Abstract
Experimental autoimmune encephalomyelitis (EAE) in rats is a highly valuable model of multiple sclerosis (MS) because it mimics major hallmarks of the human disease. EAE induced with myelin-oligodendrocyte-glycoprotein (MOG) in DA rats is relapsing/remitting, and lesions in the central nervous system show inflammation, demyelination, and axonal and neuronal loss. Recently, bone marrow transplantation (BMT) was introduced as a novel strategy to treat MS, but its efficiency and the underlying mechanism are debatable. In MOG-induced EAE we found that BMT at the peak of EAE but not in the chronic phase leads to disease attenuation. In both settings, rats receiving bone marrow (BM) transplants were protected from subsequently induced relapses. These findings could be confirmed by histopathology in which rats receiving BM transplants did not have lesions compared with controls not receiving transplants. Importantly, the protective effect was achieved by allogeneic, syngeneic, and BM grafts from diseased rats. BMT resulted in increased numbers of CD4+CD25bright regulatory T cells, increased Foxp3 expression, a shift in T-cell epitope recognition, and a strong reduction of autoantibodies even after rechallenge with MOG. Thus, our results indicate potential mechanisms of how BMT may contribute to the improvement of MS and provide a rationale for its application in patients suffering from various autoimmune diseases. (Blood. 2005;106:1875-1883)
Introduction
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system (CNS) that is characterized by demyelinating plaques and axonal loss.1 Although MS is highly prevalent in Northern Europe and the United States,2 no cure is presently available and the clinical management of the disease is unsatisfactory.1 The etiology of MS is unknown. However, it is generally believed that both genetic and environmental factors contribute to the development of its pathology.3 The onset of MS is presumably characterized by autoreactive T cells crossing the blood brain barrier (BBB) and initiating an inflammatory response that results in an opening of the BBB. This allows influx of additional immune cells such as granulocytes, macrophages, natural killer (NK) cells, and B cells as well as antibodies and complement.4 Subsequently, this process leads to myelin destruction, induction of oligodendrocyte death, axonal degeneration and, ultimately, to the functional deficits seen in MS patients.5 Experimental autoimmune encephalomyelitis (EAE) is a frequently used animal model for MS that can be induced in rodents and monkeys.6 In particular, myelin-oligodendrocyte-glycoprotein (MOG)-induced EAE in DA rats mimics major hallmarks of the human disease. These include the relapsing/remitting type of disease course, the occurrence of demyelinated plaques in the brain and spinal cord, axonal loss, and the involvement of both antibodies and complement in the pathogenesis.7-11
Autologous bone marrow transplantation (BMT) and hematopoietic stem cell transplantation (HSCT) are presently discussed as novel options for the treatment of patients with MS with fast progressive disease courses that do not respond to conventional treatment.12,13 Clinical trials were to some extent inconclusive because they arrived at divergent results.14-16 Preclinical studies were performed in EAE that assessed certain aspects of BMT like timing and source of grafts to some extent in rats and mice.17-24 To lay further ground for clinical studies, we conducted BMT using MOG-induced EAE in the rat. We performed all possible combinations of BMT in susceptible DA and resistant ACI rats that share the RT1av1 haplotype (RT1 is major histocompatibility complex [MHC] in rats).8 Also, we analyzed the effect of BMT in acute versus chronic disease and the specificity of the protection after BMT in this model. Importantly, we were able to investigate the immunologic mechanisms operative during BMT regarding the T-cell recognition, autoantibodies, and T regulatory cells in the treatment of EAE. This allows us to suggest a strategy of how BMT could be most efficiently employed in the therapy of MS and how clinical markers could be defined to assess treatment success.
Materials and methods
Rats
Female rats 10 to 14 weeks of age were used in all experiments. DA rats were obtained from Harlan Winkelmann (Borchen, Germany) and ACI rats from Harlan USA (Indianapolis, IN). Rats were kept under specific pathogen-free (SPF) conditions and obtained food and water ad libitum. Generation and characterization of enhanced green fluorescent protein (eGFP) transgenic LEW rats25 (line UGC) have been described elsewhere. All animal experiments were approved by the regional boards in Tübingen and Würzburg, Germany.
Synthetic peptides and antigens
MOG 73-90 (KESIGEGKVALRIQNVRF), MOG 91-108 (SDEGGYTCFFRDHSYQEE), and myelin basic protein (MBP) 63-88 (HTRTTHYGSLPQKSQRTQDENPVVHF) were prepared by Karl-Heinz Wiesmüller (EMC microcollections, Tübingen, Germany) by solid phase peptide synthesis using F-moc/tBu chemistry. Peptides were purified by preparative high-performance liquid chromatography (HPLC) (Abimed, Langenfeld, Germany). The identity of the purified peptides was confirmed by electrospray mass spectrometry. The purity of peptides was more than 95% as determined by analytical HPLC (Abimed). Recombinant rat MOG, corresponding to the N-terminal sequence of MOG (amino acids 1-125, MOG 1-125) was expressed in Escherichia coli and purified. The purified protein in 6 M urea was dialyzed against phosphate-buffered saline (PBS) and stored at -20°C.
BMT
For total body irradiation (TBI), 6 mV photons at a dose rate of approximately 1 Gy/min delivered from a linear accelerator (Elekta, Hamburg, Germany) were used. Rats were irradiated with a lethal dose of 10 Gy. Bone marrow (BM) was flushed out of the bones of naive or diseased DA or ACI rats and resuspended in PBS. A total of 2 × 107 viable cells were injected 1 day after irradiation intravenously into recipient rats. BM from eGFP transgenic LEW25 rats was harvested and transplanted into LEW rats.
Determination of chimerism
DNA was isolated from blood using a Qiagen (Hilden, Germany) DNeasy kit. Primers for the microsatellite marker 21RHAP115FF526 (forward: 6-FAM AGGAGAGCTCGTGAGCTGAG; and reverse: ACAAGGAATGACACCCAAGG) were used in a polymerase chain reaction (PCR). Products were separated and quantified with a polyacrylamide gel on an ABI prism 377 DNA sequencer running with Genescan software (Applied Biosystems, Foster City, CA) Results were set in relation to a standard obtained by mixing known amounts of ACI and DA blood.
Blood cell enumeration
To assess reconstitution of cell lineages in rats undergoing transplantation, blood samples from the tail vein were analyzed in an automated blood analyzer (VetABC; Scil, Viernheim, Germany).
Induction and scoring of EAE
EAE was induced by administration of 200 μL inoculum intradermally at the base of the tail. The inoculum consisted of 50 μg MOG 1-125 in PBS emulsified with complete Freund adjuvant (CFA) (Sigma, St Louis, MO) (1:1) containing 200 μg heat-inactivated Mycobacterium tuberculosis (strain H 37 RA; Difco, Detroit, MI). Clinical signs were scored as follows: grade 1, tail weakness or tail paralysis; grade 2, hind-leg paraparesis or hemiparesis; grade 3, hind-leg paralysis or hemiparalysis; grade 4, complete paralysis, tetraplegia, moribund state, or death. Rats that died after transplantation were excluded from the experiment, and rats that died of EAE after reimmunization were scored with a score of 4.
Isolation of mononuclear cells (MNCs) from lymph nodes (LNs) and spleens
Draining inguinal lymph nodes (LNs) and spleens were dissected out under deep anesthesia. LNs were disrupted and mononuclear cells (MNCs) washed twice in Dulbecco modified eagle medium (DMEM) (Life Technologies, Paisley, United Kingdom), resuspended in complete medium (CM) containing DMEM supplemented with 5% fetal calf serum (FCS) (PAA Laboratories, Linz, Austria), 1% penicillin/streptomycin (Life Technologies), 1% glutamine (Life Technologies), and 50 μM 2-mercaptoethanol (Life Technologies), and flushed through a 70 μm plastic strainer (Falcon; BD Biosciences, Franklin Lakes, NJ). MNCs from spleen were prepared in the same way as from LNs with the difference that red blood cells (RBCs) were lysed with lysis buffer consisting of 0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2 EDTA (ethylenediaminetetraacetic acid) adjusted to pH 7.4.
Enzyme-linked immunospot (ELISPOT)
Nitrocellulose-bottomed 96-well plates (MAHA; Millipore, Molsheim, France) were coated with anti-interferon-γ (anti-IFN-γ) mouse monoclonal antibody (mAb) DB1 (a generous gift from Peter van der Meide, TNO Primate Center, Rijswijk, The Netherlands). Following washing with PBS, plates were blocked with 5% FCS in DMEM (Life Technologies). MNCs (4 × 105 per well) in 200 μL CM and antigen were added to the plates and incubated for 48 hours at 37°C in a humidified atmosphere containing 5% CO2 For each antigen, triplicate determinations were performed. MOG 1-125 was used at a concentration of 3 μg/mL and peptide at a concentration of 10 μg/mL. Concanavalin A (conA) was purchased from Sigma and used at a concentration of 1 μg/mL. Cells were discarded, and plates were washed 4 times with PBS. Secreted and bound IFN-γ was visualized with biotinylated DB12 mAb against rat IFN-γ (also a generous gift from Peter van der Meide), avidin-biotin peroxidase (Vector Laboratories, Burlingame, CA), and subsequently by staining with carbazole (Sigma).
ELISA
Blood samples for antibody measurements were taken at the end of experiments. Enzyme-linked immunosorbent assay (ELISA) plates (96 well; Nunc, Roskilde, Denmark) were coated with 2.5 μg/mL (100 μL per well) MOG 1-125 overnight at 4°C. Plates were washed with PBS/0.05% Tween 20 and blocked with milk powder for 1 hour at room temperature. After washing, diluted serum samples were added and plates were incubated for 1 hour at room temperature. Then, plates were washed and rabbit antirat antiserum (immunoglobulin G [IgG], IgG1, IgG2a, IgG2b, IgG2c; Nordic, Tilburg, The Netherlands) was added and incubated for 1 hour at room temperature. Plates were washed prior to the addition of peroxidase-conjugated goat antirabbit antiserum (Nordic) diluted in PBS/0.05% Tween 20. After 30 minutes of incubation, plates were washed and bound Abs were visualized by addition of 2,2′-azino-bis-(3-benzthiazoline-6-sulfonic acid) (ABTS) (Roche Diagnostics, Mannheim, Germany). After 15 minutes of incubation, optical density was read at 405 nm.
Quantitative real-time RT-PCR
Total RNA was extracted from splenocytes using RNeasy Mini kit (Qiagen). To avoid amplification/detection of contaminating genomic DNA, extracted RNA was treated with Rnase free DNase (Promega, Madison, WI). Subsequently, cDNA was synthesized by reverse transcription with Moloney murine leukemia virus reverse transcriptase (RT) and random pdN6 primers in the presence of RNase inhibitor (Promega). Amplification was performed on an Applied Biosystems Prism 7000 Sequence Detection System (Applied Biosystems) using a SYBR green protocol. The primer sequences 5′ to 3′ were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward: GGTTGTCTCCTGTGACTTCAA, reverse: CATACCAGGAAATGAGCTTCAC; Foxp3: forward: CCACACCTCCTCTTCTTCCTT, reverse: TGACTAGGGGCACTGTAGGC. Results were expressed as 2-ΔΔCT values.
Antibodies and flow cytometry
Fluorescein isothiocyanate (FITC)-conjugated mAb against CD45RA (OX-33), CD25 (OX-39), and T-cell receptor alpha beta (TCRAB) (R73) and phycoerythrin (PE)-conjugated mAb against CD4 (OX-35) and CD8 (OX-8) and appropriate isotype controls were purchased from Becton Dickinson (Heidelberg, Germany). Flow cytometry was performed on a FACScalibur running with Cellquest software (Becton Dickinson). Cells were gated on the lymphocyte population in the forward scatter-side scatter (FSC-SSC) dot plot.
Enrichment of CD4+CD25+ T cells
A single cell suspension of MNCs from spleens was obtained as described in “Isolation of mononuclear cells (MNCs) from lymph nodes (LNs) and spleens.” The cell suspension was incubated with OX42-FITC (Becton Dickinson) recognizing the complement type 3 receptor. After washing, cells were incubated with OX33 (anti-CD45 RA), OX8 (anti-CD8), and anti-FITC magnetic bead-labeled antibodies (Miltenyi Biotec, Bergisch Gladbach, Germany). The cell suspension was then applied to an LD column (Miltenyi Biotec) to deplete granulocytes/macrophages, B cells, and CD8+ cells. The remaining CD4+ T cells (purity more than 94% determined by flow cytometry) were then incubated with OX39 FITC (anti-CD25), incubated with anti-FITC magnetic bead-labeled antibodies, and finally enriched on an LS column (Miltenyi Biotec). Success of the enrichment procedure was determined by double staining with OX35-PE and OX39-FITC; 1 × 106 of these cells were injected intravenously into recipient rats.
Histopathologic evaluation
Histologic evaluation was performed on paraformaldehyde-fixed, paraffin-embedded sections of brains and spinal cords. Paraffin sections were stained with hematoxylin and eosin, Luxol fast blue (LFB), and Bielschowsky silver impregnation to assess inflammation, demyelination, and axonal pathology, respectively, as described.8-10 Immunohistochemistry was performed using a modified avidin-biotin-based technique using diaminobenzidine as chromogen.27 After microwave pretreatment, sections were incubated overnight with antibodies against ED1 (Serotec, Raleigh, NC), CD3 (Serotec), rat immunoglobulin (Amersham, Arlington Heights, IL), rat C9 (kindly provided by B. P. Morgan, University of Cardiff, United Kingdom), and amyloid precursor protein (APP) (Chemicon, Temecula, CA) followed by appropriate biotinylated secondary antibodies (Amersham). Sections incubated without primary antibody, with isotype control antibodies, and with preimmune sera did not show any immunoreactivity. Sections were counterstained with hemalaun.
Data analysis
Statistical significance was tested by Student t test or analysis of variance (ANOVA) as indicated. For post hoc analysis we used multiple t test with an α level of 0.05. All statistical analyses were performed with JMP for Windows (release 5.0). Error bars in the graphs represent standard error of mean (SEM).
Results
Chimerism and engraftment
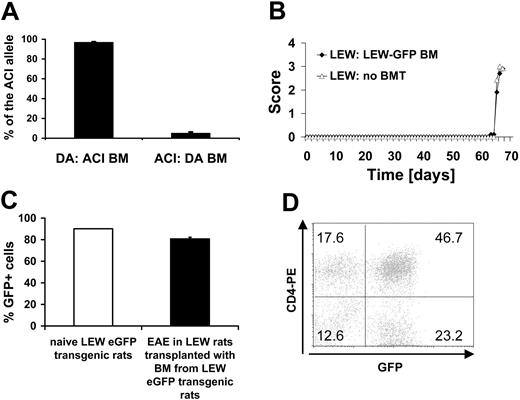
We chose DA and ACI rats as a source for the BM grafts because these rat strains are MHC matched (RT1av1) while differing in their non-MHC genome and their susceptibility to MOG 1-125-induced EAE: DA rats are EAE susceptible while ACI rats are resistant.8,28 We determined the degree of engraftment of donor BM after BMT on day 107 after transplantation. To differentiate between cells of ACI or DA rat origin we analyzed the relative intensity of a microsatellite marker in a quantitative PCR reaction of rats undergoing transplantation. DA rats with ACI BM (n = 6) had a high percentage of cells with the ACI allele in their blood (96.9% ± 0.55%), while the ACI allele in the blood of ACI rats with DA BM (n = 8) was low (5.1% ± 1.19%) (Figure 1A). We determined the number of leukocytes, platelets, and erythrocytes as well as hematocrit and hemoglobin concentration in DA rats undergoing BMT on day 107 after transplantation. We did not detect any difference compared with animals not receiving transplants except for the leukocyte counts (P < .001, ANOVA) confirming complete reconstitution of the recipient rats (Table 1).
Blood cell parameters in rats undergoing and not undergoing BMT
Group . | No. . | Lymphocytes, × 109/L . | Erythrocytes, × 1012/L . | Hemoglobin level, g/L . | Hematocrit . | Platelets, × 109/L . |
|---|---|---|---|---|---|---|
| DA BM | 8 | 8.9 ± 0.3 | 7.9 ± 0.1 | 135 ± 8 | 0.368 ± 0.033 | 763.4 ± 24 |
| ACI BM | 6 | 12.4 ± 1.0 | 7.6 ± 0.3 | 132 ± 5 | 0.376 ± 0.013 | 784.5 ± 11 |
| No BMT | 9 | 11.2 ± 0.4 | 8.2 ± 0.1 | 142 ± 1 | 0.405 ± 0.003 | 775.9 ± 17.8 |
Group . | No. . | Lymphocytes, × 109/L . | Erythrocytes, × 1012/L . | Hemoglobin level, g/L . | Hematocrit . | Platelets, × 109/L . |
|---|---|---|---|---|---|---|
| DA BM | 8 | 8.9 ± 0.3 | 7.9 ± 0.1 | 135 ± 8 | 0.368 ± 0.033 | 763.4 ± 24 |
| ACI BM | 6 | 12.4 ± 1.0 | 7.6 ± 0.3 | 132 ± 5 | 0.376 ± 0.013 | 784.5 ± 11 |
| No BMT | 9 | 11.2 ± 0.4 | 8.2 ± 0.1 | 142 ± 1 | 0.405 ± 0.003 | 775.9 ± 17.8 |
All rats were DA.
To further verify the quality of the transplantation procedure, we transplanted BM from eGFP transgenic LEW rats25 into syngeneic LEW rats (n = 16). Susceptibility to MBP 63-88-induced EAE was unchanged in LEW rats reconstituted with eGFP transgenic BM (n = 5) compared with naive LEW rats (n = 5) (Figure 1B). We confirmed the successful reconstitution of host lymphatic tissues (LNs, thymus) and BM using our grafting protocol (Figure 1C). LN cells in LEW rats receiving BM transplants from eGFP transgenic LEW rats were comparable in eGFP expression compared with naive eGFP transgenic LEW rats (pool of 5 naive eGFP transgenic LEW rats and 5 LEW rats receiving BM transplants from eGFP transgenic LEW rats). Cells eluted from the CNS in the course of EAE were also highly eGFP positive (Figure 1D).
Chimerism and engraftment. (A) Chimerism was determined in DA rats grafted with ACI BM (n = 6) and ACI rats grafted with DA BM (n = 8) on day 107 after BMT from blood by a microsatellite as described in “Materials and methods.” (B) EAE was induced with MBP 63-85 in the LEW/eGFP-LEW bone marrow chimeras on day 54 after BMT. The LEW/eGFP-LEW chimeric rats with EAE (n = 5) showed a similar disease course compared with a LEW rat control group not undergoing transplantation (n = 5). (C) eGFP-expressing cells in LNs in naive eGFP-expressing LEW rats (pool of 5) and LEW rats receiving transplants with BM from eGFP transgenic rats and subsequently induced with EAE (n = 5). (D) Example of the numbers of eGFP-expressing CNS-infiltrating cells eluted from the CNS of a LEW rat with MBP-induced EAE that had undergone transplantation with BM from an eGFP transgenic LEW rat. Numbers in graph represent percentages of gated lymphocytes in each quadrant.
Chimerism and engraftment. (A) Chimerism was determined in DA rats grafted with ACI BM (n = 6) and ACI rats grafted with DA BM (n = 8) on day 107 after BMT from blood by a microsatellite as described in “Materials and methods.” (B) EAE was induced with MBP 63-85 in the LEW/eGFP-LEW bone marrow chimeras on day 54 after BMT. The LEW/eGFP-LEW chimeric rats with EAE (n = 5) showed a similar disease course compared with a LEW rat control group not undergoing transplantation (n = 5). (C) eGFP-expressing cells in LNs in naive eGFP-expressing LEW rats (pool of 5) and LEW rats receiving transplants with BM from eGFP transgenic rats and subsequently induced with EAE (n = 5). (D) Example of the numbers of eGFP-expressing CNS-infiltrating cells eluted from the CNS of a LEW rat with MBP-induced EAE that had undergone transplantation with BM from an eGFP transgenic LEW rat. Numbers in graph represent percentages of gated lymphocytes in each quadrant.
BMT studies in naive rats
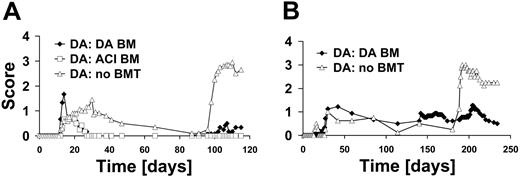
DA rats develop a relapsing/remitting disease course after immunization with MOG 1-125 (n = 5). To address the question of whether BMT by itself alters the susceptibility to EAE, we performed TBI with 10 Gy in naive DA rats and gave them transplants with DA BM (n = 4). On day 60 after BMT after BM reconstitution, rats were immunized with MOG 1-125. Similar to rats that had not received BMT, the disease course in the rats receiving transplants was of the relapsing/remitting disease type (Figure 2A). Confirmatory experiments gave the same results (naive DA undergoing transplantation with DA BM [n = 5], data not shown). In contrast, DA rats undergoing transplantation with BM from the EAE-resistant ACI rat strain developed a progressive type of EAE (n = 7).
In a second set of experiments, ACI rats that are resistant to EAE induced with MOG 1-1258 were used as recipients of the BM graft (n = 4). When ACI rats were treated with DA BM and subsequently EAE was induced on day 60 after BMT, they developed a mild relapsing/remitting disease course (n = 8). Unexpectedly, EAE induction after engraftment of ACI BM into ACI rats (n = 4) resulted in severe EAE (Figure 2B). Repeated experiments gave the similar results (naive ACI undergoing transplantation with ACI BM [n = 11] and naive ACI undergoing transplantation with DA BM [n = 4], data not shown).
Effects of BMT on established disease and induced relapses
To evaluate the effect of TBI and subsequent BMT on EAE, we divided DA rats in 3 groups: DA rats grafted with DA BM, DA rats grafted with ACI BM, and not irradiated ungrafted DA rats. At the peak of acute disease on day 17 after immunization, rats were treated with 10 Gy TBI and received BM of either DA (n = 6) or ACI (n = 6) origin. Importantly, this treatment led to a significant clinical improvement as compared with DA rats that had not received transplants (n = 10) (cumulative score day 19 to 87 after immunization, P = .036, ANOVA) (Figure 3A). On day 90 after immunization, rats were reimmunized with MOG 1-125. While the rats that had not received transplants relapsed, rats treated with BM were largely protected from induced relapses (cumulative score day 91 to 115 after immunization, P < .001, ANOVA). It is further noteworthy that the difference between the groups treated with syngeneic DA and allogeneic ACI BM was not significant ([NS] ANOVA) (Figure 3A). To confirm our results we conducted a second experiment under similar conditions (data not shown). This time DA rats were irradiated on day 16 after immunization and received BM of either DA (n = 8) or ACI (n = 6) origin on the following day. Controls were left untreated (n = 9). Again, there was a significant effect of the treatment on the disease course (cumulative score day 17 to 131 after immunization, P = .027, ANOVA). Furthermore, rats were also strongly protected from induced relapse when reimmunized on day 131 after immunization (cumulative score day 140 to 154 after immunization, P < .001, ANOVA). Again, there was no difference in the efficiency of BMT in its ability to protect from EAE with regard to the source of BM (DA versus ACI, NS, ANOVA).
BMT of naive rats and subsequent EAE induction with MOG. (A) DA rats (n = 5) without BMT develop a relapsing/remitting type of disease course after immunization with MOG 1-125. Naive DA rats undergoing transplantation with DA BM graft (n = 4) or with ACI BM graft (n = 7) were immunized with MOG 1-125 on day 60 after irradiation. MOG-EAE was induced in all groups. (B) ACI rats without BMT do not develop EAE (n = 4). ACI rats with DA BM graft (n = 8) develop a mild relapsing/remitting disease course if induced 68 days after BMT. Engraftment of ACI BM into ACI rats (n = 4) equally resulted in susceptibility to EAE.
BMT of naive rats and subsequent EAE induction with MOG. (A) DA rats (n = 5) without BMT develop a relapsing/remitting type of disease course after immunization with MOG 1-125. Naive DA rats undergoing transplantation with DA BM graft (n = 4) or with ACI BM graft (n = 7) were immunized with MOG 1-125 on day 60 after irradiation. MOG-EAE was induced in all groups. (B) ACI rats without BMT do not develop EAE (n = 4). ACI rats with DA BM graft (n = 8) develop a mild relapsing/remitting disease course if induced 68 days after BMT. Engraftment of ACI BM into ACI rats (n = 4) equally resulted in susceptibility to EAE.
To investigate whether BMT also had a positive effect on the disease course when performed in the chronic phase of EAE, we chose a later time point (day 140 after immunization) for BMT. Interestingly, we did not observe any improvement of EAE after transplantation with DA BM (n = 9) in this setting. To further evaluate whether rats were protected from following relapses under these conditions, we reimmunized them with MOG 1-125 after reconstitution (day 181 after immunization). While we were able to demonstrate partial improvement of EAE using this protocol (n = 4), protection induced by BMT in the chronic phase was slightly weaker as compared with the situation when BMT was performed at the peak of EAE (cumulative score day 188 to 234 after immunization, P = .021, t test) (Figure 3B).
Histopathology
We performed a histopathological examination of brains and spinal cords at the end of the experiments (Figure 3A and repetition of experiment). Thirteen of 15 DA rats without BMT had strong inflammation and widespread demyelination in the CNS (Figure 4A-H). In contrast, only 3 of 14 DA rats treated with DA BM and none of the 11 DA rats treated with ACI BM showed inflammation or/and demyelination in the CNS (Figure 4I-O). Lesion pathology in untreated animals was characterized by large, confluent demyelination (Figure 4A), macrophage infiltration (Figure 4B), and deposition of immunoglobulin and C9 indicative of antibody-mediated demyelination (Figure 4C-D). CD3+ T cells were found scattered in the lesions (Figure 4E). Inflammatory infiltrates were accompanied by acute axonal damage (Figure 4F-G) and a reduction in axonal density. No pathology was observed in a representative DA rat treated with DA BM (Figure 4I-O).
BMT as a treatment for EAE. (A) DA rats were irradiated on day 17 after immunization and received BM transplants of either DA (n = 6) or ACI (n = 6) origin. Controls were not irradiated and did not receive BM transplants (n = 10). Differences between the groups receiving BM transplants compared with the control were significant (cumulative score day 19 to 87 after immunization, P = .036, ANOVA). Rats were reimmunized for EAE on day 90. Rats receiving BM transplants had significantly lower disease scores upon reimmunization compared with controls not receiving transplants (P < .001, ANOVA). A second confirmatory experiment gave similar results (data not shown). (B) Rats were immunized on day 0 with MOG 1-125 and underwent transplantation on day 140 with BM of DA origin (n = 9) or did not receive BM transplants (n = 4). There was no effect of BMT at a late time point. Rats were then reimmunized on day 181 with MOG 1-125. After reimmunization only a slight exacerbation of disease was observed in the group receiving transplants compared with the group without BMT (cumulative score day 188 to 234 after immunization, P = .021, t test).
BMT as a treatment for EAE. (A) DA rats were irradiated on day 17 after immunization and received BM transplants of either DA (n = 6) or ACI (n = 6) origin. Controls were not irradiated and did not receive BM transplants (n = 10). Differences between the groups receiving BM transplants compared with the control were significant (cumulative score day 19 to 87 after immunization, P = .036, ANOVA). Rats were reimmunized for EAE on day 90. Rats receiving BM transplants had significantly lower disease scores upon reimmunization compared with controls not receiving transplants (P < .001, ANOVA). A second confirmatory experiment gave similar results (data not shown). (B) Rats were immunized on day 0 with MOG 1-125 and underwent transplantation on day 140 with BM of DA origin (n = 9) or did not receive BM transplants (n = 4). There was no effect of BMT at a late time point. Rats were then reimmunized on day 181 with MOG 1-125. After reimmunization only a slight exacerbation of disease was observed in the group receiving transplants compared with the group without BMT (cumulative score day 188 to 234 after immunization, P = .021, t test).
Syngeneic BMT with BM from diseased rats
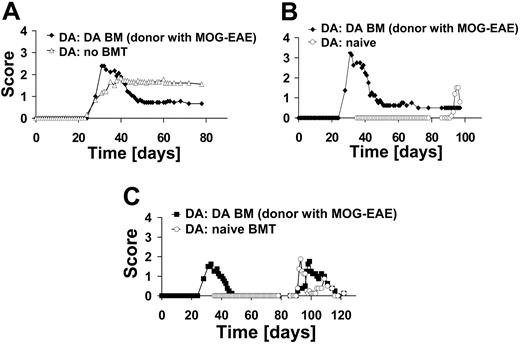
To mimic the clinical setting more closely, we conducted an experiment in which rats were treated in the acute phase of EAE with syngeneic BM of rats with EAE. This transplantation regimen led to an amelioration of the disease course (n = 9) as compared with those that had not received transplants (n = 9) (Figure 5A). Syngeneic BM from rats with EAE also protected from induced relapses with MOG 1-125 (Figure 5B).
Histopathology of the rats receiving transplants fromFigure 3Aand repeated experiment. (A-H) Characteristics of MOG-induced EAE in a DA rat not undergoing BM transplantation. Large, confluent areas of demyelination and macrophage infiltration in the dorsal funiculus of a representative untreated DA rat. Macrophages containing LFB-positive myelin degradation products indicative of ongoing demyelination are shown in the inset (A; LFB-periodic acid-Schiff [PAS] staining). Infiltration by foamy macrophages/activated microglia cells shown by immunohistochemistry for ED1 (B; brown reaction product). Dense deposits of immunoglobulin (C) and C9 (D) in the lesions (plaque edge indicated by arrows). CD3+ T cells scattered in the lesional area (E). APP-positive axonal profiles indicative of acute axonal damage (F; plaque edge indicated by arrows). Acutely damaged axons indicated by arrows (G [enlarged from panel F]; brown reaction product). Reduction in axonal density as demonstrated by Bielschowsky silver impregnation; plaque edge indicated by arrows (H). (I-N) DA rat immunized with MOG and receiving DA BM transplants. No evidence for demyelination (I), macrophage infiltration (ED1) (J), immunoglobulin (K) and C9 deposition (L), acute axonal damage (APP) (M), or axonal loss (N) in a representative immunized and treated DA rat. A,B,I-N: bar, 100 μm; C,D,F,H: bar, 50 μm; E,G: bar, 20 μm. Light microscopic images were taken using a Color View digital camera (Soft Imaging System, Muenster, Germany) mounted on an Olympus BX51 microscope (Olympus, Tokyo, Japan). Panels A, B, and I-N were taken at a magnification of 100 × (10 ×/0.3 NA objective); C, D, F, H, at 200 × (20 ×/0.5 NA objective); and E, G, and inset of A, at 400 × (40 ×/0.75 NA objective). Images were imported using Analysis software (Soft Imaging System), and Adobe Photoshop CS 8.0.1 (Adobe Systems, San Jose, CA) was used to create the color plate.
Histopathology of the rats receiving transplants fromFigure 3Aand repeated experiment. (A-H) Characteristics of MOG-induced EAE in a DA rat not undergoing BM transplantation. Large, confluent areas of demyelination and macrophage infiltration in the dorsal funiculus of a representative untreated DA rat. Macrophages containing LFB-positive myelin degradation products indicative of ongoing demyelination are shown in the inset (A; LFB-periodic acid-Schiff [PAS] staining). Infiltration by foamy macrophages/activated microglia cells shown by immunohistochemistry for ED1 (B; brown reaction product). Dense deposits of immunoglobulin (C) and C9 (D) in the lesions (plaque edge indicated by arrows). CD3+ T cells scattered in the lesional area (E). APP-positive axonal profiles indicative of acute axonal damage (F; plaque edge indicated by arrows). Acutely damaged axons indicated by arrows (G [enlarged from panel F]; brown reaction product). Reduction in axonal density as demonstrated by Bielschowsky silver impregnation; plaque edge indicated by arrows (H). (I-N) DA rat immunized with MOG and receiving DA BM transplants. No evidence for demyelination (I), macrophage infiltration (ED1) (J), immunoglobulin (K) and C9 deposition (L), acute axonal damage (APP) (M), or axonal loss (N) in a representative immunized and treated DA rat. A,B,I-N: bar, 100 μm; C,D,F,H: bar, 50 μm; E,G: bar, 20 μm. Light microscopic images were taken using a Color View digital camera (Soft Imaging System, Muenster, Germany) mounted on an Olympus BX51 microscope (Olympus, Tokyo, Japan). Panels A, B, and I-N were taken at a magnification of 100 × (10 ×/0.3 NA objective); C, D, F, H, at 200 × (20 ×/0.5 NA objective); and E, G, and inset of A, at 400 × (40 ×/0.75 NA objective). Images were imported using Analysis software (Soft Imaging System), and Adobe Photoshop CS 8.0.1 (Adobe Systems, San Jose, CA) was used to create the color plate.
Antigen specificity of the protection from EAE mediated by BMT
All our experiments described so far suggested that protection from EAE is due to induction of tolerance to MOG-induced EAE relapses. To assess whether protection is antigen specific, we rechallenged rats that had received a DA BM transplant from DA rats with EAE at the peak of MOG-EAE with the immunodominant MBP 63-88 peptide (n = 4) (Figure 5C). As controls, rats that received BM transplants as naive rats were challenged with MBP 63-88 (n = 4). Both groups were not protected against MBP 63-88-induced EAE (Figure 5C). These results indicate that the tolerance achieved by BMT is specific for the antigen used to induce disease.
BMT with BM from diseased rats and specificity of protection. (A) DA rats were immunized with MOG 1-125 in CFA on day 0 and boosted with MOG 1-125 in incomplete Freund adjuvant (IFA) on day 18. Subsequently, they received a BM transplant on day 36 from DA rats with EAE (n = 9). Control rats received no BM transplant (n = 9). (B) A subgroup of rats after transplantation of BM from diseased rats (n = 4) and naive rats (naive BMT; n = 5) were immunized with MOG 1-125. Naive rats developed EAE while rats that had received BM from diseased rats did not relapse. (C) DA rats after BMT (DA BM from diseased rats; n = 4) and rats that were naive at the time of transplantation (naive BMT; n = 4) were immunized with MBP 63-88 on day 79. Both groups developed EAE after immunization with MBP 63-88, indicating specificity of BMT-induced tolerance.
BMT with BM from diseased rats and specificity of protection. (A) DA rats were immunized with MOG 1-125 in CFA on day 0 and boosted with MOG 1-125 in incomplete Freund adjuvant (IFA) on day 18. Subsequently, they received a BM transplant on day 36 from DA rats with EAE (n = 9). Control rats received no BM transplant (n = 9). (B) A subgroup of rats after transplantation of BM from diseased rats (n = 4) and naive rats (naive BMT; n = 5) were immunized with MOG 1-125. Naive rats developed EAE while rats that had received BM from diseased rats did not relapse. (C) DA rats after BMT (DA BM from diseased rats; n = 4) and rats that were naive at the time of transplantation (naive BMT; n = 4) were immunized with MBP 63-88 on day 79. Both groups developed EAE after immunization with MBP 63-88, indicating specificity of BMT-induced tolerance.
Autoantibodies
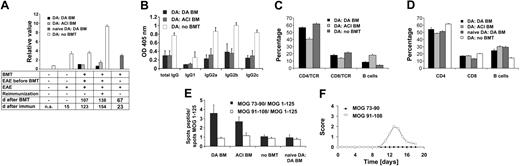
Antibodies against MOG play an important role in mediating widespread demyelination in DA rats.11 Therefore, we assessed total IgG levels against MOG 1-125. Upon immunization the antibody levels increased to MOG (n = 6) compared with naive controls (n = 4) (P < .002). On day 123 after immunization, 107 days after BMT, DA rats undergoing DA BMT (n = 8) and DA rats undergoing ACI BMT (n = 6) showed a strong reduction of autoantibody titers compared with the control group with EAE not receiving transplants (n = 8) (P < .001). This effect was even more pronounced on day 154 after reimmunization (no BMT [n = 6], DA BM [n = 6], ACI BM [n = 5]) (P < .001) (Figure 6A).
To test whether BMT by itself leads to a reduced antibody response, we measured serum antibody levels in rats that underwent transplantation as naive rats and were immunized with MOG 1-125. These rats developed an antibody response comparable to rats without BMT and significantly higher than the rats undergoing transplantation during acute EAE (P < .001) (Figure 6A).
Immunologic changes in rats undergoing BMT. (A) Anti-MOG IgG titers in different immunization and transplantation settings. BMT leads to strong reduction of autoantibody titers, which persist also after secondary challenge (time point day 0: naive DA rats n = 4; time point day 15: EAE without BMT n = 6; time point day 123: DA BM n = 8, ACI BM n = 6, no BM n = 8; time point day 154: DABM n = 6,ACI BM n = 5, no BM n = 6). EAE leads to an increase in anti-MOG antibody titers (P < .002). On day 123 and day 154, groups undergoing BMT showed strongly reduced autoantibody titers compared with rats not receiving transplants (each P < .001). Rats that received transplants as naive animals (day 23 after immunization) and were subsequently induced with EAE did not show a reduction of autoantibodies compared with controls not receiving transplants (day 15 after immunization) (NS). (B) Reduction of anti-MOG 1-125 IgG, IgG1, IgG2a, and IgG2c levels in both groups receiving transplants at day 115 after immunization (day 98 after BMT: DA BM graft n = 6, ACI BM graft n = 5) compared with the group not receiving transplants (n = 6) (each P < .001, ANOVA). No significant differences for IgG2b between the DA/ACI BM treatment groups were observed. Only the group not undergoing BMT had higher levels of IgG2b and IgG2c antibodies (P < .001, ANOVA). (C) In lymphocytes derived from blood the percentage of B cells was increased while the percentage of CD4+ and CD8+ T cells was reduced when comparing rats receiving transplants and controls. Significant changes were seen for B cells (P < .001,ANOVA), CD4+ cells (P < .001,ANOVA), and CD8+ cells (P = .04,ANOVA) between rats receiving transplants and those not receiving transplants. (D) Lymphocytes isolated from draining lymph nodes of rats were analyzed by fluorescence-activated cell sorting (FACS) (DA BM graft n = 5, ACI BM graft n = 6, naive BMT n= 5, no BMT n = 6). The relative size of the CD4+ T-cell compartment was reduced in rats undergoing BMT if compared with controls, while the relative size of the B-cell compartment was enlarged compared with controls. The differences between the group not receiving transplants and the groups receiving transplants were significant in the T-cell (CD4 and CD8) and B-cell compartment (for all P < .001, ANOVA). (E) DA BM and ACI BM grafted rats at the height of EAE had an increase in numbers of MOG 73-90-specific IFN-γ-secreting cells related to numbers of MOG 1-125-specific IFN-γ-secreting cells compared with the other investigated groups (P < .001, ANOVA) (DA BM n = 15, ACI BM n = 10, no BMT n = 16, naive DA BMT n = 5). In contrast, the numbers of MOG 91-108-specific IFN-γ-secreting cells in relation to MOG 1-125-specific IFN-γ-secreting cells did not differ between the groups (NS, ANOVA). (F) Only DA rats immunized with MOG 91-108 (n = 5) developed EAE but not DA rats immunized with MOG 73-90 (n = 5) (cumulative score day 0 to 18 after immunization, P < .001, t test).
Immunologic changes in rats undergoing BMT. (A) Anti-MOG IgG titers in different immunization and transplantation settings. BMT leads to strong reduction of autoantibody titers, which persist also after secondary challenge (time point day 0: naive DA rats n = 4; time point day 15: EAE without BMT n = 6; time point day 123: DA BM n = 8, ACI BM n = 6, no BM n = 8; time point day 154: DABM n = 6,ACI BM n = 5, no BM n = 6). EAE leads to an increase in anti-MOG antibody titers (P < .002). On day 123 and day 154, groups undergoing BMT showed strongly reduced autoantibody titers compared with rats not receiving transplants (each P < .001). Rats that received transplants as naive animals (day 23 after immunization) and were subsequently induced with EAE did not show a reduction of autoantibodies compared with controls not receiving transplants (day 15 after immunization) (NS). (B) Reduction of anti-MOG 1-125 IgG, IgG1, IgG2a, and IgG2c levels in both groups receiving transplants at day 115 after immunization (day 98 after BMT: DA BM graft n = 6, ACI BM graft n = 5) compared with the group not receiving transplants (n = 6) (each P < .001, ANOVA). No significant differences for IgG2b between the DA/ACI BM treatment groups were observed. Only the group not undergoing BMT had higher levels of IgG2b and IgG2c antibodies (P < .001, ANOVA). (C) In lymphocytes derived from blood the percentage of B cells was increased while the percentage of CD4+ and CD8+ T cells was reduced when comparing rats receiving transplants and controls. Significant changes were seen for B cells (P < .001,ANOVA), CD4+ cells (P < .001,ANOVA), and CD8+ cells (P = .04,ANOVA) between rats receiving transplants and those not receiving transplants. (D) Lymphocytes isolated from draining lymph nodes of rats were analyzed by fluorescence-activated cell sorting (FACS) (DA BM graft n = 5, ACI BM graft n = 6, naive BMT n= 5, no BMT n = 6). The relative size of the CD4+ T-cell compartment was reduced in rats undergoing BMT if compared with controls, while the relative size of the B-cell compartment was enlarged compared with controls. The differences between the group not receiving transplants and the groups receiving transplants were significant in the T-cell (CD4 and CD8) and B-cell compartment (for all P < .001, ANOVA). (E) DA BM and ACI BM grafted rats at the height of EAE had an increase in numbers of MOG 73-90-specific IFN-γ-secreting cells related to numbers of MOG 1-125-specific IFN-γ-secreting cells compared with the other investigated groups (P < .001, ANOVA) (DA BM n = 15, ACI BM n = 10, no BMT n = 16, naive DA BMT n = 5). In contrast, the numbers of MOG 91-108-specific IFN-γ-secreting cells in relation to MOG 1-125-specific IFN-γ-secreting cells did not differ between the groups (NS, ANOVA). (F) Only DA rats immunized with MOG 91-108 (n = 5) developed EAE but not DA rats immunized with MOG 73-90 (n = 5) (cumulative score day 0 to 18 after immunization, P < .001, t test).
Moreover, we determined IgG isotype distribution against MOG 1-125 (IgG1, IgG2a, IgG2b, and IgG2c) on day 115 after immunization or day 98 after transplantation. Total IgG against MOG 1-125 as well as all IgG subtypes (IgG1, IgG2a, IgG2b, IgG2c) were strongly reduced in the groups undergoing BMT (DA BM graft [n = 6], ACI BM graft [n = 6]) compared with the group not receiving transplants (n = 8) (IgG1, P < .004; IgG2a, IgG2b, IgG2c, each P < .001, ANOVA) (Figure 6B). Based on the isotype distribution, we did not find evidence for a T-helper 2 (Th2) shift.
Lymphocyte subset distribution
To evaluate whether differences in the lymphocyte subset distribution between EAE-protected rats undergoing transplantation (DA BM graft [n = 6], ACI BM graft [n = 6]) and controls (no BMT [n = 6]) were present, cell surface expression of CD4, CD8, and CD45RA (B cells) was analyzed in the blood before reimmunization and in LNs after reimmunization. In the blood, the percentage of B cells was increased while the percentage of CD4+ and CD8+ T cells was reduced when comparing rats receiving transplants and controls. Significant changes were seen for B cells (P < .001, ANOVA), CD4+ cells (P < .001, ANOVA), and CD8+ cells (P = .04, ANOVA) between rats receiving transplants and not receiving transplants (Figure 6C).
Similar changes were detected in the lymphocyte subpopulation distribution after BMT in LNs (DA BM graft [n = 5], ACI BM graft [n = 6]) and controls (no BMT [n = 6], naive BMT [n = 5]). Here the group of naive rats undergoing BMT showed a pattern comparable to the BMT groups (DA BM graft and ACI BM graft). This indicates that the observed changes are linked to the BMT. The differences between the group that had not undergone BMT and the groups receiving transplants were significant in both the T-cell (CD4+ and CD8+) and the B-cell compartment (for all P < .001, ANOVA) (Figure 6D).
T-cell responses toward MOG peptides
We used the ELISPOT method for quantification of MOG-specific IFN-γ-producing cells. The numbers of MOG 73-90- or MOG 91-108- (dominant determinants)29 specific IFN-γ-secreting cells in relation to those specific for the MOG 1-125 were determined. The 2 groups that had undergone BMT (DA BM graft or ACI BM graft) showed an increase of MOG 73-90-specific IFN-γ-secreting cells but no changes in numbers of MOG 91-108-specific IFN-γ-secreting cells in relation to MOG 1-125-specific cells (IFN-γ-secreting cells of DA and ACI BM rats undergoing BMT treated on the peak of disease were different from IFN-γ-secreting cells of naive rats treated with BMT and IFN-γ-secreting cells of rats without BMT, P < .001, ANOVA). In contrast, naive control rats and MOG 1-125-immunized rats that had not received a BM graft (no BMT) had about equal numbers of MOG 73-90- and MOG 91-108-specific cells in relation to MOG 1-125-specific cells (NS, ANOVA) (DA BM graft [n = 15], ACI BM graft [n = 10], no BMT [n = 16], naive DA BMT [n = 5]). These results indicate that the relative increase of IFN-γ-secreting cells in response to MOG 73-90 is directly related to BMT in context of ongoing MOG-EAE (Figure 6E).
Encephalitogenicity of MOG 73-90 and MOG 91-108 peptides
Next, we tested the encephalitogenicity of MOG 73-90 by immunizing groups of DA rats (each n = 5) with MOG 73-90 or MOG 91-108 in CFA. Only DA rats immunized with MOG 91-108 developed disease while DA rats immunized with MOG 73-90 did not develop any signs of EAE (cumulative score day 0 to 18 after immunization, P < .001, t test) (Figure 6F).
Induction of CD4+CD25bright regulatory T cells in rats undergoing BMT. (A) Representative FACS blots of DA rats without BMT and with BMT with DA or ACI BM. (B) CD4+CD25bright cells in LNs of DA rats without BMT and with BMT (DA rats without BMT [n = 8], DA rats after BMT with BM from DA rats [n = 6] or ACI [n = 6] rats). Numbers in graphs represent percentages of gated lymphocytes in each quadrant. There were differences between the groups receiving and not receiving transplants (*P < .01, ANOVA). (C) Foxp3 expression in spleen cells from DA rats without BMT (n = 7) and with BMT with syngeneic BM (n = 7). There were differences between the groups receiving and not receiving transplants (*P = .05, t test). (D) Transfer of CD4+CD25+ T cells results in protection from MOG-induced EAE in DA rats as compared with rats transferred with CD4+ T cells depleted of CD25+ cells (n = 7 each group, P < .001, t test). Repeated experiments showed similar results (n = 3 each group).
Induction of CD4+CD25bright regulatory T cells in rats undergoing BMT. (A) Representative FACS blots of DA rats without BMT and with BMT with DA or ACI BM. (B) CD4+CD25bright cells in LNs of DA rats without BMT and with BMT (DA rats without BMT [n = 8], DA rats after BMT with BM from DA rats [n = 6] or ACI [n = 6] rats). Numbers in graphs represent percentages of gated lymphocytes in each quadrant. There were differences between the groups receiving and not receiving transplants (*P < .01, ANOVA). (C) Foxp3 expression in spleen cells from DA rats without BMT (n = 7) and with BMT with syngeneic BM (n = 7). There were differences between the groups receiving and not receiving transplants (*P = .05, t test). (D) Transfer of CD4+CD25+ T cells results in protection from MOG-induced EAE in DA rats as compared with rats transferred with CD4+ T cells depleted of CD25+ cells (n = 7 each group, P < .001, t test). Repeated experiments showed similar results (n = 3 each group).
Induction of T cells with a regulatory phenotype by BMT
Finally, cytofluorometric analysis of CD25 expression of CD4+ T cells in LNs revealed that CD4+CD25bright cells were increased in DA rats undergoing transplantation with either DA BM (n = 6) or ACI BM (n = 6) compared with rats not undergoing transplantation (n = 8) (P < .01, ANOVA) (Figure 7A-B). Also, the mRNA of transcription factor Foxp3 was increased in lymphocytes from spleen of rats undergoing BMT (n = 7) compared with controls (n = 7) measured by quantitative real-time PCR (P = .05, t test) (Figure 7C). Transfer experiments with enriched CD4+CD25+ T cells and CD4+ T cells depleted of CD25+ cells from naive DA (n = 10) into DA recipients (n = 7 each) resulted in protection from EAE (P < .001, t test) (Figure 7D).
To find out if CD4+CD25+ regulatory T cells are also responsible for the EAE resistance of ACI rats, we stained LN cells from naive DA and ACI rats for CD4+CD25+ T cells (each n = 5). DA rats had higher numbers of CD4+CD25+ T cells (5.1% ± 0.2%) compared with ACI rats (2.9% ± 0.1%, P < .001, t test). These data indicate that CD4+CD25+ regulatory T cells are not the reason for protection in naive ACI rats.
Discussion
Autologous BMT is a novel option for treatment of fast progressive patients with MS, but initial clinical trials have proven inconclusive.14-16 Even though there have been a number of studies regarding BMT in EAE,17-24 our studies on BMT therapy in a well-characterized animal model for MS represent a comprehensive analysis of this innovative therapy and provide a means to investigate its characteristics in detail with regard to the timing of BMT, the source of the graft, and the mechanisms of its action.
MOG-induced EAE in rats is currently one of the best models for MS because it largely reproduces the immunologic complexity of MS, which is characterized by a combination of T cell-mediated effector mechanisms and the action of autoantibodies, macrophages, and NK cells in lesion development.11 In this sense MOG-induced EAE in DA rats used in our studies contrasts purely T cell-mediated models (eg, T-cell transfer studies) widely used for investigations on mechanisms operating in MS and BMT.24,30,31 The similarity of our model to MS is shown in histopathology where widespread demyelination and axonal loss are found.8-10 In this study we also show by histopathology large demyelinated plaques and axonal pathology with the presence of T cells, macrophages, antibodies, and complement factors. Therefore, the model described here can be considered to be highly relevant for the preclinical testing of novel strategies for the treatment of MS. Furthermore, it might contribute to a better understanding of the pathogenesis of MS.29,32-34
In agreement with earlier studies we demonstrate that BMT leads to disease attenuation and protects from further induced relapses with the disease-inducing antigen, strongly suggesting that this therapy is a promising option for the treatment of MS in humans.17-24 For the first time we demonstrate in direct comparisons that protection can be achieved by MHC-matched allogeneic, syngeneic, and BM grafts from EAE-diseased DA rats. The protective effect was strongest when BM was transplanted in the acute phase of EAE. BMT was not effective in attenuating the disease course and severity when performed during the chronic phase of EAE. Similar observations have been done in a transfer EAE model and in humans.16,24 Importantly, in the acute and chronic phase of the disease, BMT prevented subsequently induced relapses. For BMT in the acute phase of EAE this has been shown before.18,23 BMT in the chronic phase and subsequent demonstration of protection from relapses has not been shown before. We conclude that BMT in clinical trials should be preferentially performed at peak of disease, while it is still worth considering also in later stages of disease progression as long as inflammatory activity is present.
As to the mode of action of BMT, 3 novel interconnected mechanisms for protection could be found: (1) induction of CD4+CD25bright regulatory T cells; (2) an increased reactivity of T cells responsive to a nonencephalitogenic determinant, namely MOG 73-90; and (3) reduced autoantibody levels.
T-cell tolerance is achieved by positive and negative selection in the thymus, peripheral induction of anergy, and by the action of regulatory T cells.35 Our data using MBP 63-88 for rechallenge in MOG-induced EAE suggest that the protection from EAE conferred by BMT is specific for the disease-inducing antigen and not a general immunosuppressive effect. CD4+CD25+ regulatory T cells play an important role in the maintenance of immune tolerance to self.36 CD4+CD25+ cells have an antigen-specific regulatory function in vivo in EAE.37 By transfer studies we show that CD4+CD25+ cells are also protective when transferred in naive rats. Further studies regarding the role of regulatory T cells in EAE and BMT are ongoing in our laboratory. In the present study we demonstrate increased numbers of CD4+CD25bright T cells in rats after BMT as compared with control rats not undergoing BMT. Foxp3 is a transcription factor that is necessary for the function of these regulatory T cells.38 Male scurfy mice with a mutation in Foxp3 and Foxp3 knock-out mice develop a lethal lymphoproliferative disorder, stressing the importance of this gene in immune homeostasis.38,39 Foxp3 acts as a T regulatory cell lineage specification factor and mediator of the genetic mechanisms of dominant tolerance.40 We observed an increased expression of Foxp3 in splenocytes of rats undergoing BMT compared with controls. These data strongly indicate that regulatory T cells are suppressing subsequent relapses in rats undergoing BMT. The potential of purified CD4+CD25+ T regulatory cells should be evaluated in MS in humans.
B-cell tolerance is predominantly mediated by negative selection in the BM. An altered homing behavior and a reduced life span of autoreactive B cells contribute to peripheral B-cell tolerance. We show that BMT leads to a reduction of autoantibody levels while the relative size of the B-cell compartment increases. Moreover and most interestingly, rechallenge with the autoantigen does not lead an increase in autoantibody titers in rats undergoing BMT. These data underscore that long-lasting B-cell tolerance is induced by BMT. These findings are novel and could be very important in regard to the clinical effects of BMT. Such analysis has not been performed in EAE and in patients with MS up to now. There are strong indications that anti-MOG antibodies are of clinical importance and can be used as novel biomarkers for treatment effects.41 Analysis of IgG isotypes did not indicate a BMT procedure-mediated shift in Th1/Th2 balance.42
Naive irradiated and DA rats receiving transplants with BM from resistant ACI rats were not protected from disease induction, and BM from resistant rats transplanted into resistant rats did not protect from EAE. These data in connection with BMT studies in established EAE clearly show that specific protection is only achieved in the context of ongoing inflammation and suggest that availability of the autoantigen during reconstitution of the immune system is important for subsequent protection. MOG-EAE-resistant ACI rats become susceptible when irradiated and BM that is of either ACI or DA rat origin is transplanted in a naive state. These data reinforce the concept that autoantigen or mimicking antigens need to be present during the establishment of tolerance.43 Furthermore, impaired homeostasis could be of great importance and lead to increased disease susceptibility in nondiseased rats undergoing transplantation.37,44 Reduction of numbers of regulatory cells could be a further factor affecting susceptibility in naive rats undergoing BMT. MOG is sequestered behind the BBB in the CNS as an immunoprivileged site and not easily accessible to the immune system.11 The fetal and postnatal period during which orchestrated gene expression of most self antigens takes place is necessary for long-lasting tolerance induction to various autoantigens.45 Obviously, the same quality of tolerance cannot be achieved after BMT in adult life. Therefore, we propose that the eradication of lymphocytes after irradiation leads to an altered thymic output in the adult rat in context with inflammation and the exposure to autoantigen, which leads to the induction and production of regulatory T cells with the ability to suppress EAE. Importantly, it has to be considered in the clinical therapy that BMT, even though effective in the context of a specific autoimmune disease, could therefore result in increased susceptibility to other autoimmune diseases.
We found a reduction of the CD4 compartment in rats receiving transplants. This was an effect of the transplantation procedure because we measured the same reduction in rats that had undergone transplantation in the naive state as compared with rats that had undergone transplantation in context of EAE. It has been claimed that the reduction of the CD4 compartment could be of importance in the effect of BMT in autoimmune conditions.15 Our data would challenge this hypothesis, because we observed in MOG- and MBP-induced EAE in naive BMT rats unaltered or increased susceptibility.
Others have analyzed the influence of BMT on the T-cell repertoire and on autoantigen-specific T-cell responses.46,47 Recently it was shown that BMT in humans can change the T-cell receptor (TCR) repertoire.47 In another study a transient reduction and change in MBP-specific T-cell responses to different MBP determinants after BMT could be demonstrated.46 We define for the first time that BMT leads to a change in the functional outcome of autoantigen recognition: Although the response to the encephalitogenic determinant MOG 91-108 remained similar, an increased response was observed to the nonencephalitogenic determinant MOG 73-90. This was substantiated by immunization with the encephalitogenic and nonencephalitogenic determinants. Our data could be of relevance for the effects of BMT in humans and warrant further studies in analyzing responses to different autoantigens.
Taken together, our data clearly indicate that BMT arrests the progression of neuroinflammatory autoimmune diseases, which is best achieved in an early phase of disease. Given the relevance and good comparability of the MOG-induced EAE model for human MS, these data are highly promising and argue for a continuation of clinical trials using autologous BMT considering our findings on the timing of BMT therapy and the source of the BM grafts. Nevertheless, despite these optimistic results, BMT should be used with caution because it may result in increased susceptibility to other autoimmune diseases. The increased size of the CD4+CD25bright population and the up-regulation of Foxp3 after BMT as a treatment for ongoing EAE give important evidence for the involvement of regulatory T cells in the process of the observed tolerance after induced relapses. The efficacy of BMT is not only due to a transient immunosuppression but, as we demonstrate, due to multiple interconnected mechanisms of immunomodulation.
Prepublished online as Blood First Edition Paper, May 17, 2005; DOI 10.1182/blood-2004-12-4607.
Supported by German Research Foundation (DFG) (R.W.) (DFG We 1947/2-3 and SFB 510) and fortüne. R.W. holds a Heisenberg fellowship of DFG (We 1947/4-1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kerstin Stuck for excellent technical assistance.


![Figure 4. Histopathology of the rats receiving transplants from Figure 3A and repeated experiment. (A-H) Characteristics of MOG-induced EAE in a DA rat not undergoing BM transplantation. Large, confluent areas of demyelination and macrophage infiltration in the dorsal funiculus of a representative untreated DA rat. Macrophages containing LFB-positive myelin degradation products indicative of ongoing demyelination are shown in the inset (A; LFB-periodic acid-Schiff [PAS] staining). Infiltration by foamy macrophages/activated microglia cells shown by immunohistochemistry for ED1 (B; brown reaction product). Dense deposits of immunoglobulin (C) and C9 (D) in the lesions (plaque edge indicated by arrows). CD3+ T cells scattered in the lesional area (E). APP-positive axonal profiles indicative of acute axonal damage (F; plaque edge indicated by arrows). Acutely damaged axons indicated by arrows (G [enlarged from panel F]; brown reaction product). Reduction in axonal density as demonstrated by Bielschowsky silver impregnation; plaque edge indicated by arrows (H). (I-N) DA rat immunized with MOG and receiving DA BM transplants. No evidence for demyelination (I), macrophage infiltration (ED1) (J), immunoglobulin (K) and C9 deposition (L), acute axonal damage (APP) (M), or axonal loss (N) in a representative immunized and treated DA rat. A,B,I-N: bar, 100 μm; C,D,F,H: bar, 50 μm; E,G: bar, 20 μm. Light microscopic images were taken using a Color View digital camera (Soft Imaging System, Muenster, Germany) mounted on an Olympus BX51 microscope (Olympus, Tokyo, Japan). Panels A, B, and I-N were taken at a magnification of 100 × (10 ×/0.3 NA objective); C, D, F, H, at 200 × (20 ×/0.5 NA objective); and E, G, and inset of A, at 400 × (40 ×/0.75 NA objective). Images were imported using Analysis software (Soft Imaging System), and Adobe Photoshop CS 8.0.1 (Adobe Systems, San Jose, CA) was used to create the color plate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2004-12-4607/6/m_zh80170583440004.jpeg?Expires=1767847356&Signature=DpKUsE~5rOn9EVc6BY~wG2zVPv65vqzkY8KF~ilLflT10-4CpRb6u3mwnyOL-AIey9FdDGbMzNIRvaRiG3Ovh8t5Ns5oQDC-fLgX3I86BQXI7T75H9pArSM7GullQiGeBNlAH5FunX-SlhGzRXpNWVyZnEqN0tEL55GRTXWdyJ1QHpGOGDC~gW2lbZC-IvOXuYPkpbf1zA-NnAsjcM71SQkSOSd8xq1gbfrURn~rhwmbwuoP0npMRXW~lAn9vjN11nkIFbxBr38qkglaid74VNTTivHL0077mynmw2Y8Su~vYmcLknS~8CyQgf6cWzNTw2eDrX3fwcSYtHRH5hWBHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Induction of CD4+CD25bright regulatory T cells in rats undergoing BMT. (A) Representative FACS blots of DA rats without BMT and with BMT with DA or ACI BM. (B) CD4+CD25bright cells in LNs of DA rats without BMT and with BMT (DA rats without BMT [n = 8], DA rats after BMT with BM from DA rats [n = 6] or ACI [n = 6] rats). Numbers in graphs represent percentages of gated lymphocytes in each quadrant. There were differences between the groups receiving and not receiving transplants (*P < .01, ANOVA). (C) Foxp3 expression in spleen cells from DA rats without BMT (n = 7) and with BMT with syngeneic BM (n = 7). There were differences between the groups receiving and not receiving transplants (*P = .05, t test). (D) Transfer of CD4+CD25+ T cells results in protection from MOG-induced EAE in DA rats as compared with rats transferred with CD4+ T cells depleted of CD25+ cells (n = 7 each group, P < .001, t test). Repeated experiments showed similar results (n = 3 each group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2004-12-4607/6/m_zh80170583440007.jpeg?Expires=1767847356&Signature=BoHFbd4xexjwqku9LXOI0XZCnd7RblRubNABbhPTxcX4tNCm-uoEtOrULSJdPhOR1r7dafmAixjJaOdm7ftcDVoXoF9VYkU5xAl5hxGX32pKk4UCWSxihCe~JY0fIlXRwm3uKBbHAuEESxDa6DaFOnj2QwpOcixolAphqCVNYYCLLgX9DKEWTXSusaA5uKQWkRSglyDkDsyeBtyoF4O0M0wFcs24OOmLNRkyO0xRwR6iOk4NqcLegiRmBWOXYqYZknzc29E57vPzIUDUekpUOcCYzI1OCUSdpHMsnrBC8CKjH76SEXnZ0gnqBqSiysYCZ7e-AB9kljIeGpyzrn~vPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal