Abstract
We tested the effects of small-molecule XIAP antagonists based on a polyphenylurea pharmacophore on cultured acute myelogenous leukemia (AML) cell lines and primary patient samples. X-linked inhibitor of apoptosis protein (XIAP) antagonist N-[(5R)-6-[(anilinocarbonyl)amino]-5-((anilinocarbonyl){[(2R)-1-(4-cyclohexylbutyl)pyrrolidin-2-yl]methyl}amino)hexyl]-N-methyl-N′-phenylurea (1396-12), but not a structurally related control compound, induced apoptosis of primary leukemia samples with a lethal dose (LD50) of less than 10 μM in 16 of 27 (60%) samples. In contrast, XIAP antagonist 1396-12 was not lethal to the normal hematopoietic cells in short-term cytotoxicity assays. Response of primary AML specimens to XIAP inhibitor correlated with XIAP protein levels, with higher levels of XIAP associated with sensitivity. The XIAP antagonist 1396-12 induced activation of downstream caspases 3 and 7 prior to the activation of upstream caspase 8 and caspase 9. Apoptosis induction was also independent of B-cell lymphoma protein-2 (Bcl-2) or caspase 8, indicative of a downstream effect on apoptotic pathways. Thus, polyphenylurea-based XIAP antagonsists directly induce apoptosis of leukemia cells and AML patient samples at low micromolar concentrations through a mechanism of action distinct from conventional chemotherapeutic agents.
Introduction
At a fundamental level, patients with acute leukemia do not respond to treatment because the malignant blasts are not eradicated by current chemotherapy. In part, this failure is due to defects in apoptosis pathways.1 Therefore, agents that overcome roadblocks to apoptosis could be therapeutically useful for this disease.
Classically, apoptosis is caused by the activation of caspases, a family of intracellular cysteine proteases that cleave substrates at aspartic acid residues.2,3 Currently, at least 4 pathways for initiation of caspase activation exist: (1) the mitochondrial pathway with cytochrome c; (2) the death receptor pathway with the tumor necrosis factor (TNF) family of death receptors; (3) direct caspase activation by cytolytic T-cell protease Granzyme B; and (4) a pathway connected to the endoplasmic reticulum. These pathways launch a proteolytic cascade, in which upstream (initiator) caspases cleave and activate downstream (effector) caspases.
The inhibitor of apoptosis proteins (IAPs) are a family of endogenous caspase inhibitors that share a common baculoviral IAP repeat (BIR) domain. To date, 8 IAP family members exist in humans. Of these, XIAP is probably the best characterized with respect to its structure and biochemical mechanisms. XIAP inhibits caspases 3, 7, and 9, but not caspases 1, 6, 8, or 10.4 XIAP contains 3 tandem baculovirus IAP repeat (BIR) domains and a really interesting new gene (RING) domain. The second BIR domain of XIAP (BIR2) inhibits caspases 3 and 7, while the third BIR domain (BIR3) inhibits caspase 9. Through their ability to inhibit caspases, IAPs act as antiapoptotic proteins5-10 and are promising therapeutic targets. Inhibition of XIAP by antisense strategies or peptides that bind and inhibit the BIR3 domain of XIAP sensitizes malignant cells to chemotherapy.11-18
Based on the knowledge that XIAP directly inhibits active caspase 3, we devised an enzymatic derepression assay to screen for molecules that relieve protease inhibition. Using this assay, we screened combinatorial libraries of chemical compounds and identified active agents based on different pharmacophores. The initial report of these XIAP inhibitors described a series of compounds based on the polyphenylurea pharmacophore including the active compound N-[(5R)-6-[(anilinocarbonyl)amino]-5-((anilinocarbonyl){[(2R)-1-(4-cyclohexylbutyl)pyrrolidin-2-yl]methyl}amino)hexyl]-N-methyl-N′-phenylurea (1396-12) and structural analogues.19 Corresponding to their activity in the enzymatic assay, active polyphenylurea-based inhibitors but not inactive controls, induced rapid apoptosis of several types of tumor cell lines.19 We determined that active compounds inhibit XIAP by binding its BIR2 domain at a site distinct from the binding pocket of the endogenous XIAP inhibitor second modulator of apoptotic proteases (SMAC).20
Given the potential therapeutic utility of IAP inhibition, we tested these chemical IAP inhibitors in cultured leukemia cell lines and primary acute myelogenous leukemia (AML) patient samples. We show here that the XIAP inhibitor 1396-12 induces apoptosis of primary AML samples at low micromolar concentrations, while being less toxic to normal hematopoietic cells. In addition, this XIAP inhibitor acts via a mechanism distinct from standard chemotherapeutic agents, triggering apoptosis at a distal point in the apoptosis pathways.
Materials and methods
Chemical XIAP inhibitors
Chemical XIAP inhibitors based on the polyphenylurea pharmacophore were identified by screening a mixture-based combinatorial library for compounds that reversed XIAP-mediated inhibition of caspase 3 in a cell-free enzyme derepression assay.19 Individual compounds were synthesized by solid-phase methods and purified by high-performance liquid chromatography (HPLC). Purified compounds were analyzed by mass spectrometry to confirm identity and more than 90% purity. Compounds were dissolved in dimethyl sulfoxide (DMSO) and stored at -20°C. Compounds were subsequently diluted in phosphate-buffered saline (PBS) prior to their addition to cell cultures. In all cases, the final concentration of DMSO was less than 0.05% (vol/vol). Experiments were performed using more than one purified preparation of compound to ensure reproducibility.
Cell culture
Cell lines used for these studies included the myeloid leukemia cell lines OCI M2, HL60, K562, and U937, which were cultured in RPMI 1640 medium, and OCI-AML 2 cells that were maintained in α minimum essential medium (MEM). Jurkat lymphocytic leukemia cells were maintained in RPMI 1640 medium. All cell lines were supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 1 mM l-glutamine, and antibiotics. Cells were cultured at 37°C with 5% CO2 in a humid atmosphere.
For experiments with primary AML cells, fresh samples of heparinized peripheral blood were obtained from patients with AML who had at least 80% blasts among the mononuclear cells in the peripheral blood and who consented to donate a research sample. Normal hematopoietic cells were obtained from healthy volunteers donating their peripheral blood stem cells or bone marrow for allotransplantation. Mononuclear cells were isolated from the samples by Ficoll density-gradient centrifugation. Primary cells were cultured in RPMI 1640 medium supplemented 10% FBS, 1 mM l-glutamine, and antibiotics. The collection and use of human tissue was approved by the local ethics review board.
Apoptosis and cell-death assays
Apoptosis was measured by annexin-V surface staining. Briefly, cells (6.25 × 105/mL) were double stained with fluorescein isothiocyanate (FITC)--conjugated annexin V and propidium iodide (PI) using a kit according to manufacturer's instructions (Biovision, Mountainview, CA). Fluorescence was measured by flow cytometry (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, CA) and data analyzed by the Cell Quest program. Assays measuring reduction of the tetrazolium salt 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were performed essentially as previously described.21 To determine the effects of chemical caspase inhibitors on apoptosis, cells were treated with the XIAP inhibitors with and without the pan-caspase inhibitor z-benzyloxycarbonyl-Val-Ala-Asp (VAD)-fmk, the effector caspase inhibitor Ac-Asp-Glu-Val-Asp (DEVD)-cmk, the caspase 8 inhibitor z-Ile-Glu-Thr-Asp (IETD)-fmk, or the caspase 9 inhibitor Leu-Glu-His-Asp (LEHD)-cho (final concentration 10 μM) (Calbiochem, La Jolla, CA).
Active caspases 3/7, 8, and 9 were identified with APO LOGIX carboxyfluoroscein caspase detection kits (Cell Technologies, Minneapolis, MN) according to the manufacturer's instructions. These kits contain FITC-labeled cell permeable peptides FAM-DEVD-fmk, FAM-LETD-fmk, and FAM-LEHD-fmk, which preferentially and irreversibly bind and inhibit active caspases 3/7, 8, and 9, respectively. After incubation with caspase detection reagents for 1 hour, cells were washed and analyzed by flow cytometry.
Colony formation assays
Primary AML cells (6.25 × 105/mL) were treated with the chemical XIAP inhibitors, a structurally related inactive control compound, or buffer control for 24 hours. After treatment, cells were washed and equal volumes were plated in triplicate in MethoCult GF H4434 medium containing 1% methycellulose in Iscoves medium, 30% FBS, 1% bovine serum albumin, 3 U/mL recombinant human (rh) erythropoietin, 10-4 M 2-Mercaptoethanol, 2 mM l-glutamine, 50 ng/mL rh stem cell factor, 10 ng/mL granulocyte macrophage-colony-stimulating factor (GM-CSF), and 10 ng/mL rh interleukin 3 (IL-3; StemCell Technologies, Vancouver, BC, Canada). Seven days after plating, the number of colonies containing 20 or more cells was counted as previously described.22 To confirm that the colony contained leukemic blasts, cells were picked from the colony, stained with May-Grünwald-Giemsa, and their morphology examined as previously described.22 With this assay, a plating efficiency of 0.1% was routinely achieved.
G-CSF-mobilized peripheral blood stem cells (6.25 × 105/mL) were treated with the XIAP chemical inhibitors, the structurally related inactive control compound, or buffer control for 24 hours. After treatment, the cells were washed and equal volumes plated in triplicate in MethoCult GF H4434 medium. Fourteen days after plating, the number of colonies containing 20 or more cells was counted and the number of erythroid-burst-forming unit (BFU-E) and granulocyte macrophage-colony-forming unit (CFU-GM) colonies were enumerated by morphologic assessment. To confirm the lineage of colonies, cells were picked from individual colonies, stained with May-Grünwald-Giemsa, and their morphology examined as previously described.22 A plating efficiency of 0.1% was routinely achieved.
Immunoblotting
Cytosolic extracts were prepared from primary AML and normal cells as described previously.23 Briefly, cells were washed in PBS and then resuspended in an equal volume of hypotonic lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA [ethylenediaminetetraacetic acid], and 1 mM dithiothreitol [DTT]). Protein concentrations were determined by the Bradford assay.24 Immunoblot assays were performed as described previously.25 Briefly, equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels followed by transfer to nitrocellulose membranes. Membranes were probed with monoclonal mouse anti-human XIAP (0.25 μg/mL; Transduction Laboratories, Lexington, KY), mouse anti-cIAP1 (1 μg/mL; R&D Systems, Minneapolis, MN), mouse anti-cIAP2 (0.75 μg/mL; R&D Systems), rabbit anticaspase 3 (1:10 000 vol/vol),26 rabbit anti-B-cell lymphoma protein-2 (Bcl-2),27 rabbit anti-Bcl-xL,28 and monoclonal mouse anti-β-actin (1: 10 000 vol/vol; Sigma, St Louis, MO). Secondary antibodies consisted of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and anti-rabbit IgG (1:3000 vol/vol; Amersham, Piscataway, NJ). Detection was performed by the enhanced chemiluminescence (ECL) method (Pierce, Rockford, IL).
Statistics
Logistic regression was used to investigate if the level of XIAP was predictive of response to the XIAP inhibitor in the AML patient samples. To test whether 1396-12 enhanced Ara-C toxicity in a synergistic or additive fashion, a regression model was constructed with effects for 1396-12 and Ara-C included. An interaction effect between 1396-12 and Ara-C was then tested for whether it significantly added information to the regression model. If the interaction effect was statistically significant, then a synergistic effect was present. Statistical significance was defined as a P value of .05 or less and all tests were 2-sided. Analysis of variance (ANOVA) was used to investigate whether the XIAP antagonist preferentially activated caspase 3 before caspases 8 and 9.
Results
Polyphenylurea-based XIAP antagonists induce apoptosis of leukemia cell lines
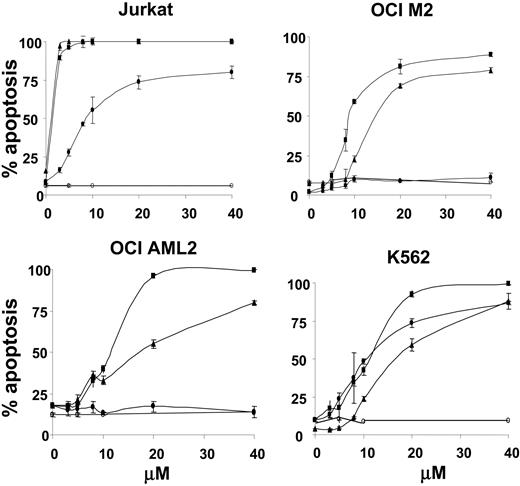
We previously identified at least 5 chemical compounds based on the polyphenylurea pharmacophore that inhibit the ability of XIAP to suppress effector caspases. To preliminarily explore their efficacy against acute leukemia, we extended the evaluation of these compounds to leukemia cell lines and primary AML patient samples. Leukemia cell lines, including acute lymphoblastic leukemia (ALL) Jurkat cells and AML cells OCI M2, OCI-AML 2, and K562, were treated with increasing concentrations of polyphenylurea-based XIAP inhibitors 1396-12, 1396-22, 1396-34, or the structurally related inactive compound 1396-28. The Jurkat lymphocytic leukemia cell line was also included as it has intact mitochondrial and death receptor pathways of caspase activation.23 At 24 hours after incubation, apoptosis was measured by annexin V surface staining. Of the XIAP inhibitors tested, 1396-12 appeared the most active, as it induced apoptosis in the majority of tested cell lines with a lethal dose (LD50) in the low micromolar range. In contrast, the inactive control compound displayed no toxicity against the leukemia cell lines (Figure 1). The cell death induced by the XIAP inhibitors was confirmed by MTT and colony formation assays (data not shown). Given the superior potency of 1396-12 against leukemia cell lines, it was selected for further study.
XIAP inhibitors induce apoptosis of primary AML cells
To evaluate the polyphenylurea-based XIAP inhibitor 1396-12 as a potential novel therapy for acute leukemia, primary leukemic blasts were isolated from patients with AML (n = 27). The characteristics of the 27 patients with AML are shown in Table 1.
XIAP antagonists induce apoptosis of leukemia cell lines. Jurkat, OCI-M2, OCI-AML 2, and K562 leukemia cells (6.5 × 105/mL) were treated with increasing concentrations of the active XIAP antagonists 1396-12 (▪), 1396-22 (▴), 1396-34 (•), or the structurally related inactive control 1396-28 (○). At 24 hours after treatment, apoptosis was measured by annexin V staining (% positivity). The mean plus or minus SD of 3 independent experiments is shown.
XIAP antagonists induce apoptosis of leukemia cell lines. Jurkat, OCI-M2, OCI-AML 2, and K562 leukemia cells (6.5 × 105/mL) were treated with increasing concentrations of the active XIAP antagonists 1396-12 (▪), 1396-22 (▴), 1396-34 (•), or the structurally related inactive control 1396-28 (○). At 24 hours after treatment, apoptosis was measured by annexin V staining (% positivity). The mean plus or minus SD of 3 independent experiments is shown.
Patient characteristics
n | 27 |
| Age at sample, y, mean ± SD | 53 ± 16 |
| Sex, % male | 56 |
| White blood cell count at sample, median (range) | 22 (2.4-312) |
| Status at evaluation | |
| Treatment naive | 21 |
| Relapsed | 6 |
| Response to induction chemotherapy, n = 14 (%) | |
| CR | 8 (57) |
| NR | 6 (43) |
| Cytogenetics, % | |
| High | 33 |
| Intermediate | 48 |
| Good | 19 |
| FAB subclass, %* | |
| M0 | 8 |
| M1 | 16 |
| M2 | 8 |
| M3 | 8 |
| M4 | 39 |
| M5 | 16 |
n | 27 |
| Age at sample, y, mean ± SD | 53 ± 16 |
| Sex, % male | 56 |
| White blood cell count at sample, median (range) | 22 (2.4-312) |
| Status at evaluation | |
| Treatment naive | 21 |
| Relapsed | 6 |
| Response to induction chemotherapy, n = 14 (%) | |
| CR | 8 (57) |
| NR | 6 (43) |
| Cytogenetics, % | |
| High | 33 |
| Intermediate | 48 |
| Good | 19 |
| FAB subclass, %* | |
| M0 | 8 |
| M1 | 16 |
| M2 | 8 |
| M3 | 8 |
| M4 | 39 |
| M5 | 16 |
Does not add up to 100 due to rounding
As a control, mononuclear cells isolated from primary normal peripheral blood stem cells (PBSCs; n = 6) or normal bone marrow (n = 1) were studied. Primary malignant and normal cells were treated with increasing concentrations of 1396-12, or the inactive control compound 1396-28. After 24 hours of incubation, apoptosis was measured by surface annexin V staining. The median LD50 among the AML patient samples tested was 6 μM (range: 2 μM to > 40 μM). The XIAP antagonist 1396-12 induced apoptosis with an average LD50 of less than or equal to 10 μM in 16 of 27 (60%) primary AML samples tested and with an LD50 of more than 40 μM in 7 of 27 (26%) samples. In contrast, 1396-12 was less toxic to normal PBSCs or marrow samples. Among the normal samples tested, the XIAP inhibitor 1396-12 induced 23% ± 5% (mean ± standard deviation [SD]) apoptosis at a final concentration of 10 μM with an LD50 of more than 40 μM in all normal samples tested. As a comparison, the inactive control compound 1396-28 was not toxic to any of the AML or normal hematopoietic samples at concentrations up to 40 μM (Figure 2A and data not shown). The XIAP inhibitor was equally active in samples from treatment-naive and relapsed patients. Likewise, it produced similar toxicity in samples from the 14 patients who did and did not achieve complete remission with induction chemotherapy (Figure 2B).
In addition to short-term cytotoxicity assays, the effects of chemical XIAP antagonists on primary AML and normal hematopoietic cells were evaluated in colony formation assays. Figure 2C shows data from 4 arbitrarily chosen AML patient samples and 2 normal PBSC samples treated with the XIAP antagonist 1396-12. The XIAP antagonist inhibited clonogenic survival in the AML samples tested with an LD50 of less than 5 μM. For 2 of the 4 AML samples, both clonogenic survival and annexin V assays were performed. By clonogenic survival, the LD50 for both samples was 4 μM, whereas the LD50 by annexin V staining after a 24-hour incubation was 4 μM for one sample, and 20 μM for the other. Treatment with XIAP antagonist 1396-12 also reduced colony formation by 2 normal PBSC samples, one with an LD50 of 8.5 ± 0.3 μM and the other with an LD50 of 5.6 ± 0.4 μM. In these 2 normal samples the LD50 after a 24-hour incubation was more than 40 μM as measured by annexin-V staining. In the normal PBSC samples, both the BFU-E and CFU-GM lineages were equally reduced after treatment with the XIAP antagonist. Treatment with the structurally related inactive control compound 1396-28 did not reduce colony growth in the AML or normal samples.
XIAP inhibitor induces apoptosis in primary AML samples. (A) Primary AML blasts were isolated from peripheral blood samples obtained from patients with AML who had more than 80% blasts in the peripheral blood. As a control, mononuclear cells were isolated from samples of normal mobilized peripheral blood cells or from bone marrow. Primary blasts or normal hematopoietic mononuclear cells were treated with increasing concentrations of the XIAP inhibitor antagonist 1396-12 for 24 hours. After treatment, apoptosis was measured by annexin V surface expression. For each sample, the percentage of apoptosis after treatment with 10 μM of 1396-12 is shown with the LD50 displayed along the x-axis. (B) XIAP inhibitor is equally effective in samples from patients with chemosensitive and chemoresistant AML. The LD50 for 1396-12 is compared between samples derived from patients who achieved complete remission with induction chemotherapy (CR), had no response to induction chemotherapy (NR), or who were in relapse. The horizontal line represents the median LD50 for the group. (C) Colony formation assay. Primary AML blasts (—) or mobilized normal peripheral blood stem cells (-) were treated with increasing concentrations of the XIAP antagonist 1396-12 or buffer control for 24 hours. After treatment, cells were washed, plated in methylcellulose cultures, and counted after one week. For each sample, the mean plus or minus SD of 3 experiments expressed as a percentage of the buffer treated control is shown. Each symbol represents an individual patient.
XIAP inhibitor induces apoptosis in primary AML samples. (A) Primary AML blasts were isolated from peripheral blood samples obtained from patients with AML who had more than 80% blasts in the peripheral blood. As a control, mononuclear cells were isolated from samples of normal mobilized peripheral blood cells or from bone marrow. Primary blasts or normal hematopoietic mononuclear cells were treated with increasing concentrations of the XIAP inhibitor antagonist 1396-12 for 24 hours. After treatment, apoptosis was measured by annexin V surface expression. For each sample, the percentage of apoptosis after treatment with 10 μM of 1396-12 is shown with the LD50 displayed along the x-axis. (B) XIAP inhibitor is equally effective in samples from patients with chemosensitive and chemoresistant AML. The LD50 for 1396-12 is compared between samples derived from patients who achieved complete remission with induction chemotherapy (CR), had no response to induction chemotherapy (NR), or who were in relapse. The horizontal line represents the median LD50 for the group. (C) Colony formation assay. Primary AML blasts (—) or mobilized normal peripheral blood stem cells (-) were treated with increasing concentrations of the XIAP antagonist 1396-12 or buffer control for 24 hours. After treatment, cells were washed, plated in methylcellulose cultures, and counted after one week. For each sample, the mean plus or minus SD of 3 experiments expressed as a percentage of the buffer treated control is shown. Each symbol represents an individual patient.
Response to XIAP antagonists correlates with levels of XIAP protein
To determine variables that predict response to the XIAP antagonists in primary AML blasts, we measured levels of XIAP, cIAP1, cIAP2, and caspase 3 proteins by immunoblotting in lysates from the patient samples. For these experiments, protein lysates were available from 21 of 27 AML samples. Immunoblot data were quantified by scanning densitometry, normalized for β-actin, and compared with an internal reference cell line (K562) and with PBSCs from normal volunteers.
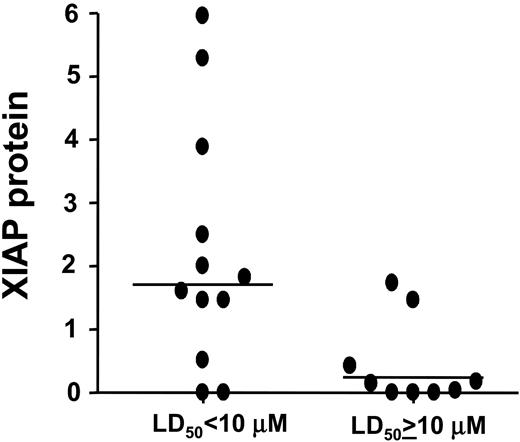
AML samples most resistant to the XIAP inhibitor 1396-12 contained low to absent levels of XIAP protein (Figure 3). The median level of XIAP was 14% of normal PBSCs among the AML samples where 1396-12 induced apoptosis with a LD50 of more than or equal to 10 μM. In contrast, the median level of XIAP was 171% of normal PBSCs among the AML samples where 1396-12 induced apoptosis with a LD50 of less than 10 μM. By logistic regression analysis, increased levels of XIAP were associated with a higher probability of response to treatment with 1396-12, where response was defined as a LD50 of less than 10 μM (P = .04). Similar correlations were observed if the cut-point for response to the inhibitor was set at the median LD50 for the population (8 μM) or the levels of XIAP among the AML samples were compared with the K562 internal control.
In contrast to the correlation with XIAP protein levels, levels of cIAP1, cIAP2, caspase 3, Bcl-2, or Bcl-xL did not correlate with response to the inhibitor. Pretreatment levels of active caspase 3 by immunoblotting or apoptosis by annexin V staining also did not correlate with response to the inhibitor. The mean level of spontaneous apoptosis by annexin V staining was 16% ± 10% (data not shown). Among the clinical parameters examined, the XIAP antagonist was equally effective across cytogenetic risk groups29 and the subtypes based on the French-American-British classification. Response also did not correlate with the white blood count or blast count, patient's age, or sex (data not shown).
Studies of XIAP antagonist 1396-12 in combination with Ara-C
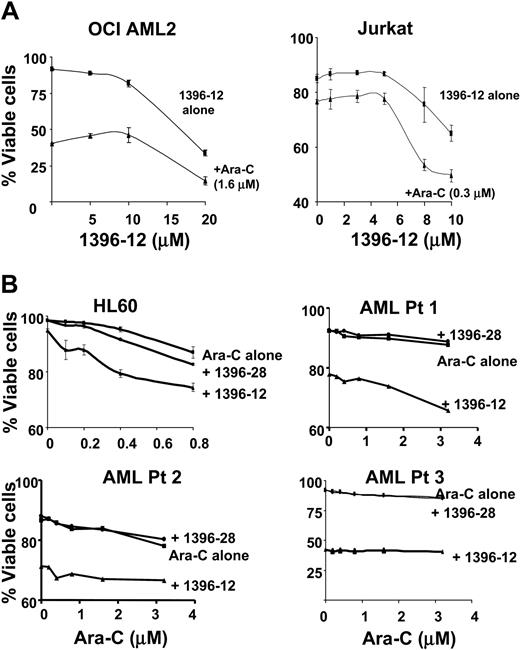
To explore whether small-molecule XIAP antagonists sensitize leukemic cells to conventional chemotherapeutic agents, OCI AML2 and Jurkat leukemic cells were treated with increasing concentrations of 1396-12, with or without Ara-C. In these leukemia cell lines, the addition of 1396-12 did not sensitize the cells to Ara-C (Figure 4). To confirm that 1396-12 did not enhance Ara-C toxicity, a regression model was constructed to assess the Ara-C and 1396-12 interaction effect. The interaction effect was not significant (P > .2) indicating that there was no enhancement of 1396-12 toxicity by Ara-C. Of note, Ara-C did not alter levels of XIAP as measured by immunoblotting (data not shown).
Correlation of XIAP levels with in vitro response to XIAP antagonists. Protein lysates were prepared from 21 of 27 AML samples. Samples were normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for XIAP and β-actin. Immunoblot data were quantified by scanning densitometry, normalized for β-actin expression, and compared with a sample of normal peripheral blood stem cells as an internal reference control. The figure shows the relative level of XIAP protein expressed as a fold increase over the reference standard for patient samples dichotomized by their response to the XIAP inhibitor (LD50 < 10 μM or LD50 ≥ 10 μM). Horizontal lines represent the median XIAP protein level for the group.
Correlation of XIAP levels with in vitro response to XIAP antagonists. Protein lysates were prepared from 21 of 27 AML samples. Samples were normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for XIAP and β-actin. Immunoblot data were quantified by scanning densitometry, normalized for β-actin expression, and compared with a sample of normal peripheral blood stem cells as an internal reference control. The figure shows the relative level of XIAP protein expressed as a fold increase over the reference standard for patient samples dichotomized by their response to the XIAP inhibitor (LD50 < 10 μM or LD50 ≥ 10 μM). Horizontal lines represent the median XIAP protein level for the group.
Studies of XIAP inhibitor 1396-12 in combination with Ara-C. (A) OCI AML2 and Jurkat leukemia cells (6.25 × 105/mL) were treated with increasing concentrations of 1396-12 with (▴) or without (▪) Ara-C. Twelve hours after incubation, apoptosis was measured by annexin V staining. (B) HL60 leukemia cells (6.25 × 105/mL) or primary AML patient samples (AML Pt 1-3) (× 105/mL) were treated with increasing concentrations of Ara-C alone (▪) or in combination with 8 μM (HL60) or 10 μM (AML patient samples) of the active XIAP inhibitor 1396-12 (▴) or the inactive control 1396-28 (•). Apoptosis was measured by annexin V staining 48 hours after incubation. The mean percentage plus or minus SD of viable cells is shown.
Studies of XIAP inhibitor 1396-12 in combination with Ara-C. (A) OCI AML2 and Jurkat leukemia cells (6.25 × 105/mL) were treated with increasing concentrations of 1396-12 with (▴) or without (▪) Ara-C. Twelve hours after incubation, apoptosis was measured by annexin V staining. (B) HL60 leukemia cells (6.25 × 105/mL) or primary AML patient samples (AML Pt 1-3) (× 105/mL) were treated with increasing concentrations of Ara-C alone (▪) or in combination with 8 μM (HL60) or 10 μM (AML patient samples) of the active XIAP inhibitor 1396-12 (▴) or the inactive control 1396-28 (•). Apoptosis was measured by annexin V staining 48 hours after incubation. The mean percentage plus or minus SD of viable cells is shown.
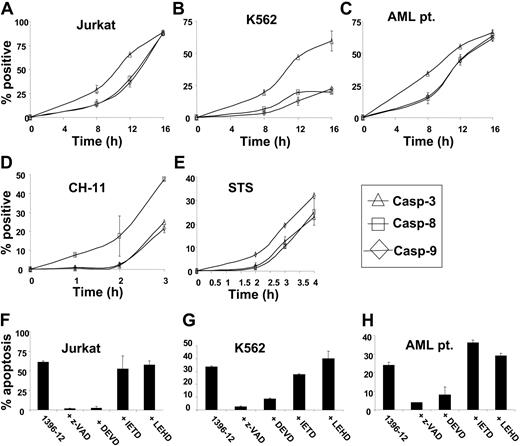
XIAP inhibitor 1396-12 activates effector caspases. XIAP inhibitor 1396-12 activates effector caspases as an early event: Jurkat (A), K562 (B), and primary AML (C) leukemic cells (6 × 105/mL) were treated with the XIAP inhibitor 1396-12 (10 μM) for increasing periods of time. As controls, Jurkat cells were treated with CH-11 anti-Fas antibody (100 ng/mL; D) or staurosporine (STS; E) for increasing periods of time. After treatment, the mean percentage plus or minus SD of cells above control with active caspase 3 (▵), caspase 8 (□), and caspase 9 (⋄) was determined. Inhibition of effector caspases blocks XIAP inhibitor-induced apoptosis: Jurkat (F), K562 (G), and primary AML leukemic cells (H) (6 × 105/mL) were treated with the XIAP inhibitor 1396-12 (10 μM) for 12 hours with or without the pan-caspase inhibitor z-VAD, the preferential effector caspase inhibitor DEVD, the preferential caspase 8 inhibitor IETD, or the preferential caspase 9 inhibitor LEHD. After treatment, apoptosis was measured by annexin V staining. The mean percentage plus or minus SD of apoptotic cells above control is shown.
XIAP inhibitor 1396-12 activates effector caspases. XIAP inhibitor 1396-12 activates effector caspases as an early event: Jurkat (A), K562 (B), and primary AML (C) leukemic cells (6 × 105/mL) were treated with the XIAP inhibitor 1396-12 (10 μM) for increasing periods of time. As controls, Jurkat cells were treated with CH-11 anti-Fas antibody (100 ng/mL; D) or staurosporine (STS; E) for increasing periods of time. After treatment, the mean percentage plus or minus SD of cells above control with active caspase 3 (▵), caspase 8 (□), and caspase 9 (⋄) was determined. Inhibition of effector caspases blocks XIAP inhibitor-induced apoptosis: Jurkat (F), K562 (G), and primary AML leukemic cells (H) (6 × 105/mL) were treated with the XIAP inhibitor 1396-12 (10 μM) for 12 hours with or without the pan-caspase inhibitor z-VAD, the preferential effector caspase inhibitor DEVD, the preferential caspase 8 inhibitor IETD, or the preferential caspase 9 inhibitor LEHD. After treatment, apoptosis was measured by annexin V staining. The mean percentage plus or minus SD of apoptotic cells above control is shown.
To further explore sensitization to Ara-C chemotherapy, leukemic cells that displayed little sensitivity to Ara-C were treated with increasing concentrations of Ara-C with or without 8 μM of the active XIAP anatagonist 1396-12 or the inactive control compound 1396-28. In cultured leukemia cell lines and primary AML patient samples that were mostly refractory to Ara-C, the addition of 1396-12 resulted in little or no additional cell killing. Thus, 1396-12 does not appear to restore sensitivity to Ara-C. Therefore, at least for Ara-C-insensitive AMLs, polyphenylurea XIAP antagonists are active as single agents, but do not appear to potentiate cytotoxic responses when combined with Ara-C, a conventional chemotherapeutic agent used in the treatment of acute leukemia.
Caspase activation is induced by treatment of AML cells with the XIAP-antagonists
To examine the mechanism of action of the polyphenylurea-based XIAP inhibitors, we assessed the sequence of caspase activation in leukemic cell lines and primary AML blasts treated with the XIAP antagonist 1396-12. At increasing times after treatment, active caspases 3, 7, 8, and 9 were detected by adding cell-permeable, FITC-labeled peptides that bind preferentially and irreversibly to the active caspases. In leukemia cell lines as well as primary AML blasts, the XIAP antagonist 1396-12 activated caspase 3 and caspase 7 prior to caspase 8 and caspase 9 (P < .01 by ANOVA; Figure 5A-C). In keeping with known amplification loops in the caspase activation pathways,30 activation of caspase 8 and caspase 9 was observed after effector caspase activation. In contrast to the XIAP inhibitor, treatment of cells with CH-11 anti-Fas antibody (100 ng/mL), a known stimulant of the death receptor pathway, activated caspase 8 before caspase 3 and caspase 9 (Figure 5D). Similarly, staurosporine, a known stimulant of the mitochondrial pathway, activated caspase 9 before caspase 3 and caspase 8 (Figure 5E). Taken together, these results suggest that the activation of the downstream caspase 3 and caspase 7 by the XIAP antagonists occurs prior to the activation of upstream caspase 8 and caspase 9. The findings are consistent with the compound targeting the BIR2 domain of XIAP, which binds and suppresses effector caspases. In contrast, death receptor ligands and mitochondrial stimulants activate upstream caspases prior to downstream effector caspases.
To confirm that the XIAP inhibitor 1396-12 induces apoptosis by activating effector caspases, we assessed the effects of selective caspase inhibitors on 1396-12-induced apoptosis. Jurkat and K562 leukemia cells as well as a primary AML sample were treated with 1396-12 (10 μM) for 12 hours with or without caspase inhibitors. After incubation, apoptosis was measured by annexin V staining. The pan-caspase inhibitor z-VAD and the preferential effector caspase inhibitor Ac-DEVD inhibited 1396-12-induced apoptosis. In contrast, the preferential caspase 8 inhibitor Ac-IETD and the caspase 9 inhibitor Ac-LEHD did not block 1396-12-induced apoptosis (Figure 5F-H). As a positive control, Ac-LEHD and Ac-IETD inhibited staurosporine- and CH-11-induced apoptosis, respectively (data not shown). These data therefore suggest 1396-12 acts at a distal point in apoptotic pathways.
XIAP antagonists induce apoptosis independent of Bcl-2 and caspase 8
To further evaluate whether chemical XIAP antagonists induce apoptosis by directly activating caspase 3 and caspase 7 independent of caspase 8 and caspase 9, we studied XIAP antagonist-induced apoptosis in leukemia cells that had defects in either the mitochondrial or death receptor pathway of caspase activation.31,32
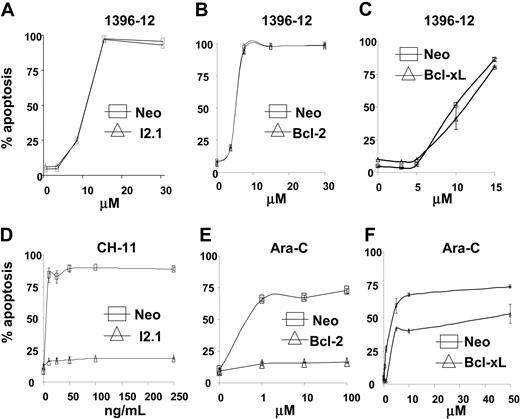
Overexpression of Bcl-2 or Bcl-XL blocks the mitochondrial pathway for caspase activation and renders leukemia cells resistant to conventional chemotherapeutic agents such as Ara-C or VP-16.33 In contrast, the absence of caspase 832 selectively blocks the death receptor pathway of caspase activation and renders cells resistant to Fas-ligand. We hypothesized that antagonists of the BIR2 domain of XIAP should induce apoptosis independent of these upstream blocks in the mitochondrial or death receptor pathways. To test our hypothesis, Jurkat cells stably overexpressing Bcl-2 or a Jurkat cell line with absent caspase 8 (I2.1),32 and a U937 cell line overexpressing Bcl-XL were treated with increasing concentrations of the XIAP antagonist 1396-12. Overexpression of Bcl-2 or Bcl-XL, or the absence of caspase 8 had no effect on the toxicity of the XIAP inhibitor 1396-12, but protected against apoptosis induced by Ara-C and CH-11 anti-Fas antibody, respectively (Figure 6). Thus consistent with our earlier reports,19,20 these data support the hypothesis that the polyphenylurea-based XIAP inhibitors induce apoptosis by activating or facilitating the downstream portion of the caspase activation pathways, independent of Bcl-2 and caspase 8, and in a manner distinct from currently available chemotherapeutic agents.
XIAP inhibitor 1396-12 induces apoptosis independent of Bcl-2, Bcl-XL, and caspase 8. Jurkat cells lacking caspase 8 (I2.1)32 (A,▵), Jurkat cells overexpressing Bcl-231 (B,▵), U937 cells overexpressing Bcl-xL (C, ▵), or the corresponding vector controls (6 × 105/mL; □) were treated with increasing concentrations of the XIAP inhibitor 1396-12. As controls, Jurkat cells lacking caspase 8 (D, ▵) were treated with increasing concentrations of CH-11 anti-Fas antibody (100 ng/mL). Also, Jurkat cells (E) and U937 cells (F) overexpressing Bcl-2 or Bcl-xL (▵), respectively, were treated with increasing concentrations of Ara-C. Apoptosis was measured by annexin V staining. The mean plus or minus SD of 3 independent experiments is shown.
XIAP inhibitor 1396-12 induces apoptosis independent of Bcl-2, Bcl-XL, and caspase 8. Jurkat cells lacking caspase 8 (I2.1)32 (A,▵), Jurkat cells overexpressing Bcl-231 (B,▵), U937 cells overexpressing Bcl-xL (C, ▵), or the corresponding vector controls (6 × 105/mL; □) were treated with increasing concentrations of the XIAP inhibitor 1396-12. As controls, Jurkat cells lacking caspase 8 (D, ▵) were treated with increasing concentrations of CH-11 anti-Fas antibody (100 ng/mL). Also, Jurkat cells (E) and U937 cells (F) overexpressing Bcl-2 or Bcl-xL (▵), respectively, were treated with increasing concentrations of Ara-C. Apoptosis was measured by annexin V staining. The mean plus or minus SD of 3 independent experiments is shown.
Discussion
XIAP is a member of the IAP family of proteins and functions as a potent downstream inhibitor of apoptosis by specifically blocking active caspases 3, 7, and 9 but not other caspases. Overexpression of IAPs contributes to chemoresistance and poor patient outcome.23,34,35 Therefore, agents that reverse IAP-mediated repression of caspases may be therapeutically useful. Recently, we identified a series of chemical XIAP inhibitors based on the polyphenylurea pharmacophore that bind and inhibit the BIR2 domain of XIAP responsible for suppressing caspase 3 and caspase 7, but do not bind or inhibit the BIR3 domain.19,20 As such, these compounds derepress IAP inhibition of caspase 3 and caspase 7 but not caspase 9.
In this report, we demonstrated that the XIAP antagonists induce apoptosis of AML cell lines and primary AML specimens at low micromolar concentrations. We determined that the most potent XIAP antagonist 1396-12 induced apoptosis with a LD50 of less than 10 μM in approximately 60% of the samples tested. The compounds were equally active in samples from treatment-naive and relapsed patients. Likewise, they demonstrated activity in samples from patients with poor risk cytogenetics. In short-term cytotoxicity assays, the XIAP antagonists were less toxic to primary normal hematopoietic cells. A preferential toxicity for malignant cells agrees with previous xenograft studies that showed the XIAP antagonists decreased tumor growth, but were not toxic to mice at doses of 30 mg/kg daily. Mice receiving single doses of up to 200 mg/kg or daily doses of 30 mg/kg for one week had no change in weight and no decrease in their red cell, white cell, or platelet counts19 (and unpublished data, A.D.S., C.P., and J.C.R., June 2003).
The differential sensitivity between malignant and normal primary cells to an overnight exposure to the XIAP antagonists suggests that there is a fundamental difference in the state of caspase activation in tumor versus normal cells. One can envision that malignant cells, through mechanisms including dysregulation of proto-oncogenes, hypoxia, and cell cycle disruption, have a drive to activate caspases. This drive may be blocked by IAP expression.36 In contrast, normal cells would be expected to have fewer drives to activate caspases. Thus, derepressing XIAP may be more toxic to malignant cells than normal cells. In support of this hypothesis, levels of XIAP and active caspase 3 tend to be higher in malignant cells than in normal cells.37 In addition, IAP knockout mice reveal little or no pathology.38
In contrast to the short-term cytotoxicity assays, clonogenic survival assays revealed small differences in the sensitivity of normal PBSCs and primary AML cells to the XIAP inhibitor. These discordant results likely reflect the unique cell population under study. In the short-term cytotoxicity assays, the entire cell population is evaluated, whereas in the colony formation assay only 0.1% of primary normal or AML cells routinely formed colonies. Thus, the colony formation assay measures the toxicity of the compound on a small subset of colony-forming malignant and normal cells. Results of colony formation assays do not always translate into clinical toxicity. For example, chemotherapeutic agents such as cytarabine or amsacrine (m-AMSA) that are used in the treatment of AML show little or no selectivity for malignant cells over normal cells in colony formation assays, but are valuable therapeutic agents.39,40 Nonetheless, the small differential sensitivity between PBSCs and primary AML cells after treatment with the XIAP antagonists raises concerns about their potential hematologic toxicity and their safety will have to be carefully evaluated in preclinical and phase 1 clinical trials.
To understand why some primary AML samples are sensitive to the polyphenylurea XIAP antagonists while others are more resistant, we correlated response to the inhibitors with intracellular levels of the IAP proteins. We determined that resistance to the compounds was primarily seen in samples with very low to absent levels of XIAP protein. In these patients, factors other than XIAP may be important contributors to disease pathogenesis or blocks in caspase activation. In that case, due to the lack of the target, derepression of XIAP would have no effect on cell death. Consequently, these findings validate the mechanisms of our polyphenylurea-based XIAP antagonists.
Variations in the sensitivity and shape of the dose response curves among cell lines could represent differences in IAP levels between the cell lines or differences in the uptake, protein binding, or degradation of the compounds. Variations in response may also represent subtle differences in the affinities of the compounds for the various IAP family members. Alternatively, it may reflect the presence of other factors important for tumor survival that influence the activity of IAPs in protecting cells against apoptosis. As not all IAP family members contain a BIR2 domain, we anticipate that the polyphenylurea XIAP inhibitors will not inhibit all of the IAPs. Currently, studies are underway to compare the binding of the compounds to the BIR domains of the different IAP family members as well as to assess the stability and solubility of the compounds. These studies may also help to explain variations in sensitivity to the inhibitors.
The polyphenylurea XIAP antagonist 1396-12 was active as a single agent, directly inducing apoptosis. In contrast, small-molecule and peptidyl SMAC-mimics sensitize cells to chemotherapy, but are generally inactive as single agents.41-44 Differences in induction of cell death by polyphenylurea XIAP antagonists versus the SMAC-mimics may relate to their different effects on caspases. Peptidyl and chemical SMAC-mimics derepress caspase 9 by binding the BIR3 domain of XIAP, but caspases 3 and 7 remain blocked as SMAC-mimics do not efficiently inhibit the BIR2 domain of XIAP.6,8,45 The polyphenylurea compounds, in contrast, do not bind or inhibit the BIR3 domain of XIAP. Rather, they inhibit the BIR2 domain, thereby relieving the repression of the downstream caspases 3 and 7.19,20 Through positive feedback loops, caspases 3 and 7 also activate upstream caspase 8 and caspase 9. Thus, the ability to derepress downstream caspases directly may explain why the polyphenylurea XIAP antagonists directly induce apoptosis while peptidyl and small-molecule SMAC-mimics are mostly chemosensitizers. Alternatively, the polyphenylurea XIAP inhibitors may have other actions related or unrelated to their role as XIAP inhibitors.
The polyphenylurea XIAP antagonists act through a mechanism distinct from conventional apoptotic stimuli. Chemotherapeutic agents such as staurosporine and VP-16 stimulate the mitochondrial pathway of caspase activation and activate caspase 9 as an early event. Agents such as Fas and TNF-related apoptosis-inducing ligand (TRAIL) stimulate the death receptor pathway and initially activate caspase 8 and caspase 10. In this report, we demonstrated that the polyphenylurea-based XIAP antagonists activated caspase 3 prior to caspase 8 and caspase 9, and their toxicity was not affected by overexpression of Bcl-2 or the absence of caspase 8. These results are consistent with a XIAP BIR2 domain-dependent mechanism of action, where derepressing downstream effector caspases accounts for our observation of caspases 3 and 7 activation before caspase 8 and caspase 9. Thus, these compounds may bypass upstream defects in the apoptosis pathway, which are common in cancers and leukemias.
In conclusion, the polyphenylurea-based XIAP antagonists we recently identified directly induce apoptosis of AML cell lines and primary AML patient samples at low micromolar concentrations. By directly derepressing downstream caspases, these agents are mechanistically distinct from conventional chemotherapeutic agents and, as such, can bypass common blocks in apoptosis pathways. These antagonists can be used as biologic tools to understand the role of IAPs in normal and malignant hematopoietic cells. In addition, they may serve as lead compounds for the development of useful therapies for the treatment of leukemia and other malignancies.
Prepublished online as Blood First Edition Paper, February 1, 2005; DOI 10.1182/blood-2004-08-3168.
Supported by grants from the Leukemia and Lymphoma Society, the Ladies Leukemia League, and the National Institutes of Health (CA-55164). A.D.S. is a Canadian Institutes of Health Research Clinician Scientist.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs. David Barth, Bill Brien, Hong Chang, Bruce Patterson, and Doug Tkachuk for their assistance in analyzing the pathology of the patient samples, and Francesca Pulice for assistance with manuscript preparation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal