Megakaryocytes and erythroid cells share a common precursor, the megakaryocyte/erythroid progenitor (MEP). Two reports in this issue of Blood provide new insights into megakaryocyte ontogeny and the plasticity of MEPs.
Shortly after gastrulation, fetuses must produce their own supply of red blood cells to transport nutrients and nourish the embryo. This is accomplished in 2 stages: primitive erythropoiesis, which initiates in the blood islands of the yolk sac, and definitive erythropoiesis, which predominantly occurs in intraembryonic sites such as the fetal liver. Although multiple studies have shown that erythroid cells share a common precursor with megakaryocytes during definitive hematopoiesis and that megakaryocytes can be generated from yolk sac tissue,1,2 the relationship between these 2 cell types during primitive blood development has remained unclear. Furthermore, the developmental stages during which embryonic platelets are produced, and the requirements for platelets in utero, have not been well defined.
Meg-CFCs and 2 novel bipotential MEPs emerge during embryonic hematopoiesis. See the complete figure in the article beginning on page 1433.
Meg-CFCs and 2 novel bipotential MEPs emerge during embryonic hematopoiesis. See the complete figure in the article beginning on page 1433.
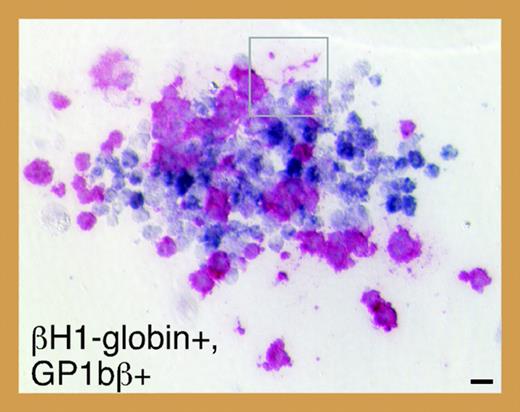
To investigate the ontogeny of megakaryocytes in mice, Tober and colleagues evaluated megakaryopoiesis in murine embryos beginning at E7.0. Using a combination of progenitor assays and immunohistochemistry, they discovered that the capacity to produce megakaryocytes coincides temporally and spatially with primitive and definitive waves of erythropoiesis. In addition, consistent with the existence of a bipotential primitive megakaryocyte/erythroid progenitor (MEP), they identified single colonies containing both primitive erythroid (βH1-globin positive) and megakaryocytic (GP1bβ-positive) cells (see figure). Serial dilution assays suggest that, similar to definitive hematopoiesis, a single progenitor cell can give rise to both lineages during primitive hematopoiesis. Do these progenitors give rise to platelets in vivo? To answer this question, Tober and colleagues assayed for megakaryocytes and platelets in both the yolk sac and the embryo proper. Rare GP1bβ-positive cells corresponding to megakaryocytes were detected in the yolk sac at E9.5 and within the fetal liver by E10.5. Surprisingly, platelets were first detected as early as E10.5 in peripheral blood pooled from multiple embryos, and subsequently in all fetuses by E11.5. These early platelets are larger than those circulating within late-stage fetuses and adults, and contain smaller α-granules and a larger open canalicular system. Also, in contrast to adult platelets, the vast majority of E11.5 platelets was reticulated. Although these differences may reflect the maturation stage of the megakaryocytes from which the platelets were derived, it is tempting to speculate that primitive platelets are morphologically distinct from their more mature counterparts in the same way that primitive erythroid cells are larger than definitive erythroid cells.
MEPs are defined by their limited potential to give rise to erythroid and megakaryocytic cells. Both of these lineages require the transcription factor GATA-1 for their terminal maturation, but the function of GATA-1 in the MEP has not been extensively studied. Mice engineered to express reduced levels of GATA-1 (GATA-1low mice) display thrombocytopenia throughout their lifetime and eventually develop anemia with myelofibrosis and extramedullary hematopoiesis.3 In addition, these mutants show defects in mast-cell development and an accumulation of unique trilineage cells committed to the erythroid, megakaryocytic, and mast-cell lineages.4 In this issue of Blood, Ghinassi and colleagues demonstrate that single immunophenotypic MEPs isolated from GATA-1low mice, which express 4-fold less GATA-1 than wild-type MEPs, have the remarkable ability to generate not only red blood cells and megakaryocytes, but also mast cells. These latter cells are normally derived from mast-cell precursors that branch off from the common myeloid progenitor (CMP) opposite to and distinct from the MEP. These observations demonstrate that GATA-1 is a key element for lineage commitment of progenitors downstream of the CMP. Furthermore, the level of GATA-1 expression in these progenitor-cell compartments appears to play an essential role in determining what progenies are generated.
Precise regulation of GATA-1 activity is essential for normal hematopoiesis, as mutations that interfere with its normal function contribute to megakaryocytic leukemia in children with Down syndrome (DS-AMKL). Likewise, in several animal models reduced expression of GATA-1 has been linked to increased progenitor-cell proliferation and hematologic abnormalities. Of great interest, mice engineered to express the mutant form of GATA-1 associated with DS-AMKL show a transient expansion of a unique yolk sac and fetal liver progenitor.5 As Tober and colleagues suggest, it is tempting to speculate that the target cell of the GATA1 mutation in this malignancy is the yolk sac megakaryocyte progenitor described in their report. Future studies focused on human fetal progenitor cells and DS-AMKL patient samples will likely provide the next wave of important new insights into the development of this disease.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal