Abstract
The purpose of this study is to compare our standard chemotherapy regimen (CHVP [cyclophosphamide, doxorubicin, teniposide, and prednisone]) plus interferon with 4 courses of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) followed by high-dose therapy with autologous stem cell transplantation (ASCT) in treatment-naive patients with advanced follicular lymphoma. Four hundred one patients were included from July 1994 to March 2001: 209 received 12 cycles of CHVP plus interferon α for 18 months (CHVP-I arm) and 192 received 4 cycles of CHOP followed by high-dose therapy (HDT) with total body irradiation and ASCT (CHOP-HDT arm). Overall response rates were similar in both groups (79% and 78% after induction chemotherapy, respectively). One hundred thirty-one of the 150 patients eligible for HDT underwent transplantation (87%). Intent-to-treat analysis after a median follow-up of 7.5 years showed that there was no difference between the 2 arms for overall survival (P = .53) or event-free survival (P = .11). Patients with a complete response at the end of the induction therapy had a statistically longer event-free survival and overall survival (P = .02 and < .001, respectively). After long-term follow-up, our study showed that there was no statistically significant benefit in favor of first-line high-dose therapy in patients with follicular lymphoma. High-dose therapy should be reserved for relapsing patients.
Introduction
Follicular lymphoma accounts for 25% to 40% of all lymphomas, and patients have an indolent course with a median survival of 7 to 10 years. This lymphoma is not curable with conventional treatments and there is a constant annual death rate of 8%.1 Objective response rates of 60% to 70% were reported with standard chemotherapy regimens such as alkylating agent-based or anthracycline-containing regimens.2,3 However, the median response duration did not exceed 1.5 to 3 years. Chemotherapy combined with interferon α (Intron A) gave an improved survival in comparison with chemotherapy alone but it did not cure patients with follicular lymphoma.4 High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) gave encouraging results in phase 2 studies on relapsing patients with follicular lymphoma.5-8 HDT was also tested as a potentially curative option in first-line treatment of the subset of patients with disseminated disease.9-11 Recently, 2 randomized studies reported a benefit in event-free survival but not in overall survival in patients with high-risk follicular lymphoma, possibly due to a short follow-up.12,13
In 1994, Groupe d'Etude des Lymphomes de l'Adulte (GELA) designed a randomized controlled trial for patients with newly diagnosed follicular lymphoma and a high tumor burden comparing CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) followed by HDT (CHOP-HDT) with a conventional chemotherapy regimen with interferon α (cyclophosphamide, doxorubicin, teniposide, and prednisone; CHVP-I). This regimen, CHVP-I, was previously found to be superior to chemotherapy alone in the same group of patients.14,15 In the analysis presented here, results are given after a median follow-up of 7.5 years, allowing definitive conclusions for overall survival.
Patients, materials, and methods
Eligibility criteria
To be eligible, patients had to (1) be younger than 61 years with untreated follicular lymphoma at bulky stage II disease or stage III or IV and (2) require therapy because of a high tumor burden. High tumor burden was defined by at least one of the following parameters using Groupe d'Etude des Lymphomas Folliculaires (GELF) criteria: systemic symptoms (> 10% weight loss, temperature ≥ 38°C for more than 5 days, abundant night sweats); performance status (PS) greater than 1 according to the Eastern Cooperative Oncology Group (ECOG) scale; elevated lactate dehydrogenase (LDH) level; β2-microglobulin level greater than 25.5 nM/L (3 μg/mL); a single lymph node larger than 7 cm; marked splenomegaly; organ failure; pleural effusion or ascites; orbital or epidural involvement; blood infiltration; or cytopenia.14-16 Exclusion criteria were as follows: previous treatment for lymphoma; diagnosis more than 3 months before; blood creatinine level above 150 μM; history of another cancer except in situ breast cancer or uterine cancer; contraindication to doxorubicin, interferon, or intensive therapy; positive serologic test for the human immunodeficiency virus; or histologic transformation into a more aggressive lymphoma. This study complied with the Declaration of Helsinki and its current amendments and was conducted in accordance with Good Clinical Practice guidelines. All patients gave written informed consent. The protocol and informed consent forms were approved by local and national institutional review boards in each participating center.
Staging
The extent of the disease was determined by a standardized evaluation including computed tomography of the chest, abdomen, and pelvis; bone marrow biopsy; bone marrow aspiration with complete blood counts; LDH level; and β2-microglobulin assay. PS was graded with the ECOG scale. A panel of 5 hematopathologists conducted a central pathology review.
Randomization
After stratification according to center, eligible patients were assigned by the study coordinating center to CHVP with interferon or to CHOP followed by HDT with ASCT.
Treatment
In the conventional chemotherapy arm (CHVP-I), patients received 6 monthly courses of CHVP, with cyclophosphamide 600 mg/m2, doxorubicin 25 mg/m2, and teniposide 60 mg/m2 on day 1 and prednisolone 40 mg/m2 on days 1 to 5. As the marketing of teniposide was stopped during this study, it was replaced by etoposide 100 mg/m2 on day 1. Interferon alpha was given subcutaneously at a dosage of 5 million units (MU) 3 times a week. Patients then achieving a complete response (CR) or partial response (PR) received 6 courses of CHVP plus interferon every 2 months for 1 year. In the event of hematologic toxicity, the next chemotherapy cycle was postponed for 1 week and the dose of interferon was decreased to 3 MU. In the event of chronic grade 3 or 4 interferon-related toxicity, the dose of interferon was reduced to 3 MU. If grade 4 toxicity occurred despite this decreased dosage, interferon was stopped and chemotherapy was continued as scheduled. In the intensive arm (CHOP-HDT), therapy consisted of 4 cycles of CHOP every 3 weeks, with cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 (maximum 2 mg) on day 1 and prednisolone 40 mg/m2 on days 1 to 5. Two weeks after the fourth course of CHOP, the response was assessed and patients with stable or progressive disease were considered to be nonresponders and were treated according to the center policy. Responding patients (CR or PR) received a single course of cyclophosphamide 4500 mg/m2, etoposide 450 mg/m2 in 3 infusions, and G-CSF 300 μg from days 4 to 12 followed by peripheral blood stem cell (PBSC) harvest. PBSCs were harvested until 4 × 108 mononuclear cells/kg were obtained. High-dose therapy (HDT) was instituted 4 weeks later and comprised cyclophosphamide 60 mg/kg/d, mesna 60 mg/kg/d, and etoposide 150 mg/m2 from day -6 to -5. Split total body irradiation (TBI) was then performed delivering 10 Gy in 5 fractions from day -3 to -1 followed by PBSC reinfusion on day 0. A change in the dose or a delay between 2 courses of CHOP was not recommended except in the case of grade 3 or 4 hematologic toxicity.
Response assessment
Response to treatment was assessed at the end of the first period of treatment (induction) and at the end of scheduled treatment in both arms. Of note, these time points were defined differently in each arm: after 6 cycles or 12 cycles of CHVP for the CHVP-I arm (6 months and 18 months, respectively), and after 4 cycles of CHOP and after transplantation for the CHOP-HDT arm (11 weeks and 6 months, respectively).
CR was defined by the disappearance of all clinical evidence of the disease and normalization of all laboratory values, radiographic findings, bone marrow biopsies, and aspiration findings as defined by an international workshop.17 PR was defined as a reduction of more than 50% in the largest diameter of each measurable site of the disease or complete nodal response with residual bone marrow involvement. Stable disease (SD) was considered if tumor regression was less than 50%. Patients with no change in bone marrow infiltration or with persistent peripheral blood involvement were considered to have stable disease. Progressive disease (PD) was defined as an increase in tumor size of more than 25% or evidence of a new site of involvement.
Statistical analyses
The primary objective was to compare event-free survival (EFS) for the 2 treatments. EFS was calculated as the time from randomization to any event. Events were defined as induction failure (SD or PD at first response assessment), institution of an alternative treatment, death regardless of cause, progression after PR, or relapse after CR. The sample size required to detect a difference of 1 year in favor of the intensive therapy arm with a β error of 10%, α error of 5%, and a one-sided test was 400 patients. Patients' characteristics before treatment and responses rates were compared with a chi-square test. Overall survival (OS) and EFS were calculated with the product-limit method, and the significance of differences between the 2 survival curves was tested using the log-rank test. All analyses were made according to the intent-to-treat principle. At the time of analysis, 93% of the surviving patients had been examined during the last 9 months and only 8 patients were last seen more than 2 years before the analysis.
Results
The study was conducted in 71 centers in France and Belgium. A total of 402 patients were enrolled between July 1994 and March 2001. One patient was found to have a benign disease on revision and was rapidly withdrawn from the study. The other 401 patients were included in the final analysis, 209 in the CHVP-I arm and 192 in the CHOP-HDT arm.
Baseline characteristics of the 401 patients
Median age was 49 years. There was no significant difference between the 2 groups for clinical and biologic characteristics (Table 1). A central pathologic review was completed for 356 patients (88% of randomized patients). The diagnosis of follicular lymphoma was confirmed in 339 cases (95% of the reviewed cases). Seventeen patients were reclassified as diffuse large cell lymphoma (n = 10), mantle cell lymphoma (n = 5), or small lymphocytic lymphoma (n = 2).
Characteristics of the 401 patients included in the study
Characteristic . | CHVP-I arm . | CHOP-ASCT arm . |
|---|---|---|
| No. of patients | 209 | 192 |
| Median age, y | 49 | 49 |
| Male sex, no. (%) | 114 (55) | 107 (56) |
| Performance status, no. (%)* | ||
| 0 | 121 (62) | 114 (63) |
| 1 | 56 (29) | 54 (30) |
| Greater than 1 | 17 (9) | 12 (7) |
| Disease stage, no. (%)* | ||
| II | 16 (8) | 11 (6) |
| III | 26 (13) | 23 (12) |
| IV | 160 (79) | 153 (82) |
| B symptoms, no. (%)* | 58 (30) | 44 (24) |
| Bone marrow involvement, no. (%)* | 143 (73) | 140 (76) |
| Elevated LDH level, no. (%)* | 68 (35) | 64 (34) |
| Elevated β2-microglobulin level, no. (%)* | 85 (46) | 81 (45) |
| Tumor larger than 7 cm, no. (%)* | 135 (67) | 131 (70) |
| FLIPI score, no. (%)* | ||
| Low risk | 63 (32) | 55 (30) |
| Intermediate risk | 67 (34) | 79 (43) |
| High risk | 69 (34) | 51 (27) |
| Histologic findings, no. (%) | ||
| Reviewed | 182 (87) | 173 (91) |
| Follicular | 172 (95) | 167 (96) |
| Grade 1 | 36 (21) | 38 (23) |
| Grade 2 | 116 (68) | 112 (67) |
| Grade 3 | 4 (2) | 3 (2) |
| Unknown | 16 (9) | 14 (8) |
| Other lymphoma subtypes | 10 (5) | 7 (4) |
Characteristic . | CHVP-I arm . | CHOP-ASCT arm . |
|---|---|---|
| No. of patients | 209 | 192 |
| Median age, y | 49 | 49 |
| Male sex, no. (%) | 114 (55) | 107 (56) |
| Performance status, no. (%)* | ||
| 0 | 121 (62) | 114 (63) |
| 1 | 56 (29) | 54 (30) |
| Greater than 1 | 17 (9) | 12 (7) |
| Disease stage, no. (%)* | ||
| II | 16 (8) | 11 (6) |
| III | 26 (13) | 23 (12) |
| IV | 160 (79) | 153 (82) |
| B symptoms, no. (%)* | 58 (30) | 44 (24) |
| Bone marrow involvement, no. (%)* | 143 (73) | 140 (76) |
| Elevated LDH level, no. (%)* | 68 (35) | 64 (34) |
| Elevated β2-microglobulin level, no. (%)* | 85 (46) | 81 (45) |
| Tumor larger than 7 cm, no. (%)* | 135 (67) | 131 (70) |
| FLIPI score, no. (%)* | ||
| Low risk | 63 (32) | 55 (30) |
| Intermediate risk | 67 (34) | 79 (43) |
| High risk | 69 (34) | 51 (27) |
| Histologic findings, no. (%) | ||
| Reviewed | 182 (87) | 173 (91) |
| Follicular | 172 (95) | 167 (96) |
| Grade 1 | 36 (21) | 38 (23) |
| Grade 2 | 116 (68) | 112 (67) |
| Grade 3 | 4 (2) | 3 (2) |
| Unknown | 16 (9) | 14 (8) |
| Other lymphoma subtypes | 10 (5) | 7 (4) |
Data were missing for performance status (27 patients), disease stage (12 patients), B symptoms (25 patients), bone marrow involvement (24 patients), elevated LDH level (19 patients), elevated β2-microglobulin level (36 patients), tumor larger than 7 cm (12 patients), and FLIPI score (17 patients)
Response to initial therapy and patients' flowchart
The first assessment of response was performed 4 weeks after the sixth monthly course of CHVP in the CHVP-I arm (week 24) and 2 weeks after the fourth course of CHOP in the CHOP-HDT arm (week 11). Response rates in the 2 arms were not statistically different (Table 2). Objective response rates were 80% in the CHVP-I arm and 79% in the CHOP-HDT arm.
Response to treatment at time of first evaluation in the 2 arms (6 months for CHVP-I arm and 12 weeks for CHOP-ABMT arm)
Responses . | CHVP-I, no. (%) . | CHOP-HDT, no. (%) . |
|---|---|---|
| CR | 35 (17) | 27 (14) |
| PR | 130 (62) | 123 (64) |
| SD | 12 (6) | 31 (16) |
| PD | 23 (11) | 6 (3) |
| Death | 3 (1) | 0 (0) |
| Treatment stopped | 2 (1) | 4 (2) |
| Not evaluable | 4 (2) | 1 (1) |
Responses . | CHVP-I, no. (%) . | CHOP-HDT, no. (%) . |
|---|---|---|
| CR | 35 (17) | 27 (14) |
| PR | 130 (62) | 123 (64) |
| SD | 12 (6) | 31 (16) |
| PD | 23 (11) | 6 (3) |
| Death | 3 (1) | 0 (0) |
| Treatment stopped | 2 (1) | 4 (2) |
| Not evaluable | 4 (2) | 1 (1) |
In the CHVP-I arm, 165 patients obtained a CR or PR after the 6 months of induction therapy, and only 129 patients (62%) fulfilled the full 18 months of therapy, with 74 patients in CR (35% of included patients) and 55 patients in PR (26%) at the end of treatment. In the transplantation arm, 131 (78%) of the 150 patients eligible for transplantation underwent ASCT (68% of the total, 87% of eligible patients). The reasons for failure to proceed with transplantation were patient refusal in 4 cases; progressive disease in 3 cases; severe concomitant disease in 3 cases; physician's decision because of persistent bone marrow involvement in 3 cases and nonfollicular histologic subtypes in 2 cases; failure of collection in 1 case; and unknown reason in 3 cases. One patient died at day 55 after transplantation from interstitial pneumonia. At the end of therapy, 128 patients (67%) were in CR or PR.
Patient outcome
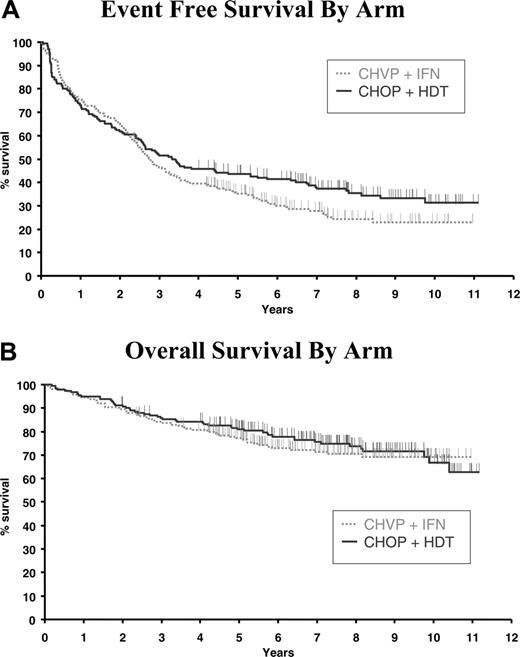
With a median follow-up of 92 months, EFS was not significantly different between the 2 arms. Median EFS was 33 months (95% confidence interval [CI], 29-40 months) in the CHVP-I arm compared with 40 months (95% CI, 31-64 months) in the CHOP-HDT arm. Seven-year EFS estimates were 28% (95% CI, 21%-34%) and 38% (95% CI, 31%-45%), respectively (P = .11; Figure 1A). Overall survival was similar in both treatment groups: median OS was not reached in the 2 groups, with an estimated 7-year OS of 71% (95% CI, 65%-71%) for the CHVP-I arm compared with 76% (95% CI, 69%-82%) for the CHOP-HDT arm (P = .53; Figure 1B).
Survival according to treatment arm. (A) Event-free survival (P = .11). (B) Overall survival (P = .53).
Survival according to treatment arm. (A) Event-free survival (P = .11). (B) Overall survival (P = .53).
After the central pathology review, diagnosis of follicular lymphoma was confirmed for 339 of the 401 patients included in the trial. For those 339 patients, EFS was better in the transplantation arm than in the chemotherapy arm, with a 7-year EFS of 40% (95% CI, 33%-48%) for the CHOP-HDT arm compared with 29% (95% CI, 21%-36%) for the CHVP-I arm (P = .05); however, overall survival was similar (P = .4).
Analysis according to prognostic indexes
The recently developed Follicular Lymphoma International Prognostic Index (FLIPI) index also gave a good prediction of survival outcome in our population18 : 7-year overall survivals were respectively 83%, 74%, and 64% in the groups with 0-1 adverse factor (117 patients), 2 adverse factors (144 patients), or more than 2 adverse factors (121 patients; P = .002); and 7-year EFSs were respectively 43%, 33%, and 23% for the same groups of patients (P = .001). When outcomes were stratified retrospectively according to the FLIPI score, there was a trend for a longer EFS in patients with an intermediate or high-risk score in the intensive arm, with a 7-year EFS of 37% (95% CI, 26%-43%) for the 130 patients in the CHOP-HDT arm compared with 29% (95% CI, 15%-30%) for the 135 patients in the CHVP-I arm (P = .057). No difference was observed between the 2 arms for overall survival.
Responding patients with a clinical CR had a longer OS and longer EFS than those only reaching a PR at the end of induction treatment: the 7-year OS was 88% (95% CI, 79%-96%) for CR patients and 77% (95% CI, 71%-82%) for PR patients (P = .023) and EFS was 56% (95% CI, 43%-69%) and 36% (95% CI, 31%-43%), respectively (P < .001; Figure 2). This favorable impact of CR after induction was observed for the subgroup of patients treated with CHVP-I but not for patients treated with CHOP-HDT who had the same outcome regardless of the quality of response.
Survival according to patient response at the end of induction. (A) Event-free survival (P < .001). (B) Overall survival (P = .023).
Survival according to patient response at the end of induction. (A) Event-free survival (P < .001). (B) Overall survival (P = .023).
Late toxicity
With a median follow-up of 92 months, 2 cases of secondary myelodysplastic syndrome and 2 of secondary acute myeloid leukemia were observed in the CHVP-I arm, whereas 2 cases of myelodysplastic syndrome were reported in the CHOP-HDT arm. Ten solid tumors were observed in the CHVP-I arm (lung, 4; oral cavity, 3; kidney, 1; bladder, 1; esophagus, 1) and 9 in the CHOP-HDT arm (oral cavity, 3; Hodgkin disease, 2; stomach, 2; breast, 1; kidney, 1).
Discussion
HDT with ASCT is feasible with an acceptable toxicity as first-line treatment for patients with advanced follicular lymphoma: 87% of patients responding to CHOP and eligible for HDT received a transplant, with only one toxic death. However, we were unable to confirm the benefit reported in 2 previous European studies12,13 ; by an intent-to-treat analysis, no difference was observed for EFS or OS. These negative results may be due to our long follow-up: 7.5 years compared with 4 years and 5 years in the 2 other studies, respectively. We have, in fact, previously reported the results of this study after a shorter follow-up and found a trend for a longer survival with HDT, mainly in high-risk patients.19 This may also reflect the fact that patients treated with chemotherapy alone were re-treated with rituximab alone or rituximab and chemotherapy after progression followed by ASCT. The impressive effect of rituximab in patients with follicular lymphoma has considerably changed the outcome in these patients.
Another reason for not obtaining better results with HDT is the fact that 4 cycles of CHOP did not provide a sufficiently good treatment before HDT. Clearly, the combination of CHOP with rituximab improves patient outcome.20,21 As the activity of standard chemotherapy is improved by a combination with rituximab,22 the role of HDT with ASCT in first-line treatment remains uncertain. HDT was advocated for patients with a higher risk of progression and death from lymphoma as defined by the FLIPI and should be tested in prospective clinical trials but, even in these selected patients, we were not able to find a better outcome with HDT. The subgroup of patients not reaching CR after induction might also benefit from this treatment, as this study showed the impact on outcome of the quality of response at the end of induction therapy.
The German randomized study showed a longer progression-free survival for patients treated with HDT, with no clear difference for overall survival.13 However, only patients responding to induction chemotherapy were randomized in this study and the results were only analyzed for those patients who effectively received their assigned treatment, whereas our results are analyzed on an intent-to-treat basis. The Groupe de l'Ouest et Est d'Etude des Leucemies Aigues et Maladies du Sang (GOELAMS) study also reported a benefit of HDT in high-risk follicular lymphoma without a benefit for overall survival.12 A major concern in these studies was the incidence of therapy-related myelodysplasia and secondary acute myeloid leukemia, mainly because of the use of high-dose etoposide for harvesting stem cells and the use of TBI in the intensification.23,24 After a median follow-up of more than 5 years, we only observed 2 cases in the transplant arm and 4 cases in the CHVP-I arm. The number of solid tumors was similar in both arms.
The recent development of monoclonal antibodies such as rituximab targeted against specific cell surface antigens represents a major change in the treatment of B-cell lymphomas.22,25,26 When associated with chemotherapy such as CHOP, rituximab provides a major improvement in the results, with an increase in the response rate of 72% to 100%. Recently reported randomized controlled trials in patients with follicular lymphoma have shown a longer time-to-treatment failure for patients receiving the rituximab combination in comparison with the same chemotherapy alone.20,21,27 If further follow-up demonstrates a longer survival, this combination will become the standard treatment for patients with advanced follicular lymphoma. In this context, the role of HDT with ASCT remains unclear and may be restricted to patients presenting a partial response at the end of treatment or patients relapsing after a combination of rituximab and chemotherapy. In the last German study,21 young patients responding to the rituximab plus CHOP regimen were randomized between HDT and autologous transplantation or interferon, since a difference had been observed when patients only received the CHOP regimen. After a short follow-up, no difference was observed between the 2 arms.21 The place of HDT in the setting of rituximab plus chemotherapy in first-line treatment remains to be confirmed.
Appendix
The following persons and institutions participated in this GELA study.
Pathologic review committee. N. Brousse, F. Charlotte, B. Fabiani, E. Labouyrie, L. Xerri.
Statistics. N. Mounier, E. Lepage, N. Nio, E. Capellani, and all clinical research assistants (CRAs) who collected the data.
Study centers. Hôpital Saint-Louis, Paris: C. Gisselbrecht; Centre Hospitalier Lyon-Sud, Pierre-Bénite: B. Coiffier; Centre Becquerel, Rouen: H. Tilly; Hôpital Henri Mondor, Créteil: F. Reyes; Centre Hospitalier Universitaire de Brabois, Nancy: P. Lederlin; Institut Paoli Calmette, Marseille: R. Bouabdallah; Centre Léon Bérard, Lyon: P. Biron; Centre Hospitalier Université Catholique de Louvain, Yvoir, Belgium: A. Bosly; Hôpital Saint-Joseph, Gilly, Belgium: P. Mineur; Hôpital Necker, Paris: C. Belanger; Hôpital Pitié Salpétrière, Paris: J. Gabarre; Institut Gustave Roussy, Villejuif: V. Ribrag; Hôpital de Hautepierre, Strasbourg: R. Herbrecht; Centre Jean Bernard, Le Mans: P. Solal-Celigny; Hôpital Kremlin-Bicêtre: G. Tertian; Centre Hospitalier Lapeyronie, Montpellier: J. F. Rossi; Centre Hospitalier R. Dubos, Pontoise: Y. Kerneis; Centre Hospitalier d'Annecy: C. Martin; Centre Alexis Vautrin, Nancy T. Conroy; Institut Curie, Paris: D. Decaudin; Centre Hospitalier de Meaux: C. Allard; Centre Hospitalier de Chambéry: M. Blanc; Hôpital Bon Secours, Metz: B. Christian; Hôpital de Valence: P. Y. Péaud; Hôpital Beaujon, Paris: J. Brière; Clinique Sainte Anne, Bruxelles: J. L. Doyen; Centre Val d'Aurelle, Montpellier: M. Fabbro; Hôpital André Mignot, Le Chesnay: S. Castaigne; Centre Hospitalier de la Durance, Avignon: E. Lepeu; Hôpital Inter Armées Percy, Clamart: G. Nedellec; Hôpital Pasteur, Colmar: B. Audhuy; Centre Médical Foch, Suresnes: E. Baumelou; Centre Hospitalier de Brive: S. Lefort; Centre Hospitalier Gilles de Corbeil: A. Devidas; Centre René Huguenin, Saint-Cloud: M. Janvier; Centre Hospitalier, Martigues: S. Caillières; Hôpital, Chalon: B. Salles; Hôpital La Fontonne, Antibes: J. F. Dor; Centre Hospitalier Saint-Vincent, Lille: C. Rose; Hôpital Emile Muller, Mulhouse: J. C. Eisenmann; Hôpital Edouard Herriot: J. Troncy; Centre Hospitalier, Bourg en Bresse: H. Orfeuvre; Centre Hospitalier, Lens: P. Morel; Centre Hospitalier Universitaire, Lille: I. Plantier; Centre Hospitalier Universitaire Clemenceau, Caen: O. Reman; Hôpital de Roanne: M. C. Goutebelle; Hôpital Jean Verdier, Bondy: O. Fain; Hôpital Saint Eloi, Montpellier: Quitet; Hôpital Béclère, Clamart: F. Boué; Centre Hospitalier Universitaire, Dijon: D. Caillot; Université Catholique de Louvain, Bruxelles: E. Van Den Neste; Centre Hospitalier Universitaire, Liège: G. Fillet; Fondation Drevon, Dijon: M. Flesch; Centre Hospitalier Bayonne: F. Bauduer; Centre Hospitalier, Martigues: A. Nezri; Centre Hospitalier Henri Duffaut, Avignon: H. Zerahzi; Centre Hospitalier, Nîmes: B. Richard; Centre Hospitalier, Libourne: K. Bouabdallah; Hôpital Marc Jacquet, Melun: C. Kulekci; Centre Hospitalier Victor Dupouy, Argenteuil: V. Pulik; Centre Hospitalier Robert Ballanger, Aulnay sous Bois: P. Agranat; Centre Hospitalier de la Citadelle, Liège: B. de Prijck; Hôpital J. Monod, Le Havre: Durant; Clinique du Méridien, Cannes: H. Naman; Hôpital Saint Joseph, Arlon: P. Pierre; CM de Bligny: C. Ferme; Centre Hospitalier, Saint Brieuc: Y. Agha; Centre Hospitalier, Valenciennes: M. Simon; and Hôpital Sainte Blandine, Metz: F. Rumilly.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-03-013193.
A complete list of the members of the Groupe d'Etude des Lymphomes de l'Adulte (GELA) appears in “Appendix.”
Supported by the French Programme Hospitalier de Recherche Clinique (PHCRC-1994) and by a grant from Schering-Plough.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal