Abstract
Unlike unanimous opinion on tissue factor (TF) expression in monocytes, the quest for TF presence in granulocytes has been going on for decades. To study the cell origin and track the blood-borne TF, we assessed TF activity and protein levels, knocked-down endogenous TF expression with small interfering RNA (siRNA), and overexpressed TF–yellow fluorescent protein (TF-YFP) fusion in immunologically isolated human monocytes and granulocytes. Monocytes and, to a much lesser extent, granulocytes isolated from lipopolysaccharide (LPS)/phorbol 12-myristate-13-acetate (PMA)–stimulated whole blood contained active TF antigen. However, only monocytes possessed significant TF activity and protein levels when stimulated with LPS/PMA in suspension. Reintroduction of TF-silenced monocytes to whole blood led to a profound reduction of LPS/PMA-stimulated TF activity in both mononuclear cell (MNC) and granulocyte fractions. No reduction in TF activity in MNC and granulocyte fractions was observed when TF-silenced granulocytes were reintroduced to whole blood. As shown by immunoblotting, flow cytometry, and confocal microscopy, granulocytes became positive for TF-YFP when isolated from whole blood reconstituted with TF-YFP–expressing monocytes. Together, we pinpoint monocytes as a major source of TF and provide solid experimental evidence for a direct transfer of TF protein from the monocytes to granulocytes in the blood.
Introduction
The source of tissue factor (TF) among circulating blood leukocytes still remains a mystery that is bolstered by several conflicting reports.1-7 TF is a 45-kDa membrane glycoprotein that binds to factor F VII (FVII)/FVIIa with high affinity.8 The formation of TF–FVIIa complexes triggers the coagulation cascade by activating both factors IX and X, which leads to thrombin generation and stimulates platelet activation and fibrin deposition.9-11 Under physiologic conditions, TF is absent in the cells and tissues located on the interface with circulating blood. It is constitutively expressed in vascular cell types that have no direct contact with blood, such as adventitial fibroblasts, pericytes, and smooth muscle cells.12 This mode of expression provides a haemostatic protection should vascular injury occur. In 1989, Drake et al created a concept of TF as being a “haemostatic envelope ready to activate coagulation when vascular integrity is disrupted.”13
Although studies demonstrating the presence of TF on circulating blood cells began in the early 1970s, the question of which blood cell type expresses TF is still a matter of considerable discussion.14,15 To date, neutrophils, eosinophils, platelets, and even lymphocytes were shown to be positive for TF protein under diverse conditions.2-7
In our present study, we studied TF procoagulant activity and antigen in highly pure resting and stimulated monocytes and granulocytes under conditions of whole blood and plating. Having used small interfering RNA (siRNA)–mediated knockdown of endogenous TF expression in freshly isolated monocytes or granulocytes, we pinpoint monocytes as the only source of TF in whole blood. Furthermore, expressing human TF tagged with yellow fluorescent protein (YFP) in monocytes, we provide solid experimental evidence for a direct transfer of TF from monocytes to granulocytes in the blood.
Methods
The complete description of materials and methods is in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Plasmids and siRNA
Double-stranded siRNA against human TF mRNA (si-TF) or control nontargeting pooled siRNAs (si-CTR) with scrambled sequences were purchased from Dharmacon RNA Technologies (Lafayette, CO). The expression construct encoding human TF, the C-terminus of which was fused to YFP, was purchased from RZPD (Berlin, Germany). Green fluorescent protein (GFP)–encoding plasmid pGFP-C1 plasmid was from Clontech (Mountainview, CA). Membrane-targeted YFP was engineered by fusing myristylation motif to N-terminus of YFP (myr-YFP). All plasmids were purified by EndoFree Plasmid Maxi Kit (Qiagen Norge, Oslo, Norway). Lipopolysaccharide (LPS) contamination of DNA preps and other reagents was assessed with CoaTest (Haemochrom Diagnostica, Fredericksberg, Denmark).
Cell preparation and magnetic labeling
Blood sampling was performed under protocols approved by the Regional Committee for Medical Research Ethics at the Medical Faculty, University of Tromsø. Informed consent was obtained in accordance with the Declaration of Helsinki. Cells of interest (mononuclear cells [MNCs] or granulocytes) were isolated by density centrifugation by Lymphoprep or Polymorphprep (Axis-Shield, Oslo, Norway) according to manufacturer instructions. For further magnetic labeling, 107 of cells were used. Magnetic labeling was performed according to the manufacturer protocols. All kits for MACS columns and MACS Separator were from Miltenyi Biotec (Auburn, CA). Immunologic isolation of untouched human monocytes was performed as a negative selection using Monocyte Isolation Kit II. Granulocytes collected from the lower Polymorphprep layer were depleted for unwanted monocytes in a single-step isolation procedure by CD14 MicroBeads kit.
Nucleofection
For TF-silencing, 3 × 106 cells were nucleofected with 3 μg siRNA using Amaxa Nucleofector II device (Amaxa Biosystems, Gaithersburg, MD) according to manufacturer instructions. For transient expression of TF-YFP or myr-YFP fusion proteins in monocytes, 3 μg plasmid DNA was used for nucleofection of 6 × 106 cells. For assessment of nucleofection efficiency in TF-silencing experiments, 1 μg pGFP-C1 plasmid was used. After nucleofection, the expression of GFP was inspected at different times after nucleofection.
Verification of nucleofection efficiency and cell death rates
Flow cytometric analysis of GFP-positive cell population was performed to ascertain nucleofection efficiency at 2, 5, 10, and 24 hours after nucleofection. Death rate after nucleofection was assessed by incubation of monocytes or granulocytes in propidium iodide (PI) buffer (Invitrogen, Oslo, Norway) and flow cytometric analysis of PI-positive cell population. Differential interference contrast (DIC) images as well as images of GFP and PI fluorescence were obtained for visual inspection using fluorescent confocal microscope.
Experimental design
To detect TF protein expression in monocytes or granulocytes, whole blood or freshly immunologically purified monocytes or granulocytes were stimulated with LPS (5 ng/mL) or a combination of LPS and phorbol 12-myristate-13-acetate (PMA; 5 ng/mL) for 2 hours in cell culture incubator. In addition, isolated granulocytes were stimulated with P-selectin (5 μg/mL) for 1 hour or with a combination of granulocyte macrophage colony-stimulating factor (GM-CSF; 50 ng/mL) and platelet-activating factor (PAF; 10 μM) for 25 minutes.
To study the effects of silencing endogenous TF expression in monocytes or granulocytes, we reintroduced immunologically purified TF-silenced monocytes or granulocytes back into the whole blood, depleted for the corresponding cell type before reintroduction. Monocyte-free MNC fraction was recombined with washed red blood cells (RBCs), TF-silenced monocytes (or si-CTR–nucleofected or non-nucleofected monocytes), and platelet-rich plasma (PRP) in a proportion resembling the physiologic blood cell count (for flowchart of the procedure, see Figure S1). Reintroduction of TF-silenced granulocytes (or si-CTR–nucleofected or non-nucleofected granulocytes) to whole blood required mixing of RBCs, MNC fraction, and PRP (for flowchart of the procedure, see Figure S2).
Whole blood reconstituted with TF-silenced monocytes or granulocytes was stimulated with a combination of LPS and PMA. MNCs or granulocytes were isolated and kept frozen for analysis of TF procoagulant activity and protein levels.
To test the possibility of TF protein transfer from monocytes to granulocytes in a whole blood environment, immunologically isolated monocytes were nucleofected with a TF-YFP or with myr-YFP. Nucleofected monocytes were reintroduced to the whole blood and stimulated with LPS. MNC and granulocyte fractions were analyzed for YFP expression by flow cytometry, confocal microscopy, and Western blotting.
Flow cytometry
The purity of all cellular fractions was assessed by flow cytometry. The yield of immunologically purified monocytes was evaluated by immunostaining with fluorescein isothiocyanate (FITC)–conjugated mouse anti–human CD14 monoclonal antibody (Southern Biotechnology Associates, Birmingham, AL). Possible contamination by nonmonocytes was assessed by counterstaining with phycoerythrin (PE)-conjugated streptavedin (Southern Biotech). The yield of immunologically enriched granulocytes was evaluated by staining of cells with FITC-conjugated mouse anti–human CD15 monoclonal antibody (BD Biosciences PharMingen, San Diego, CA). Monocyte contamination of granulocyte fraction was checked by immunostaining with PE-conjugated mouse anti–human CD14 monoclonal antibodies (BD Biosciences PharMingen). To check for monocyte contamination in Lymphoprep RBC pellet prepared for whole blood recombining experiments, immunostaining with PE-conjugated mouse anti–human CD14 monoclonal antibody was used. To evaluate the expression of TF-YFP in nucleofected immunologically purified monocytes and the transfer of monocyte-originated TF-YFP fusion protein, cells of MNC and granulocyte fractions, counterstained for CD14 or CD15, were analyzed for YFP fluorescence.
Western blotting
After applied treatments, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting of cellular lysates was performed as described previously.16 Membranes were probed with the following antibodies: mouse anti-human TF monoclonal antibody (kindly provided by Dr K.G. Mann, University of Vermont, Burlington), mouse anti–human TF monoclonal antibody (Calbiochem, San Diego, CA), rabbit anti-GFP monoclonal antibody (Abcam, Cambridge, United Kingdom), and horseradish peroxidase–conjugated goat anti-rabbit or anti-mouse antibodies (BD Biosciences PharMingen). Detection and quantification of immunopositive bands was performed using LumiImager F1 and LumiAnalyst software (Roche Diagnostics/Boehringer Mannheim, Mannheim, Germany).
Fluorescent confocal microscopy
Peripheral MNC and granulocyte fractions were isolated from whole blood reconstituted with TF-YFP– or myr-YFP–nucleofected monocytes, paraformaldehyde-fixed, and immunostained with PE-conjugated mouse anti-human CD14 monoclonal antibodies. The morphologic evaluation of granulocyte nuclei was done by use of DRAQ5 DNA dye and visual inspection (Biostatus, Shepshed, United Kingdom).
Images were acquired using an Axiovert 200 microscope (Carl Zeiss, Oslo, Norway) equipped with a 40× 1.2W C-Apochromat objective and a confocal module (LSM510 META; Carl Zeiss). The LSM5 software version 3.2 (Carl Zeiss) was used for image acquisition.
Quantification of TF activity
TF was measured in the intact or frozen/thawed preparations of cells using a 2-stage clotting assay based on the ability of TF to accelerate the activation of FX by FVIIa as described previously.17
Blood cell counting
Blood cell counting was performed on a Sysmex K1000 (TOA Medical Electronics, Kobe, Japan) before and after density centrifugations and immunologic purifications.
Statistics
Statistical comparisons were performed using SigmaPlot 8.0 (SPSS, Chicago, IL) and Microsoft Excel version 2003 software (Microsoft Norge, Oslo, Norway). The data presented are shown as mean plus SEM. Comparisons between mean values were performed using the Student paired t test. A P value below .05 was used as the measure of significance.
Results
Efficient TF knockdown in monocytes and granulocytes
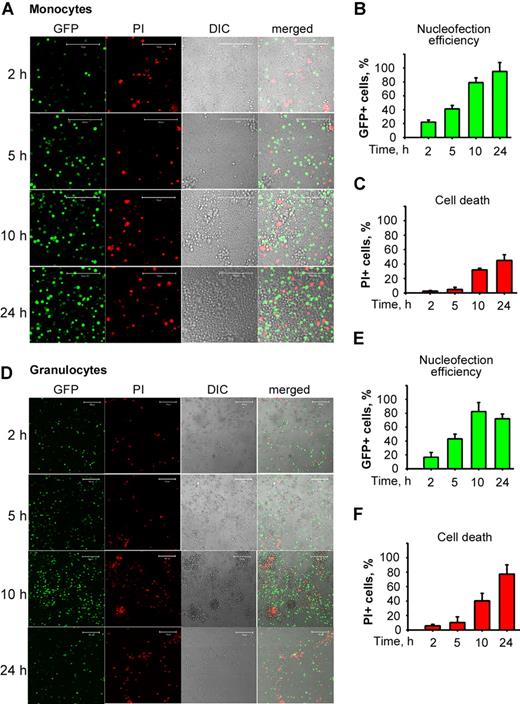
Flow cytometric analysis revealed that the percentages of GFP-expressing monocytes and granulocytes were gradually increasing over the time of incubation after electric shock (Figure 1B,E). Both monocytes and granulocytes tolerated nucleofection well during the first 5 hours after electric shock (Figure 1C,F, respectively). Furthermore, nucleofection did not bring about up-regulation of TF activity (data not shown).
Monocytes and granulocytes are readily nucleofected and tolerate nucleofection well. Monocytes or granulocytes were nucleofected with pGFPC1 and plated until analyzed at 2, 5, 10, and 24 hours for GFP expression or cell death by PI incorporation using flow cytometry and confocal microscopy. Confocal DIC and fluorescence images of nucleofected monocytes (A) and granulocytes (D) depict dynamics of GFP expression and PI positivity. Plots summarize the data on nucleofection efficiency of monocytes (B) and granulocytes (E) and rates of cell death monocytes (C) and granulocytes (F) obtained by flow cytometric analyses. Data in panels B, C, E, and F are means (± SEM). Scale bars represent 100 μm.
Monocytes and granulocytes are readily nucleofected and tolerate nucleofection well. Monocytes or granulocytes were nucleofected with pGFPC1 and plated until analyzed at 2, 5, 10, and 24 hours for GFP expression or cell death by PI incorporation using flow cytometry and confocal microscopy. Confocal DIC and fluorescence images of nucleofected monocytes (A) and granulocytes (D) depict dynamics of GFP expression and PI positivity. Plots summarize the data on nucleofection efficiency of monocytes (B) and granulocytes (E) and rates of cell death monocytes (C) and granulocytes (F) obtained by flow cytometric analyses. Data in panels B, C, E, and F are means (± SEM). Scale bars represent 100 μm.
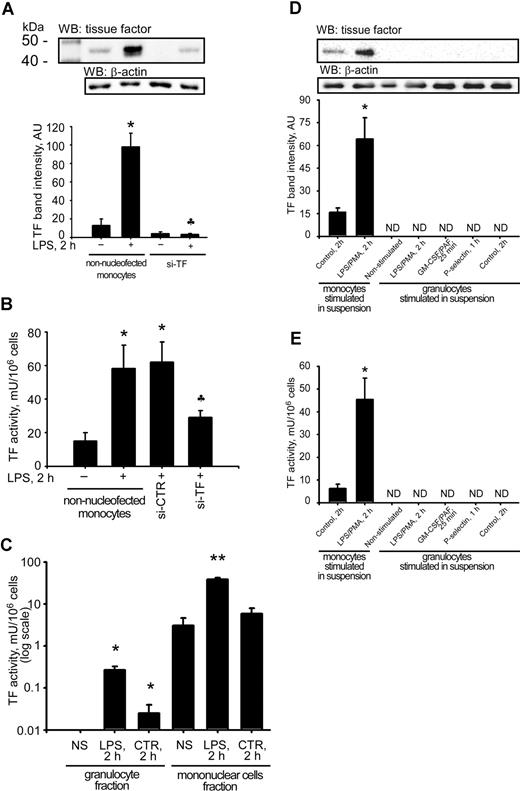
Because a several-hour timeframe is needed for activation of RNA-induced silencing complex after introduction of siRNA into the cell,18 we chose the time point of 5 hours for further use in the experiments. An efficient knockdown of LPS-induced TF expression was demonstrated by a significant reduction of TF activity and protein levels in TF-silenced MNCs (Figure 2A,B).
Major differences in TF activity and protein levels between monocytes and granulocytes. A clear reduction in TF protein levels was observed when purified si-TF–nucleofected monocytes were reintroduced into whole blood predepleted for monocytes (A). The decrease in TF protein levels was accompanied by a reduction in TF procoagulant activity in MNC lysates (B). Granulocytes, isolated from LPS-stimulated whole blood, possessed almost 200-fold weaker TF activity than MNCs from the same blood aliquot (C). Stimulation of granulocytes with LPS/PMA, GM-CSF/PAF, and P-selectin did not induce any detectable TF protein levels (D) or TF activity (E) in contrast to monocytes. Data are means (± SEM). NS indicates nonstimulated; CTR, 2h, time-matched control; and ND, nondetectable. *P < .05 compared with respective nonstimulated controls. P < .05 compared with nonsilenced LPS-stimulated monocyte samples. **P < .05 compared with samples from unstimulated MNC fraction.
Major differences in TF activity and protein levels between monocytes and granulocytes. A clear reduction in TF protein levels was observed when purified si-TF–nucleofected monocytes were reintroduced into whole blood predepleted for monocytes (A). The decrease in TF protein levels was accompanied by a reduction in TF procoagulant activity in MNC lysates (B). Granulocytes, isolated from LPS-stimulated whole blood, possessed almost 200-fold weaker TF activity than MNCs from the same blood aliquot (C). Stimulation of granulocytes with LPS/PMA, GM-CSF/PAF, and P-selectin did not induce any detectable TF protein levels (D) or TF activity (E) in contrast to monocytes. Data are means (± SEM). NS indicates nonstimulated; CTR, 2h, time-matched control; and ND, nondetectable. *P < .05 compared with respective nonstimulated controls. P < .05 compared with nonsilenced LPS-stimulated monocyte samples. **P < .05 compared with samples from unstimulated MNC fraction.
Immunologic isolation yielded highly pure cell fractions
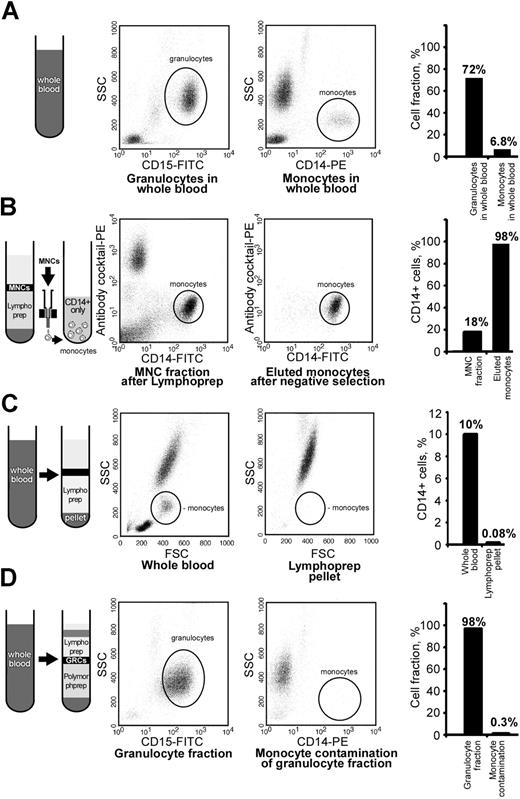
Granulocyte and monocyte fractions in whole blood were used as preisolation reference controls and constituted 72% and 6.8% of cells, respectively (Figure 3A). MNC fraction collected after Lymphoprep density centrifugation contained 18% of CD14-positive cells. We were able to enrich the fraction of monocytes to more than 98% after immunologic depletion of nonmonocytes; the remaining 2% of cells in monocyte fraction were CD14 negative (Figure 3B).
High purity of leukocyte fractions was achieved by immunologic cell separations. The purity of all cell fractions was assessed by flow cytometry using side-forward scatter and antigenic characteristics. The fraction of granulocytes in the preisolation reference control whole blood was 72% (A). SSC indicates side scatter channel. (B) Shown is the enrichment of monocytes from 18% to 98% in the Lymphoprep MNC fraction by removal of cells positive for CD3, CD7, CD16, CD19, CD56, CD123, and CD235A. Monocytes were depleted down to 0.08% in the Lymphoprep RBC pellet (C). The purity of granulocyte fraction was 98% after removal of CD14-positive cells from the lower Polymorphprep layer, and the monocyte contamination of granulocyte preparation was only 0.3% (D). Flow cytometric histograms and the plotted mean percentage of analyzed cells are presented.
High purity of leukocyte fractions was achieved by immunologic cell separations. The purity of all cell fractions was assessed by flow cytometry using side-forward scatter and antigenic characteristics. The fraction of granulocytes in the preisolation reference control whole blood was 72% (A). SSC indicates side scatter channel. (B) Shown is the enrichment of monocytes from 18% to 98% in the Lymphoprep MNC fraction by removal of cells positive for CD3, CD7, CD16, CD19, CD56, CD123, and CD235A. Monocytes were depleted down to 0.08% in the Lymphoprep RBC pellet (C). The purity of granulocyte fraction was 98% after removal of CD14-positive cells from the lower Polymorphprep layer, and the monocyte contamination of granulocyte preparation was only 0.3% (D). Flow cytometric histograms and the plotted mean percentage of analyzed cells are presented.
Lymphoprep RBC pellet was checked for the presence of remaining monocytes after the gradient centrifugation. The percentage of monocytes in whole blood was approximately 10% before isolation of MNCs by Lymphoprep. After density centrifugation, monocytes in RBC pellet were depleted down to 0.08% (Figure 3C).
Monocyte contamination of granulocyte fraction after Polymorphprep density centrifugation and depletion by CD14 MicroBeads was checked by immunostaining against CD14 and was found to be negligible, as it was only 0.3% of total cell population. After monocyte removal, granulocyte fraction contained 98% of CD15-positive cells (Figure 3D).
Monocytes express active TF antigen
Nucleofection of monocytes with si-TF successfully interfered with TF expression because the strong immunopositive band at 43 kDa corresponding to TF disappears completely in both nonstimulated and LPS-stimulated conditions (Figure 2A). This 10-fold reduction in protein levels was accompanied by more than a 2-fold reduction of LPS-induced MNC-TF activity compared with si-CTR–nucleofected monocytes reintroduced to the whole blood (Figure 2A,B). Equal protein loading of immunoblots was ascertained by the lack of differences in β-actin signal intensities between the samples.
LPS stimulation of non-nucleofected monocytes, reintroduced back to whole blood, led to a 10-fold rise in TF protein levels (Figure 2A) along with a rise in MNC-TF activity from 15 plus or minus 4 to 58 plus or minus 11 mU/106 cells (Figure 2B). A 3.5-fold induction in TF activity in MNCs isolated from LPS-stimulated whole blood containing non-nucleofected reintroduced monocytes (Figure 2B) was comparable to those obtained from the reference whole blood (Figure 2C). These data ascertained that cell handling procedures did not affect LPS-induced TF up-regulation, although a slight baseline elevation of active TF protein was observed in reconstitution experiments (Figure 2A,B). The immunoblot data obtained by use of monoclonal anti-human TF antibody (Calbiochem) was confirmed by the use of another monoclonal anti-human TF antibody (gift from Dr K.G. Mann, University of Vermont, Burlington; data are not shown).
Lack of TF expression in granulocytes
After isolation of cells from LPS-stimulated whole blood, granulocytes possessed as low as 0.3 plus or minus 0.05 mU/106 cells versus 31 plus or minus 3 mU/106 cells of TF activity in monocytes (Figure 2C). Immunoblotting of lysates of freshly isolated granulocytes stimulated in suspension with different granulocyte-specific agonists revealed the absence of TF protein in both nonstimulated and stimulated samples (Figure 2D). In the same experiments, we failed to detect any TF activity, whereas in isolated monocytes, stimulated in suspension with LPS/PMA, TF activity raised from 16 plus or minus 1 in nonstimulated to 45 plus or minus 8 mU/106 cells (Figure 2E).
Granulocytes acquire TF antigen from activated monocytes
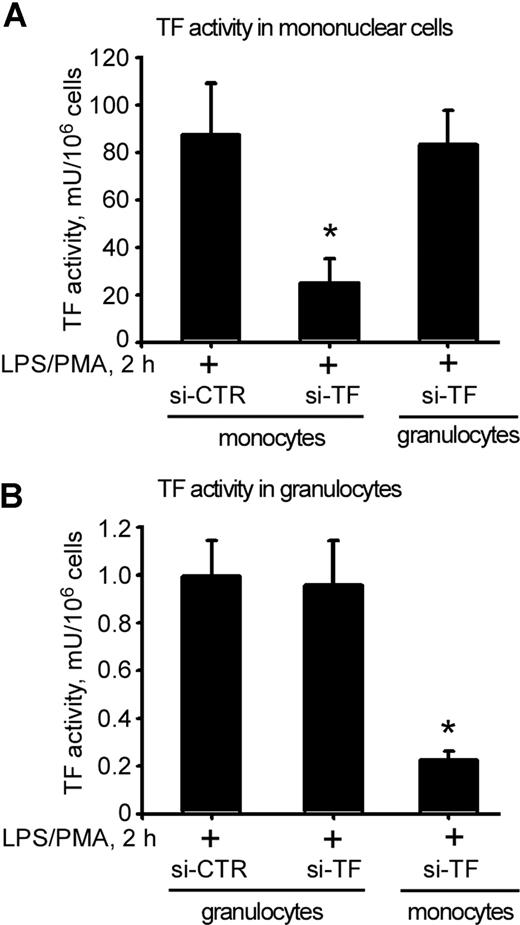
No reduction of active TF antigen was observed in Lymphoprep-isolated MNC faction when TF-silenced granulocytes were reintroduced to the whole blood stimulated with LPS/PMA (Figure 4A). In contrast, LPS/PMA stimulation of whole blood containing reintroduced TF-silenced monocytes led to 4-fold reduction of TF activity in MNC fraction (Figure 4A).
Knockdown of endogenous TF in monocytes reduces TF activity in granulocytes. MNC-TF activity was dramatically reduced when si-TF–nucleofected monocytes, but not granulocytes, were reintroduced into whole blood (A). Reintroduction of si-TF–nucleofected monocytes led to a profound reduction in LPS/PMA-stimulated TF activity in granulocytes (B). Data are presented as percentage of si-CTR–nucleofected samples; error bars are SEM. *P < .05 compared with si-CTR monocytes.
Knockdown of endogenous TF in monocytes reduces TF activity in granulocytes. MNC-TF activity was dramatically reduced when si-TF–nucleofected monocytes, but not granulocytes, were reintroduced into whole blood (A). Reintroduction of si-TF–nucleofected monocytes led to a profound reduction in LPS/PMA-stimulated TF activity in granulocytes (B). Data are presented as percentage of si-CTR–nucleofected samples; error bars are SEM. *P < .05 compared with si-CTR monocytes.
No reduction in LPS/PMA-induced TF activity was observed in Polymorphprep granulocyte fraction isolated from whole blood reconstituted with TF-silenced granulocytes (Figure 4B). Surprisingly, when TF-silenced monocytes were reintroduced to the whole blood, which was subsequently stimulated with LPS/PMA, TF activity in Polymorphprep isolated granulocyte fraction was reduced by nearly 5-fold (Figure 4B). Thus, the knockdown of endogenous TF expression in monocytes, which brought about a significant reduction in TF procoagulant activity in granulocytes, has suggested the transfer of active TF antigen from one cell type to another.
To test the possibility of a direct transfer of TF from activated monocytes to granulocytes, TF-YFP fusion protein was overexpressed in immunologically isolated monocytes that were subsequently reintroduced to whole blood. After LPS stimulation of whole blood, granulocyte fraction was isolated and tested for YFP fluorescence by flow cytometry, confocal microscopy, and Western blotting.
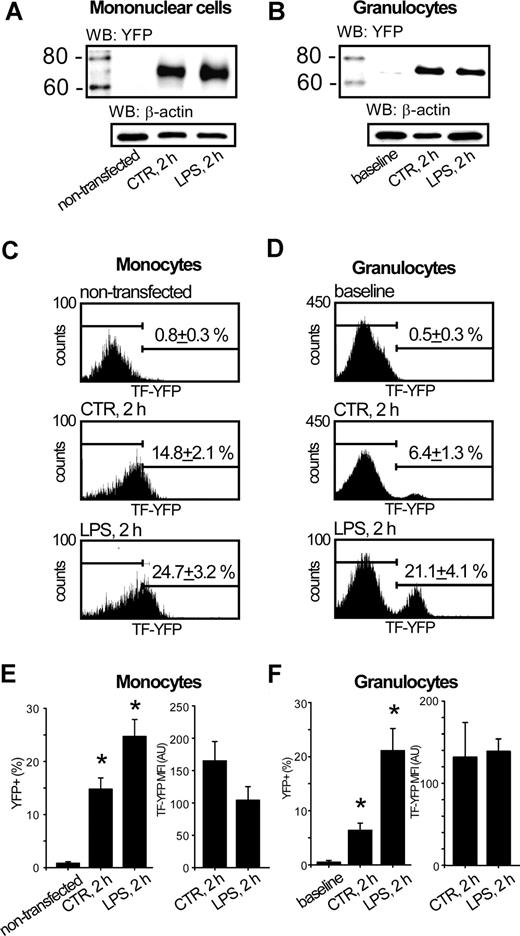
Immunoblotting of lysates of nucleofected control MNCs with anti-GFP antibodies revealed a strong band at 70 kDa, corresponding to TF-YFP fusion protein (Figure 5A second lane). The levels of TF-YFP were slightly higher after stimulation of whole blood with LPS (Figure 5A third lane). Surprisingly, the lysates of granulocyte fraction, isolated from control whole blood reconstituted with TF-YFP–expressing monocytes, also contained TF-YFP fusion protein (Figure 5B second lane). Stimulation of whole blood with LPS led to a dramatic elevation of TF-YFP band intensity in granulocyte fraction, pointing toward an increased accumulation of the fusion protein in granulocytes (Figure 5B third lane).
Granulocytes acquire TF expressed in monocytes. Immunoblotting of MNC lysates against YFP demonstrates that TF-YFP fusion protein is well expressed in nucleofected monocytes regardless of LPS stimulation (A). Representative immunoblot shows that TF-YFP fusion protein is acquired by granulocytes, isolated from whole blood reconstituted with TF-YFP–expressing monocytes. WB indicates Western Blot (B). The population of TF-YFP–positive monocytes was increased under LPS stimulation of whole blood, reconstituted with TF-YFP–expressing monocytes (C). The population of CD15 + granulocytes, acquired TF-YFP in whole blood reconstituted with TF-YFP–expressing monocytes, is increased by LPS stimulation (D). Plots summarize the mean percentage and MFIs of TF-YFP + cells from the total CD14 + MNC population (E) or TF-YFP + cells from the total CD15 + granulocyte population (F). Error bars represent SEM. *P < .05 compared with nontransfected monocytes or baseline conditions of granulocytes, respectively.
Granulocytes acquire TF expressed in monocytes. Immunoblotting of MNC lysates against YFP demonstrates that TF-YFP fusion protein is well expressed in nucleofected monocytes regardless of LPS stimulation (A). Representative immunoblot shows that TF-YFP fusion protein is acquired by granulocytes, isolated from whole blood reconstituted with TF-YFP–expressing monocytes. WB indicates Western Blot (B). The population of TF-YFP–positive monocytes was increased under LPS stimulation of whole blood, reconstituted with TF-YFP–expressing monocytes (C). The population of CD15 + granulocytes, acquired TF-YFP in whole blood reconstituted with TF-YFP–expressing monocytes, is increased by LPS stimulation (D). Plots summarize the mean percentage and MFIs of TF-YFP + cells from the total CD14 + MNC population (E) or TF-YFP + cells from the total CD15 + granulocyte population (F). Error bars represent SEM. *P < .05 compared with nontransfected monocytes or baseline conditions of granulocytes, respectively.
Flow cytometrical analysis of whole blood, containing TF-YFP–expressing monocytes, revealed that LPS stimulation led to the increased population of monocytes expressing TF-YFP to 24.7 plus or minus 3.2% compared with 14.8 plus or minus 2.1% in control samples (Figure 5C middle, bottom; Figure 5E left). Interestingly, monocytes isolated from LPS-stimulated whole blood bore less TF-YFP than monocytes obtained from time-matched control samples because mean fluorescence intensity (MFI) of YFP in LPS-stimulated cells was decreased by 1.7-fold. This suggests the loss of TF antigen upon stimulation with LPS (Figure 5E right). In the same blood aliquots, stimulation with LPS led to an increase of TF-YFP–positive population of granulocytes to 21.1 plus or minus 4.1% compared with 6.4 plus or minus 1.3% in time-matched control samples (Figure 5D middle, bottom; Figure 5F left). Interestingly, the MFI of YFP in granulocytes was not changed by LPS stimulation (Figure 5F right). This result pointed to the saturable ability of granulocytes to acquire limited amounts of exogenous TF-YFP.
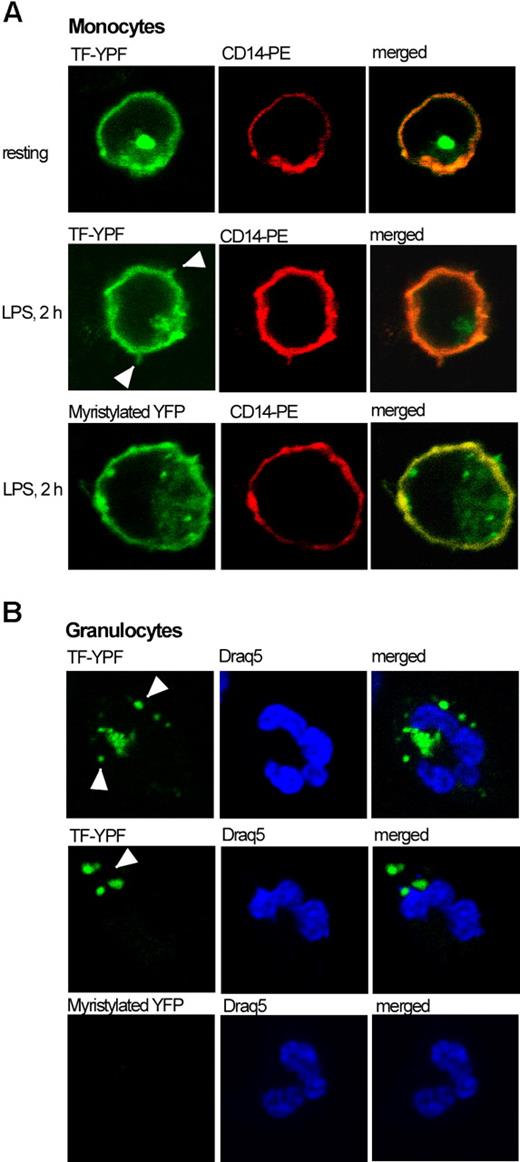
Observed differences in distribution of TF-YFP fusion protein between monocyte and granulocyte fractions after stimulation with LPS led us to verify our data by confocal microscopy. The pattern of TF-YFP distribution in monocytes, isolated from nonstimulated whole blood, could be characterized by even fluorescence of TF-YFP on monocyte membrane and a strong TF-positive dot unilaterally from the nucleus, reflecting most probably the newly synthesized TF protein (Figure 6A top). After 2 hours of LPS stimulation, monocytes had their membranes enriched with TF antigen. Moreover, we found TF-positive membrane protrusions extending from activated monocytes upon the LPS stimulation (Figure 6A middle). Interestingly, exogenous TF-YFP acquired by granulocytes is localized intracellularly within several cytoplasmic speckles of heterogeneous size (Figure 6B top, middle). These speckles are too large to be endosomes but may represent fusions of several endosomes containing detectable amounts of TF-YFP.
Intracellular distribution of TF-YFP fusion protein in monocytes and granulocytes. TF-YFP is evenly distributed throughout the membrane of nonstimulated monocytes and is also accumulated in the cytoplasm as a single speckle (panel A top). LPS stimulation enriches TF-YFP localization onto the monocyte membrane, which extends the protrusions (panel A middle). Myristylated YFP localized in the plasma membrane on monocytes (panel A bottom). TF-YFP fusion protein, acquired by granulocytes, localizes within cytoplasmic speckles (panel B top, middle). Granulocytes did not acquire myristylated YFP (panel B bottom). Draq5 indicates DNA fluorescent marker.
Intracellular distribution of TF-YFP fusion protein in monocytes and granulocytes. TF-YFP is evenly distributed throughout the membrane of nonstimulated monocytes and is also accumulated in the cytoplasm as a single speckle (panel A top). LPS stimulation enriches TF-YFP localization onto the monocyte membrane, which extends the protrusions (panel A middle). Myristylated YFP localized in the plasma membrane on monocytes (panel A bottom). TF-YFP fusion protein, acquired by granulocytes, localizes within cytoplasmic speckles (panel B top, middle). Granulocytes did not acquire myristylated YFP (panel B bottom). Draq5 indicates DNA fluorescent marker.
To address the question of whether the transfer of TF-YFP is specific to TF, we have imaged monocytes and granulocytes for YFP positivity and its intracellular localization in control experiments when membrane-targeted myr-YFP was introduced to the monocytes. We found myr-YFP to be located exclusively in the plasma membrane of monocytes and within few intracellular speckles. Surprisingly, we could not detect any transferred myr-YFP in granulocytes (Figure 6A,B bottom).
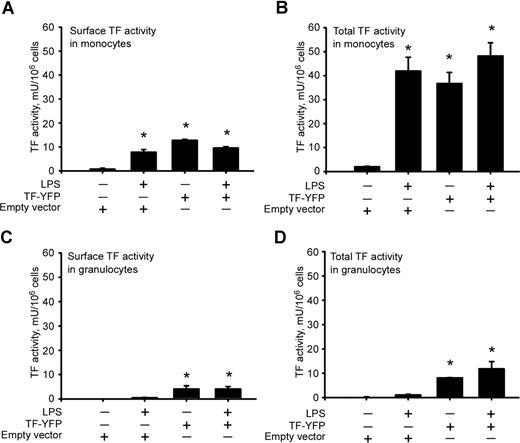
Because we detected most of the TF-YFP transferred from monocytes to granulocytes in the cell interior, we addressed the question of whether TF acquired by granulocytes was functionally active. Surface and total TF activity in nonstimulated monocytes expressing TF-YFP was as high as in LPS-stimulated monocytes transfected with a control empty vector (Figure 7A,B). Reintroduction of TF-YFP–expressing monocytes to the whole blood led to an increase of surface TF activity on granulocytes even in the absence of LPS stimulation (Figure 7C). The surface TF activity on granulocytes constituted almost one half of their total TF activity (Figure 7C,D).
Intracellular and surface distribution of TF-YFP–mediated activity in monocytes and granulocytes. Overexpression of TF-YFP leads to increase in surface (A) and total (B) TF activity in monocytes regardless of LPS stimulation. Reintroduction of TF-YFP–expressing monocytes increases surface (C) and total (D) TF activity in granulocytes. *P < .05 compared with nonstimulated controls transfected with empty vector. Error bars represent SEM.
Intracellular and surface distribution of TF-YFP–mediated activity in monocytes and granulocytes. Overexpression of TF-YFP leads to increase in surface (A) and total (B) TF activity in monocytes regardless of LPS stimulation. Reintroduction of TF-YFP–expressing monocytes increases surface (C) and total (D) TF activity in granulocytes. *P < .05 compared with nonstimulated controls transfected with empty vector. Error bars represent SEM.
Discussion
In our present study, using siRNA-mediated knockdown of endogenous TF expression and overexpression of YFP-tagged TF in monocytes, we demonstrated that monocyte-synthesized TF is acquired by granulocytes in the whole blood settings. This novel approach enabled us to pinpoint monocytes as the only cellular origin of TF antigen. Our data rule out granulocytes as the source of TF and provide experimental explanation for detectable levels of TF antigen in granulocytes.
To date, all studies searching for TF origin in peripheral blood cells were merely based on the detection of TF-positive cells by measuring TF mRNA or protein levels. None of these studies have ever used silencing of endogenous TF expression as well as overexpression of fluorescently labeled TF in primary isolated human blood cells. Certain difficulties were associated with the technical obstacles because it was impossible to introduce foreign nucleic acids into freshly isolated leukocytes via the routine transfection protocols. The latter would invariably necessitate prolonged culturing of transfected cells to achieve a sufficient knockdown of gene expression, leading to changes in cell phenotypes and significant activation of white blood cells. In our study, we circumvented these adversities by applying nucleofection delivery of nucleic acids. Isolated human blood leukocytes were successfully nucleofected with both siRNAs and plasmid DNA. We achieved rapid silencing of endogenous TF expression even under conditions of LPS stimulation. Nucleofection route of plasmid DNA delivery led to desirable levels of TF-YFP fusion protein expression within a short time after the electric pulse. In our control experiments, we demonstrated that nucleofection or other cell handling did not produce additional activation of leukocytes.
Furthermore, none of the previously published studies systematically used immunologic purification of several types of human leukocytes, which enables simultaneous comparison of several putative TF sources in one study. Immunologic cell purification provides a high power to the study because conclusions are based on data produced from highly pure cell fractions devoid of contamination with other cell types. Application of immunologic cell purification also enabled us to perform experiments not only in the cell culturing conditions, but, most important, in the conditions of whole blood environment. Reconstitution of predepleted whole blood with siRNA- or TF-YFP–nucleofected monocytes made it possible to answer the most important question of our present study, whether granulocytes produce TF or acquire it from elsewhere.
Our study opposes the concept created in early 1970s that granulocytes synthesize TF in vivo19-21 and in vitro.22-24 It has been shown that TF promoter in neutrophils contains cytosine-phosphate-guanosine CpG islands, which are not heavily methylated. This indicates that TF gene in neutrophils is not epigenetically silenced, and TF gene can be transcribed upon the activation of neutrophils.15,25 Indeed, the study by Giesen et al demonstrated TF-positive neutrophils attached to the collagen-coated glass slides exposed to the flow of human blood.1 Although TF-bearing neutrophils were detected, the authors did not answer the question of whether TF antigen was produced in neutrophils or acquired from other cell types. In our study, we show that granulocytes, isolated from stimulated whole blood, contain nearly undetectable levels of TF activity, whereas plated resting or LPS/PMA-stimulated granulocytes did not possess any TF activity or antigen. This may suggest that granulocytes acquire TF but do not synthesize it themselves. We are able to give a definitive answer to the question of where this TF activity on granulocytes comes from. Our data demonstrate that in experiments, when whole blood was reconstituted with TF-silenced monocytes and stimulated with LPS, the minute TF activity in granulocytes was even further reduced to nearly nondetectable levels. Acquisition of monocyte-expressed TF-YFP fusion protein by granulocytes in whole blood further confirms the validity of the transfer hypothesis of TF from monocytes to granulocytes in the blood. Furthermore, using the membrane-targeted myr-YFP, which was not transferred from monocytes to granulocytes under conditions of LPS-stimulated whole blood, we suggest that TF-YFP transfer is specific to TF and is not a random shedding/fusion of monocyte membranes to/with other cells.
Therefore, we conclude that granulocytes do not express TF but acquire it from monocytes via transfer mechanism. Our data are supported by previous published reports that neutrophils are capable of fusion with microparticles derived from other cell types.26-29 Furthermore, our present study is in line with a previous study by B.Ø. et al, who failed to show any TF activity or antigen in granulocytes recombined with plasma and stimulated with LPS/PMA.30
The positioning of monocytes at the nexus between innate immunity and blood coagulation further substantiates the clinical importance of our findings. Thus, the recent study by Swirski et al demonstrates that hypercholesterolemia induces monocytosis by elevation of Ly6Chi, the subset of murine monocytes, which corresponds to CD14highCD16− cells in humans. The enlargement of this population leads to enhanced monocyte adhesion and extravasation at the sites of vascular injury.31 The same study shows that fat-rich diets favor overloading of atherosclerotic plaques with monocyte-derived macrophages, which are known to express major quantities of TF antigen.32 Furthermore, according to the recent report by Liu et al, cholesterol activates monocytes, which leads to the increased TF synthesis and its release with membrane microparticles into the systemic circulation.33 Monocytosis and increased levels of TF on circulating monocytes are also characteristic hematologic findings in patients with antiphospholipid syndrome associated with recurrent thromboses.34-36 Thus, our data give novel insights into better understanding of several clinical conditions associated with high monocyte counts and thrombogenic potential of the blood.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Margo Price for her help in preparing the manuscript.
Authorship
Contribution: E.M.E. and M.A.S. designed and performed the experiments, analyzed the data, and wrote the paper. J.O.O. participated in performing experiments. B.Ø. participated in data analysis and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elena M. Egorina, Department of Biochemistry, Institute of Medical Biology, University of Tromsø, N-9037, Tromsø, Norway; e-mail: egorina@fagmed.uit.no.
References
Author notes
E.M.E. and M.A.S. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal