Abstract
Forced dimerization or oligomerization has emerged as a powerful mechanism for unleashing the oncogenic properties of chimeric transcription factors in acute leukemias. Fusion of transcriptional regulators with a variety of heterologous partner proteins as a consequence of chromosomal rearrangements induces inappropriate self-association, leading to aberrant transcriptional properties and leukemogenesis. Forced dimerization/oligomerization may alter the association of a DNA-binding protein for its transcriptional cofactors, or the dimerization motifs themselves may constitutively recruit transcriptional effector molecules. Oligomerized chimeras may also sequester essential partners or cofactors to exert dominant-negative effects on target gene expression. A key mechanistic feature, and one with major clinical implications, is the nature of the transcriptional cofactors that are recruited by the dimerized oncoprotein. Chimeric RARα and acute myeloid leukemia 1 (AML1) proteins induce constitutive repression after the recruitment of corepressors, whereas inappropriate maintenance of target gene expression by mixed-lineage leukemia (MLL) chimeras may result from the recruitment of coactivators or the basal transcriptional machinery. Molecular therapies directed at enzymatic activities of the aberrantly recruited cofactors, or antagonism of dimerization itself, represent promising avenues of current and future investigation.
Introduction
Homodimerization has emerged as a powerful and prevalent mechanism for activating the oncogenic properties of a wide range of proto-oncogenic proteins in hematopoietic malignancies. This paradigm was originally established by studies of protein tyrosine kinases (receptor and nonreceptor), whose signaling properties are normally controlled by regulated dimerization, such as that induced by ligand engagement of cell surface receptors. Acquired oncogenic mutations induce inappropriate dimerization and constitutive kinase signaling, which leads to the genesis of human chronic leukemias, myeloproliferative syndromes, and other malignances.1,2 In these neoplastic disorders, tyrosine kinases are frequently activated by chromosomal rearrangements that result in their fusion with heterologous protein partners containing homodimerization moieties (Table 1). In contrast to the critical roles of chimeric kinases in chronic myeloid disorders, the pathogenesis of acute leukemias is often initiated by the actions of fusion proteins with features of chimeric transcription factors. Recent studies reveal a recurrent theme whereby forced dimerization or oligomerization of these factors, induced by the fusion partner moiety, confers aberrant transcriptional properties hypothesized to be responsible for their oncogenic potentials.3
Forced dimerization of tyrosine kinases and transcription factors associated with various human malignancies
. | Molecular abnormality . | Disease subtype . |
|---|---|---|
| Chimeric fusions | ||
| Tyrosine kinases | BCR-ABL | CML, ALL |
| BCR-FGFR1 | CML | |
| TEL-ABL | ALL | |
| TEL-PDGFRβ | CMML | |
| FIP1L1-PDGFRα | Hypereosinophilic syndrome, chronic eosinophilic leukemia | |
| FOP-FGFR1 | EMS or stem cell leukemia lymphoma syndrome | |
| ZNF198-FGFR1 | EMS or stem cell leukemia lymphoma syndrome | |
| CEP110-FGFR1 | EMS or stem cell leukemia lymphoma syndrome | |
| NPM-ALK | Non-Hodgkin lymphoma | |
| Transcription factors | ||
| AML1-ETO | AML-M2 | |
| AML1-MTG16 | AML, MDS | |
| TEL-AML1 | ALL | |
| CBFβ-SMMHC | AML-M4eo | |
| NPM-RARα | AML-M3 | |
| PML-RARα | AML-M3 | |
| PLZF-RARα | AML-M3 | |
| NuMA-RARα | AML-M3 | |
| Stat5b-RARα | AML-M3 | |
| MLL-GAS7 | AML | |
| MLL-AF1p | AML | |
| Subset of MLL fusions | AML, ALL, ABL |
. | Molecular abnormality . | Disease subtype . |
|---|---|---|
| Chimeric fusions | ||
| Tyrosine kinases | BCR-ABL | CML, ALL |
| BCR-FGFR1 | CML | |
| TEL-ABL | ALL | |
| TEL-PDGFRβ | CMML | |
| FIP1L1-PDGFRα | Hypereosinophilic syndrome, chronic eosinophilic leukemia | |
| FOP-FGFR1 | EMS or stem cell leukemia lymphoma syndrome | |
| ZNF198-FGFR1 | EMS or stem cell leukemia lymphoma syndrome | |
| CEP110-FGFR1 | EMS or stem cell leukemia lymphoma syndrome | |
| NPM-ALK | Non-Hodgkin lymphoma | |
| Transcription factors | ||
| AML1-ETO | AML-M2 | |
| AML1-MTG16 | AML, MDS | |
| TEL-AML1 | ALL | |
| CBFβ-SMMHC | AML-M4eo | |
| NPM-RARα | AML-M3 | |
| PML-RARα | AML-M3 | |
| PLZF-RARα | AML-M3 | |
| NuMA-RARα | AML-M3 | |
| Stat5b-RARα | AML-M3 | |
| MLL-GAS7 | AML | |
| MLL-AF1p | AML | |
| Subset of MLL fusions | AML, ALL, ABL |
BCR-ABL indicates breakpoint-cluster region-Abelson leukemia; FGFR1, fibroblast growth factor receptor 1; PDGFRβ, platelet-derived growth factor receptor β; CML, chronic myeloid leukemia; ALL, acute lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; EMS, 8p11 myeloproliferative syndrome.
Dimerization and corepressor recruitment by RARα oncoproteins
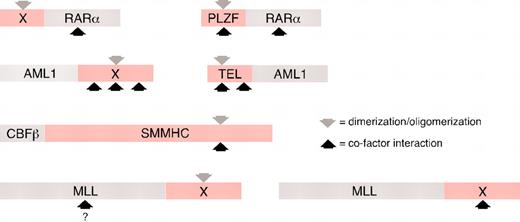
In acute myeloid leukemia (AML), the most prevalent chimeric transcriptional proteins arise from mutations of the retinoic acid receptor α (RARα), the core binding factor (CBF) (either of its 2 subunits AML1 or CBFβ), or the mixed-lineage leukemia (MLL) protein. Interestingly, some of the proteins that serve as fusion partners for RARα and AML1 in acute leukemias have also been identified as partners that induce homodimerization of activated tyrosine kinases in chronic leukemias (eg, nucleophosmin [NPM] and translocation ETS leukemia [TEL], respectively) (Table 1). For the chimeric RARα proteins of acute promyelocytic leukemia (APL), all known fusion partners contain oligomerization domains, which include the coiled-coil domains of PML, signal transducer and activator of transcription 5b (Stat5b), and nuclear mitotic apparatus (NuMA) and the pox-virus and zinc finger (POZ) domain of promyelocytic leukemia zinc finger (PLZF). These self-association domains are retained in the respective fusion proteins (Figure 1) and are necessary and sufficient to induce aberrant transcriptional repression of target genes by retinoic acid receptor α (RARα), which is critical for leukemogenesis.4-11 However, with the exception of the POZ domain in PLZF and the coiled-coil domain of Stat5b, none of these heterologous domains has inherent transcriptional repression properties.
Correlation of transcriptional cofactor interaction domains and dimerization/oligomerization motifs in leukemia-associated fusion proteins. Chimeric oncoproteins are illustrated schematically with fusion partner moieties indicated in pink. X indicates that diverse proteins have been identified as fusion partners. Downward gray arrows indicate sites of dimerization/oligomerization. Upward black arrows indicate sites of transcriptional cofactor interaction. For dimerization-dependent MLL chimeras, the question mark indicates that the site of cofactor recruitment has not been determined.
Correlation of transcriptional cofactor interaction domains and dimerization/oligomerization motifs in leukemia-associated fusion proteins. Chimeric oncoproteins are illustrated schematically with fusion partner moieties indicated in pink. X indicates that diverse proteins have been identified as fusion partners. Downward gray arrows indicate sites of dimerization/oligomerization. Upward black arrows indicate sites of transcriptional cofactor interaction. For dimerization-dependent MLL chimeras, the question mark indicates that the site of cofactor recruitment has not been determined.
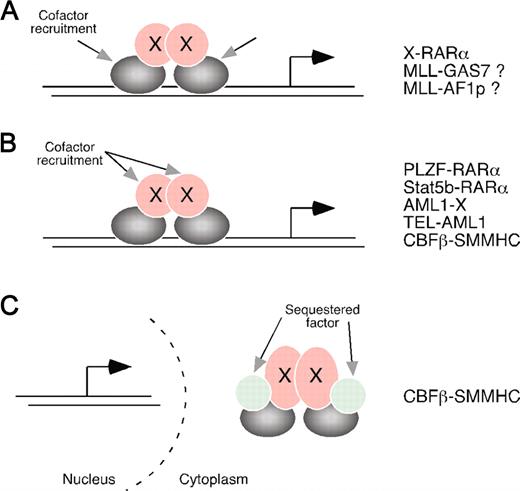
Rather, their enforced dimerization of RARα appears to enhance its affinity for transcriptional corepressors such as Sin3A, SMRT, and Nco-R (Figure 2A). Support for this scenario is provided by synthetic fusions of RARα containing heterologous oligomerization modules, which display transcriptional repression properties that mimic those of bona fide leukemia fusion proteins and facilitate RARα recruitment of transcriptional corepressors.12,13 Enhanced RARα corepressor association is reversed by pharmacologic levels of retinoic acid, which potentially accounts for its therapeutic efficacy in APL. The POZ domain of PLZF and the coiled-coil domain of Stat5b not only induce homodimerization and enhanced corepressor association by RARα but also independently interact with transcriptional corepressor complexes (Figures 1, 2B). The latter interactions are resistant to reversal by pharmacologic levels of retinoic acid, thus accounting for retinoic acid's lack of therapeutic efficacy in these rare, molecularly distinct subtypes of APL.11,14-18
General mechanisms for oncogenic activation of chimeric transcription factors by dimerization/oligomerization in acute leukemias. (A) Forced homotypic interactions mediated by the fusion partner trap the DNA-binding component of the chimera in a conformation with altered association for transcriptional cofactors. For RARα, the cofactors mediate transcriptional repression, but, for dimerized MLL fusion proteins, they likely mediate activation. (B) In a second mechanistic category, the dimerization/oligomerization moiety itself serves as a platform for recruitment of transcriptional cofactors, as best illustrated by AML1 fusion proteins. This scenario relies on conformational features of the dimerization moiety that favor preferential assembly of corepressors (or coactivators) on multimerized protein interfaces. (C) A third mechanistic category invokes competitive sequestration of a DNA-binding partner (eg, AML1) by the oligomerized chimera, a model proposed for the potential role of CBFβ/SMMHC. Undoubtedly, some chimeras simultaneously function through more than one of these mechanisms. Examples are the dual roles of PLZF/RARα, Stat5b/RARα, and possibly CBFβ/SMMHC.
General mechanisms for oncogenic activation of chimeric transcription factors by dimerization/oligomerization in acute leukemias. (A) Forced homotypic interactions mediated by the fusion partner trap the DNA-binding component of the chimera in a conformation with altered association for transcriptional cofactors. For RARα, the cofactors mediate transcriptional repression, but, for dimerized MLL fusion proteins, they likely mediate activation. (B) In a second mechanistic category, the dimerization/oligomerization moiety itself serves as a platform for recruitment of transcriptional cofactors, as best illustrated by AML1 fusion proteins. This scenario relies on conformational features of the dimerization moiety that favor preferential assembly of corepressors (or coactivators) on multimerized protein interfaces. (C) A third mechanistic category invokes competitive sequestration of a DNA-binding partner (eg, AML1) by the oligomerized chimera, a model proposed for the potential role of CBFβ/SMMHC. Undoubtedly, some chimeras simultaneously function through more than one of these mechanisms. Examples are the dual roles of PLZF/RARα, Stat5b/RARα, and possibly CBFβ/SMMHC.
Putative roles of dimerization by AML1 and CBFβ oncoproteins
Dimerization also is a feature of the CBF leukemia fusion proteins. Characterized fusion partners of AML1, including TEL, ETO, and MTG16, contain homodimerization domains that are invariably retained in the respective AML1 fusion proteins (Figure 1).19 Structure/function analyses reveal that these dimerization motifs (eg, the pointed domain of TEL and the NHR2 motif in ETO1/2) serve dual roles by also mediating transcriptional repression through direct recruitment of transcriptional corepressors, analogous to the POZ domain of PLZF20-24 (Figure 2B). However, TEL and ETO contain multiple corepressor interaction sites, and not all are required for repression in transient transcriptional assays. Furthermore, the transcriptional repression activities of individual domains are dependent on cell content.25 The dimerization domains of TEL and ETO seem to be sufficient for repression, but it has not been possible to separate dimerization from corepressor recruitment. It is not yet clear whether dimerization is required for the function of AML1 fusion proteins, but domain-swapping experiments suggest that it may not be sufficient.26
CBFβ is a DNA-binding partner of AML1 that undergoes protein fusion with smooth muscle myosin heavy chain (SMMHC) in a subset of AML. The CBFβ/SMMHC chimera displays potent dominant-negative effects on the expression of AML1 target genes in vitro and phenocopies loss of AML1 function in vivo during embryonic development.27 Two alternative, but not necessarily mutually exclusive, models for the transcriptional effects of CBFβ/SMMHC invoke either multimerization-induced sequestration of AML1 in the cytoplasm (sequestration model; Figure 2C)28-30 or SMMHC-mediated recruitment of transcriptional corepressor complexes to AML1 target genes (repressor model; Figure 2B).31,32 Both mechanisms account for the overlapping locations within SMMHC of corepressor interaction motifs and multimerization domains (the so-called assembly competence domain) (Figure 1) required for the dominant-negative effects of CBFβ/SMMHC on AML1 target gene expression.
Studies on AML1 and CBFβ fusion proteins raise the possibility that aberrant dimerization or multimerization may contribute to their transcriptional repression properties; however, the role for self-association in leukemia pathogenesis has not been clearly demonstrated. Hug et al25 showed that the NHR2 domain of ETO, which mediates homodimerization and heterodimerization when fused with AML1, is sufficient but not absolutely necessary for AML1-ETO to inhibit differentiation and proliferation of murine bone marrow cells. Conversely, recent unpublished observations reveal that the NHR2 domain is necessary (S. D. Nimer, personal oral communication, 2003) and may be sufficient (D. E. Zhang, personal oral communication, 2003) for the growth-promoting effects of AML1-ETO on human and murine hematopoietic progenitors, respectively. Further structure/function analyses using appropriate hematopoietic cell transformation assays are necessary to establish unequivocally whether homodimerization/multimerization is a prerequisite for efficient transcriptional repression and leukemogenesis induced by CBF fusion proteins. Circumstantial evidence of a pathogenetic role for forced dimerization in AML1-associated leukemias may be the frequent deletion of the wild-type TEL gene in acute lymphoblastic leukemias expressing TEL-AML1, which would effectively circumvent antagonism of TEL-AML1 transcriptional function resulting from heterodimerization with TEL.33
Forced dimerization of MLL as a novel oncogenic mechanism
MLL is a histone methyltransferase that undergoes heterologous fusion with a wide variety of partner proteins (more than 40) in acute leukemias and myelodysplastic syndromes (MDS).34 A subset of MLL fusion partners displays transcriptional activation potential required for leukemia pathogenesis by the respective MLL fusion protein.35,36 These MLL oncoproteins, which are not known to homodimerize, appear to inappropriately maintain transcription, likely by recruiting or tethering transcriptional coactivator or chromatin remodeling factors at MLL target genes through the fusion partner moiety of the MLL chimera (Figure 3). This contrasts with recruitment of corepressors by RARα, AML1, and CBFβ fusion proteins, leading to constitutive transcriptional repression.
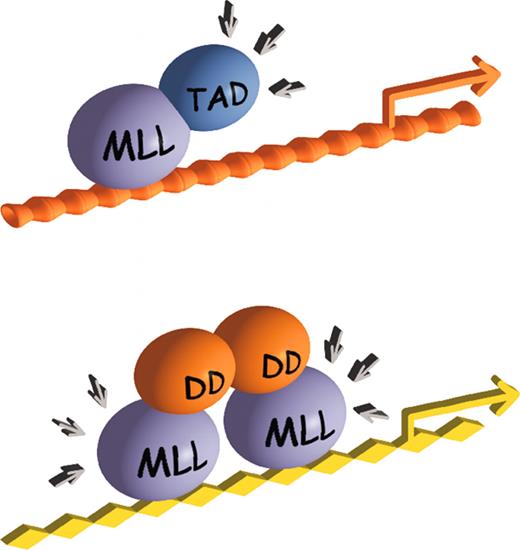
Alternative mechanisms for oncogenic activation of MLL in hematologic malignancies. (A) Direct fusion of MLL with transcriptional effector domains results in the recruitment of transcription activation complexes to MLL target genes through the fusion partner moiety. (B) Fusion partner–induced dimerization/oligomerization of MLL results in the aberrant maintenance of target gene expression, possibly by the recruitment of cofactors or basal transcriptional machinery through the MLL moiety of the chimera. TAD indicates transactivation domain; DD, dimerization domain.
Alternative mechanisms for oncogenic activation of MLL in hematologic malignancies. (A) Direct fusion of MLL with transcriptional effector domains results in the recruitment of transcription activation complexes to MLL target genes through the fusion partner moiety. (B) Fusion partner–induced dimerization/oligomerization of MLL results in the aberrant maintenance of target gene expression, possibly by the recruitment of cofactors or basal transcriptional machinery through the MLL moiety of the chimera. TAD indicates transactivation domain; DD, dimerization domain.
A different subset of MLL fusion partners consists of cytoplasmic proteins that do not have inherent transcriptional activities.34 Some of these are notable for containing homodimerization or oligomerization domains (Figure 1). Recent studies demonstrate that coiled-coil oligomerization domains of 2 MLL fusion proteins (MLL-GAS7 and MLL-AF1p) are necessary and sufficient for the induction of leukemia in a murine transplantation model, albeit with reduced disease penetrance and longer latency.37 Furthermore, experimental fusion of MLL with synthetic dimerization modules creates a strong transcriptional activator and induces transformation of hematopoietic progenitors in vitro.37,38 Taken together, these studies support at least 2 distinct pathways (Figure 3), one of which appears to be dimerization dependent, for oncogenic activation of MLL, and both pathways lead to inappropriate maintenance, rather than repression, of transcription.
It remains unclear how homodimerization activates the transcriptional properties of MLL. One possibility is that self-association mediated by the fusion partner traps MLL in a conformation with altered association for key effector molecules, similar to the model for RARα chimeras. Another possibility is that the dimerization domains of MLL fusion partners independently recruit transcriptional effectors to MLL target genes, but they display no significant inherent transactivation activity in transient transcriptional assays.37 The possibility that MLL dimers may exert dominant-negative effects to sequester or dislocate critical cofactors seems unlikely, though immunofluorescence analyses demonstrate a subnuclear localization pattern for MLL-AF1p that differs from that of wild-type MLL.39
Transcription cofactors are promising therapeutic targets
A key mechanistic feature, with major clinical implications, is the nature of the transcriptional cofactors recruited by the dimerized oncoprotein. Constitutive repression of critical target genes results from the recruitment of corepressors, whereas inappropriate maintenance of target gene expression would result from the recruitment of coactivators or possibly the basal transcriptional machinery. Important components of the repression machinery are histone deacetylases (HDACs), whose enzymatic activities assist in rendering chromatin nonpermissive for transcription. Several HDAC inhibitors are under evaluation in clinical trials to determine their efficacy in treating leukemias with AML1-ETO or PML-RARα mutations. Notably, their effectiveness in MLL leukemias has not been demonstrated,40 which likely reflects the biologic and functional differences among these chimeric transcriptional factors. In contrast to AML1/CBFβ or RARα, dimerization of MLL results in constitutive transcriptional activation of known MLL targets such as Hox genes,37,38 though the pathogenic roles of individual Hox genes in MLL-mediated leukemias may vary.41-43 An effective therapeutic strategy in MLL leukemias, therefore, would require targeting of cofactors that maintain rather than repress transcription.
Additional studies are needed to refine the pathogenic contribution and underlying mechanisms of chimeric transcription factors in leukemogenesis. Nevertheless, current data indicate that constitutive dimerization is a fundamental regulatory mechanism for the activation of transcriptional and signaling pathways, which are frequently disrupted in human malignancies and constitute a potential molecular target for oncogenic suppression.
Prepublished online as Blood First Edition Paper, May 6, 2004; DOI 10.1182/blood-2004-03-0992.
Supported by the Children's Health Initiative and the National Cancer Institute. C.W.S. is a special fellow of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Stephen Nimer and Dong-Er Zhang for sharing unpublished data, Scott Hiebert and Shuo Dong for useful discussion, and Caroline Tudor and Erica Tse for artwork assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal