Abstract
Due to high frequency of side effects caused by high-dose total body irradiation (TBI) the nonmyeloablative regimen together with cytotoxic agents is currently used especially for elderly patients. However, immediate and long-term effects of low-dose irradiation used in allogeneic transplantation on stem cells is less well known. We have studied the effect of low-dose 3 Gy TBI on the number of hematopoietic stem cell (HSC) clones contributing simultaneously to granulocyte production in rhesus macaque. The number of clones after 3 Gy TBI decreased markedly by 2 to 3 weeks after 3 Gy TBI, followed by a period of clonal instability, and recovery to almost pre–3 Gy TBI clonal diversity. The clones accounting for this recovery contributed before 3 Gy TBI, suggesting the profound initial impact of TBI was on a pool of progenitor cells, whereas most of the more primitive HSCs remained unaffected and were able to again contribute to hematopoiesis after recovery. Clonal fluctuation may indirectly suggest the presence of short-term/long-term HSC populations in rhesus macaque bone marrow as reported in a mouse model. The results indicate that even low-dose irradiation affects hematopoietic clonal dynamics and have implications for design of conditioning regimens for transplantation purposes.
Introduction
Total body irradiation (TBI) is used therapeutically in the treatment of hematopoietic malignancies and to condition recipients for hematopoietic stem cell (HSC) transplantation, in order to decrease competition from endogenous HSCs, suppress the immune system in order to prevent graft rejection, and eradicate residual tumor cells.1 TBI doses of 10 Gy or more are used to ablate residual hematopoiesis in patients who require autologous or allogeneic stem cell transplantation in order to recover hematopoiesis. Doses in the range of 1 to 5 Gy are much less well studied, but are beginning to be used in a wide variety of clinical applications, most notably in nonablative allogeneic stem cell transplantation.2 Since the side effects of irradiation in these dose ranges are much milder than following high-dose TBI, this approach has been used to extend allografting to older patients or those with serious organ dysfunction.3 However, further optimization and prediction of toxicity for this and other applications would be greatly aided by an understanding of the impact of TBI on stem cell dynamics in vivo.
We have developed an approach that allows direct tracking of individual stem and progenitor cell clones in a large-animal rhesus macaque model with direct relevance to human stem cell biology and transplantation.4 Animals received transplants of autologous primitive CD34+ hematopoietic cells transduced with standard helper-free retroviral vectors containing marker DNA sequences. After engraftment, animals can be followed long term for the presence of vector-containing progeny of primitive progenitor and stem cells. Since each vector insertion is stable and semirandom in the genome, a sensitive and specific linear amplification-mediated polymerase chain reaction (LAM-PCR) technique can be used to identify and track specific vector-genomic DNA junctions.5 Each individual junction is a unique tag for a stem or progenitor cell and all its progeny. We have previously reported that hematopoiesis is highly polyclonal and stably derived from many multilineage clones after transplantation in rhesus macaques.6 We have used this approach to directly investigate the impact of irradiation on in vivo hematopoietic dynamics. The results suggest that TBI, even at relatively low doses not resulting in significant hematopoietic suppression, profoundly but temporarily impacts on the number of stem and progenitor cell clones contributing to mature peripheral blood cell production. These findings have important implications for design of conditioning regimens prior to transplantation and also offer insights into the in vivo behavior of stem cells.
Materials and methods
Rhesus macaque transplantation and care
Rhesus macaques (Macaca mulatta) were housed and handled in accordance with the guidelines set by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (Department of Health and Human Services [DHHS] publication no. NIH 85-23). All protocols were approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. Stem cell factor/granulocyte-stimulating factor–mobilized peripheral blood CD34+ cells were collected from 2 animals (96E019 and 96E025) by aphaeresis, transduced with the retroviral vectors G1Na and/or LNL6, containing the neomycin phosphotransferase gene, and reinfused following 10 Gy TBI.7-9 While transduction conditions were the same in both animals, CD34+ enrichment and total colony-forming units (CFUs) were 31% and 35% lower, respectively, in animal 96E025 compared with animal 96E019. Details of the vectors, transduction procedures, and gene transfer efficiency in the first year following transplantation have been previously published for both animals.10 In the current study, 3 Gy total body gamma irradiation was delivered by a cesium source at a rate of 8.8 cGy/min. No stem cell rescue or transfusion support was given following this dose of TBI. Blood samples were collected periodically for blood cell counts. Granulocytes and mononuclear cells were separated via centrifugation over Lymphocyte Separation Medium (Organon Teknika, Durham, NC), with purities of more than 95%. DNA isolation of each cell fraction was carried out using the QIAamp DNA blood Midi kit (QIAGEN, Valencia, CA).
Linear amplification-mediated PCR (LAM-PCR)
The number of retrovirally marked progenitor or stem cell clones contributing to granulocyte production was determined using a modification of the previously described LAM-PCR technique.6 During the second round of exponential PCR, the long terminal repeat (LTR) III primer was labeled at the 5′ end with 5-carboxyfluorosein (HEX). From each sample, 2 μL PCR product was mixed with 12 μL formamide, and 0.5 μL GeneScan 1000 ROX Size Standards (Applied Biosystems, Warrington, United Kingdom). After denaturation for 2 minutes at 90°C, the products were applied to a sequencing capillary and separated, and automatic fluorescence quantification was used to size and determine the intensity of each band using GeneScan 672 Software, allowing precise sizing of amplification products (Applied Biosystems). Criteria for identification of true peaks on GeneScan analysis were set based on reproducible visualization of the same LAM-PCR product bands on Spreadex gel (Elchrom Scientific, Cham, Switzerland) electrophoresis, and confirmation that bands of an area under the curve of more than 10 000 represented true vector insertion sites as shown by sequencing of bands cut out from the gel.11
Cloning and sequencing
LAM-PCR–amplified fragments were isolated from Spreadex EL 1200 gels using the BandPick device (Elchrom Scientific, Cham, Switzerland). The isolated fragments were amplified for 30 cycles using a primer pair 5′-CCTTGCAAAATGGCGTTACT-3′ and 5′-AGTGGCACAGCAGTTAGG-3′. The amplification conditions were the same as in LAM-PCR nested amplification. The isolated fragment was cloned into TOPO pCR4 vector (Invitrogen, Carlsbad, CA) for sequencing.
Tracking of individual clones
The individual retrovirus integration clones identified by sequencing were tracked using specific PCR primers for the 5′LTR and flanking genomic sequence. Retrovirus integration clones were amplified from genomic DNA using nested LTR-primers 5′-CCTGTTTGGCCCATATTC-3′ and 5′-GCTAGCTTGCCAAACCTAC-3′. For the clone located at 3q26.2, nested flanking DNA sequence specific primers were 5′-GAAGGCCAAGGAACTTCACA-3′ and 5′-CTTGACTTGCCAGGCTAACT-3′. Flanking DNA sequence-specific nested primers for the clone located at 1p36.13 were 5′-GGCACATCTGCATGAGCTATGG-3′ and 5′-AGAGCTTGAGTGACCGGTGTGA-3′. For the clone 18q21.31, the flanking DNA-specific nested primers were 5′-GGAACACCAGGATAACCAGTAG-3′ and 5′-ATGGGTGAAGTTGGCTACAG-3′. The annealing temperature (TA) in the first reaction was 50°C and in the latter, 60°C. Both amplifications had 35 cycles. For the amplification of the integration clone at 18q21.2, the latter LTR primer was used together with 5′-AGCACTTCTTCTTAAAGCCTG-3′. The TA was 50°C and the reaction had 35 cycles.
Semiquantitative analysis
The analysis for relative contribution of the clones having integrations at 3q26.2 and 1p36.13 was done using the primers mentioned for tracking of individual clones. For both analyses, 250 ng genomic DNA was first amplified 18 cycles (TA 60°C), the PCR product was purified with Microspin S-400 HR columns (Amersham Biosciences, Freiburg, Germany), and 5 μL was used for semiquantitative amplification (40 cycles TA 60°C) with the ABI Prism 7700 Sequence Detector (Applied Biosystems) using SYBR Green PCR Core Reagents (Applied Biosystems). The signal representing the amount of integrated clones was normalized against the β-actin signal.
Marking level analysis
The retrovirus marking level (relative ratio of retrovirus genome containing cells versus total cells, assuming one insertion per transduced cell) was measured as previously described using PCR amplifying retroviral neomycin phosphotransferase and cellular β-actin sequences in DNA isolated from peripheral blood granulocytes at different time points before and after TBI.12 Nested PCR was run using 100 ng DNA as template. Band intensity after autoradiography was determined by PhosphoImager (Molecular Dynamics, Sunnyvale, CA), and copy number was calculated by linear regression analysis in comparison with a set of known copy number controls amplified simultaneously.
Statistical analysis
The statistical analysis was done using paired t tests for clone numbers between different time points in each animal.
Results
The rhesus macaque model has proved predictive of human stem cell behavior and transduction capabilities with potential impact on understanding human hematopoiesis.4 For the current study, we chose 2 animals that had previously received transplants of retrovirally transduced CD34+ cells (Figure 1). Standard amphotropic retroviral vectors were used for the transduction, carrying a neomycin phosphotransferase expected to have no impact on stem or progenitor cell behavior.13 We studied clonal contributions in granulocytes, which have a brief life span of fewer than 3 to 4 days in the blood and rapid maturation from primitive precursor cells, in both animals, since they best reflect ongoing hematopoiesis from stem or progenitor cells. We also studied clonal contributions in lymphocytes, which may have life spans of months to years, in one animal following 3 Gy TBI. We chose a dose of 3 Gy TBI, since this dose is within the range used in nonablative allogeneic transplantation protocols.14-16 Since the animals had previously undergone transplantation, we used a dose lower than naive animals had tolerated with moderate count suppression in our previous studies.17
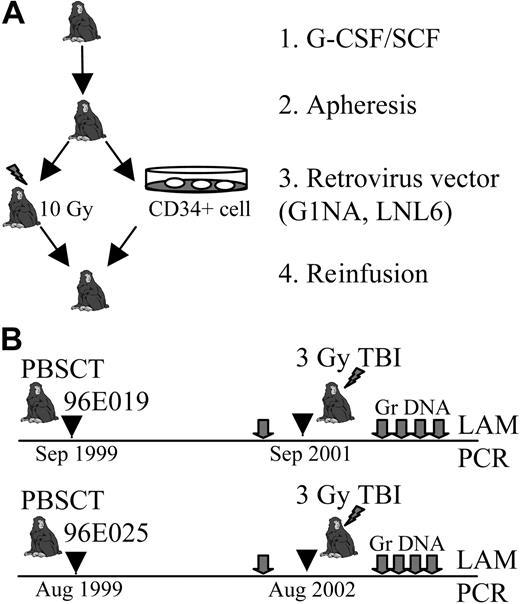
Study design. (A) Autologous peripheral blood stem cell transplantation (PBSCT). Cytokine-mobilized CD34+ cells were collected by apheresis from 2 animals, 96E019 and 96E025. Harvested cells were transduced with neomycin phosphotransferase retrovirus vectors (LNL6 and G1Na) and reinfused to monkeys after 10 Gy conditioning. G-CSF indicates granulocyte colony-stimulating factor; SCF, stem cell factor. (B) Timeline of the study. The animals received 3 Gy irradiation dose 2 years (96E019) and 3 years (96E025) after PBSCT. Blood samples (gray arrows) were collected for blood cell counts and granulocyte DNA (Gr DNA) isolation. The number of transduced clones contributing granulocyte production at each time point was determined by LAM-PCR analysis.
Study design. (A) Autologous peripheral blood stem cell transplantation (PBSCT). Cytokine-mobilized CD34+ cells were collected by apheresis from 2 animals, 96E019 and 96E025. Harvested cells were transduced with neomycin phosphotransferase retrovirus vectors (LNL6 and G1Na) and reinfused to monkeys after 10 Gy conditioning. G-CSF indicates granulocyte colony-stimulating factor; SCF, stem cell factor. (B) Timeline of the study. The animals received 3 Gy irradiation dose 2 years (96E019) and 3 years (96E025) after PBSCT. Blood samples (gray arrows) were collected for blood cell counts and granulocyte DNA (Gr DNA) isolation. The number of transduced clones contributing granulocyte production at each time point was determined by LAM-PCR analysis.
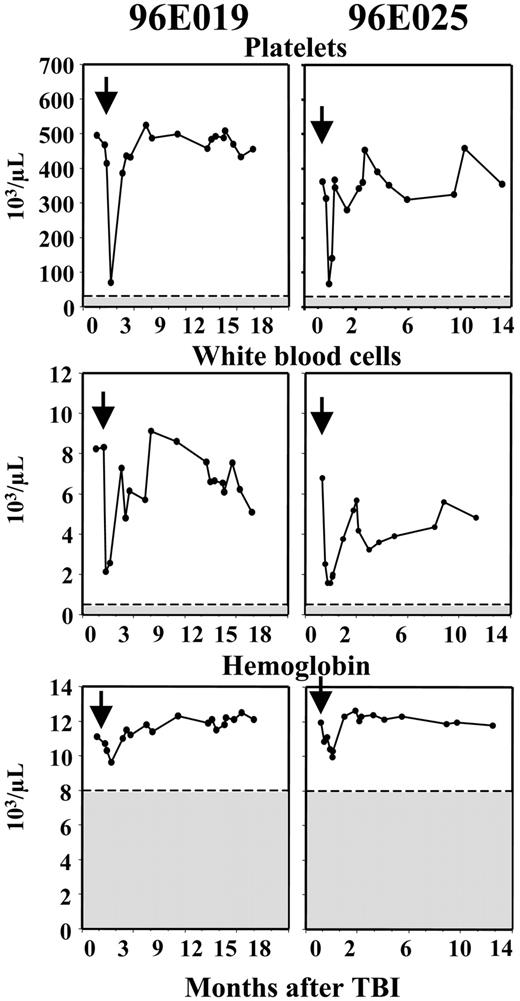
The blood cell counts showed a pattern of moderate and transient suppression following a single dose of 3 Gy TBI (Figure 2). There was no significant impact on red blood cell counts or hemoglobin concentrations, likely reflecting the prolonged life span of red blood cells in the circulation. The number of platelets and leukocytes decreased by one week following irradiation to approximately 25% of their baseline values and recovered to normal within one month. The decrease in leukocytes was primarily due to a marked drop in neutrophils, to only 5% of baseline, and a much less pronounced (minimum value 50% of baseline) and more transient drop in circulating lymphocyte numbers. These decreases were not associated with any clinical toxicity, and did not require transfusions or antibiotic support.
Blood cell counts following 3Gy TBI. Arrows indicate the administration of 3 Gy TBI. Shadowed areas indicate clinically significant thresholds for cytopenias. The nadir counts for 96E019 and 96E025, respectively, were 74 × 103/μL and 66 × 103/μL (platelets), 2.13 × 103/μL and 1.43 × 103/μL (white blood cells), and 9.62 × 103/μL and 9.58 × 103/μL (hemoglobin). The decrease in blood cell counts was mild and transient in both animals and did not cause any discernable symptoms.
Blood cell counts following 3Gy TBI. Arrows indicate the administration of 3 Gy TBI. Shadowed areas indicate clinically significant thresholds for cytopenias. The nadir counts for 96E019 and 96E025, respectively, were 74 × 103/μL and 66 × 103/μL (platelets), 2.13 × 103/μL and 1.43 × 103/μL (white blood cells), and 9.62 × 103/μL and 9.58 × 103/μL (hemoglobin). The decrease in blood cell counts was mild and transient in both animals and did not cause any discernable symptoms.
Semirandom integration of retroviruses into host genome enables identification of the presence of progeny of individual stem or progenitor cell clones, using the LTR-genomic DNA insertion site junctions as unique tags. The number of individual retrovirally marked stem and progenitor cell clones contributing to granulocyte and lymphocyte production was quantified using LAM-PCR followed by GeneScan analysis to identify individual junctions present in a polyclonal population of granulocytes and lymphocytes.6,11 Because the animals have polyclonal hematopoiesis with as many as 50 to 100 different clones contributing to overall marking levels of 1% to 10%, sampling of a single 100-ng sample of DNA will not detect all contributing clones, even assuming 100% efficiency of LAM-PCR amplification. Prior analysis has shown that 5 copies of a single vector insertion can be detected within a 100-ng sample containing nontransduced cells or cells with different vector insertions. However, 100 ng DNA corresponds to only 10 000 cells at most, and with a marking level of 5% and 100 clones contributing, 100 ng DNA contains close to the detectable limit of each individual clone, even if each clone contributes equally and amplification is maximally efficient. Increasing the amount of DNA per reaction to more than 100 ng results in interference, thus replicates of 100-ng samples were tested, and we have previously reported that by increasing the number of replicates, a more complete representation of all clones present could be obtained, with a maximum number of clones detected after approximately 18 replicates, in animals with marking levels of 0.5% to 10%.11 Thus for each sample, 3 independent amplifications of 6 replicates were performed to determine contributing clone numbers.
As expected based on our previous reports, both animals had polyclonal and stable clonal contributions to granulocyte and lymphocyte production at time points preceding the TBI treatment. In animal 96E019, there was a rapid and significant (P < .05) decrease in the number of independent clones contributing to granulocyte production within 1 to 3 weeks of 3 Gy TBI. This decrease was followed by a 6-month period of marked clonal fluctuation, finally succeeded by a stable recovery (P < .01) with contributing clone numbers approaching the pre–3 Gy TBI levels (Figure 3A). In the second animal, 96E025, a similar decline (P < .001) in contributing clones was seen following 3 Gy TBI, with a more extended period of clonal disequilibrium. At least partial recovery close to baseline polyclonality was finally observed at 1 year 2 months after 3 Gy TBI (Figure 3B). The clonal contributions to lymphocyte production were studied in animal 96E025. Before TBI, the mononuclear cell fraction, consisting of 90% T lymphocytes, showed similar clonal diversity as did granulocytes before TBI. There was no significant change in clone numbers contributing to lymphocytes before and after 3 Gy TBI (Figure 3C).
The average number of transduced stem/progenitor clones contributing to blood cell production. (A) Animal 96E019 granulocytes. (B) Animal 96E025 granulocytes. (C) Animal 96E025 mononuclear cells (> 95% lymphocytes). Arrows indicate the time of 3 Gy TBI. P values are determined by comparing the time point immediately before 3 Gy TBI with subsequent time points after 3 Gy TBI as shown. The clone number refers to the average number of clones from 3 independent LAM-PCRs consisting of 6 replicates. (A) In animal 96E019, the clone numbers immediately after TBI were decreased significantly (P < .05), and the recovery from the lowest value after TBI to the last follow-up was significantly increased (P < .01). (B) In the animal 96E025, the immediate decrease was more severe (P < .001) and clonal fluctuation has persisted for the entire follow-up period. There was at least a partial recovery in clonal diversity (P < .05) by 14 months after 3 Gy TBI. (C) Lymphocyte fraction (mononuclear cell [MNC]) from animal 96E025 did not show any significant changes in clonal numbers (P = not significant [ns]) after 3 Gy low-dose TBI despite the significant impact on clonal derivation of granulocytes as shown in panel B. Error bars represent standard deviation.
The average number of transduced stem/progenitor clones contributing to blood cell production. (A) Animal 96E019 granulocytes. (B) Animal 96E025 granulocytes. (C) Animal 96E025 mononuclear cells (> 95% lymphocytes). Arrows indicate the time of 3 Gy TBI. P values are determined by comparing the time point immediately before 3 Gy TBI with subsequent time points after 3 Gy TBI as shown. The clone number refers to the average number of clones from 3 independent LAM-PCRs consisting of 6 replicates. (A) In animal 96E019, the clone numbers immediately after TBI were decreased significantly (P < .05), and the recovery from the lowest value after TBI to the last follow-up was significantly increased (P < .01). (B) In the animal 96E025, the immediate decrease was more severe (P < .001) and clonal fluctuation has persisted for the entire follow-up period. There was at least a partial recovery in clonal diversity (P < .05) by 14 months after 3 Gy TBI. (C) Lymphocyte fraction (mononuclear cell [MNC]) from animal 96E025 did not show any significant changes in clonal numbers (P = not significant [ns]) after 3 Gy low-dose TBI despite the significant impact on clonal derivation of granulocytes as shown in panel B. Error bars represent standard deviation.
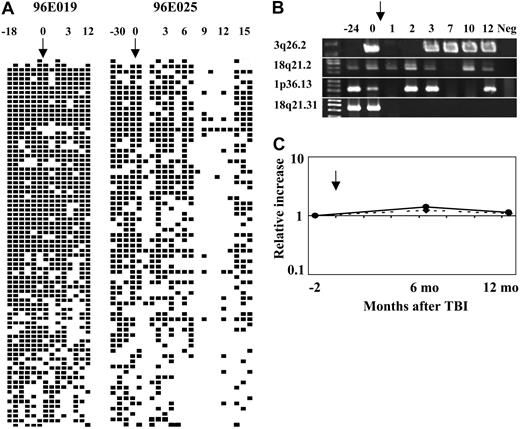
GeneScan analysis and confirmation with tracking primers indicated that recovery of clone numbers after 3 Gy TBI did not generally represent activation of previously completely quiescent or undetectable stem cell clones, but instead represented recovery of contribution from clones also detected before 3 Gy TBI (Figure 4A). The clonal responses to 3 Gy TBI were confirmed by individual tracking of 4 individual independent retroviral integration sites using insertion site–specific primers for representative clones (Figure 4B). Clones having integrations at 3q26.2, 18q21.2, 1p36.13, and 18q21.31 were amplified from different time points and showed persistent presence and variable detection correlating with findings on LAM-PCR and GeneScan analysis. Semiquantitative analysis of the clones integrated at 3q26.2 and 1p36.13 indicates that the relative contributions from these clones were relatively constant after recovery from TBI (Figure 4C).
Schematic summary of the detection of individual clones contributing to granulocyte production before and after 3 Gy TBI. (A) The left-hand panel shows results from animal 96E019 and right-hand panel results from animal 96E025, and includes all clones detected at least once in a total of 18 separate LAM-PCR reactions (3 independent sets of 6 replicates). ▪ represents the detection of a specific clone at least once from a given time; □, no detection of a specific clone; and ↓, the time of 3 Gy TBI. Each column represents a separate time point: animal 96E019 6 months after transplantation, 7 months after transplantation, 9 months after transplantation, 1 year after transplantation, 1 year 11 months after transplantation, 2 years after transplantation, 1 week after 3 Gy TBI, 1 month after 3 Gy TBI, 2 months after 3 Gy TBI, 4 months after 3 Gy TBI, 5 months after 3 Gy TBI, 6 months after 3 Gy TBI, 9 months after 3 Gy TBI, 1 year after 3 Gy TBI; animal 96E025 7 months after transplantation, 9 months after transplantation, 1 year after transplantation, 3 years after transplantation, 1 week after 3 Gy TBI, 2 weeks after 3 Gy TBI, 3 weeks after 3 Gy TBI, 1 month after 3 Gy TBI, 2 months after 3 Gy TBI, 3 months after 3 Gy TBI, 4 months after 3 Gy TBI, 5 months after 3 Gy TBI, 6 months after 3 Gy TBI, 7 months after 3 Gy TBI, 8 months after 3 Gy TBI, 9 months after 3 Gy TBI, 10 months after 3 Gy TBI, 11 months after 3 Gy TBI, 1 year after 3 Gy TBI, 1 year 2 months after 3 Gy TBI, 1 year 3 months after 3 Gy TBI, 1 year 4 months after 3 Gy TBI. The arrows indicate the time of 3 Gy TBI. Numbering above panels refers to time in months before and after 3 Gy TBI. (B) Use of insertion-specific PCR tracking primers to verify presence or absence of individual clones. The arrow indicates the time of 3 Gy TBI, 3 years after initial transplantation in animal 96E025. There were 4 insertion-specific primers designed to amplify individual insertion site clones from animal 96E025, initially identified from LAM-PCR on the pre-TBI sample. Each insertion-specific primer was used along with an LTR primer to amplify an individual insertion. Those clones chosen included 3q26.2 within the mds1 gene, 18q21.2 within the dcc gene, 1p36.13 within a repeat element, and 18q21.31 within the hak gene. The time points for amplification were 1 (1 year after transplantation); 2 (2 years 10 months after transplantation); 3 (3 weeks after 3 Gy TBI); 4 (2 months after 3 Gy TBI); 5 (3 months after 3 Gy TBI); 6 (7 months after 3 Gy TBI); 7 (10 months after 3 Gy TBI); 8 (1 year after 3 Gy TBI); 9 (negative control normal rhesus macaque DNA). This clone-specific PCR confirms the clonal instability seen by LAM-PCR, with many clones disappearing and reappearing, and a minority not detectable at the most recent follow-up, possibly indicating complete deletion. (C) Semiquantitative analysis of the level of contribution of clones 3q26.2 (straight line) and 1p36.13 (dashed line) to granulocytes from animal 96E025. The arrow indicates the time of 3 Gy TBI. The relative clonal contribution was normalized against β-actin, and the first time point (3 months before 3 Gy TBI) was chosen as a baseline level. By 6 and 12 months after TBI, the relative contribution to granulopoiesis from these clones was stable and not significantly changed compared with baseline.
Schematic summary of the detection of individual clones contributing to granulocyte production before and after 3 Gy TBI. (A) The left-hand panel shows results from animal 96E019 and right-hand panel results from animal 96E025, and includes all clones detected at least once in a total of 18 separate LAM-PCR reactions (3 independent sets of 6 replicates). ▪ represents the detection of a specific clone at least once from a given time; □, no detection of a specific clone; and ↓, the time of 3 Gy TBI. Each column represents a separate time point: animal 96E019 6 months after transplantation, 7 months after transplantation, 9 months after transplantation, 1 year after transplantation, 1 year 11 months after transplantation, 2 years after transplantation, 1 week after 3 Gy TBI, 1 month after 3 Gy TBI, 2 months after 3 Gy TBI, 4 months after 3 Gy TBI, 5 months after 3 Gy TBI, 6 months after 3 Gy TBI, 9 months after 3 Gy TBI, 1 year after 3 Gy TBI; animal 96E025 7 months after transplantation, 9 months after transplantation, 1 year after transplantation, 3 years after transplantation, 1 week after 3 Gy TBI, 2 weeks after 3 Gy TBI, 3 weeks after 3 Gy TBI, 1 month after 3 Gy TBI, 2 months after 3 Gy TBI, 3 months after 3 Gy TBI, 4 months after 3 Gy TBI, 5 months after 3 Gy TBI, 6 months after 3 Gy TBI, 7 months after 3 Gy TBI, 8 months after 3 Gy TBI, 9 months after 3 Gy TBI, 10 months after 3 Gy TBI, 11 months after 3 Gy TBI, 1 year after 3 Gy TBI, 1 year 2 months after 3 Gy TBI, 1 year 3 months after 3 Gy TBI, 1 year 4 months after 3 Gy TBI. The arrows indicate the time of 3 Gy TBI. Numbering above panels refers to time in months before and after 3 Gy TBI. (B) Use of insertion-specific PCR tracking primers to verify presence or absence of individual clones. The arrow indicates the time of 3 Gy TBI, 3 years after initial transplantation in animal 96E025. There were 4 insertion-specific primers designed to amplify individual insertion site clones from animal 96E025, initially identified from LAM-PCR on the pre-TBI sample. Each insertion-specific primer was used along with an LTR primer to amplify an individual insertion. Those clones chosen included 3q26.2 within the mds1 gene, 18q21.2 within the dcc gene, 1p36.13 within a repeat element, and 18q21.31 within the hak gene. The time points for amplification were 1 (1 year after transplantation); 2 (2 years 10 months after transplantation); 3 (3 weeks after 3 Gy TBI); 4 (2 months after 3 Gy TBI); 5 (3 months after 3 Gy TBI); 6 (7 months after 3 Gy TBI); 7 (10 months after 3 Gy TBI); 8 (1 year after 3 Gy TBI); 9 (negative control normal rhesus macaque DNA). This clone-specific PCR confirms the clonal instability seen by LAM-PCR, with many clones disappearing and reappearing, and a minority not detectable at the most recent follow-up, possibly indicating complete deletion. (C) Semiquantitative analysis of the level of contribution of clones 3q26.2 (straight line) and 1p36.13 (dashed line) to granulocytes from animal 96E025. The arrow indicates the time of 3 Gy TBI. The relative clonal contribution was normalized against β-actin, and the first time point (3 months before 3 Gy TBI) was chosen as a baseline level. By 6 and 12 months after TBI, the relative contribution to granulopoiesis from these clones was stable and not significantly changed compared with baseline.
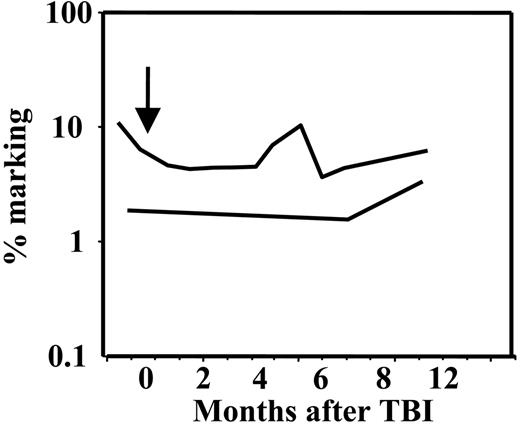
The level of vector-containing cells was stable in both animals before and after 3 Gy TBI treatment. At 2 years after the initial transplantation, the retroviral marking level was 4% to 10% of circulating cells in 96E019, and remained at the same overall level after 3 Gy TBI despite clonal fluctuation and overall fewer clones contributing immediately following 3 Gy TBI. The marking level in the second monkey was lower, 1.5% to 3.0%, and remained at this level after 3 Gy TBI (Figure 5). This suggests that the transduced clones were not inherently more susceptible to radiation effects.
Overall retroviral marking levels. The arrow indicates the time of 3 Gy TBI. The upper line corresponds to animal 96E019 and the lower line, to animal 96E025. The marking was stable in both animals after low-dose 3 Gy TBI, despite marked clonal fluctuation. The marking level for the animal 96E025 was 4% to 10% and for the animal 96E025, 1.5% to 3%.
Overall retroviral marking levels. The arrow indicates the time of 3 Gy TBI. The upper line corresponds to animal 96E019 and the lower line, to animal 96E025. The marking was stable in both animals after low-dose 3 Gy TBI, despite marked clonal fluctuation. The marking level for the animal 96E025 was 4% to 10% and for the animal 96E025, 1.5% to 3%.
Discussion
The exquisite sensitivity of many tumor cells to radiation and the clear suppressive impact of radiation on bone marrow and lymphocyte function led to the pioneering and successful development of high-dose total body irradiation (TBI) as a conditioning regimen prior to transplantation of allogeneic or autologous bone marrow.18 More recently, there has been a growing realization that the therapeutic benefit of allogeneic transplantation for hematologic malignancies is related more to an immunologic graft-versus-tumor effect instead of to direct tumor killing by conditioning regimens. This has stimulated development of less toxic “nonmyeloablative” conditioning regimens, including low-dose TBI as a single agent or in combination with chemotherapy.19 Low-dose irradiation has also been explored as conditioning prior to infusion of autologous genetically modified or otherwise marked hematopoietic cells. Doses of 4 to 5 Gy appear almost as effective as ablative TBI in allowing engraftment of transduced cells in monkey models, but 1 to 2 Gy was insufficient for significant in vivo long-term engraftment with transduced cells.15,16 However, the impact of nonablative doses of TBI on stem cell behavior and survival is poorly understood, since allogeneic engraftment is determined not only by donor-versus-recipient stem cell competition but more importantly by the balance of graft-versus-marrow and host-versus-graft immune responses.
In our rhesus macaque model, we have the unique ability to identify and then follow the behavior of individual repopulating units over time, based on retroviral insertion sites serving as clonal tags for each transduced cell and its progeny.6 We have investigated the impact of 3 Gy TBI on hematopoiesis. This dose range was chosen based on evidence from murine models that it can allow engraftment of syngeneic cells, suggesting a significant impact on marrow progenitor and stem cell dynamics, as well as the prior nonhuman primate studies indicating tolerability and a significant impact of doses of 4 to 5 Gy in naive animals.15,17,20 The individual surviving progenitor clones were able to rapidly respond and expand, resulting in only transient and relatively mild peripheral blood cytopenias. Even 1 or 2 more cell divisions of committed progenitors will result in an exponential increase in the marrow output of mature cells.
Asignificant decrease in the number of contributing clones, followed by a period of clonal instability, was observed in both animals, with marked fluctuations in the number of clones contributing to production of mature granulocytes. Previous studies in murine models have shown that primitive long-term repopulating cells located in the bone marrow compartment are significantly less radiation sensitive than less quiescent short-term repopulating cells.21 Even though the presence of short-term and long-term repopulating cells has not been definitively proved in large-animal models, the clonal fluctuation could indirectly suggest that the same hierarchy documented in mice22 is also present in larger animals. The 3 Gy TBI may have affected these less primitive cells in the bone marrow, with loss of a significant number of short-term repopulating cells, which are able to rapidly respond to blood cell production needs, eventually followed by recovery and contribution from long-term HSCs. The fluctuations seen during recovery likely result from stochastic activation over time of quiescent very primitive cells, with eventual detection of their contributions. This is particularly evident in the setting of a small number of contributing clones, as previously demonstrated by Abkowitz et al in a computer modeling model of feline hematopoiesis following transplantation or busulfan administration.23 If the clonal fluctuation indicates the presence of short-term/long-term HSC populations, this would in turn suggest a stabilizing role in hematopoiesis for short-term HSCs in conditions when large amounts of mature blood cells are needed. Similar clonal fluctuation as seen with granulocyte production was not detected in lymphocytes. The dose of 3 Gy only very transiently and minimally decreased lymphocyte numbers, and the long life span of mature lymphocytes in the blood or lymph node most likely masked any impact on more primitive lymphoid progenitors.
The clones contributing to hematopoiesis at the time of recovery are virtually all the same clones detected as contributing prior to the 3 Gy TBI, ruling out recruitment of a previously completely quiescent population of HSCs, and in agreement with murine DNA labeling experiments showing that virtually all stem cells present in the marrow eventually cycle and contribute to hematopoiesis.24 Even though some of the clones may have disappeared at the time of low-dose TBI, there is no evidence for complete deletion of significant numbers of primitive long-term repopulating clones. However, we cannot rule out a permanent impact of low-dose irradiation on the self-renewal or stress-responsiveness of these primitive cells. In a murine study, doses as low as 1.6 Gy TBI produced profound defects in competitive repopulation ability, suggesting that even if HSCs survive, they may have defects in homing or expansion upon reaching the marrow.25
The prolonged time period before recovery of a more normal clonal pattern, even with this relatively low dose of irradiation, helps to explain the significant boost in engraftment seen after transplantation of donor cells following even very low-dose TBI (0.5-1.6 Gy) compared with no radiation in the murine syngeneic transplantation model.20,26 In this setting, we would hypothesize that normal nonirradiated HSCs home to the marrow and begin to cycle, proliferate, and self-renew in response to open marrow niches, and out-compete the remaining damaged and irradiated recipient HSCs.
In conclusion, our current results indicate that low-dose 3 Gy TBI can profoundly impact on hematopoietic activity in vivo without depleting hematopoietic stem cells, and thus should continue to be pursued, along with agents such as busulfan, as effective and safe conditioning prior to transplantation.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-04-1498.
M.O.L. was supported by the Academy of Finland, Sigrid Juselius Foundation, Paivikki and Sakari Sohlberg Foundation, and Leiras Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staff at 5 Research Court for excellent primate care during this study.

![Figure 3. The average number of transduced stem/progenitor clones contributing to blood cell production. (A) Animal 96E019 granulocytes. (B) Animal 96E025 granulocytes. (C) Animal 96E025 mononuclear cells (> 95% lymphocytes). Arrows indicate the time of 3 Gy TBI. P values are determined by comparing the time point immediately before 3 Gy TBI with subsequent time points after 3 Gy TBI as shown. The clone number refers to the average number of clones from 3 independent LAM-PCRs consisting of 6 replicates. (A) In animal 96E019, the clone numbers immediately after TBI were decreased significantly (P < .05), and the recovery from the lowest value after TBI to the last follow-up was significantly increased (P < .01). (B) In the animal 96E025, the immediate decrease was more severe (P < .001) and clonal fluctuation has persisted for the entire follow-up period. There was at least a partial recovery in clonal diversity (P < .05) by 14 months after 3 Gy TBI. (C) Lymphocyte fraction (mononuclear cell [MNC]) from animal 96E025 did not show any significant changes in clonal numbers (P = not significant [ns]) after 3 Gy low-dose TBI despite the significant impact on clonal derivation of granulocytes as shown in panel B. Error bars represent standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/3/10.1182_blood-2004-04-1498/6/m_zh80030573420003.jpeg?Expires=1765972292&Signature=YehJi0Ru1zhlBm58AfTJDL2cCRmFgBXrV9HVB~g5PoRHfVSUXysYU6-cuSGEkIDgteodr~HiuMA3JmdVNkwDJp1rcGd~tlTPTGfpk6DUQX7fsBK55R5na5dramxOHV~4D44n0cKXQfWkznjYh74G40H3E4Ij-1-HUvuufbtzlpcC4tu~SpUEvX-Dg0i7Xt3kCeU~LXDBMAcFEokMQ20RSV-1HKxXvlvOTzoeRLkKwu0x9TIjZVWKdqcwABO1bnkL-nKxdaolg-qX4O-ENR~O-iiuUJ2fYAJLYm~FGUD5nrNxqebljeKqCex-ralhCtH8I2oh5yXm2R6ia7LSSgFZ6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal