Abstract
The impact of timing of antigen introduction into fetus and neonates leads to the suggestion that pre-existing antigens are tolerogenic to immunocompetent cells generated thereafter. This hypothesis predicts that in patients with cancer who are undergoing bone marrow transplantation, newly produced T cells with specificity for pre-existing tumor cells will be inactivated by the tumor antigens in the host. Because the effect of tumor cells on developing cancer-reactive T cells has not been investigated, we set out to systematically analyze the impact of tumor cells in the periphery on the development of tumor-reactive T cells in the thymus and their immunocompetence in the periphery. Our data demonstrate that in the host in which a tumor is established in the periphery, the cancer-reactive T cells develop normally, remain fully immunocompetent, become activated in the periphery, and cause regression of large established tumors. The immunocompetence of T cells generated in an antigen-bearing host is also confirmed in a skin graft transplantation model.
Introduction
Classic studies by Medawar et al established a fundamental principle that antigens presented to an immature immune system are tolerogenic.1 This discovery forms the cornerstone of immunology because it is the basic tenet of the clonal deletion theory proposed by Burnet,2 Talmage,3 and Lederberg.4 Brestcher and Cohn formulated their 2-signal theory in part based on the consideration that this process can be continuous throughout the life span of the animal.5 For antigens that are present throughout ontogeny, numerous studies have demonstrated the tolerogenic effect of pre-existing antigens on immunocompetent cells.6,7,8 What was less clear was the fate of self-reactive T cells produced de novo in adults to pre-existing antigens in the periphery, such as cancer antigens. Interestingly, studies with chimera mice suggested that for the same antigen and T-cell combination, transgenic T cells specific for allogeneic antigen expressed in the skin were tolerized in neonatal but not in adult mice.9
In recent years bone marrow transplantation has become a promising cancer immunotherapy. Thus, T-cell tolerance in adult animals to antigens not expressed in the thymus is a fundamental issue considering the implications for immunity generated subsequent to bone marrow transplantation. In addition to a wealth of clinical data showing increased patient survival following bone marrow transplantation, it has also been demonstrated that tumor antigen-specific T cells have been produced after bone marrow transplantation.10 Likewise, donor cells transplanted into virally infected baboons generated virus-specific cytotoxic T lymphocytes (CTLs)11 ; however, it remains unclear whether the antigen-specific T cells were derived from mature T cells in the bone marrow or from T cells produced de novo in the antigen-bearing host.
To definitively address whether developing T cells in adult animals remain competent to peripheral tumor antigens, we investigated the development of transgenic T cells specific for tumor antigen P1A in tumor-bearing adult mice and analyzed their immune competence, antigen-induced activation, and effector function. Here we report that cancer-specific cells produced de novo in tumor-bearing mice survive thymic deletion and remain competent when encountering tumor cells causing the regression of large established tumors.
Materials and methods
Experimental animals and cell lines
BALB/c and C57BL/6 mice were purchased from Charles River Labs (Wilmington, MA) under contract with the National Cancer Institute. BALB/c Rag2−/− and BALB/c nude mice were purchased from Taconic Farms (Germantown, NY) and bred in our facility. Mice containing a transgenic T-cell receptor (TCR) for the tumor antigen P1A (P1CTL mice) have been described previously.12 Mice were housed in a specific pathogen-free environment.
Bone marrow transplantation
Bone marrow from donor mice was obtained by flushing out marrow from the femur, tibia, and humerus. Red blood cells were lysed with ammonium chloride (Sigma, St Louis, MO). Mature T cells were depleted by incubation with rat anti–mouse CD4 and CD8 monoclonal antibodies, followed by goat anti–rat IgG Dynabeads (Dynal, Brown Deer, WI). T cells bound by Dynabeads were removed by magnetic separation, and T cell–depleted bone marrow was injected intravenously. For some treatment groups, T-depleted bone marrow from P1CTL mice was mixed with T-depleted bone marrow from BALB/c mice at a 3:2 ratio before injection.
Nude P1CTL bone marrow transplantation
Athymic nude mice were bred with P1CTL mice to obtain F1 mice. F1 mice were screened for the presence of the TCR transgene by staining peripheral blood for the Vα8.3 TCR. F1 mice were bred together to produce F2 mice. Nude pups were screened for the presence or absence of the TCR transgene by polymerase chain reaction (PCR) of tail DNA. Bone marrow from 3- to 4-week-old TCR transgene-positive or transgene-negative mice was harvested. Red blood cells were lysed and bone marrow was injected intravenously.
Tumorigenicity experiments
J558 cells (1-5 × 106) were injected subcutaneously in the lower abdomen. In experiments using mixed bone marrow from BALB/c and P1CTL donors, bone marrow was given at days 6 and 13 after tumor challenge. In the nude P1CTL experiment, bone marrow was given in a single dose on day 2 after tumor challenge. In some experiments, mice were killed after 1 month to analyze spleen and thymus. Tumor size was measured in 2 perpendicular dimensions.
Flow cytometry
Fluorochrome-labeled antibodies against cell-surface markers and H-2Ld dimeric fusion proteins were purchased from BD PharMingen (San Diego, CA).
Allogeneic skin graft experiments
BALB/c mice were anesthetized and a 1- to 2-cm patch of flank skin was removed and replaced with a similar size patch of C57BL/6 skin. Grafts were bandaged for approximately 1 week, and mice possessing healthy intact grafts after at least 2 weeks were used for experiments. T-depleted bone marrow (prepared as described) or undepleted spleen cells from BALB/c mice were injected intravenously (bone marrow) or intraperitoneally (spleen cells) in 2 doses at 1-week intervals. Mice were observed for signs of graft rejection, and grafts were considered rejected when completely removed from mice.
In vivo cytotoxicity assay
Spleen cells from BALB/c mice were pulsed with 10 μg/mL of either P1A (LPYLGWLVF) or a control peptide (YPHFMPTNL) in the presence of either 5 mM or 0.5 mM CFSE, respectively. After mixing at a 1:1 ratio, the labeled cells were injected intravenously into recipients and spleen cells were harvested 4 to 6 hours later and analyzed by flow cytometry for the relative abundance of the CFSEhi and CFSElow populations.
Results
Tumor growth did not cause clonal deletion of tumor-specific T cells in the thymus
Because mice with a targeted mutation of Rag2 lack endogenous T and B cells, tumors that will not induce adaptive immune response prior to bone marrow transplantation can be established in these mice. Taking advantage of this, we began by injecting plasmacytoma J558 into syngeneic Rag2−/− mice. Once the tumors became palpable, the tumor-bearing mice were reconstituted with a mixture of T cell-depleted bone marrow from both syngeneic wild-type (WT) mice and transgenic mice expressing a TCR specific for the unmutated tumor antigen P1A.12 As a control for the effect of tumor, we also generated chimera mice that were not challenged with tumor.
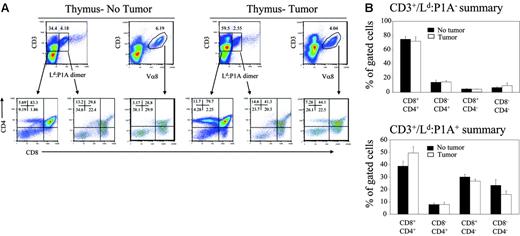
We have previously demonstrated that more than 95% of transgenic T cells express high levels of transgenic TCR,12,13 and, therefore, have the ability to bind to their cognate antigen. Based on this, we used the Ld:P1A dimer to identify transgenic T cells.14 As shown in Figure 1A(upper panels), despite a 3:2 advantage of bone marrow from transgenic mice, the majority of T cells produced are from WT bone marrow as revealed by the fact that most of the thymocytes are Ld:P1A−. To determine whether established tumors caused deletion of tumor-specific T cells, we compared the composition of cells derived from TCR-transgenic bone marrow in mice with or without pre-existing tumor. As shown in Figure 1A (lower panels), thymocytes from the non-transgenic donors have developed normally regardless of whether the recipients have tumor. Perhaps owing to deletion of P1CTL as a result of expression of P1A antigen in the thymic medullary epithelial cells,15 the phenotype of transgenic T cells is consistent with that of a partial clonal deletion, characterized by an increased proportion of CD4−CD8− T cells and a decreased proportion of CD4+CD8+ T cells (Figure 1A lower panels). However, the profiles of thymocytes from tumor-free and tumor-bearing mice are indistinguishable (Figure 1A-B). Moreover, in contrast to non-transgenic mature T cells, which consist of more CD4+CD8− subset, there is a significant skewing toward CD8+CD4− subset in both groups. This is consistent with a normal positive selection in both groups.
Development of P1A-reactive T cells in RAG-2–deficient chimera mice reconstituted with a mixture of bone marrow cells from BALB/c and BALB/c P1CTL. Rag2−/− BALB/c mice were given subcutaneous injections with either PBS or J558 tumor cells. Six days later, when the tumors were palpable, a mixture of T-depleted BALB/c P1CTL and WT BALB/c bone marrow cells (3:2) were injected intravenously in 2 doses at a 1-week interval. At 3 weeks after the second injection, when tumor rejection was observed in the periphery, the mice were killed and the T-cell subsets in the thymus were analyzed by 4-color flow cytometry using Ld:P1A dimer, and anti-CD3, -CD4, and -CD8. (A) Representative fluorescence-activated cell sorting (FACS) profiles of thymocytes from control (left panels) and tumor-bearing (right panels) mice are shown. The profiles of non-transgenic T cells are shown on the left of each group, whereas that of the transgenic T cells, as revealed by their specific binding to the P1A:Ld dimer or with anti–Vα8.3 TCR antibody, are shown on the right. (B) Summary of the T-cell subsets among polyclonal (top panel) and transgenic P1A-reactive T cells (bottom panel) in tumor-bearing and unchallenged mice. Graphs depict the mean ± SEM of 6 to 7 mice/group and are representative of 2 independent experiments.
Development of P1A-reactive T cells in RAG-2–deficient chimera mice reconstituted with a mixture of bone marrow cells from BALB/c and BALB/c P1CTL. Rag2−/− BALB/c mice were given subcutaneous injections with either PBS or J558 tumor cells. Six days later, when the tumors were palpable, a mixture of T-depleted BALB/c P1CTL and WT BALB/c bone marrow cells (3:2) were injected intravenously in 2 doses at a 1-week interval. At 3 weeks after the second injection, when tumor rejection was observed in the periphery, the mice were killed and the T-cell subsets in the thymus were analyzed by 4-color flow cytometry using Ld:P1A dimer, and anti-CD3, -CD4, and -CD8. (A) Representative fluorescence-activated cell sorting (FACS) profiles of thymocytes from control (left panels) and tumor-bearing (right panels) mice are shown. The profiles of non-transgenic T cells are shown on the left of each group, whereas that of the transgenic T cells, as revealed by their specific binding to the P1A:Ld dimer or with anti–Vα8.3 TCR antibody, are shown on the right. (B) Summary of the T-cell subsets among polyclonal (top panel) and transgenic P1A-reactive T cells (bottom panel) in tumor-bearing and unchallenged mice. Graphs depict the mean ± SEM of 6 to 7 mice/group and are representative of 2 independent experiments.
Because the P1A:Ld dimer has relatively low affinity for TCR in comparison with monoclonal antibodies, it is possible that it may not be able to detect P1CTL TCR at lower levels. To overcome this caveat, we also used anti–Vα8.3 TCR antibody, which provides about 10-fold stronger staining of the T cells. As expected, the anti–Vα8.3 TCR antibodies marked T cells with broader levels of TCR/CD3 and thus a higher proportion of T cells. Nevertheless, the relative abundance of each subset was unaltered irrespective of which reagents were used to mark transgenic T cells (Figure 1A). Therefore it appears that established tumors in the periphery have little impact on the development of cancer-reactive T cells in the thymus.
Activation of T cells generated de novo in tumor-bearing mice
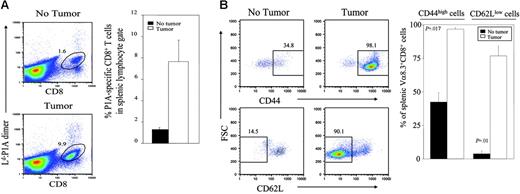
It has been established that self-reactive T cells that exit the thymus are also controlled by peripheral mechanisms, including functional inactivation and activation-induced cell death.6,15,16 We compared the peripheral T cells from tumor-bearing and non–tumor-bearing mice for their frequency and phenotype. As shown in Figure 2A, the number of P1A-reactive T cells is significantly increased in tumor-bearing mice. Since the number of transgenic T cells in the thymus is similar between tumor and non–tumor-bearing hosts, it is likely that the T cells have expanded in the periphery. Consistent with this notion, essentially all splenic transgenic T cells in tumor-bearing mice exhibit a phenotype of activated T cells, as revealed by up-regulation of CD44 and down-regulation of CD62L (Figure 2B).
Activation of P1A-reactive T cells in the periphery of tumor-bearing mice. Spleen cells from the chimera mice reconstituted with bone marrow from BALB/c P1CTL and BALB/c mice as described in Figure 1 were analyzed for the phenotype and number of P1A-specific T cells. (A) Expansion of P1A-specific T cells in the spleens of tumor-bearing mice. Spleens from chimera mice were stained with anti-CD8, and Ld:P1A dimer or anti–Vα8 TCR. FACS plots are representative profiles of cells within the lymphocyte gate. Graph depicts mean ± SEM of 6 to 7 mice/group. This trend of expanded P1A-specific T cells in tumor-bearing mice is representative of 2 independent experiments. (B) Phenotype of P1A-specific T cells from tumor-bearing and nonbearing mice. Data shown are CD44 and CD62L profiles of gated CD8+Vα8 TCR+ T cells. A representative profile from each group is presented on the left, whereas the graph on the right depicts the mean ± SEM of 3 mice/group and is representative of 2 independent experiments.
Activation of P1A-reactive T cells in the periphery of tumor-bearing mice. Spleen cells from the chimera mice reconstituted with bone marrow from BALB/c P1CTL and BALB/c mice as described in Figure 1 were analyzed for the phenotype and number of P1A-specific T cells. (A) Expansion of P1A-specific T cells in the spleens of tumor-bearing mice. Spleens from chimera mice were stained with anti-CD8, and Ld:P1A dimer or anti–Vα8 TCR. FACS plots are representative profiles of cells within the lymphocyte gate. Graph depicts mean ± SEM of 6 to 7 mice/group. This trend of expanded P1A-specific T cells in tumor-bearing mice is representative of 2 independent experiments. (B) Phenotype of P1A-specific T cells from tumor-bearing and nonbearing mice. Data shown are CD44 and CD62L profiles of gated CD8+Vα8 TCR+ T cells. A representative profile from each group is presented on the left, whereas the graph on the right depicts the mean ± SEM of 3 mice/group and is representative of 2 independent experiments.
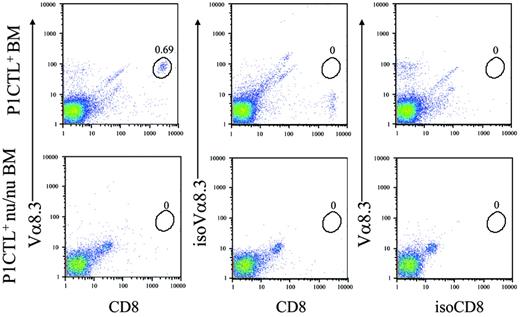
Although we have depleted mature T cells from the bone marrow to barely detectable levels (data not shown), it remains possible that the cells responding to tumor antigens in the periphery are expanded from undepleted mature T cells. To rule out this possibility, we bred the P1CTL transgene into the nude (nu/nu) background and used the P1CTL+nu/nu bone marrow cells to reconstitute tumor-bearing Rag2−/− hosts. As shown in Figure 3, the bone marrow from young P1CTL+nu/nu mice had no transgenic T cells.
P1CTL nu/nu bone marrows do not have mature transgenic T cells. One-month-old P1CTL nu/nu bone marrow cells were stained with anti-CD8, anti–Vα8.3 TCR, or isotype controls. WT P1CTL bone marrow was used as positive control. Data indicate a complete lack of T cells in the P1CTL+ nu/nu bone marrow.
P1CTL nu/nu bone marrows do not have mature transgenic T cells. One-month-old P1CTL nu/nu bone marrow cells were stained with anti-CD8, anti–Vα8.3 TCR, or isotype controls. WT P1CTL bone marrow was used as positive control. Data indicate a complete lack of T cells in the P1CTL+ nu/nu bone marrow.
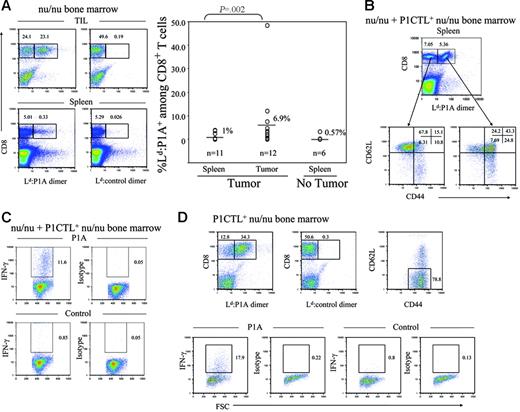
Because both donor and recipient mice were incapable of producing mature T cells, all T cells in the chimera mice were produced de novo after bone marrow reconstitution of the tumor-bearing host. One can therefore investigate the responsiveness of the T cells generated in the presence of tumors. In mice that received non-transgenic nu/nu bone marrow, all but one mouse had scarcely detectable levels of P1A-reactive T cells. Expansion of T cells in this mouse can be caused by abnormal up-regulation of P1A antigen, which is expressed at low levels under normal circumstances.12 However, it is interesting to note that a high level of P1A-reactive T cells was found among tumor-infiltrating cells, although considerable variation was observed in different recipients (Figure 4A) These data indicate that even in nontransgenic mice P1A-reactive T cells are generated de novo and preferentially expanded in tumor-bearing hosts.
Immune competence of T cells produced de novo in tumor-bearing mice. RAG-2–deficient BALB/c mice were challenged with 5 × 106 J558 tumor cells or PBS as control. Two days later, the mice received 500 rad irradiation and infusion of nu/nu bone marrow cells or a mixture of bone marrow cells from P1CTL+ nu/nu and nu/nu mice. The recipient mice were killed at the fourth week and tumor-specific T cells analyzed for their phenotype and response to P1A antigenic peptide. (A) High frequency of P1A-specific T cells among the TILs of nu/nu bone marrow recipients. Left panels show representative FACS profiles depicting highly expanded tumor-specific T cells among the TILs (top left panel) but not in the spleen (bottom left panel). Middle panels depict control stains. A summary graph of P1A-reactive CD8 T cells among the splenocytes and TIL of nu/nu recipients is shown in the right panel. (B) Enhanced activation of P1A-specific CD8 T cells. cell-surface phenotype of P1A-reactive and nonreactive CD8 T cells in the spleens of tumor-bearing mice reconstituted with a 4:3 mixture of bone marrow from nu/nu and P1CTL+nu/nu mice. (C) Cytokine response of spleen cells in tumor-bearing mice. Spleen cells from tumor-bearing mice reconstituted with a mixture of nu/nu and P1CTL+nu/nu bone marrow were stimulated with P1A (left panels) or control peptides (right panels) in the presence of Golgi blocker for 6 hours and stained with anti–IFN-γ antibodies after fixation and membrane permeablization. Data shown are gated CD8+ cells and are representative of 3 independent experiments. (D) Accumulation and immune competence of P1A-specific T cells in the tumors. Top panels show accumulation (left) and activation status (right) of tumor-reactive T cells; the bottom panels show cytokine response to tumor antigenic peptide P1A. Tumor single-cell suspension from chimera mice reconstituted with P1CTL+nu/nu bone marrow were stimulated with P1A or control peptides in the presence of Golgi blocker for 6 hours and stained with anti–IFN-γ antibodies after fixation and membrane permeablization. Cytokine response is shown for gated CD8+ T cells. T-depleted splenocytes from syngeneic WT mice were used as antigen-presenting cells (APCs). All data presented in this figure have been repeated 2 to 3 times.
Immune competence of T cells produced de novo in tumor-bearing mice. RAG-2–deficient BALB/c mice were challenged with 5 × 106 J558 tumor cells or PBS as control. Two days later, the mice received 500 rad irradiation and infusion of nu/nu bone marrow cells or a mixture of bone marrow cells from P1CTL+ nu/nu and nu/nu mice. The recipient mice were killed at the fourth week and tumor-specific T cells analyzed for their phenotype and response to P1A antigenic peptide. (A) High frequency of P1A-specific T cells among the TILs of nu/nu bone marrow recipients. Left panels show representative FACS profiles depicting highly expanded tumor-specific T cells among the TILs (top left panel) but not in the spleen (bottom left panel). Middle panels depict control stains. A summary graph of P1A-reactive CD8 T cells among the splenocytes and TIL of nu/nu recipients is shown in the right panel. (B) Enhanced activation of P1A-specific CD8 T cells. cell-surface phenotype of P1A-reactive and nonreactive CD8 T cells in the spleens of tumor-bearing mice reconstituted with a 4:3 mixture of bone marrow from nu/nu and P1CTL+nu/nu mice. (C) Cytokine response of spleen cells in tumor-bearing mice. Spleen cells from tumor-bearing mice reconstituted with a mixture of nu/nu and P1CTL+nu/nu bone marrow were stimulated with P1A (left panels) or control peptides (right panels) in the presence of Golgi blocker for 6 hours and stained with anti–IFN-γ antibodies after fixation and membrane permeablization. Data shown are gated CD8+ cells and are representative of 3 independent experiments. (D) Accumulation and immune competence of P1A-specific T cells in the tumors. Top panels show accumulation (left) and activation status (right) of tumor-reactive T cells; the bottom panels show cytokine response to tumor antigenic peptide P1A. Tumor single-cell suspension from chimera mice reconstituted with P1CTL+nu/nu bone marrow were stimulated with P1A or control peptides in the presence of Golgi blocker for 6 hours and stained with anti–IFN-γ antibodies after fixation and membrane permeablization. Cytokine response is shown for gated CD8+ T cells. T-depleted splenocytes from syngeneic WT mice were used as antigen-presenting cells (APCs). All data presented in this figure have been repeated 2 to 3 times.
To study the immune competence of P1A-reactive T cells, we analyzed the expression of cell-surface markers and cytokine response of P1A-reactive T cells derived from P1CTL+nu/nu bone marrow. As shown in Figure 4B, in mice that received a mixture of both transgenic and non-transgenic nu/nu bone marrow, the priming of P1A-reactive T cells is suggested by the down-regulation of CD62L and up-regulation of CD44. To test whether transgenic T cells have been primed in the tumor-bearing host, we stimulated the spleen cells from tumor-bearing recipients with tumor antigenic peptide P1A35-43 and analyzed the synthesis of IFN-γ following a short in vitro stimulation. As shown in Figure 4C, the transgenic T cells in the spleens were highly responsive to antigenic peptide as judged by the considerable production of IFN-γ.
The fact that tumor-specific T cells were primed in vivo indicates that they have come into contact with tumor antigens. To determine the functionality of these T cells, we analyzed the tumor-infiltrating T cells from mice that received P1CTL+nu/nu bone marrow cells. As shown in Figure 4D (top right), essentially all P1A-specific T cells exhibit an activated phenotype. Moreover, after P1A peptide stimulation, a high percentage of tumor-infiltrating T cells were capable of producing IFN-γ (Figure 4D bottom). Because the tumor-infiltrating lymphocytes (TILs) remained immune competent in an antigen-rich milieu, contacting pre-existing antigen did not tolerize the newly produced T cells.
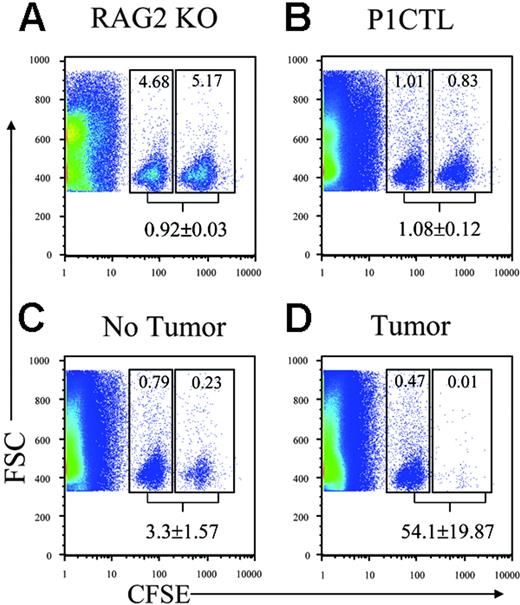
To determine whether T cells generated in tumor-bearing mice are cytotoxic, we carried out an in vivo cytotoxicity assay comparing the efficiency of elimination of P1A-pulsed versus control peptide-pulsed syngeneic T cells. As shown in Figure 5A, in Rag2–deficient hosts, the P1A-pulsed and control peptide-pulsed targets are presented at approximately 1:1 ratio. A slight, but statistically insignificant, reduction of P1A-pulsed target was observed in one of the naive WT P1CTL transgenic mice (Figure 5B). In the unchallenged recipients of T-depleted P1CTL bone marrow, the ratio of control to P1A-pulsed targets is approximately 3:1, which is consistent with activation of P1CTL either by homeostatic proliferation or by endogenous P1A antigen. Most remarkably, in tumor-bearing mice the ratio has increased to 54:1. These data provide clear evidence that tumor growth activates rather than silences T cells generated de novo in tumor-bearing hosts.
In vivo cytotoxicity of T cells produced in tumor-bearing hosts. Rag2− /− mice were challenged with tumor cells or left unchallenged. Two days later, the mice were reconstituted with T-depleted bone marrow cells from P1CTL mice. At 4 weeks after reconstitution, they received an intravenous injection of a mixture of P1A-pulsed (CFSEhi) and control peptide-pulsed (CFSElo) syngeneic BALB/c spleen cells (5 × 106 each). The spleens were harvested and analyzed by flow cytometry for distribution of fluorescence. Data shown are dot plots, showing the forward scatters and fluorescence intensity. CFSEhi and CFSElo cells are gated as indicated. The number shown in the gates are the percent of gated cells, whereas the number shown underneath the gates are means and SDs of the ratios of CFSElo/CFSEhi cells. The data indicate drastically stronger cytolysis of P1A-pulsed targets by CTLs in tumor-bearing hosts. The recipients used were: (A) control RAG-2–deficient mice without tumor challenge; (B) P1CTL mice without tumor challenge; (C) unchallenged RAG-2–deficient mice reconstituted with T-depleted P1CTL bone marrow; and (D) tumor-challenged RAG-2–deficient mice reconstituted with T-depleted P1CTL bone marrow. Data shown are representative of 2 independent experiments involving a total of 3 to 5 mice per group. Two-sided Student t tests indicate a very significant difference between the cytolysis found in tumor-bearing hosts versus all other groups (P < .001). None of the other differences are statistically significant.
In vivo cytotoxicity of T cells produced in tumor-bearing hosts. Rag2− /− mice were challenged with tumor cells or left unchallenged. Two days later, the mice were reconstituted with T-depleted bone marrow cells from P1CTL mice. At 4 weeks after reconstitution, they received an intravenous injection of a mixture of P1A-pulsed (CFSEhi) and control peptide-pulsed (CFSElo) syngeneic BALB/c spleen cells (5 × 106 each). The spleens were harvested and analyzed by flow cytometry for distribution of fluorescence. Data shown are dot plots, showing the forward scatters and fluorescence intensity. CFSEhi and CFSElo cells are gated as indicated. The number shown in the gates are the percent of gated cells, whereas the number shown underneath the gates are means and SDs of the ratios of CFSElo/CFSEhi cells. The data indicate drastically stronger cytolysis of P1A-pulsed targets by CTLs in tumor-bearing hosts. The recipients used were: (A) control RAG-2–deficient mice without tumor challenge; (B) P1CTL mice without tumor challenge; (C) unchallenged RAG-2–deficient mice reconstituted with T-depleted P1CTL bone marrow; and (D) tumor-challenged RAG-2–deficient mice reconstituted with T-depleted P1CTL bone marrow. Data shown are representative of 2 independent experiments involving a total of 3 to 5 mice per group. Two-sided Student t tests indicate a very significant difference between the cytolysis found in tumor-bearing hosts versus all other groups (P < .001). None of the other differences are statistically significant.
Transplantation of transgenic P1CTL bone marrow provides therapeutic effect to tumor-bearing mice
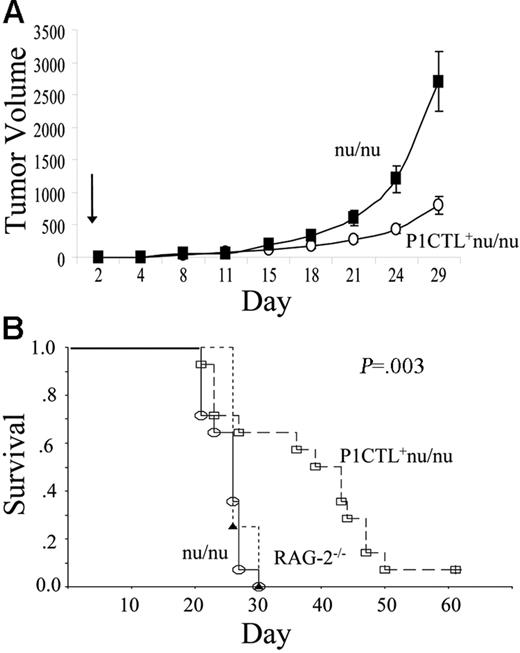
An important issue is whether tumor-specific T cells generated de novo in tumor-bearing hosts are capable of causing the rejection of tumor cells. As shown in Figure 6, with a single dose of bone marrow, we observed a substantial survival advantage and delay in tumor growth in mice receiving P1CTL+ nu/nu bone marrow, compared to mice receiving non-transgenic nu/nu bone marrow. The efficacy of the bone marrow cells is comparable to that of mature T cells, as we and others have reported.14,17,18,19
Bone marrow therapy of established tumors. (A) Delayed tumor growth in RAG-2–deficient mice receiving bone marrow from P1CTL+ nu/nu mice compared to those receiving bone marrow from nu/nu mice. The growth kinetics of tumors is shown in panel A (mean ± SEM), and the survival of tumor-bearing mice (as defined by time to reach tumor size of 4000 mm3) is shown in panel B. Growth kinetics are representative of 3 independent experiments; the survival data of tumor-bearing mice are cumulative data from 2 independent experiments.
Bone marrow therapy of established tumors. (A) Delayed tumor growth in RAG-2–deficient mice receiving bone marrow from P1CTL+ nu/nu mice compared to those receiving bone marrow from nu/nu mice. The growth kinetics of tumors is shown in panel A (mean ± SEM), and the survival of tumor-bearing mice (as defined by time to reach tumor size of 4000 mm3) is shown in panel B. Growth kinetics are representative of 3 independent experiments; the survival data of tumor-bearing mice are cumulative data from 2 independent experiments.
Transplantation of T-depleted bone marrow causes rejection of established allogeneic skin graft
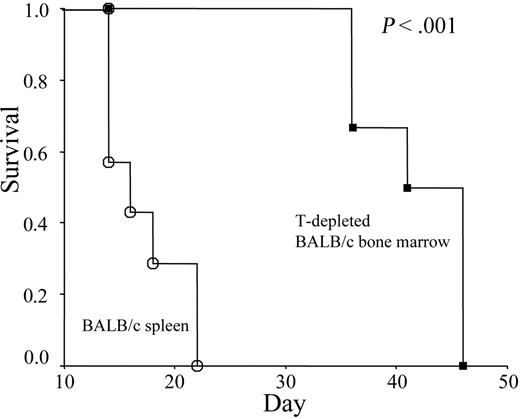
Previous work by Alferink et al9 demonstrated that transgenic T cells were neither tolerized nor activated in adult hosts that exclusively expressed specific antigen in the skin. To test whether polyclonal T cells developed in hosts bearing established allogeneic grafts are immunocompetent, we transferred T-depleted bone marrow cells from immune competent BALB/c mice into syngeneic BALB/c RAG-2−/− mice bearing an established skin graft from C57BL/6 mice. As shown in Figure 7, graft rejection was observed in all chimera mice that received T-depleted bone marrow cells. The significant delay in rejection in comparison to the mice that received allogeneic spleen cells is consistent with the time frame required for the maturation of T cells. Thus, immune competence of the T cells generated in an antigen-bearing host is not restricted to those that are specific for tumor antigen.
T-depleted allogeneic bone marrow cells cause rejection of pre-established skin grafts. RAG-2–deficient mice were given skin grafts from C57BL/6j mice. Survival of the skin grafts was monitored over a 2- to 3-month period. Kaplan-Meier curves depict skin graft survival in mice treated with either T-depleted bone marrow (n = 6; ▪) or undepleted spleen cells (n = 7; ○). The rejection of allogeneic skin grafts after treatment with T-depleted bone marrow has been observed in 2 independent experiments.
T-depleted allogeneic bone marrow cells cause rejection of pre-established skin grafts. RAG-2–deficient mice were given skin grafts from C57BL/6j mice. Survival of the skin grafts was monitored over a 2- to 3-month period. Kaplan-Meier curves depict skin graft survival in mice treated with either T-depleted bone marrow (n = 6; ▪) or undepleted spleen cells (n = 7; ○). The rejection of allogeneic skin grafts after treatment with T-depleted bone marrow has been observed in 2 independent experiments.
Discussion
Our data demonstrate that T cells generated de novo in antigen-bearing hosts are activated rather than inactivated. Our results provide a new explanation for the substantial clinical benefit of bone marrow transplantation into cancer patients.20,21 Previous studies demonstrated the function of donor T cells following bone marrow transplantation,10,11 although it is less clear in these earlier studies whether the antigen-specific T cells were derived from mature T cells in the bone marrow or from T cells produced de novo in the antigen-bearing host. Using bone marrow from T cell-deficient hosts, we have demonstrated unequivocally the immune competence of antigen-specific CD8 T cells generated in tumor-bearing hosts.
Our data also indicated that it is necessary to achieve a high frequency of antigen-specific T cells to reject rapidly growing tumors. Because the technical barrier of genetic reprogramming has been overcome recently,22 antigen-specific T cells can be produced en masse in tumor-bearing hosts to convey protection against established tumors.
The absence of tumor-induced clonal deletion, as reported here, differs from the clonal deletion in the setting of neonatal tolerance. Thus, previous studies using viral antigen and viral superantigens demonstrated that neonatal tolerance often involves clonal deletion in the thymus.23,24 One potential explanation is that the antigens in the peripheral tissues in neonates may be expressed or presented in the thymus, whereas those produced in the adult mice are not. Such a difference in antigen expression may explain why tumors established in adults do not cause clonal deletion.
Previous work by Aferink et al9 demonstrated that T cells produced in the adult host are ignorant of allogeneic antigens expressed as a transgene. On the other hand, our data demonstrate that tumor antigens and allogeneic skin grafts are capable of priming T cells. The fact that substantial numbers of tumor-reactive T cells (around 7% of CD8 T cells) can be detected in tumor-bearing mice reconstituted with non-transgenic nu/nu bone marrow indicate that the T cells have undergone substantial clonal expansion. Moreover, in another non-transgenic model we show that newly generated T cells cause complete rejection of MHC-mismatched skin grafts. As such, the naïve antigen-specific T cells were vigorously primed. These differences between our results and the conclusions of Aferink can be attributed to differences in experimental design. First, Aferink et al expressed the allogeneic MHC transgene by a keratin promoter. Therefore, the allogeneic MHC (H-2Kb) is expressed in nonhematopoietic cells. Because the alloantigen is not cross-presented, it will likely be presented to T cells by nonhematopoietic cells. In contrast, the P1A antigen is readily cross-presented, as we have reported.25 Secondly, an elegant study by Barker and Billingham26 has established a critical role for afferent lymphatics in skin graft rejection. In this regard, transgenically expressed Kb on nonhematopoietic cells also differs immunologically from the alloantigen expressed naturally in the skin that contains Langerhans cells that migrate into lymphoid organs to cause T-cell priming.
Taken together, our main conclusion of immunocompetence of cancer-reactive T cells produced in tumor-bearing mice demonstrates the utility of stem cell-based immunotherapy. This approach differs from T-cell adoptive therapy in an important way, because stem cells can continuously supply immune competent cells that are educated, but not tolerized, in the cancer-bearing host.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: K.F.M., K.L., P.Z., and Y.L. designed the experiments and wrote the manuscript; and K.F.M., K.L., E.K., S.A., L.Y., O.L., and Z.G. carried out the experiments. G.P. carried out part of the statistical analysis.
K.F.M. and K.L. contributed equally to this study.
Acknowledgments
This work was supported by grants from National Institutes of Health and Department of Defense Prostate Cancer Program. We thank Lynde Shaw for secretarial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal