfunction, possibly of viral origin; 1 acute graft-versus-host disease; and 1 cerebral hemorrhage), with 74% of patients alive in CR. Four patients experienced relapse at 2.7, 10.9, 10, and 31.4 months after alloBMT administered according to the protocol. At the time of the analysis performed on an intent-to-treat basis on 41 patients, the OS and DFS rates observed at 6 years were both 75% (Table 3).
Characteristics of patients according to their treatment (no. patients alive in CR = 156)
. | . | Patients alive in CR . | . | . | ||
|---|---|---|---|---|---|---|
| Parameter . | Overall . | ABMT . | ABMT younger than 50 y . | AlloBMT . | ||
| Age, y | 198 | 115 | 106 | 41 | ||
| Median age, y (range) | 33 (15-59) | 27 (15-57) | 26 (15-50) | 34 (15-52) | ||
| Younger than 35 y, no. (%), P = .02 | 110 (55.6) | 78 (67.8) | 78 (73.6) | 23 (56.1) | ||
| At least 35 y, no. (%) | 88 (44.4) | 37 (32.2) | 28 (26.4) | 18 (43.9) | ||
| WBC count, no. (%) | ||||||
| Less than 30 × 109/L* | 115 (58) | 70 (61) | 66 (62) | 25 (61) | ||
| More than 30 × 109/L | 83 (42) | 45 (39) | 40 (38) | 16 (39) | ||
| Cytogenetic factors, no. (%) | 104 | 96 | 33 | — | ||
| Poor risk, P = .01 | 51 (29) | 19 (18) | 15 (16) | 11 (33) | ||
| Ph1, P = .037 | 37 (21) | 14 (13) | 11 (11) | 8 (24) | ||
| t(4;11) | 8 (5) | 3 (3) | 2 (2) | 1 (3) | ||
| t(1;19) | 6 (3) | 2 (2) | 2 (2) | 2 (6) | ||
| Complex | 22 (13) | 16 (15) | 15 (16) | 2 (6) | ||
| Normal | 44 (25) | 33 (32) | 33 (34) | 7 (21) | ||
| Others | 51 (29) | 36 (35) | 33 (34) | 13 (39) | ||
| Failure | 24 (14) | 11 (11) | 10 (10) | 8 (20) | ||
| No. poor-risk factors, no. (%) | ||||||
| 1* | 82 | 57 (50) | 56 (53) | 18 (44) | ||
| 2 | 64 | 41 (36) | 38 (36) | 12 (29) | ||
| 3 | 36 | 13 (11) | 11 (10) | 9 (22) | ||
| 4 | 16 | 4 (4) | 1 (1) | 2 (5) | ||
| No. cycles needed for CR, no. (%) | ||||||
| 1* | 159 | 109 (95) | 100 (95) | 37 (90) | ||
| 2 | 11 | 6 (5) | 6 (6) | 4 (10) | ||
| B-ALL*† | 48 | 38 (33) | 35 (33) | 10 (24) | ||
| T-ALL*‡ | 10 | 7 (6) | 6 (6) | 3 (7.3) | ||
| OS | ||||||
| Median months, no., P = .0027 | 29 | 31.0 | 31.2 | NR | ||
| At 5 y, % (SE) | 44 (4) | 43 (5) | 46 (5) | 75 (7) | ||
| At 6 y, % (SE) | 41 (4) | 39 (6) | 40 (7) | 75 (7) | ||
| DFS | ||||||
| Median months, no., P = .0004 | 15.8 | 18.3 | 20.9 | NR | ||
| At 5 y, % (SE) | 45 (4) | 38 (5) | 41 (5) | 75 (7) | ||
| At 6 y, % (SE) | 41 (5) | 31 (9) | 33 (6) | 75 (7) | ||
. | . | Patients alive in CR . | . | . | ||
|---|---|---|---|---|---|---|
| Parameter . | Overall . | ABMT . | ABMT younger than 50 y . | AlloBMT . | ||
| Age, y | 198 | 115 | 106 | 41 | ||
| Median age, y (range) | 33 (15-59) | 27 (15-57) | 26 (15-50) | 34 (15-52) | ||
| Younger than 35 y, no. (%), P = .02 | 110 (55.6) | 78 (67.8) | 78 (73.6) | 23 (56.1) | ||
| At least 35 y, no. (%) | 88 (44.4) | 37 (32.2) | 28 (26.4) | 18 (43.9) | ||
| WBC count, no. (%) | ||||||
| Less than 30 × 109/L* | 115 (58) | 70 (61) | 66 (62) | 25 (61) | ||
| More than 30 × 109/L | 83 (42) | 45 (39) | 40 (38) | 16 (39) | ||
| Cytogenetic factors, no. (%) | 104 | 96 | 33 | — | ||
| Poor risk, P = .01 | 51 (29) | 19 (18) | 15 (16) | 11 (33) | ||
| Ph1, P = .037 | 37 (21) | 14 (13) | 11 (11) | 8 (24) | ||
| t(4;11) | 8 (5) | 3 (3) | 2 (2) | 1 (3) | ||
| t(1;19) | 6 (3) | 2 (2) | 2 (2) | 2 (6) | ||
| Complex | 22 (13) | 16 (15) | 15 (16) | 2 (6) | ||
| Normal | 44 (25) | 33 (32) | 33 (34) | 7 (21) | ||
| Others | 51 (29) | 36 (35) | 33 (34) | 13 (39) | ||
| Failure | 24 (14) | 11 (11) | 10 (10) | 8 (20) | ||
| No. poor-risk factors, no. (%) | ||||||
| 1* | 82 | 57 (50) | 56 (53) | 18 (44) | ||
| 2 | 64 | 41 (36) | 38 (36) | 12 (29) | ||
| 3 | 36 | 13 (11) | 11 (10) | 9 (22) | ||
| 4 | 16 | 4 (4) | 1 (1) | 2 (5) | ||
| No. cycles needed for CR, no. (%) | ||||||
| 1* | 159 | 109 (95) | 100 (95) | 37 (90) | ||
| 2 | 11 | 6 (5) | 6 (6) | 4 (10) | ||
| B-ALL*† | 48 | 38 (33) | 35 (33) | 10 (24) | ||
| T-ALL*‡ | 10 | 7 (6) | 6 (6) | 3 (7.3) | ||
| OS | ||||||
| Median months, no., P = .0027 | 29 | 31.0 | 31.2 | NR | ||
| At 5 y, % (SE) | 44 (4) | 43 (5) | 46 (5) | 75 (7) | ||
| At 6 y, % (SE) | 41 (4) | 39 (6) | 40 (7) | 75 (7) | ||
| DFS | ||||||
| Median months, no., P = .0004 | 15.8 | 18.3 | 20.9 | NR | ||
| At 5 y, % (SE) | 45 (4) | 38 (5) | 41 (5) | 75 (7) | ||
| At 6 y, % (SE) | 41 (5) | 31 (9) | 33 (6) | 75 (7) | ||
P values are between patients 50 years or younger who received alloBMT or ASCT.
SE indicates standard error; NR, not reached. See Tables 1 and 2 for other abbreviations.
P is nonsignificant.
Patients with WBC less than 30 × 109/L, no poor-risk cytogenetic factors, and in CR after the first induction course.
Patients older than 35 years, without poor-risk cytogenetic factors, and in CR after the first induction course.
Autologous BMT. Age older than 50 years or lack of HLA-identical sibling donor excluded 115 patients from alloBMT; 91 of them underwent ASCT (bone marrow, 72; peripheral stem cell, 19). Twenty-four patients did not receive HDT according to the protocol because of early relapse (n = 13), toxicity or poor general condition (n = 7), or unrelated alloBMT (n = 4). Five of these patients were alive in CR after unrelated alloBMT (n = 1), alloBMT in second CR (n = 1), or maintenance chemotherapy (n = 3) because of cardiac insufficiency, pulmonary aspergillosis, or poor hematopoietic recovery. The flow of patients according to their age is described in Figure 3. Thirteen patients experienced relapse before ASCT at a median of 3.1 months after CR (range, 5-7.5 months). Hyperleukocytosis exceeding 30 × 109/L was more frequent in these 13 patients than in patients who actually underwent ASCT (8 of 13 [61%] versus 31 of 86 [36%]; P = .03), while the prevalence of other poor-risk factors was similar in these 2 populations as well as in the patients who had received allografts (Table 3). ASCT was performed after a median of 5.7 months after CR (range, 2-10.8 months). The OS and DFS are 39% and 31%, respectively, at 6 years when the data from 115 patients were analyzed on an intent-to-treat basis.
Three (3%) patients died of early infectious complications (2 septic shock syndromes, 1 pulmonary aspergillosis). Late transplantation-related toxicity included 2 secondary myeloid malignancies (1 myelodysplasia and 1 acute myeloid leukemia–6 [AML6], at 40 and 58 months, respectively) and 1 fatal unexplained cachexia and peripheral neuropathy 5 months after ASCT. Relapses occurred in 49% of these 115 patients at a median of 5.7 months after ASCT (range, 1.9-75 months); 5 of the patients were still alive in CR after unrelated alloBMT (14 and 41 months CR+) or alternative chemotherapy (6, 32, and 48 months CR+). The relapse rate was similar after bone marrow or peripheral stem cell transplantation (8 of 19 versus 36 of 72; P = .53).
Maintenance interferon-α after ASCT. Between 3 and 6 months after ASCT and hematopoietic recovery, 49 patients were randomized to receive (23 patients) or not to receive (26 patients) interferon-α maintenance for 2 years. Forty-three patients (46%) could not be randomized for interferon-α maintenance because of patient refusal (n = 11), relapse (n = 15), persistent cytopenia (n = 13), or toxic death (n = 4). Age, WBC count, immunopheno-type, poor-risk cytogenetic factors, or response after the first induction course were similar for patients randomized for maintenance with interferon-α and for patients who could not be randomized. The tolerance of this low-dose interferon-α maintenance was acceptable, with 78% of patients receiving more than 95% of the planned dose up to 2 years or until relapse. Despite dose reduction, interferon-α for 4 patients had to be stopped for hepatic dysfunction (n = 1), poor general tolerance (n = 2), or thrombopenia (n = 1) after 14, 5, 3, and 4 months of treatment, respectively. Another patient received a reduced dose for 1 year (3 MIU twice a week) because of neutropenia and then received the full dose for the second year. Maintenance was briefly interrupted 3 times for herpes zoster infection (1 month), for hepatic dysfunction (.5 month), or to improve general tolerance at treatment initiation (1 month). Relapse occurred in 10 patients (43%) after a median of 3.9 months of interferon-α treatment (range, 0.2-8.4 months) and a median of 5 months (range, 1-10 months) after ASCT. None of the patients who experienced relapse had received reduced interferon-α doses for poor tolerance. Two late relapses occurred among the interferon-α–treated patients, 39 and 66 months after ASCT, and interferon-α had been stopped for these patients because of poor general tolerance and thrombopenia after 3 and 4 months of treatment, respectively. Eight of the 26 patients not receiving interferon-α experienced relapse, all but 1 during the first year after ASCT (median, 7 months; range, 5-30 months). Overall, no differences between the 2 groups were observed for OS or DFS, with respective 5-year OS and DFS rates of 56% and 52% in the interferon-α group and 77% and 69% in the control group (P = .15 and P = .2, respectively).
The 3 patients with CNS involvement at diagnosis achieved CR. One of them, who was Ph+, died in CR after alloBMT because of septic shock syndrome, while the 2 others are alive in CR after alloBMT (86 months) or ASCT without interferon-α maintenance (43 months). Despite our CNS prophylaxis regimen, 1 instance of CNS disease and hematologic relapse occurred 2 months after ASCT in a patient with T-lineage ALL.
Outcome according to cytogenetic findings
Outcome according to cytogenetic factors is described in Tables 2 and 3. Thirty-seven patients (median age, 39 years; range, 20-55 years) had either a Philadelphia chromosome (n = 35) or a BCR-ABL gene rearrangement (n = 2). Their median WBC count was 22 × 109/L (range, 2-188 × 109/L). Among the 7 ASCT patients in CR, 1 was still in molecular remission 82 months after intensification while the molecular biology of another became positive 1 year ago, after 3 years of molecular CR. Six patients (median age, 32 years; range, 17-39 years) had t(1;19). Their median WBC count was 49 × 109/L (range, 8-139 109/L). All had B-lineage ALL. The CR rate was 100%; 3 patients are alive (2 after alloBMT, 1 after maintenance chemotherapy) and 3 died (2 relapse after ASCT, 1 after the induction course while in CR). Eight patients (median age, 43 years; range, 19-57 years) had t(4;11). All of them had WBC count exceeding 30 × 109/L; 7 who were centrally reviewed had B-lineage ALL with aberrant myeloid antigen expression for 6 of these. CR was obtained for 6 of these patients (75%). The only patient who had an allograft survived while the others died of relapse (3 patients) or aspergillosis (2 patients).
Prognostic factors for remission and survival
CR rates after the first and second induction courses were significantly higher in younger patients (88% and 94%, respectively, for patients younger than 35 years old versus 71% and 75% for those at least 35 years old; P < 10–4 and P < 10–5) (Table 2). The remission-induction rate did not vary significantly according to size of the center enrolling the patients, sex, performance status (0-1 versus 2-4), presence or absence of fever or infection, splenomegaly, hepatomegaly, mediastinal mass, lymphadenopathy, initial platelet count (exceeding 50 × 109/L), WBC count (exceeding 30 × 109/L), or immunophenotypic lineage. Poor-risk cytogenetic abnormalities as defined previously were associated with a lower CR rate (63% after the first induction course), mostly because of the low CR rate of patients with Ph+ (54% in 20 of 37 patients versus 87% in 19 of 137; P < 10–5). According to multivariate analysis, age and poor-risk cytogenetics were the only prognostic factors for CR after the first induction course (P = .033 and P = .002, respectively).
Age older than 35, poor-risk cytogenetic factors, and CR obtained after 2 induction courses were also significantly associated with poorer survival (Table 2) while immunophenotype (B, T, presence of aberrant myeloid antigen expression) and size of center enrolling the patients had no impact on survival (data not shown). Two subgroups had a better outcome than the overall population (Table 2): patients with B-ALL and WBC count below 30 × 109/L, without poor-risk cytogenetic factors, and achieving CR after the first induction course exhibited a median OS of 72 months, while patients with T-ALL, older than 35, without poor-risk cytogenetic factors, and in CR after the first induction course had a median OS of 72 months. Survival was analyzed according to the presence of 1, 2, 3, 4, or 5 risks factors as defined previously (age at least 35; WBC count at least 30 × 109/L; non–T-ALL; t(9;22), t(1;19), or t(4;11); and failure to achieve CR at day 35) (Table 2). The effects of these poor-risk factors are clearly additive (P = 10–5) (Table 2; Figure 2B). Multivariate analysis demonstrated the independent influence of only 2 factors that significantly affected survival: age and poor-risk cytogenetics.
To properly compare alloBMT and ASCT, performed according to genetic randomization (donor versus no donor),19 the ASCT results were restricted to the 106 patients younger than 50 years, the age limit at that time for alloBMT. No significant difference in the distribution of adverse prognostic factors such as age of at least 35 years, WBC count exceeding 30 × 109/L, and failure to achieve CR after the first induction course was observed for patients who received alloBMT or ASCT (Table 3), except for a higher ratio of poor-risk cytogenetic findings in patients who received alloBMT compared with ASCT (poor risk karyotype in 33% versus 16%, respectively; P = .01). This analysis clearly demonstrated the clinical benefit of alloBMT over ASCT. Indeed, at 6 years, the OS and DFS after ASCT are 39% (SE, 6.7%) and 33% (SE, 6.2%), respectively, while after alloBMT, the OS and DFS are 75% (SE, 6.8%) and 72% (SE, 6.8%) (P = .0027 and P = .0004 for OS and DFS, respectively) (Table 3; Figure 2C). In addition, the cumulative incidence of relapse clearly showed a lower rate after ABMT (56% after ASCT versus 12% after alloBMT at 6 years; P = .0001) (Figure 2D). The prognostic value of poor-risk cytogenetic factors on OS remained after alloBMT (55% versus 87% for poor and good risk, respectively; P = .02) and after ASCT (20% versus 35% for poor and good risk, respectively; P = .03). while the prognostic value of age older than 35 disappeared after high-dose therapy.
Discussion
The GOELAL02 trial was designed to evaluate early allogeneic stem cell transplantation in adults with ALL and delayed HDT with ASCT followed by randomized maintenance interferon-α therapy. The median OS is 29 months with an OS and a DFS of 41% at 6 years. These results compared favorably with those of other studies,4,17,20-22 especially as patients with good-risk prognostic factors, defined by age younger than 35 with T-ALL, WBC count lower than 30 × 109/L, no poor-risk cytogenetic factors, and in CR after the first induction course, were not included in this study. However, our study does not focus only on poor-risk patients. Indeed, according to criteria admitted in the literature at the beginning of this protocol,6 patients with T-ALL and WBC count exceeding 30 × 109/L or patients with B-ALL, in CR after the first induction, without hyperleukocytosis or cytogenetic abnormality, but older than 35 years were included in this study, while their relatively good outcome was reported later.
The GOELAL02 induction regimen was derived from the BFM,6 yet used idarubicin (5 mg/m2 × 4) instead of daunorubicin (25 mg/m2 × 4) and lower doses of l-asparaginase (7500 IU/m2 × 6 instead of 5000 IU/m2 × 14) because of the poor tolerance of higher doses in a pilot study (N.I., unpublished results, 1992). After this first induction course, the high CR rate (80%) was similar to rates obtained in previous studies.5,6,17,20-24 CR following initial failure was achieved after the second induction course in 52% of patients, thereby increasing the overall CR rate to 86%. The mortality rates were 1.5% and 5% after the first and second induction courses, respectively, mainly because of infections. The reduced doses of l-asparaginase were not complicated by fatal hemorrhagic or thromboembolic events.25 No cardiac insufficiency occurred, confirming that idarubicin can safely replace daunorubicin.26,27 The achievement of CR was influenced by age and cytogenetic features.8,28 Translocation t(9;22) or BCR/ABL rearrangement decreased the CR rate (54%) as reported.6,17,21,29,30 The prognostic impact of T-lineage ALL31-33 could not be ascertained, as young patients who had T-ALL with WBC count less than 30 × 109/L were not included.
In this multicentric study, alloBMT was highly successful, with a 6-year DFS rate of 75% for the 41 patients younger than 50 years with HLA-identical sibling donors. This is higher than the 30% to 60% 5-year DFS reported in previous multicentric studies.3,34-39 Our results are not related to a biased selection of good-risk patients, as the distribution of poor-risk factors in the alloBMT group was similar to that of the overall population. We believe that this good outcome of alloBMT results both from a high antileukemic effect of the intensified conditioning regimen40 and from a low transplantation-related mortality (TRM) due to the early performance, around day 90, of alloBMT without cumulative toxicities before conditioning. Indeed, in a previous multicentric study,36 we observed a higher transplantation-related mortality rate after an AML-like induction regimen followed by a high-dose consolidation regimen (PAME [prednisone, intermediate-dose cytarabine, mitoxantrone, and etoposide]). Similarly, TRM was decreased when alloBMT was performed after 1 low-intensity consolidation course (cyclophosphamide, vincristine and prednisone [CVP])35,39,41 or after 2 months of consolidation including high-dose methotrexate.38 Nevertheless, alloBMT does not totally overcome the pejorative prognostic value of poor-risk cytogenetic abnormalities, particularly t(9;22), as reported in other studies.1,8,17,29,30,35,42-44
For patients without a matched sibling donor, randomized interferon-α maintenance therapy did not improve the OS or DFS rates. Despite the small number of patients randomized to interferon-α, it is unlikely that a significant benefit exists since the interferon arm actually fared worse. Interferon-α (3 MIU × 3/wk was introduced as early as possible after hematopoietic recovery. The general and hematologic tolerance was acceptable as the dose had to be lowered for only 3 (13%) and 2 (9%) patients, respectively. The main cause of death for these patients was relapse, not toxicity, suggesting that the absence of benefit is not secondary to a toxic effect and does not support the need for further evaluation. Similarly disappointing results were reported with interferon-α maintenance therapy given with or without concomitant chemotherapy in first CR of ALL.45,46 However, the latter study included patients older than 50 years, with a higher percentage of Ph+ patients.
Since maintenance interferon-α therapy had no effect on outcome, patients who received it and those who did not were analyzed together to evaluate the benefit of ASCT as compared with alloBMT. Our aim was to compare a strategy of early alloBMT with a strategy of delayed ASCT performed after an enhanced cytoreduction. Indeed, in our hands, ASCT performed early after CR was followed by a high rate of relapse.17,36 Despite a similar distribution of poor-risk factors, the relapse rate after delayed ASCT was not decreased, as the 6-year DFS rate for patients younger than 50 years was 33%, and the relapse rate was 48%. Our results after ASCT are in agreement with the 2 largest studies,17,38 which reported 3-year DFS of 28% and 39%, with relapse rates of 60% and 57%, respectively. In addition in the GOELAL02 study, as in others35,38,47 using the so-called genetic randomization,19 alloBMT is associated with a clear benefit over ASCT, indicating that it should be preferred for patients with ALL in first CR. The transplantation-related toxicity of the GOELAL02 treatment schedule after ASCT is low, unlike regimens that include intensive consolidation therapy before conditioning for ASCT.36 Nevertheless, late toxicity such as secondary myeloid disorders, which occurred in 2 patients (1 myelodysplasia, 1 AML6), have already been reported after chemotherapy for ALL,22 but with a lower frequency than after ASCT for follicular lymphoma.48 The ASCT procedure clearly needs to be improved. BM purging procedures have been disappointing.41,49 Since the GOELAL02 study was conducted between 1994 and 1998, bone marrow was mostly used as the source of hematopoietic stem cells, and the benefit of using peripheral blood stem cells in terms of rapid engraftment50 and lower leukemic contamination51 could not be properly evaluated.
In conclusion, the GOELAL02 trial demonstrated that alloBMT performed around day 90 for ALL in first CR provides a high DFS with low transplantation-related mortality, even in a multicentric study. In contrast, it is unlikely that ASCT-based strategies are optimal for adult ALL patients, who should be considered for alternative-donor allograft procedures.52 A large trial being conducted by the French Cooperative Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) group, which stratifies patients to receive different alloBMT strategies according to the main known prognostic factors, will hopefully clarify these points. The clear relapse-free survival as well as the overall survival advantage observed with alloBMT suggests that mechanisms other than chemotherapy dose-intensity may play important roles in the improved outcome of these patients. Better understanding of a putative graft-versus-leukemia effect, in addition to designing strategies aimed at amplifying it, and potentially applying it to maintenance may further improve the outcome of adult ALL. However maintenance interferon-α therapy was not able to induce such an antileukemic effect.
Appendix
The following French centers and investigators from the GOELAMS group participated in this study: Amiens (B. Desablens, R. Garidi, I. Vaida, F. Claisse, J. C. Capiod, C. Bastard), Angers (N. Ifrah, M. Hunault, M. Truchan-Graczyk, O. Blanchet, L. Baranger, M. Zandecki), Besançon (J. Y. Cahn, E. Deconninck, P. Darodes de Tailly, F. Mugneret, M. A. Collonge-Rame), Bobigny (P. Casassus), Brest (C. Berthou), Colmar (B. Audhuy, P. Raby, P. Moschovtchenko), Dijon (D. Caillot, E. Solary, F. Mugneret, O. Casasnovas, M. Maynadier), Grenoble (J. J. Sotto, F. Garban, P. Mossuz, D. Leroux, M. C. Jacob), Le Mans (P. Solal-Celigny, J. Dugay), Lens (P. Morel, L. Stalnikiewicz), Lille (J. P. Jouet, B. Cazin, C. Preudhomme), Nancy (F. Witz, B. Witz, S. Bologna, J. Buisine, M. J. Grégoire, M. C. Béné), Nantes (J. L. Harousseau, P. Moreau, N. Milpied), Poitiers (F. Guilhot, A. Sadoun), Reims (B. Pignon, C. Himberlin, J. P. Vilque, A. M. Blaise, S. Daliphard, P. Cornillet), Rennes (T. Lamy, M. Bernard, B. Drenou), Saint-Etienne (D. Guyotat, L. Campos, C. Mounier, J. Jaubert, N. Nadal, C. Vasselon, M. F. Bertheas), Strasbourg (B. Lioure, A. Falkenrodt, S. Struski, C. Fohrer), and Tours (P. Colombat, M. Delain, C. Barin, A. Petit).
Prepublished online as Blood First Edition Paper, July 15, 2004; DOI 10.1182/blood-2003-10-3560.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Janet Jacobson for editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal