Abstract
This multicenter phase 2 study evaluated the use of tipifarnib (R115777) in patients with poor-risk myelodysplastic syndrome (MDS; French-American-British classification). Patients (n = 82) received tipifarnib 300 mg orally twice daily for the first 21 days of each 28-day cycle. Twenty-six patients (32%) responded to tipifarnib: 12 (15%) complete responses (CRs) and 14 (17%) hematologic improvements; 37 patients (45%) had stable disease (modified International Working Group criteria, 2006). Among the 12 CRs, the median response duration was 11.5 months (range, 2.0-21.9 months), the median time to progression was 12.4 months (range, 3.9-23.8 months), and 7 were still alive at time of analysis (all > 3 years). Median overall survival was 11.7 months (95% CI, 9.4-15.0). Grade 3-4 neutropenia (18%) and thrombocytopenia (32%) were the most common treatment-related adverse events; severe nonhematologic adverse events were rarely reported. In this study, durable responses and acceptable side effects were observed. Tipifarnib is an active agent for the treatment of patients with intermediate- to high-risk MDS.
Introduction
Myelodysplastic syndromes (MDSs) are clonal stem cell disorders characterized by ineffective hematopoiesis leading to blood cytopenias and by a high risk of progression to acute myeloid leukemia (AML).1–4 Older patients, which constitute the large majority, generally receive supportive care only, low-dose chemotherapy or investigational agents. The United States Food and Drug Administration (FDA) recently approved 5-azacytidine (Vidaza, Pharmion, Boulder, CO), lenalidomide (Revlimid, Celgene, Summit, NJ), and decitabine (Dacogen, MGI Pharma, Bloomington, MN; SuperGen, Dublin, CA; Johnson & Johnson Pharmaceutical Research and Development LLC, Raritan, NJ) as new therapeutic options for patients with MDS, but treatments with improved safety, especially those that target new disease-related growth pathways, are needed to improve patient outcomes.
Tipifarnib (Zarnestra, R115777) is a potent and specific inhibitor of farnesyltransferase (FTase), a key enzyme that regulates cancer cell growth and is involved in multiple cellular functions (eg, cell signaling, proliferation, and differentiation).5–8 After a 30% response rate (complete remission [CR] + partial remission [PR]) was observed in a phase 1 study in patients with advanced leukemias,9 a single-center phase 2 study was started with tipifarnib at 600 mg orally twice a day for 4 weeks followed by a 2-week rest period in 28 patients with MDS. This study demonstrated activity with 2 CRs, lasting 7 and 14 months, one PR, and 5 hematologic improvements (HIs). However, in 11 patients, dose reduction to 300 mg orally twice a day was needed because of toxicity. The lower dose was better tolerated and responses were maintained.10
To determine a more tolerable regimen for patients with MDS, a single-center phase 1 dose-escalation study was conducted among 21 patients with MDS.11 Responses to tipifarnib were seen in 12 of the 20 evaluable patients (3 CRs, 2 PRs [25%], 7 HIs [35%] of which 2 were in all 3 lineages), and occurred at all dose levels (range, 300-900 mg/d). Median duration of response was 19+ months. The maximum tolerated dose in this study was 400 mg orally twice a day, but 300 mg orally twice a day was the recommended phase 2 dose, because at this dose the target enzyme, FTase, was consistently inhibited, durable responses occurred or were maintained, and 300 mg orally twice daily was better tolerated. The international multicenter phase 2 study in higher-risk MDS reported here was conducted to better determine the response rate at this recommend dose and schedule. The hypothesis was that a regimen associated with less drug-induced myelosuppression would result in better treatment tolerability and durable clinical responses.
Patients and methods
Study design
This single-arm, open-label, multicenter phase 2 study evaluated the effect of oral tipifarnib 300 mg twice a day for the first 21 days of each 28-day cycle for the treatment of patients with intermediate- to high-risk MDS.
Eligibility criteria
Patients 18 years of age or older were eligible for the study if they presented with pathologic evidence of MDS of the following French-American-British (FAB) subtypes: refractory anemia with excess blasts (RAEB) with bone marrow blasts more than 10%, RAEB in transition (RAEB-t), chronic myelomonocytic leukemia (CMML) with bone marrow blasts more than 10% or with bone marrow blasts 5% to 10%, and one of the following poor prognostic indicators: splenomegaly at least 10 cm below the costal margin, any splenomegaly with leukocytosis more than 10 000/mm3, serous effusion or other extramedullary sites, unfavorable karyotype (complex [≥ 3] or chromosome 7 abnormalities), transfusion-dependent anemia, leukocytosis more than 15 000/mm3, or platelet count less than 50 000/mm3.12–14 Patients could have received no more than one prior cytotoxic treatment for MDS. Previous therapy with noncytotoxic treatments for MDS was allowed; these included erythropoietin, granulocyte growth factors, danazol, thalidomide, antithymocyte globulin (ATG), cyclosporin, retinoids, and IL-11. Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1 was allowed. All patients were required to have serum creatinine less than or equal to 1.5 × the upper limit of normal (ULN) and serum transaminases less than or equal to 2.5 × ULN. Total bilirubin could not exceed 2 mg/dL, unless the increase was due to hemolysis. In accordance with the Declaration of Helsinki, the study was approved by each participating institution's ethics committee and all patients were required to provide written informed consent prior to study enrollment.
Patients with treatment-related MDS were excluded from the study unless they had been disease free from the primary malignancy for 3 years or longer. In addition, they could not have received chemotherapy for the prior malignancy within the last 3 years. Patients who had previously received extensive radiation therapy (> 25% of the bone marrow reserve) or prior treatment with FTase inhibitors were ineligible, as were patients who had received an investigational agent within the 30 days prior to study entry.
Treatment plan and evaluation
The starting dose of tipifarnib was 300 mg orally twice a day, given on the first 21 days of every 28-day cycle. In the absence of clinically significant toxic effects, the dose could be increased at 100-mg twice-a-day intervals, every 2 or more cycles, to a maximum of 600 mg orally twice daily at the discretion of the investigator for patients who had a PR, HI, or stable disease (SD). All patients were to undergo weekly to biweekly peripheral blood hematology assessment and bone marrow aspiration (and biopsy if indicated) at baseline, at the end of cycle 1, and every second cycle thereafter until reaching AML (blast count > 30%), death, or start of subsequent therapy. Patients with a CR were to continue tipifarnib treatment for 6 months or longer beyond the onset of that response. All other patients were to continue treatment until unacceptable toxicity or disease progression. After tipifarnib treatment was discontinued, patients were followed for disease progression, leukemic transformation, and overall survival.
The primary objective of this study was to assess the complete and partial response rate (CR and PR) among patients with higher-risk MDS. Secondary objectives included evaluation of HI rate (based on improvement in hemoglobin concentration and neutrophil or platelet counts), duration of outcome, overall survival, time to leukemia or death (TTLeu/D), and the safety profile of tipifarnib.
The final analysis was performed using all data at the clinical cut-off of September 30, 2004; a survival and response status update was performed in April-May 2006. All analyses were performed using the WHO classification for MDS and AML and response was defined according to the modified International Working Group (IWG) criteria, first published online in April 2006.15
Response definitions
CR was defined as a bone marrow with less than 5% myeloblasts with normal maturation of all cell lines. In addition, karyotypic abnormalities, if present at baseline, must have disappeared, and peripheral blood values had to reach the following cut-off values for at least 4 consecutive weeks: hemoglobin more than 11 g/dL (without transfusion or erythropoietin), neutrophils at least 1000/mm3 (without myeloid growth factor), and platelets equal to or more than 100 000/mm3 (without thrombopoietic agents or transfusions), and no peripheral blasts could be present. Criteria for PR were similar to CR, except that marrow blasts were required to have decreased by at least 50%. Bone marrow cellularity and morphology were not taken into account for PR. Progression was defined as loss of CR or PR either because of 50% or more increased bone marrow blast count or 50% or more decrement from best peripheral blood counts. Response duration for CR and PR is defined as the time from first assessment of response to the time of first observation of loss of response, either on the bone marrow or on peripheral blood counts. Time to progression is defined as the time from start of therapy to the time of first observation of progression. SD was referred to as the failure to achieve at least a PR, but with no evidence of disease progression for at least 2 months. In the absence of a CR or PR, patients were classified as having HI based on hemoglobin level and platelet or neutrophil counts according to the IWG criteria.15 Bone marrow CR was defined as a decrease in bone marrow blasts to less than 5% at least once, irrespective of peripheral counts. The TTLeu/D was the time from the first day of treatment until first observation of 30% or more bone marrow blasts or death.
Final analyses were based on the intent-to-treat population (n = 82). Summary statistics and plots based on Kaplan-Meier estimates were calculated.
Results
Baseline demographic and clinical characteristics
Eighty-two patients were enrolled in the study and treated with tipifarnib. The study recruited patients at 19 sites in 7 countries between September 2002 and March 2003. Therefore, inclusion criteria were based on the more widely used FAB classification. Patients with MDS defined as RAEB with marrow blasts more than 10%, RAEB-t, or CMML with marrow blasts more than 10%, or 5% to10% with additional risk factors, were eligible for this study. Because of the increased use of WHO classification and International Prognostic Scoring System (IPSS) scores, patients were reclassified using these more recent criteria. Moreover, response to therapy was reassessed using the modified IWG response criteria. Baseline demographic and clinical characteristics are shown in Table 1. Eleven patients with CMML with 10% or fewer marrow blasts had additional poor prognostic factors not taken into account by the IPSS, but shown to have poor prognosis in large CMML series.4,12–14 Two patients had RAEB-1 with 6% to 10% bone marrow blasts; they were included in the intent-to-treat analysis.
Baseline demographic and clinical characteristics of 82 patients
| Characteristic . | Patients . |
|---|---|
| Median age, y (range) | 67 (39-86) |
| Sex, no. (%) | |
| Male | 56 (68) |
| Female | 26 (32) |
| ECOG performance status, no. (%) | |
| 0 | 40 (49) |
| 1 | 42 (51) |
| Median time since MDS diagnosis, mo (range) | 8.8 (0-128) |
| Prior therapy for MDS, no. (%) | 30 (37) |
| Transfusion dependence at baseline, no. (%) | |
| Red blood cells | 57 (70) |
| Platelets | 11 (13) |
| MDS classification, no. (%) | |
| FAB classification | |
| RAEB | 40 (49) |
| RAEB-t | 23 (28) |
| CMML | 19 (23) |
| WHO classification | |
| RAEB-1 | 2 (2) |
| RAEB-2 | 40 (49) |
| CMML-1 | 8 (10) |
| CMML-2 | 9 (11) |
| AML-TLD | 18 (22) |
| AML | 5 (6) |
| IPSS score | |
| High | 32 (39) |
| Int-2 | 29 (35) |
| Int-1 | 8 (10) |
| Not applicable* | 13 (16) |
| Characteristic . | Patients . |
|---|---|
| Median age, y (range) | 67 (39-86) |
| Sex, no. (%) | |
| Male | 56 (68) |
| Female | 26 (32) |
| ECOG performance status, no. (%) | |
| 0 | 40 (49) |
| 1 | 42 (51) |
| Median time since MDS diagnosis, mo (range) | 8.8 (0-128) |
| Prior therapy for MDS, no. (%) | 30 (37) |
| Transfusion dependence at baseline, no. (%) | |
| Red blood cells | 57 (70) |
| Platelets | 11 (13) |
| MDS classification, no. (%) | |
| FAB classification | |
| RAEB | 40 (49) |
| RAEB-t | 23 (28) |
| CMML | 19 (23) |
| WHO classification | |
| RAEB-1 | 2 (2) |
| RAEB-2 | 40 (49) |
| CMML-1 | 8 (10) |
| CMML-2 | 9 (11) |
| AML-TLD | 18 (22) |
| AML | 5 (6) |
| IPSS score | |
| High | 32 (39) |
| Int-2 | 29 (35) |
| Int-1 | 8 (10) |
| Not applicable* | 13 (16) |
IPSS was not applicable because of no baseline karyotype (n = 2), secondary MDS (n = 4), or proliferative CMML (n = 7).
Tipifarnib treatment exposure
Patients received tipifarnib therapy for a median of 113 days (range, 6-1154 days). Dose escalation was performed in 8 patients. No improved responses resulted from dose increases. Treatment was discontinued for progressive disease in 42 patients and for adverse events in 22 patients; for 12 of these, the adverse advents were considered related to treatment.
Efficacy
CR was achieved in 12 of 82 patients (14.6%; 95% CI, 8.1%-24.6%). Patients reached the response after a median of 4 weeks, with 10 of 12 reaching their response within 2 months. In addition, 14 patients (17.1%) achieved HI for longer than 2 months: 12 monolineage (1E, 4N, 7P), 1 bilineage (EN), and 1 trilineage. In those patients, no deterioration in any cell lineages occurred during improvement in the other lineages. Thus, overall hematologic response to tipifarnib, demonstrated by improvement in peripheral blood count, was documented in 26 patients (31.7%). Thirty-seven patients (45.1%) had SD; this lasted for at least 1 year in 8 patients. In total, bone marrow CRs were seen in 32 patients: the 12 patients with overall CRs, 8 patients with concurrent HI, and 12 patients without reaching HI.
The median duration of CR was 11.5 months (range, 2.0-21.9 months), and the median time to progression was 12.4 months (range, 3.9-23.8 months) in these patients. At the time of the updated survival analysis (May 2006), 7 of 12 complete responders were still alive. Five died at 4.0, 15.0, 20.1, 23.3, and 25.7 months, whereas those still alive had survival ranging from 36+ to 43+ months. Four responders discontinued in CR, 3 discontinued due to adverse events, and 5 discontinued due to progressive disease. Three patients were retreated with tipifarnib at the time of relapse. One patient had a 7-month therapy-free period prior to relapse and did not attain a meaningful response on retreatment. A second patient had a 4-month therapy-free period prior to relapse and obtained a SD for 3+ months on retreatment. The third patient had a 5-month therapy-free period prior to relapse, reached a new CR for 22 cycles, and progressed recently (March 2006). Median duration of HI was 18 weeks, ranging from 6 to 76 weeks. CRs were seen in all WHO classes: RAEB (6 of 42; 14.3%), CMML-1 (1 of 8; 12.5%), CMML-2 (2 of 9; 22.2%), and AML/AML–trilineage dysplasia (TLD) (3 of 23; 13.0%) and irrespective of IPSS score: intermediate-1 (int-1; 2 of 8; 25%), int-2 (5 of 29; 17.2%), high (4 of 32; 12.5%), or not applicable (1 of 13; 7.7%).
Baseline cytogenetic assessment was successfully performed in 80 patients (98%), and repeat cytogenetic assessment was performed in 53 patients, including all 13 responding patients with CR. Sixteen of 53 patients had abnormal karyotype at baseline. Two responders had normalization of baseline karyotype (abnormal chromosome 8; trisomy 11). One patient, with SD, had a normalization from del21. Thirty-four patients, including 10 responders, maintained a normal karyotype, whereas 3 patients progressed from a normal baseline. Eight patients had no change from abnormal baseline karyotype. Progressive abnormalities were observed in 5 patients, 4 of whom had abnormal chromosome 8 at baseline.
Survival
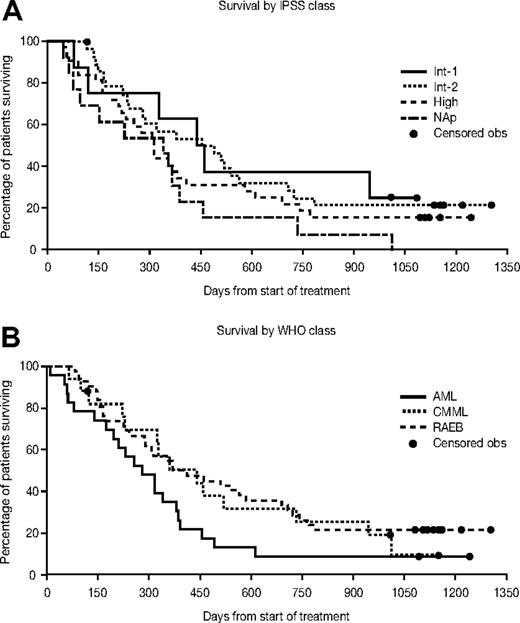
Median overall survival was 11.7 months (95% CI, 9.4-15.0 months). The median survival for patients with RAEB was 12.9 months (95% CI, 9.4-22.7 months), with CMML 14.5 months (95% CI, 7.5-24.1 months), and with AML/AML-TLD 9.2 months (95% CI, 6.4-12.5 months). The overall time to leukemic transformation or death was 7.1 months (95% CI, 4.8-9.4 months). The overall survival and the median time to leukemic transformation or death for each WHO and IPSS group are presented in Figures 1 and 2. The change in bone marrow blast count from baseline to transformation is shown in Figure 3. Median survival according to response was as follows: for CR not reached (7 of 12 alive; all for > 3 years), 10.4 months (95% CI,: 7.9-13.5) for HI/SD, and 10.4 months (95% CI, 3.2-12.5) for patients who did not respond.
Kaplan-Meier estimates for overall survival by WHO classification and IPSS class. Obs indicates observation; NAp, not applicable.
Kaplan-Meier estimates for overall survival by WHO classification and IPSS class. Obs indicates observation; NAp, not applicable.
Change in bone marrow blast count from baseline to time of transformation. BM indicates bone marrow.
Change in bone marrow blast count from baseline to time of transformation. BM indicates bone marrow.
Transfusion requirements
Prior to treatment with tipifarnib, 11 patients (13%) were dependent on platelet transfusions, and 57 patients (70%) required red blood cell (RBC) transfusions. For patients who needed transfusion at study entry, transfusion independence was defined as 2 consecutive cycles (at least 56 days, excluding the last cycle) without transfusion. Based on this definition, 3 of 11 patients (27%) became platelet transfusion independent, and 6 of 57 patients (11%) became RBC transfusion independent.
Safety and tolerability
The most common treatment-related adverse events were related to myelosuppression and its direct effects, infection or bleeding (Table 2). Grade 3-4 neutropenia was observed at baseline in 52% and during treatment in 74%. Baseline grade 3-4 thrombocytopenia was observed in 39% and during treatment in 74% of patients. Thirteen percent of patients had grade 3-4 anemia at baseline, whereas 62% reached such a grade during study. Grade 3-4 neutropenia, thrombocytopenia, and anemia were reported as possibly drug-related adverse events in 15 patients (18%), 26 patients (32%), and 15 patients (18%), respectively.
Most common drug-related grade 3 or 4 adverse events
| Adverse event . | Patients, no. (%) . | |
|---|---|---|
| Grade 3 . | Grade 4 . | |
| Hematologic events and their effects | ||
| Anemia | 7 (9) | 8 (10) |
| Neutropenia | 4 (5) | 11 (13) |
| Thrombocytopenia | 9 (11) | 17 (21) |
| Fever | 3 (4) | 0 (0) |
| Neutropenic fever* | 2 (2) | 0 (0) |
| Infection | 7 (9) | 1 (1) |
| Epistaxis | 2 (2) | 0 (0) |
| Purpura | 2 (2) | 0 (0) |
| Nonhematologic events | ||
| Rash | 3 (4) | 0 (0) |
| Fatigue | 2 (2) | 0 (0) |
| Adverse event . | Patients, no. (%) . | |
|---|---|---|
| Grade 3 . | Grade 4 . | |
| Hematologic events and their effects | ||
| Anemia | 7 (9) | 8 (10) |
| Neutropenia | 4 (5) | 11 (13) |
| Thrombocytopenia | 9 (11) | 17 (21) |
| Fever | 3 (4) | 0 (0) |
| Neutropenic fever* | 2 (2) | 0 (0) |
| Infection | 7 (9) | 1 (1) |
| Epistaxis | 2 (2) | 0 (0) |
| Purpura | 2 (2) | 0 (0) |
| Nonhematologic events | ||
| Rash | 3 (4) | 0 (0) |
| Fatigue | 2 (2) | 0 (0) |
N = 82.
Neutropenic fever not followed by period of infection.
Seven percent of patients experienced nonhematologic drug-related grade 3 or 4 adverse events: rash and fatigue (Table 2). Of 6 patients treated for longer than 16 months, 2 patients experienced mild peripheral sensory neuropathy (mild tingling) during the last cycle (cycles 22 and 23, respectively), leading to treatment discontinuation, reversible in one and still ongoing, 6 months after discontinuation, in the other patient.
Thirty-six patients (44%) were hospitalized because of adverse events, mostly related to infection: febrile neutropenia (11%), pneumonia (7%), fever (6%), bacterial infection (4%), sepsis (4%), or unspecified infection (2%). The reason for hospitalization was considered to be drug-related for only 15 patients (18%).
Ten patients died during the treatment period or up to 30 days after the last dose: 5 patients died due to progressive disease and 5 patients died due to an adverse event, of which one was considered drug-related. This latter patient died of coronary insufficiency triggered by anemia and severe internal bleeding in the context of nonresponsive MDS with persistent grade 4 thrombocytopenia.
Discussion
The reported multicenter international study evaluates the efficacy and safety of tipifarnib at 300 mg orally twice a day for the first 21 days of each 28-day cycle, among patients with intermediate- to high-risk MDS. Because responses had been observed at higher doses, too, the study allowed for dose escalation by increments of 200 mg/d up to 600 mg twice daily in patients with non-CRs who did not experience significant drug-related toxicity. Because this dose increase, performed in 8 patients, yielded only some improvement in response in one patient, it is plausible to regard the dose used, 300 mg twice a day, as the optimal dose using the schedule of days 1 to 21 every 4 weeks for this patient group. This is corroborated by the good tolerance of the regimen, the acceptable dose intensity, and the observed clinical activity. Tipifarnib therapy in this intermediate- to high-risk MDS population resulted in durable CRs in 15% of all patients treated. HI was seen in an additional 17% of patients, for an overall 32% clinical benefit rate. Important to note is that in any patient showing an HI in 1 or 2 lineages, there was no worsening in the other lineages. Responses were observed in both therapy-naïve and previously treated patients. Furthermore, 10% of patients had SD for over 1 year. The overall median survival for all patients was 11.7 months and the median time to leukemia or death 7.1 months. Of the patients who required platelet or RBC transfusions at baseline, 27% of patients became platelet transfusion independent and 11% became RBC transfusion independent.
The results of the present study are in line with or superior to those obtained in previous single-center studies of tipifarnib for the treatment of MDS using a dose range and different dosing schedules compared to the dose and schedule of this study. A higher dose, 600 mg twice a day in a protracted schedule of 4 weeks on drug followed by 2 weeks of rest, based on a phase 1 study in advanced hematologic malignancies, is considered too high for MDS patients, leading to early drop-outs because of toxicity.10 In a subsequent phase 1 study, the dose and schedule tested in the present study was reported to yield 25% CR + PR in 20 patients. Duration of response ranged from 10 to over 32 months. An additional 7 patients had HI (35%). Median overall survival was 14.6 months.11 A still-ongoing phase 1-2 dose-escalation study is intended to assess the use of an alternative schedule—that is, 1 week on study drug alternating with 1 week rest. To date, responses have been seen at several doses and tolerance has been excellent.17
Treatment for higher-risk MDS includes, in the relatively rare younger patients with MDS, allogeneic stem cell transplantation or cytarabine/anthracycline-based chemotherapy.18,19 Older patients, who constitute the large majority, generally receive supportive care only, low-dose chemotherapy, or investigational agents. The United States FDA recently approved 5-azacytidine (Pharmion, Boulder, CO) for the treatment of patients with MDS based on study results showing a significantly higher overall response (CR + PR) rate than supportive care alone (15.7% versus 0.0%).16 Lenalidomide (Celgene, Summit, NJ) was approved by the FDA for a subgroup of MDS patients, those with IPSS low or int-1 and the specific cytogenetic abnormality 5q−. This approval was based on a single-arm, open-label study, with 67% of patients becoming RBC transfusion independent for at least 8 weeks (range, 8-75+ weeks). In this study, no CR or PR nor HI in the leukocytic or thrombocytic lineages was reported.20
Most recently, decitabine (MGI Pharma, Bloomington, MN and SuperGen, Dublin, CA) was approved for treatment of MDS with IPSS scores int-1, int-2, or high, based on a randomized study versus best supportive care. Decitabine yielded a 17% response rate (CR 9%, PR 8%) with a median time to response of 93 days and a median response duration of 288 days.22
The patients treated in the present study all had higher-risk MDS, with either IPSS int-2 or high, or they had CMML with marrow blasts 5% to 10% with other poor-risk factors not accounted for in the IPSS. The complete remissions observed with tipifarnib therapy in patients with this higher-risk MDS population (15%) is similar to that observed with 5-azacytidine treatment in patients with all FAB subgroups including lower-risk MDS (15.7% or 11% when re-examined using IWG criteria),16,21 and with decitabine in patients with high-risk MDS (17%).22 In poor-risk CMML-2 (n = 9), tipifarnib yielded a 22% CR rate, with an overall median survival of 11.9 months (95% CI, 7.1-17.1). In CMML, decitabine yielded one response of 6 patients,22 whereas lonafarnib therapy in 35 patients resulted in one CR.23
The duration of response observed with tipifarnib in patients with intermediate- to high-risk MDS (11.5 mo) is at least as durable as responses observed with decitabine in patients with high-risk MDS (9.5 months).24 Obtaining durable CR and PR in patients with intermediate- to high-risk MDS, in all studies performed to date, indicates significant activity for tipifarnib in MDS. Responses are also observed in patients with a long-standing history of otherwise treated MDS (> 2 years since original diagnosis, and 1-4 lines of prior therapy): 4 CRs. Moreover, case reports from the early studies as well as the history of 3 patients in this study indicate that tipifarnib can be used to successfully reinduce responses on retreatment at the time of relapse after a previous CR to tipifarnib.
Myelosuppression was, as could be expected in this population, the most common adverse event, with grade 3-4 neutropenia and thrombocytopenia occurring each in 74% of patients. This was considered drug-related in 18% and 32%, respectively. It is important to note that 52% and 39% of these patients had grade 3-4 neutropenia and thrombocytopenia, respectively, at baseline. Grade 3-4 potentially drug-related infections or bleeding events were rarely seen and very few patients experienced nonhematologic drug-related grade 3 or 4 adverse events. This finding, the lower incidence of major infections (10%), and certainly major bleeding events (4%) contrasts with the natural history of the disease as well as with the reported toxicity to other compounds studied in this patient population. In a study of subcutaneous 5-azacytidine (75 mg/m2/d for 7 days every 28 days) in patients with all subtypes of MDS, grade 3-4 neutropenia, and thrombocytopenia were reported in 81% and 70% of patients and infection related to 5-azacytidine treatment occurred in 20% of patients.16 With decitabine, grade 3-4 neutropenia occurred in 87% of patients, grade 3-4 thrombocytopenia in 85%, and febrile neutropenia and pneumonia in 23% and 15%, respectively.22
The observed activity of tipifarnib in MDS throughout the program is in line with the demonstrated clinical activity in patients with a variety of hematologic diseases. In a phase 1 study of 35 patients with relapsed or refractory AML, the response rate was 32% in patients receiving tipifarnib.9 In a multicenter phase 2 study of tipifarnib administered at a dose of 600 mg orally twice daily for 21 days of each 28-day cycle in patients with previously untreated poor-risk hematologic malignancies (AML, n = 160; high-risk MDS, n = 4; CMML, n = 6) an overall response rate (CR + PR) of 34% was observed in 148 of 170 patients who were evaluable for response.25 Significant biologic activity with single-agent tipifarnib was reported in multiple myeloma, with 64% disease stabilization in heavily pretreated patients,26 in myelofibrosis with myeloid metaplasia,27 with 61% symptomatic decrease in organomegaly, and in myeloproliferative disorders28 with 65% biologic responses. In chronic myeloid leukemia (CML), tipifarnib has single-agent activity29 as well as activity in combination with imatinib in patients with CML refractory to imatinib.30,31
In the current study, tipifarnib 300 mg orally twice a day for the first 21 days of each 28-day cycle appears to be an active oral outpatient therapy that results in durable responses and HI with transfusion independence. Of interest is the activity in poor-risk CMML-2. With few major infections or bleeding events reported, and only rarely nonhematologic toxicity, this treatment is very tolerable in patients with intermediate- to high-risk MDS. Additional studies are warranted to further explore the place tipifarnib can have in the armamentarium for MDS therapy.
Authorship
Contribution: P.F., A.R., G.M., C.A., U.G., H.K., L.C., and R. Kurzrock. performed the research, collected data, and contributed to the writing of the paper; P.D.P. and R. Kerstens designed the research, analyzed the data, and wrote the paper. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: P.D.P. and R. Kerstens are employed by the company whose product was studied.
The complete list of participants is presented in Document S1, available on the Blood website; see the Supplemental Document link at the top of the online article.
Correspondence: Razelle Kurzrock, Phase I Program, Division of Cancer Medicine, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 422, Houston, TX 77230; e-mail: rkurzroc@mdanderson.org.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We gratefully acknowledge the many patients and families who made this study possible. We also acknowledge Aby Buchbinder, MD, and Youn C. Park for their assistance with data analysis and editing of the manuscript.
P.D.P. and R. Kerstens are employed by the company whose product was studied in the present work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal