Abstract
Many of the cellular responses that occur in activated platelets resemble events that take place following activation of cell-death pathways in nucleated cells. We tested the hypothesis that formation of the mitochondrial permeability transition pore (MPTP), a key signaling event during cell death, also plays a critical role in platelet activation. Stimulation of murine platelets with thrombin plus the glycoprotein VI agonist convulxin resulted in a rapid loss of mitochondrial transmembrane potential (Δψm) in a subpopulation of activated platelets. In the absence of cyclophilin D (CypD), an essential regulator of MPTP formation, murine platelet activation responses were altered. CypD-deficient platelets exhibited defects in phosphatidylserine externalization, high-level surface fibrinogen retention, membrane vesiculation, and procoagulant activity. Also, in CypD-deficient platelet-rich plasma, clot retraction was altered. Stimulation with thrombin plus H2O2, a known activator of MPTP formation, also increased high-level surface fibrinogen retention, phosphatidylserine externalization, and platelet procoagulant activity in a CypD-dependent manner. In a model of carotid artery photochemical injury, thrombosis was markedly accelerated in CypD-deficient mice. These results implicate CypD and the MPTP as critical regulators of platelet activation and suggest a novel CypD-dependent negative-feedback mechanism regulating arterial thrombosis.
Introduction
Platelets play a key role in the hemostatic response to vascular injury by rapidly accumulating to form a platelet plug. Excessive platelet accumulation and activation at sites of plaque rupture, however, can contribute to arterial thrombotic events, such as stroke and myocardial infarction. Coactivation of adherent platelets by soluble agonists, such as thrombin, and extracellular matrix components, such as collagen, results in dramatic changes in platelet structure and function. Activated platelets undergo shape change,1 granule release,2 and a conformational change in the fibrinogen receptor αIIbβ3 that results in aggregation.3 In a subset of activated platelets, a regulated membrane rearrangement occurs that results in phosphatidylserine externalization,4 microparticle release,5 and the surface retention of high levels of fibrinogen and other α-granule proteins.6
Although platelets are anucleate, certain cellular aspects of the platelet activation response resemble processes that occur during cell death in nucleated cells. This process has been referred to as “platelet apoptosis.”7 Phosphatidylserine externalization occurs on both activated platelets and apoptotic cells, and platelet microparticle release resembles the membrane fragmentation that occurs in necrotic cells.8 It is not known, however, if the cellular mechanisms responsible for these events are shared between platelets and nucleated cells.
The mitochondrial permeability transition pore (MPTP) is a nonselective multiprotein pore that spans the inner and outer mitochondrial membranes. Formation of the MPTP plays a critical role in the regulation of some forms of cell death.9 Deletion of the MPTP regulator cyclophilin D (CypD) causes marked impairment of MPTP formation.10-13 In contrast to the effects of CypD's absence on MPTP formation, neither mitochondrial structure10,13 nor the ability of mitochondria to supply energy through oxidative phosphorylation11,12 was altered in tissues obtained from CypD−/− mice.
A rapid loss of mitochondrial transmembrane potential (Δψm), one of the consequences of MPTP formation, has been demonstrated in some subpopulations of activated platelets,7,14,15 but CypD's role in platelet activation and the hemostatic consequences of platelet MPTP formation have not been investigated. In this study, we tested the hypothesis that MPTP formation and CypD regulate platelet activation and thrombosis.
Methods
Mice
Animal protocols were approved by the University of Iowa and Veterans Affairs Animal Care and Use committees. All mice were housed in pathogen-free conditions at the University of Iowa Animal Care Facility under National Institutes of Health guidelines and approved animal care protocols. C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). CypD−/− mice, which are homozygous for a targeted deletion of the Ppif gene,10 and control CypD+/+ mice were generated as described previously10 and maintained on an inbred SV129 background. Age- and sex-matched mice were used for each experiment.
Platelet isolation
Washed platelets were isolated as described previously.16 Platelet counts were measured using a HemaVet cell counter (Drew Scientific, Oxford, CT).
Flow cytometry
Flow cytometry of murine platelets was performed as described previously.17 Washed platelets were suspended at a concentration of 107/mL in Tyrode's buffer containing 2.1 mM CaCl2. Platelets were either left unstimulated or stimulated with thrombin (0.5 U/mL; Haematological Technologies, Essex Junction, VT), convulxin (250 ng/mL; Centerchem, Norwalk, CT), thrombin plus convulxin, thrombin plus the indicated concentration of H2O2, or ionomycin (3 μM; Sigma, St Louis, MO). Fluorescein isothiocyanate (FITC)–labeled anti–mouse CD62P (P-selectin), and FITC and phycoerythrin (PE)–labeled annexin V were obtained from BD PharMingen (San Diego, CA). FITC–labeled sheep antifibrinogen antibody (ab8845) was obtained from Abcam (Cambridge, MA). PE-labeled JON/A antibody was obtained from Emfret Analytics (Würzburg, Germany), and tetramethylrhodamine methyl ester (TMRM) was obtained from Molecular Probes (Eugene, OR).
For experiments evaluating loss of Δψm, platelets were incubated with 500 nM TMRM for 15 minutes prior to stimulation with the indicated agonist(s). Five minutes after stimulation, labeled platelets were evaluated by flow cytometry. For 2-color flow cytometry with TMRM, the FITC-labeled probe was added immediately after agonist stimulation. For experiments without TMRM, platelets were stimulated for 5 minutes with the indicated agonist(s), followed by addition of the indicated labeled probe for 1 minute and fixation in 1% paraformaldehyde in phosphate-buffered saline (PBS). Labeled platelets were analyzed on a Becton Dickinson FACScan (San Diego, CA) as described previously.17 Platelets were gated by forward and side scatter. Appropriate compensation was performed for experiments using 2-color flow cytometry. Fibrinogenhigh platelets were defined as activated platelets with high surface levels of fibrinogen and low PE-JON/A binding. For experiments evaluating the effects of cyclosporin A (Calbiochem, La Jolla, CA), platelets were incubated for 15 minutes with cyclosporin A prior to the addition of agonist(s).
Platelet procoagulant activity
Platelet procoagulant activity was measured in a prothrombinase assay as described previously.17
Electron microscopy
Activation of fibrinogen-adherent platelets was performed using a method modified from Leo et al.18 Glass coverslips (12 mm) were incubated overnight at 4°C in 100 μg/mL human fibrinogen (Sigma), 150 mM NaCl, 50 mM NaH2PO4, and 20 mM Na2HPO4, pH 8.0. After removal of the fibrinogen solution, the coverslips were blocked with 5 mg/mL bovine serum albumin in PBS for 2 hours. Following removal of the blocking reagent, washed murine platelets (107/mL) in modified Tyrode's were allowed to adhere for 30 minutes at 37°C. Nonadherent platelets were removed, and the adherent platelets were stimulated either with thrombin (0.5 U/mL) or with thrombin (0.1 U/mL) plus convulxin (50 ng/mL) in modified Tyrode's buffer with 2.1 mM CaCl2. Two minutes following stimulation, the platelets were fixed with glutaraldehyde. The coverslips were prepared for scanning electron microscopy and imaged with a Hitachi (Tokyo, Japan) S-4800 field emission scanning electron microscope. The images were taken at room temperature, with integrated digital images captured within the Hitachi S-4800 and acquired with the PC-based SEM software by Hitachi S-4800. Images were processed using Adobe Photoshop Elements 3.0 software (Adobe Systems, Mountain View, CA).
For transmission electron microscopy, platelets were fixed in a similar manner, sectioned, and imaged using a JEOL (Tokyo, Japan) JEM-1230 transmission electron microscope. Platelets were either unstimulated or examined following stimulation with thrombin plus convulxin for 2 minutes.
Platelet aggregation
Platelet aggregation in response to thrombin (0.5 U/mL), convulxin (250 ng/mL), or thrombin plus convulxin was performed using a Chrono-log (Havertown, PA) whole blood aggregometer (model 560-VS) as described previously.19
Clot retraction
Clot retraction was performed using a method modified from Schoenwalder et al.20 Whole blood was diluted 1:2 in modified Tyrode's buffer containing 1 mM ethyleneglycol bis (2-amino ethyl ether)-N, N, N′ tetraacetic acid (EGTA) (Sigma). Diluted platelet-rich plasma (PRP) was obtained by differential centrifugation, and the platelet count of the PRP was adjusted to 108/mL with modified Tyrode's with 1 mM EGTA. A loop was prepared by wrapping a galvanized steel wire around a 3D nail. To aid in visualization of clot formation, 5 μL sedimented red blood cells were added per milliliter of diluted PRP. Clot formation was initiated by the addition of CaCl2 (2.1 mM) and thrombin (0.5 U/mL), convulxin (250 ng/mL), or thrombin plus convulxin to the PRP in a polypropylene tube. Following agonist addition, the reaction mixture was stirred with the steel loop for 20 seconds. Clot formation and retraction were allowed to proceed for 2 hours. For imaging, clots were removed and placed on a Parafilm (Pechiney Plastic Packaging, Menasha, WI) surface, and the image was acquired with a Canon A540 Powershot digital camera (Canon, Lake Success, NY) and processed using Adobe Photoshop Elements 3.0 software Clot volume and weight were determined following gentle removal of the formed clot (clot volume = initial plasma volume − residual plasma volume).
Tail transection bleeding time
Tails of anesthetized 6- to 8-week-old mice were clipped 3 mm from the distal end and placed in 37°C normal saline for 10 minutes. The time to cessation of bleeding and any rebleeding were recorded.
Carotid artery thrombosis
Carotid artery thrombosis was induced in mice at 3 months of age by photochemical injury as described previously.21 Blood flow was monitored continuously for 90 minutes or until stable occlusion occurred, at which time the experiment was terminated. No significant differences in baseline carotid artery blood flow were observed between CypD+/+ and CypD−/− mice (data not shown). First occlusion was defined as the time at which blood flow first decreased to 0 for at least 10 seconds, and stable occlusion was defined as the time at which blood flow remained absent for 10 minutes or longer.
Statistical analysis
Two-way analysis of variance (ANOVA) with the Tukey test for multiple comparisons was used for evaluation of the effects of agonists and genotype on annexin V binding, fibrinogen retention, P-selectin exposure, platelet procoagulant activity, and clot retraction. The unpaired 2-tailed t test was used to compare the effects of genotype on bleeding and occlusion times. Statistical significance was defined as a P value less than .05. Values are reported as means plus or minus SE.
Results
Loss of mitochondrial transmembrane potential (Δψm) during activation of murine platelets
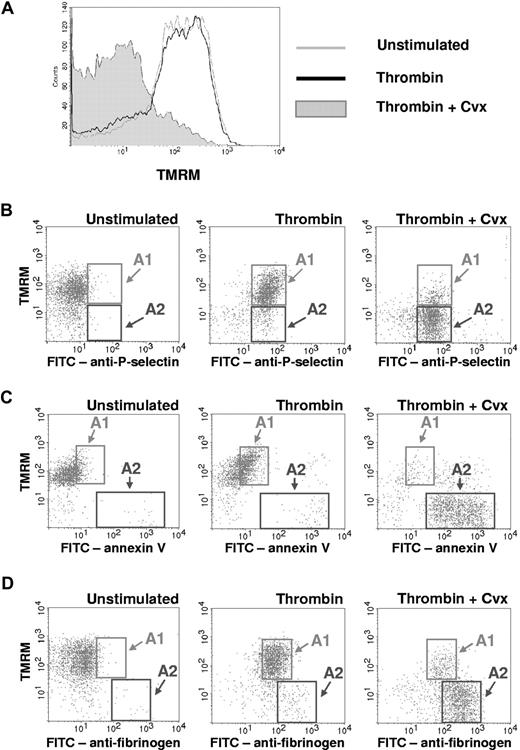
Loss of Δψm has been demonstrated previously in subpopulations of activated human and rabbit platelets.7,14,15,22 Using the cationic dye TMRM, we sought to determine whether loss of Δψm also occurs during activation of murine platelets (Figure 1).23 When washed platelets were stimulated with either thrombin (0.5 U/mL) (Figure 1A) or the glycoprotein VI agonist convulxin (250 ng/mL) (data not shown), minimal changes in TMRM staining were observed. In contrast, simultaneous stimulation of murine platelets with thrombin plus convulxin resulted in a marked loss of TMRM staining within 5 minutes (Figure 1A).
Loss of Δψm occurs in a subpopulation of activated platelets. Platelets were left unstimulated or stimulated with thrombin (0.5 U/mL), or thrombin plus convulxin (Cvx) (250 ng/mL), for 5 minutes. (A) Platelet Δψm was assessed by flow cytometry using the cationic dye TMRM. TMRM retention within mitochondria is dependent on the maintenance of Δψm, and loss of Δψm results in decreased TMRM fluorescence. (B-D) Two-color flow cytometry with TMRM and (B) FITC-labeled anti-P-selectin, (C) FITC-labeled annexin V, or (D) FITC-labeled antifibrinogen is demonstrated. Region A1 indicates the subpopulation of activated platelets that retained TMRM staining. Region A2 indicates the subpopulation of activated platelets that lost TMRM staining. Plots are representative of 3 separate experiments.
Loss of Δψm occurs in a subpopulation of activated platelets. Platelets were left unstimulated or stimulated with thrombin (0.5 U/mL), or thrombin plus convulxin (Cvx) (250 ng/mL), for 5 minutes. (A) Platelet Δψm was assessed by flow cytometry using the cationic dye TMRM. TMRM retention within mitochondria is dependent on the maintenance of Δψm, and loss of Δψm results in decreased TMRM fluorescence. (B-D) Two-color flow cytometry with TMRM and (B) FITC-labeled anti-P-selectin, (C) FITC-labeled annexin V, or (D) FITC-labeled antifibrinogen is demonstrated. Region A1 indicates the subpopulation of activated platelets that retained TMRM staining. Region A2 indicates the subpopulation of activated platelets that lost TMRM staining. Plots are representative of 3 separate experiments.
To determine whether activation-dependent loss of Δψm is associated with other platelet activation responses, 2-color flow cytometry was performed. Platelet granule release, measured by surface expression of P-selectin, occurred to a similar extent in thrombin-activated platelets, which retained the TMRM dye (region A1 in Figure 1B), and thrombin plus convulxin-activated platelets, which lost TMRM staining (region A2 in Figure 1B). In contrast, both phosphatidylserine externalization, measured by annexin V binding (Figure 1C), and high-level fibrinogen retention (Figure 1D) were observed almost solely on the subpopulation of activated platelets with loss of TMRM staining (region A2 in Figure 1C,D).
The MPTP regulatory component, CypD, is required for normal platelet activation
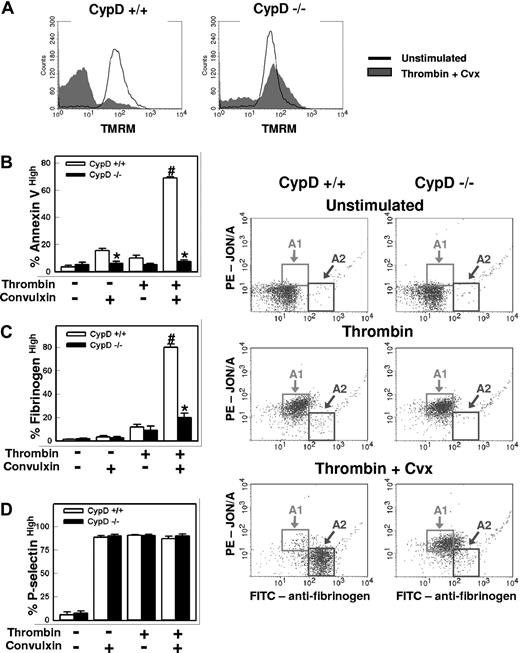
Because rapid loss of Δψm occurs following MPTP formation, we examined activation responses in platelets from homozygous CypD-deficient (CypD−/−) mice. Platelet counts, hemoglobin levels, and leukocyte counts were similar in CypD−/− and wild-type (CypD+/+) mice (data not shown). Following stimulation with thrombin plus convulxin, activation-dependent loss of TMRM staining was markedly decreased in CypD−/− platelets, which suggests that the loss of Δψm that occurs during platelet activation is mediated by CypD-dependent MPTP formation (Figure 2A).
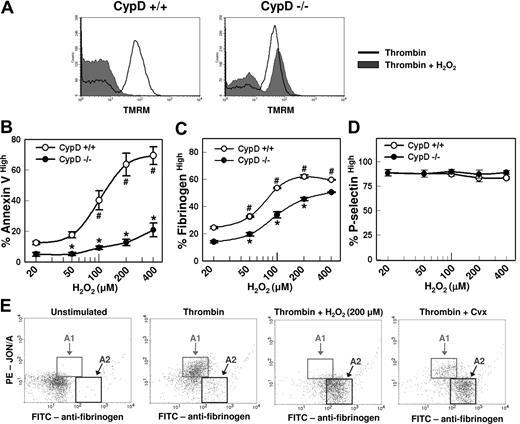
The MPTP regulatory component, CypD, is required for normal platelet activation. Platelets isolated from CypD+/+ or CypD−/− mice were left unstimulated or stimulated with thrombin (0.5 U/mL) plus convulxin (Cvx) (250 ng/mL) for 5 minutes. (A) Platelet Δψm was assessed by flow cytometry using the cationic dye TMRM (n = 3). (B-D) Percentages of (B) annexin Vhigh, (C) fibrinogenhigh, and (D) P-selectinhigh platelets (n = 4) are shown; #P < .05 compared with unstimulated CypD+/+ platelets; *P < .05 for comparison between CypD+/+ and CypD−/− platelets. (E) Fibrinogen (Fbg) and JON/A binding are shown. Platelets were stimulated with the indicated agonist(s) and examined using FITC-labeled antifibrinogen and PE-labeled JON/A. Gates are drawn to highlight activated platelets with high levels of JON/A binding and moderate fibrinogen binding (A1), and activated platelets with low levels of JON/A binding and high-level fibrinogen binding (A2) (n = 4). Error bars represent SE.
The MPTP regulatory component, CypD, is required for normal platelet activation. Platelets isolated from CypD+/+ or CypD−/− mice were left unstimulated or stimulated with thrombin (0.5 U/mL) plus convulxin (Cvx) (250 ng/mL) for 5 minutes. (A) Platelet Δψm was assessed by flow cytometry using the cationic dye TMRM (n = 3). (B-D) Percentages of (B) annexin Vhigh, (C) fibrinogenhigh, and (D) P-selectinhigh platelets (n = 4) are shown; #P < .05 compared with unstimulated CypD+/+ platelets; *P < .05 for comparison between CypD+/+ and CypD−/− platelets. (E) Fibrinogen (Fbg) and JON/A binding are shown. Platelets were stimulated with the indicated agonist(s) and examined using FITC-labeled antifibrinogen and PE-labeled JON/A. Gates are drawn to highlight activated platelets with high levels of JON/A binding and moderate fibrinogen binding (A1), and activated platelets with low levels of JON/A binding and high-level fibrinogen binding (A2) (n = 4). Error bars represent SE.
In the absence of CypD, both platelet phosphatidylserine externalization (Figure 2B) and high-level fibrinogen retention (Figure 2C,E) were markedly abrogated following stimulation with thrombin plus convulxin (P < .001 vs CypD+/+ platelets). A decrease in phosphatidylserine externalization was also seen in CypD−/− platelets after stimulation with convulxin alone (P < .05). In contrast, granule release was unaffected by the absence of CypD (Figure 2D). We have demonstrated previously that the subpopulation of activated murine platelets with high-level fibrinogen retention exhibits a characteristic loss in binding by JON/A, an antibody that recognizes the activated form of αIIbβ3.17 Two-color flow cytometric experiments demonstrated that this dual-agonist–mediated antigenic modulation of αIIbβ3 was also markedly impaired in CypD−/− platelets (Figure 2E). Pretreatment of CypD+/+ platelets with cyclosporin A (2 μM), a pharmacologic inhibitor of CypD, resulted in a similarly marked abrogation of antigenic modulation of αIIbβ3, phosphatidylserine externalization, and high-level fibrinogen retention (data not shown).
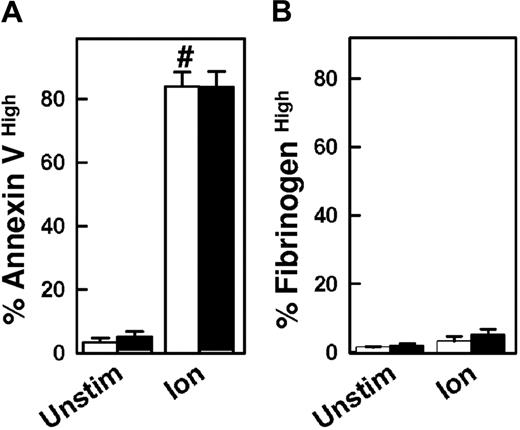
The calcium ionophore ionomycin is a potent activator of platelet phosphatidylserine externalization.4 Following ionomycin stimulation, phosphatidylserine externalization was markedly increased in CypD+/+ and CypD−/− platelets (Figure 3A). High-level fibrinogen retention was not increased in either CypD+/+ or CypD−/− platelets following stimulation with ionomycin (Figure 3B), which is consistent with previous reports.17,24
Platelet activation by ionomycin is unaffected in the absence of CypD. Platelets isolated from CypD+/+ (□) or CypD−/− (■) mice were left unstimulated (Unstim) or stimulated with ionomycin (Ion) (3 μM) for 5 minutes. Percentages of (A) annexin Vhigh and (B) fibrinogenhigh platelets are shown (n = 4). #P < .05 compared with unstimulated CypD+/+ platelets. Error bars represent SE.
Platelet activation by ionomycin is unaffected in the absence of CypD. Platelets isolated from CypD+/+ (□) or CypD−/− (■) mice were left unstimulated (Unstim) or stimulated with ionomycin (Ion) (3 μM) for 5 minutes. Percentages of (A) annexin Vhigh and (B) fibrinogenhigh platelets are shown (n = 4). #P < .05 compared with unstimulated CypD+/+ platelets. Error bars represent SE.
Vesiculation and blebbing of fibrinogen-adherent platelets are impaired in the absence of CypD
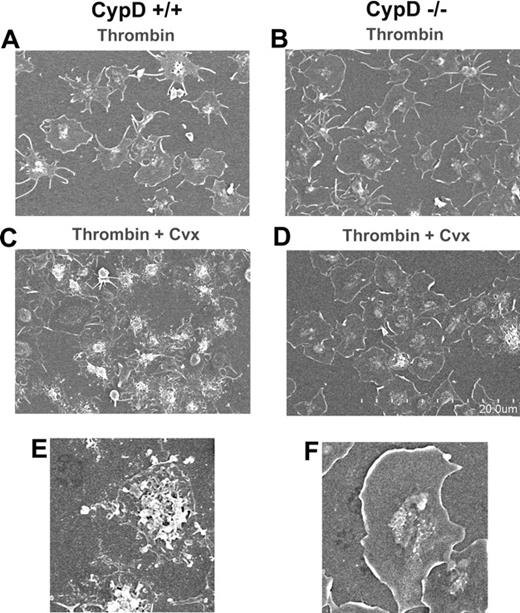
To determine whether CypD is essential for platelet shape change or membrane vesiculation, CypD+/+ or CypD−/− platelets were allowed to adhere to fibrinogen, and then stimulated with thrombin or thrombin plus convulxin. Two minutes after stimulation with thrombin, fibrinogen-adherent CypD+/+ platelets had a spread appearance with peripheral flattening, lamellipodia, and filopodial extensions (Figure 4A). The appearance of thrombin-stimulated CypD−/− platelets was similar to that of CypD+/+ platelets (Figure 4B). After stimulation with thrombin plus convulxin, the majority of CypD+/+ platelets demonstrated marked central mounding with peripheral blebbing and vesiculation. Membrane fragments, frequently arranged in a concentric pattern, surrounded these mounded and vesiculated platelets. (Figure 4C,E). In marked contrast, the large majority of thrombin-plus-convulxin–stimulated CypD−/− platelets had a spread and flattened appearance with lamellipodia and minimal central blebbing or vesiculation, and only a few CypD−/− platelets underwent the complex process of membrane blebbing and vesiculation (Figure 4D,F).
Vesiculation and blebbing of fibrinogen-adherent platelets is impaired in the absence of CypD. Fibrinogen-adherent CypD+/+ or CypD−/− platelets were stimulated with thrombin (0.5 U/mL) (A,B) or thrombin (0.1 U/mL) plus convulxin (Cvx) (50 ng/mL) (C-F) for 2 minutes, fixed, and evaluated by scanning electron microscopy. Scale of the low-magnification images is indicated in panel D. Panels E and F show higher-magnification images of a vesiculated CypD+/+ (E) and spread CypD−/− (F) platelet. Images are representative of 4 separate experiments. Total magnification for panels A, B, C, and D is 2500×; total magnification for panels E and F is 7000×. Complete microscopy information is provided in “Electron microscopy.”
Vesiculation and blebbing of fibrinogen-adherent platelets is impaired in the absence of CypD. Fibrinogen-adherent CypD+/+ or CypD−/− platelets were stimulated with thrombin (0.5 U/mL) (A,B) or thrombin (0.1 U/mL) plus convulxin (Cvx) (50 ng/mL) (C-F) for 2 minutes, fixed, and evaluated by scanning electron microscopy. Scale of the low-magnification images is indicated in panel D. Panels E and F show higher-magnification images of a vesiculated CypD+/+ (E) and spread CypD−/− (F) platelet. Images are representative of 4 separate experiments. Total magnification for panels A, B, C, and D is 2500×; total magnification for panels E and F is 7000×. Complete microscopy information is provided in “Electron microscopy.”
Platelet mitochondria from CypD+/+ and CypD−/− platelets at baseline and after stimulation with thrombin and convulxin did not appear structurally different and had no evidence of swelling when examined in cross-section using transmission electron microscopy (data not shown).
Platelet activation responses are modulated by H2O2
In nucleated cells, reactive oxygen species (ROS), such as H2O2, can trigger MPTP formation and cell-death pathways.10,12,25-27 Therefore, we examined the effects of H2O2 on platelet activation responses in CypD+/+ and CypD−/− platelets. Similar to the effects observed following costimulation with thrombin plus convulxin, costimulation with thrombin plus H2O2 resulted in loss of Δψm in a large subpopulation of CypD+/+ platelets but not CypD−/− platelets (Figure 5A). Activation of CypD+/+ platelets with thrombin (0.5 U/mL) in the presence of increasing concentrations of H2O2 caused a marked potentiation of phosphatidylserine externalization (Figure 5B) and high-level fibrinogen retention (Figure 5C) (P < .001) but had no effect on α-granule release (Figure 5D). Stimulation with thrombin plus 200 μM H2O2 produced a 6-fold increase in phosphatidylserine externalization relative to stimulation with thrombin alone (64% ± 7% vs 10% ± 2%; P < .001), a level of phosphatidylserine externalization similar to that observed following stimulation with thrombin plus convulxin (69% ± 1%). Costimulation with thrombin plus H2O2 also caused antigenic-modulation of αIIbβ3 (Figure 5E). Stimulation with 500 μM H2O2 alone had no effects on fibrinogen binding, phosphatidylserine externalization, or granule release (data not shown). Phosphatidylserine externalization (Figure 5C), high-level fibrinogen retention (Figure 5D), and antigenic modulation of αIIbβ3 (data not shown) initiated by thrombin plus H2O2 were all significantly blunted in CypD−/− platelets compared with CypD+/+ platelets.
Platelet activation responses are modulated by H2O2 in a CypD-dependent manner. (A) Platelets were stimulated with thrombin (0.5 U/mL) or thrombin plus H2O2 (200 μM) for 5 minutes. Platelets Δψm was assessed by flow cytometry using the cationic dye TMRM (n = 3). (B-D) Flow cytometry was performed to evaluate the percentage of (B) annexin Vhigh, (C) fibrinogenhigh, and (D) P-selectinhigh platelets (n = 4). #P < .05 compared with thrombin-stimulated CypD+/+ platelets; *P < .05 for comparison between CypD+/+ and CypD−/− platelets. (B) Platelets were left unstimulated or stimulated for 5 minutes with thrombin (0.5 U/mL), thrombin plus convulxin (Cvx) (250 ng/mL), or thrombin plus H2O2. Error bars represent SE. (E) Two-color flow cytometry was performed using FITC-labeled antifibrinogen and PE-labeled JON/A. Gates are drawn to highlight activated platelets with high levels of JON/A binding and moderate fibrinogen binding (A1), and activated platelets with low levels of JON/A binding and high-level fibrinogen binding (A2) (n = 5).
Platelet activation responses are modulated by H2O2 in a CypD-dependent manner. (A) Platelets were stimulated with thrombin (0.5 U/mL) or thrombin plus H2O2 (200 μM) for 5 minutes. Platelets Δψm was assessed by flow cytometry using the cationic dye TMRM (n = 3). (B-D) Flow cytometry was performed to evaluate the percentage of (B) annexin Vhigh, (C) fibrinogenhigh, and (D) P-selectinhigh platelets (n = 4). #P < .05 compared with thrombin-stimulated CypD+/+ platelets; *P < .05 for comparison between CypD+/+ and CypD−/− platelets. (B) Platelets were left unstimulated or stimulated for 5 minutes with thrombin (0.5 U/mL), thrombin plus convulxin (Cvx) (250 ng/mL), or thrombin plus H2O2. Error bars represent SE. (E) Two-color flow cytometry was performed using FITC-labeled antifibrinogen and PE-labeled JON/A. Gates are drawn to highlight activated platelets with high levels of JON/A binding and moderate fibrinogen binding (A1), and activated platelets with low levels of JON/A binding and high-level fibrinogen binding (A2) (n = 5).
CypD−/− platelets have impaired platelet procoagulant activity
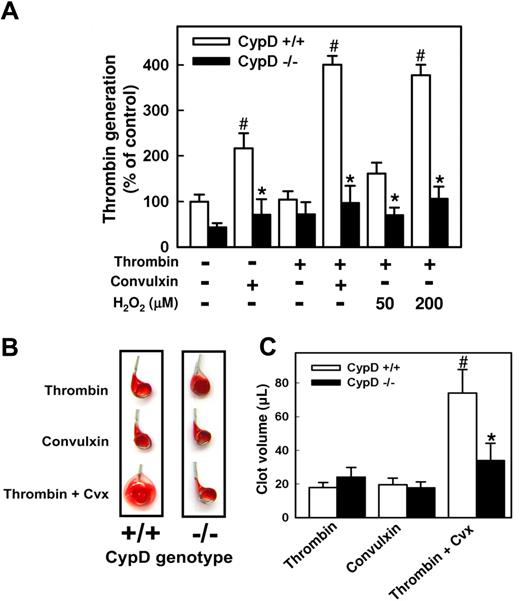
Because phosphatidylserine externalization is closely related to platelet procoagulant activity, we examined the ability of activated CypD+/+ and CypD−/− platelets to support thrombin generation by the prothrombinase complex.4 Activation of CypD+/+ platelets with thrombin plus convulxin, or thrombin plus H2O2, resulted in a marked increase in platelet prothrombinase activity relative to that observed after activation with thrombin alone (P < .001; Figure 6A). A smaller increase in platelet prothrombinase activity was observed when CypD+/+ platelets were stimulated with convulxin alone (P < .01). In contrast, CypD−/− platelets failed to support prothrombinase activity in response to any of the agonist(s) tested (Figure 6A).
Platelet function is altered in the absence of CypD. (A) Procoagulant activity is shown. Platelets were left unstimulated or stimulated with thrombin (0.5 U/mL), convulxin (250 ng/mL), thrombin plus convulxin, or thrombin plus the indicated concentrations of H2O2. Thrombin generation was measured in a prothrombinase assay and is presented relative to unstimulated CypD+/+ platelets (n = 5). #P < .05 compared with unstimulated CypD+/+ platelets; *P < .05 for comparison between CypD+/+ and CypD−/− platelets. (B,C) Clot retraction in diluted platelet-rich plasma is demonstrated. Clot formation was initiated by the addition of calcium together with thrombin (0.5 U/mL), convulxin (250 ng/mL), or thrombin plus convulxin. (B) Images of clots removed from serum 2 hours after initiation of clot formation. (C) The volume of the clot was determined 2 hours after initiation of clot formation by the indicated agonist(s) (n = 6). #P < .05 compared with thrombin-initiated CypD+/+ plasma; *P < .05 for comparison between CypD+/+ and CypD−/− plasma. Error bars represent SE.
Platelet function is altered in the absence of CypD. (A) Procoagulant activity is shown. Platelets were left unstimulated or stimulated with thrombin (0.5 U/mL), convulxin (250 ng/mL), thrombin plus convulxin, or thrombin plus the indicated concentrations of H2O2. Thrombin generation was measured in a prothrombinase assay and is presented relative to unstimulated CypD+/+ platelets (n = 5). #P < .05 compared with unstimulated CypD+/+ platelets; *P < .05 for comparison between CypD+/+ and CypD−/− platelets. (B,C) Clot retraction in diluted platelet-rich plasma is demonstrated. Clot formation was initiated by the addition of calcium together with thrombin (0.5 U/mL), convulxin (250 ng/mL), or thrombin plus convulxin. (B) Images of clots removed from serum 2 hours after initiation of clot formation. (C) The volume of the clot was determined 2 hours after initiation of clot formation by the indicated agonist(s) (n = 6). #P < .05 compared with thrombin-initiated CypD+/+ plasma; *P < .05 for comparison between CypD+/+ and CypD−/− plasma. Error bars represent SE.
Clot retraction is altered in the absence of CypD
To begin to investigate the functional significance of antigenic modulation of αIIbβ3, we examined the aggregation and clot retraction responses of CypD+/+ and CypD−/− platelets. Platelet aggregation occurred in less than 2 minutes using either CypD+/+ or CypD−/− platelets. No differences in the rate or amplitude of the aggregation response were observed between CypD+/+ and CypD−/− platelets stimulated with thrombin or thrombin plus convulxin (data not shown).
Next, we examined clot retraction using diluted platelet-rich plasma obtained from either CypD+/+ or CypD−/− mice (Figure 6B,C). No differences in clot retraction were observed between CypD+/+ and CypD−/− mice plasma when clot formation was initiated by either thrombin alone or convulxin alone. After stimulation with thrombin plus convulxin, clot retraction was markedly attenuated in CypD+/+ plasma (P < .001) but not in CypD−/− plasma. Clot volume was 2-fold lower in CypD−/− plasma than in CypD+/+ plasma after stimulation with thrombin plus convulxin (P = .001; Figure 6C). This result suggests a functional role for a CypD-dependent process in the regulation of clot retraction.
In vivo hemostasis and thrombosis in CypD−/− mice
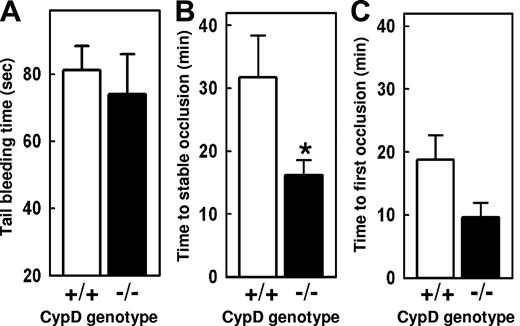
Tail transection bleeding times were measured in CypD+/+ and CypD−/− mice. No significant differences in the time to cessation of bleeding (Figure 6A) were observed. Next, we compared the thrombotic responses of CypD+/+ and CypD−/− mice using an experimental model of carotid artery thrombosis induced by photochemical injury (Figure 7B,C). The development of a stable occlusive thrombus occurred more rapidly in CypD−/− mice (16 ± 2 minutes) than in CypD+/+ mice (32 ± 7 minutes; P < .05). A trend toward a decreased time to first occlusion also was observed in CypD−/− mice (10 ± 2 minutes) compared with CypD+/+ mice (19 ± 4 minutes; P = .06).
In vivo thrombotic and hemostatic responses are altered in the absence of CypD. (A) Tail-transection bleeding time (n = 8). (B,C) Carotid artery thrombosis was induced by photochemical injury, and the times to stable (B) and first occlusion (C) were determined (n = 8). *P < .05 for comparison between CypD+/+ and CypD−/− mice. Error bars represent SE.
In vivo thrombotic and hemostatic responses are altered in the absence of CypD. (A) Tail-transection bleeding time (n = 8). (B,C) Carotid artery thrombosis was induced by photochemical injury, and the times to stable (B) and first occlusion (C) were determined (n = 8). *P < .05 for comparison between CypD+/+ and CypD−/− mice. Error bars represent SE.
Discussion
In this study, we have demonstrated a critical role for CypD in the regulation of platelet activation. In the absence of CypD, several platelet activation responses, including phosphatidylserine externalization, high-level fibrinogen retention, αIIbβ3 antigenic modulation, membrane vesiculation, procoagulant activity, and relaxation of clot retraction, were all markedly impaired. The influence of CypD deficiency was most notable following dual-agonist platelet stimulation with thrombin plus convulxin or thrombin plus H2O2.
CypD's localization to the mitochondrial matrix,28 together with the effects of CypD's absence on the activation-dependent loss of Δψm, points to a critical role for a mitochondrial event in the regulation of platelet activation. Relatively few studies have examined the role of mitochondria in platelet activation. Early reports primarily examined the role that mitochondria played in meeting the energy demands required for platelet aggregation and granule release.29 More recently, a rapid loss of Δψm has been demonstrated to occur in some activated platelet subpopulations after stimulation with certain agonists.7,14,15 Pharmacologic studies evaluating the mechanism of this rapid loss of Δψm indicated a critical role for MPTP formation as the mediator of this and other platelet activation events.15
We found that conditions that altered the potential for MPTP formation, such as the MPTP activator H2O2 or the absence of CypD, had marked effects on a defined set of platelet activation responses, those that occurred primarily following platelet stimulation with thrombin plus convulxin. Together, these data strongly suggest a critical role for MPTP formation in the regulation of the platelet activation response. Coappearance of several CypD-dependent responses, including activation-dependent loss of Δψm, phosphatidylserine externalization, high-level fibrinogen retention, and antigenic modulation of αIIbβ3, was demonstrated to occur in a distinct subpopulation of activated platelets using 2-color flow cytometry. Similar subpopulations of activated platelets have been demonstrated in previous studies following activation of human platelets with strong agonists.6,7,30-33
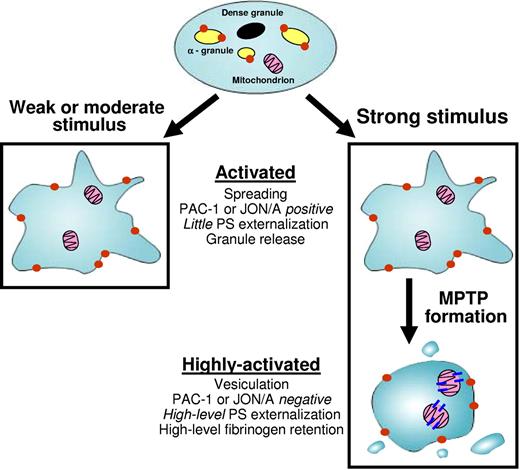
Our results suggest a model in which MPTP formation plays a critical role in the regulation of the transition of a platelet from an “activated” to a “highly activated” state (Figure 8). This transition is regulated by both the quantity and type of agonists(s) present. In response to a weak or moderate stimulus, such as a low concentration of thrombin, MPTP formation does not occur, and the stimulated platelet exhibits a CypD-independent activated phenotype. This activated phenotype is characterized by granule release, inside-out activation of αIIbβ3 (detected by activation-dependent antibodies such as JON/A or PAC-1), and platelet spreading without vesiculation.
In response to a strong stimulus, such as a high concentration of thrombin7,33 or combinations of agonists (thrombin plus collagen or thrombin plus H2O2), MPTP formation is favored. Under these conditions, the initial CypD-independent responses that result in the generation of the activated phenotype are followed by a CypD-dependent transition to a highly activated phenotype. The highly activated platelet is characterized by high-level phosphatidylserine (PS) externalization, high-level fibrinogen retention, antigenic modulation of αIIbβ3 (characterized by loss of PAC-1 or JON/A staining), and marked membrane vesiculation. As a result of their marked impairment of MPTP formation, CypD−/− platelets cannot undergo the transition from activated to highly activated and are therefore arrested in the activated state.
Several previous studies support this model of sequential and graded platelet activation. We have found that murine platelets stimulated with thrombin plus convulxin exhibit rapid αIIbβ3 activation (within 30 seconds).17 Between 1 and 3 minutes after agonist stimulation, a subpopulation with features of highly activated platelets, including high-level fibrinogen retention and αIIbβ3 antigenic modulation, emerges and then predominates. Sequential and graded platelet activation has also been observed in human platelets.34,35 Upon activation, platelets rapidly underwent spreading and inside-out activation of αIIbβ3. Subsequently, a subpopulation of activated platelets emerged that had high-level phosphatidylserine externalization, low binding of an αIIbβ3-activation–dependent antibody (PAC-1), and membrane contraction, fragmentation, and vesiculation.
Although our results indicate a critical role for CypD and MPTP function in regulating the platelet activation response to strong agonists, the nature of the upstream pathways that mediate MPTP formation, as well as the pathways leading to phosphatidylserine externalization and other downstream events, remain largely unknown. Because one of the consequences of MPTP formation is disruption of the mitochondrion's calcium-buffering capacity,13,36 one possibility is that a rise in cytoplasmic calcium levels may initiate downstream events. Consistent with this hypothesis, increased calcium levels have been demonstrated in phosphatidylserine-expressing platelets,30,37 and we have found that activation of platelets with a calcium ionophore can bypass the defect in phosphatidylserine externalization in CypD−/− platelets. Alternatively, MPTP formation may initiate downstream signaling pathways that are independent of effects on cytoplasmic calcium. Because MPTP formation can cause outer mitochondrial membrane rupture,36 release of molecules from mitochondria, such as cytochrome C, could also mediate downstream responses.
MPTP formation is redox-sensitive,26,27 and one of the mechanisms by which the oxidant H2O2 initiates cell death is by facilitating MPTP formation.10,12,25 Our results suggest a role for H2O2 as a modulator of the platelet activation response to thrombin. Stimulation of platelets with thrombin together with H2O2, at low micromolar concentrations, caused a CypD-dependent increase in phosphatidylserine externalization and high-level fibrinogen retention. Previously, ROS have been demonstrated to have variable effects on platelet activation.38 However, most of these earlier studies focused on the effects of ROS on platelet aggregation or granule release, neither of which was altered by H2O2 or by the absence of CypD. Platelets, endothelial cells, and leukocytes all produce ROS following their activation.39-41 Further evaluation of the potential role of vascular cell–derived H2O2 as a modulator of platelet activation may provide novel insights into mechanisms that regulate thrombosis in vivo.
To begin to assess the functional significance of CypD-dependent platelet activation responses, we examined the effect of CypD-deficiency on platelet procoagulant activity (Figure 6A). In the absence of CypD, platelet prothrombinase activity was markedly impaired. This finding is consistent with the observed decrease in phosphatidylserine externalization and α-granule protein retention of CypD−/− platelets, both of which have been proposed to facilitate prothrombinase complex assembly on the surface of activated platelets.4,6
We also examined the effects of dual-agonist stimulation and CypD deficiency on clot retraction. Initiation of clot formation by thrombin plus convulxin resulted in a CypD-dependent attenuation, or “relaxation,”20 of clot retraction relative to clots initiated by single agonists. Because clot retraction depends on αIIbβ3,42 these results suggest a role for a CypD-dependent process in the regulation of αIIbβ3 function, a finding consistent with our flow cytometric data demonstrating antigenic modulation of αIIbβ3 in dual agonist–stimulated platelets. Further supporting this hypothesis, a direct correlation between relaxation of clot retraction and calpain-mediated cleavage of the αIIbβ3-associated protein, talin,20 has been demonstrated in human platelets following platelet stimulation with thrombin plus the calcium ionophore A23187, a condition that strongly favors highly activated platelet formation.24 It is possible, however, that other CypD-dependent events may also contribute to the attenuation of clot retraction observed in the presence of dual agonists. Because MPTP formation disrupts ATP production by oxidative phosphorylation,9 a decrease in mitochondrial ATP production in highly activated platelets may impede the energy-dependent process of clot retraction.43
In a model of carotid-artery photochemical injury, we found that thrombosis occurred more rapidly in CypD−/− mice than in CypD+/+ mice. We have considered several potential explanations for this observation. First, because CypD is expressed in multiple tissues,10-13 the shortened occlusion time may be related to an effect of CypD deficiency in cells other than platelets. Such an effect might mask potential antithrombotic effects of platelet CypD deficiency, including effects occurring as a result of impairment of platelet prothrombinase activity. This possibility could be addressed in future experiments using adoptive transfer of platelets or bone marrow transplantation approaches.
Alternatively, impaired formation of highly activated platelets might contribute directly to accelerated thrombosis in CypD−/− mice. Although some features of highly activated platelets, such as phosphatidylserine externalization, would be expected to result in a prothrombotic phenotype, other features, such as antigenic modulation of αIIbβ3 and relaxation of clot retraction, would be expected to be antithrombotic. Moreover, highly activated platelets have high surface levels of anticoagulant proteins, such as tissue factor pathway inhibitor,44 as well as procoagulant proteins. Recent studies by Kulkarni and Jackson34 and Munnix et al35 using in vitro flow-based models of coagulation have also demonstrated that human platelets with a highly activated phenotype (PAC-1low, annexin Vhigh) have a markedly decreased ability to support platelet-platelet adhesive interactions and thrombus growth.
Taken together, our findings in CypD−/− mice, along with the observations of Kulkarni and Jackson34 and Munnix et al35 in human platelets, suggest a negative-feedback mechanism in which thrombus growth is limited by the CypD-dependent formation of highly activated platelets in response to strong agonists. Additional studies will be needed to clarify the functional role of platelet mitochondrial signaling and highly activated platelet formation in normal hemostasis and in arterial and venous thrombosis. Variations in the potential to form highly activated platelets have previously been demonstrated between individuals in human populations.6 It will be interesting to examine whether this variation might correlate with thrombotic risk. Several pharmacologic agents have potential effects on MPTP formation.45 Closer examination of these agents could result in the identification of novel therapeutics for the treatment of thrombotic disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a National Hemophilia Foundation Clinical Fellowship Award (S.M.J.), an American Heart Association Fellow-to-Faculty Transition Award (S.M.J.), and National Institutes of Health grants T-32 HL 07734 (S.M.J.), HL 04460 (J.D.P.), and HL 63943 (S.R.L.).
Authorship
Contribution: S.M.J. designed and performed research, analyzed data, and wrote the manuscript; K.M.W., L.L., and A.R. performed research; J.D.M. contributed vital experimental tools; and S.R.L. and J.D.P. designed research, analyzed data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shawn M. Jobe, 412 Emory Children's Center, Emory University, 2015 Uppergate Drive, Atlanta, GA 30322; e-mail: shawn.jobe@emory.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal