Abstract
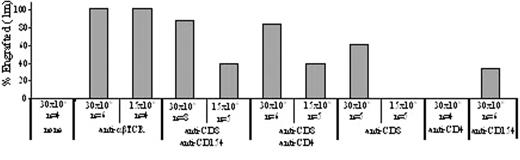
Allosensitization resulting from transfusion therapy is a major challenge for the use of bone marrow transplantation to treat sickle cell disease. Prior exposure to foreign major histocompatibility complex (MHC) antigens through transfusion or transplantation is associated with an increased rate of solid organ graft rejection. Using a mouse model for sensitization, we recently found that humoral immunity plays a dominant role in sensitization to MHC alloantigens and concomitant graft rejection. The relative contribution of cellular versus humoral adaptive immune responses has not been fully characterized. In this study, we explored the role of host T cells in rejection of allografts by sensitized recipients using μ MT mice which are defective in producing mature B cells and antibody. Therefore, the barrier from preformed antibodies in sensitized normal mice would not exist in μ MT mice. μ MT (H-2b) mice were sensitized with BALB/c (H-2d) skin grafts. Although no anti-MHC alloantibody was detected in the μ MT mice after rejection, the skin grafts were rejected with a kinetic similar to normal controls (MST = 14.1 ± 1.2 days versus 13.6 ± 1.5 days). The prompt rejection of skin allografts by B cell deficient mice suggests that T cell activation and function are normal in vivo and that T cells alone are sufficient to reject allogeneic skin grafts. BMT was subsequently performed in presensitized μ MT mice 5 weeks after sensitization as well as in naïve μ MT mice. All naïve μ MT mice (n = 6) engrafted with 700 cGy TBI and 30 x 106 bone marrow cells, but none of the presensitized μ MT mice engrafted. Since humoral immunity is absent in sensitized μ MT mice, the increased barrier in these mice therefore must be mediated by the primed T cells. To further define the relative contribution of T cell subpopulations to alloresistance, we targeted these populations in vivo as preconditioning using monoclonal antibodies (mAb). Sensitized μ MT mice were treated with anti-α β-TCR, anti-CD8, anti-CD4 or anti-CD154 mAbs alone or in combination and 850 cGy of total body irradiation, then transplanted with untreated BALB/c donor bone marrow cells (Figure). Engraftment did not occur in the sensitized μ MT mice without mAb treatment, while nearly all animals engrafted with preconditioning with anti-α β-TCR alone, anti-CD8 plus anti-CD154, or anti-CD8 plus anti-CD4 (100%, 87.5%, and 100% respectively) and transplantation with 30 x 106 donor BM cells. Moreover, anti-α β-TCR preconditioning promoted engraftment in all sensitized μ MT mice with transplantation of as low as 15 x 106 BM cells. These data demonstrate that T cell mediated cellular immunity contributes to rejection in sensitized recipients, and that successful therapies to achieve allogeneic engraftment using nonmyeloablative conditioning in sensitized recipients will need to address both arms of the adaptive antigen-specific immune system: cellular and humoral.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal