Abstract
The metalloprotease ADAMTS13 is assumed to regulate the functional levels of von Willebrand factor (VWF) appropriate for normal hemostasis in vivo by reducing VWF multimer size, which directly represents the thrombogenic activity of this factor. Using an in vitro perfusion chamber system, we studied the mechanisms of ADAMTS13 action during platelet thrombus formation on a collagen surface under whole blood flow conditions. Inhibition studies with a function-blocking anti-ADAMTS13 antibody, combined with immunostaining of thrombi with an anti-VWF monoclonal antibody that specifically reflects the VWF-cleaving activity of ADAMTS13, provided visual evidence for a shear rate–dependent action of ADAMTS13 that limits thrombus growth directly at the site of the ongoing thrombus generation process. Our results identify an exquisitely specific regulatory mechanism that prevents arterial occlusion under high shear rate conditions during mural thrombogenesis.
Introduction
The adhesive protein von Willebrand factor (VWF) plays a major role in platelet thrombogenesis, a process crucial for hemostasis. However, the excessive function of VWF is thought to increase the risk of fatal arterial thrombosis.1,2 The thrombogenic activity of VWF is strictly dependent upon its multimeric structure, which is thought to be regulated in vivo by the metalloprotease ADAMTS13 through its cleavage of the A2 domain of the VWF subunit.3,4 Indeed, patients with congenital deficiency of ADAMTS13 suffer repeated thrombotic complications attributed to excessive function of the ultra-large VWF (ULVWF) multimer, which is not found in normal blood circulation.3-6 This concept was recently confirmed by knock-out mouse studies, in which ADAMTS13−/− mice exhibited enhanced thrombogenicity in the ex vivo or in vitro experimental blood flow conditions tested.7,8
The mechanisms by which ADAMTS13 regulates VWF remain poorly understood. However, recent studies showing that ADAMTS13 under flow conditions can rapidly cleave ULVWF secreted from and anchored to cultured endothelial cell layers9,10 have raised the possibility that blood flow is critical in activating ADAMTS13.11 Indeed, the VWF-cleaving activity of ADAMTS13 cannot be reproduced in vitro under static conditions unless the substrate VWF molecule is somewhat modified (eg, denatured by guanidine-HCl or urea).3,4 Further, the question arises of whether ADAMTS13, in addition to its known action on ULVWF freshly released from endothelial cells, might also act directly at the local sites of thrombus generation to regulate thrombus growth.
To address these issues, we analyzed the role and mechanisms of ADAMTS13 action in mural platelet thrombogenesis on a collagen-coated glass surface in an in vitro perfusion chamber system. Our visual evidence demonstrates that ADAMTS13 cleaves VWF and down-regulates mural thrombus growth at the site of ongoing thrombus generation in a shear rate–dependent manner under whole blood flow conditions.
Methods
Blood collection
The present work was approved by the institutional review board of Nara Medical University, and informed consent was obtained in accordance with the Declaration of Helsinki. Using 200 μM argatroban as an anticoagulant, blood was collected from 10 nonsmoking healthy volunteers who had not taken any medications in the previous 2 weeks.
Monoclonal antibodies
A function-blocking anti-ADAMTS13 monoclonal antibody (A10), which completely inhibits plasma ADAMTS13 activity at the concentration of 20 μg/mL,12 was used as a divalent (ab')2 fragment in inhibition studies. An anti-VWF monoclonal antibody (N10) was used that reacts with an epitope within the VWF A2 domain (10-amino acid VWF peptide; D1596REQAPNLVY1605) only after cleavage by ADAMTS13 exposes the epitope; thus, reactivity of antibody N10 specifically reflects the VWF-cleaving activity of ADAMTS13, as described.13
In vitro perfusion studies
Thrombus generation on a type I collagen-coated (Sigma-Aldrich, Tokyo, Japan) glass surface was studied under various shear rates in a parallel plate flow chamber system as described.14-17 Surface coverage and volume of thrombi generated at the indicated time points during whole blood perfusion were evaluated based on images obtained by confocal laser scanning microscopy (CLSM; FV300; Olympus, Tokyo, Japan), as described.15-17 Immunohistochemical staining of thrombi using anti-VWF antibodies was performed as described.15-17 Briefly, thrombi on a glass surface were fixed with paraformaldehyde and incubated with a mixture of anti–whole VWF rabbit polyclonal antibody (30 μg/mL; DAKO Cytomation, Kyoto, Japan) and N10 antibody (60 μg/mL) or with the negative control IgG mixture (rabbit; 30 μg/mL, mouse; 60 μg/mL; DAKO Cytomation) for 90 minutes at 37°C. Samples were then stained with a mixture of fluorescein isothiocyanate (FITC)–conjugated anti–rabbit IgG (3.3 μg/mL; BioSource International, Camarillo, CA) and Cy3-conjugated anti–mouse IgG (3.3 μg/mL; Sigma-Aldrich) as secondary fluorescent antibodies for 90 minutes at 37°C, and viewed by CLSM. These conditions were determined in preliminary experiments to confirm the sufficient infiltration of both primary and secondary fluorescent antibodies into thrombi.
Results and discussion
To address the potential role of ADAMTS13 in the ongoing process of mural thrombus generation, we compared the size of thrombi generated in the presence or absence of a function-blocking antibody against ADAMTS13 in a perfusion chamber system, using blood from the same donor. This relatively simple experimental approach is able to precisely evaluate ADAMTS13 function in uniform blood conditions, avoiding the individual heterogeneity of sample blood conditions including VWF and platelets that might otherwise seriously affect the size of thrombi generated in this type of flow experiment.
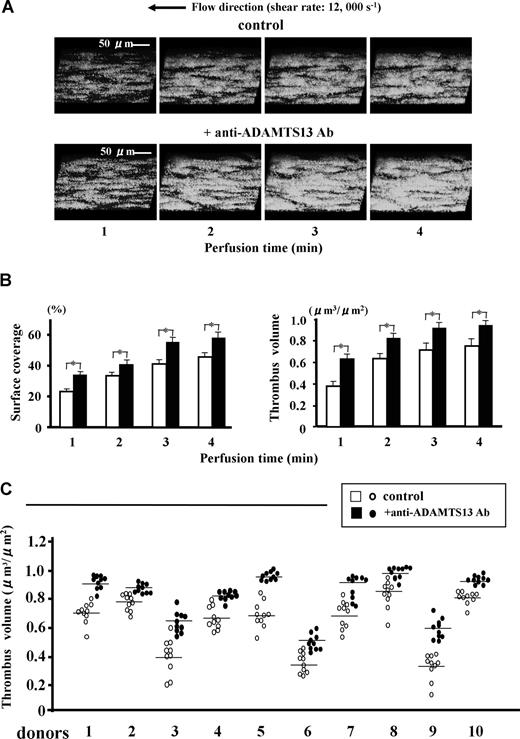
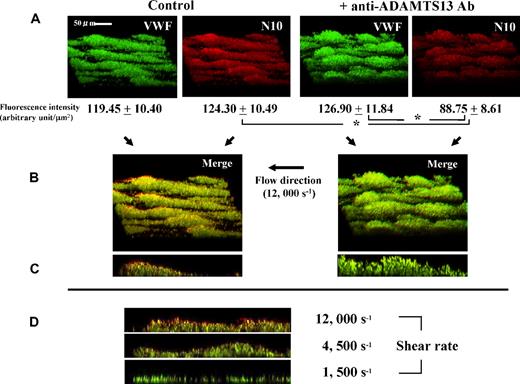
Under a very high shear rate of 12 000 s−1, thrombus growth was significantly accelerated by addition of anti-ADAMTS13 antibody (Figure 1). This enhanced thrombogenesis most likely reflects a block in ADAMTS13 activity rather than nonspecific effects of antibody on platelets, since immunostaining of thrombi with N10 antibody, which reacts only with VWF cleaved by ADAMTS13, visually confirmed the reduced VWF cleavage within thrombi by the anti-ADAMTS13 antibody (Figure 2A). Thus, these results clearly point to the regulatory role of ADAMTS13 during thrombus generation.
Effects of a function-blocking antiADAMTS13 monoclonal antibody (A10) on mural thrombus generation under very high shear rate conditions. Whole blood from healthy volunteers containing DiOC6 (1 μM)-labeled platelets, anticoagulated with argatroban, was perfused over a type I collagen-coated glass surface under very high shear rate (12 000 s−1) with anti-ADAMTS13 antibody A10 or with control mouse IgG (each 20 μg/mL). (A) Time-course changes of 3-dimensional images of thrombi (original magnification, ×600), which were constructed by the image-analyzing system of confocal laser scanning microscopy (CLSM) based on successive horizontal slices at identical portions, are representative of 10-pair flow experiments using blood from 10 independent donors. (B) Statistical analyses corresponding to the above images; bars represent mean (+ SD) surface coverage or total thrombus volume in 10 areas (each 133 × 100 μm) randomly selected in each perfusion using a single donor blood (donor number 1 in panel C). Note that thrombus generation is significantly (*; P < .01) accelerated in the presence of the anti-ADAMTS13 antibody. (C) Thrombus volume at 3 minutes' perfusion in 10-pair flow experiments using 10 independent donors; data points represent values of 10 areas randomly selected in each perfusion with (●) or without (○) anti-ADAMTS13 antibody, and transverse lines indicate mean values for each group. Note also that thrombus volumes generated in the presence of anti-ADAMTS13 antibody are significantly (P < .01; asterisks not included in the figure) greater than control thrombi in all 10-pair experiments.
Effects of a function-blocking antiADAMTS13 monoclonal antibody (A10) on mural thrombus generation under very high shear rate conditions. Whole blood from healthy volunteers containing DiOC6 (1 μM)-labeled platelets, anticoagulated with argatroban, was perfused over a type I collagen-coated glass surface under very high shear rate (12 000 s−1) with anti-ADAMTS13 antibody A10 or with control mouse IgG (each 20 μg/mL). (A) Time-course changes of 3-dimensional images of thrombi (original magnification, ×600), which were constructed by the image-analyzing system of confocal laser scanning microscopy (CLSM) based on successive horizontal slices at identical portions, are representative of 10-pair flow experiments using blood from 10 independent donors. (B) Statistical analyses corresponding to the above images; bars represent mean (+ SD) surface coverage or total thrombus volume in 10 areas (each 133 × 100 μm) randomly selected in each perfusion using a single donor blood (donor number 1 in panel C). Note that thrombus generation is significantly (*; P < .01) accelerated in the presence of the anti-ADAMTS13 antibody. (C) Thrombus volume at 3 minutes' perfusion in 10-pair flow experiments using 10 independent donors; data points represent values of 10 areas randomly selected in each perfusion with (●) or without (○) anti-ADAMTS13 antibody, and transverse lines indicate mean values for each group. Note also that thrombus volumes generated in the presence of anti-ADAMTS13 antibody are significantly (P < .01; asterisks not included in the figure) greater than control thrombi in all 10-pair experiments.
Visual evaluation of ADAMTS13 activity within thrombi generated under high shear rate conditions using a monoclonal antibody (N10) that specifically detects ADAMTS13-cleaved VWF. Experimental conditions were as described in Figure 1, except that platelets were not labeled. Thrombi generated on a collagen-coated glass surface at 3 minutes' perfusion with or without an anti-ADAMTS13 antibody under 12 000 s−1 shear were fixed, reacted with both N10 antibody and anti–whole VWF antibody, double-stained with Cy3 (red)- and FITC (green)-fluorescence, and viewed by CLSM. (A) Three-dimensional images of thrombi, representative of 5 independent flow experiments, and the corresponding fluorescence intensity (mean ± SD of 5 areas randomly selected in a single perfusion) corrected for background value (negative control IgGs), indicate that VWF cleavage by ADAMTS13 (red color) within thrombi is significantly (*; P < .01) reduced in the presence of anti-ADAMTS13 antibody as compared with control thrombi (original magnification; ×600). (B) Merged 3-dimensional images and (C) the corresponding longitudinal views of thrombi; in the merged images, portions stained with both green and red fluorescence basically show the color of the higher pixel value, whereas a yellowish color is seen when both pixel values are nearly equal. Thus, the predominantly reddish appearance of the surface portions of control thrombi suggests that ADAMTS13 is more active on the surface of thrombi forming under very high shear rate conditions, while this tendency is barely visible in the presence of anti-ADAMTS13 antibody. (D) Longitudinal views of thrombi, generated at 3 minutes' perfusion without an anti-ADAMTS13 antibody under various shear rates and double-stained, are representative of 5 separate experiments. Note the prominent red color, especially at the surface portions of thrombi, indicating higher ADAMTS13 activity under higher shear rates.
Visual evaluation of ADAMTS13 activity within thrombi generated under high shear rate conditions using a monoclonal antibody (N10) that specifically detects ADAMTS13-cleaved VWF. Experimental conditions were as described in Figure 1, except that platelets were not labeled. Thrombi generated on a collagen-coated glass surface at 3 minutes' perfusion with or without an anti-ADAMTS13 antibody under 12 000 s−1 shear were fixed, reacted with both N10 antibody and anti–whole VWF antibody, double-stained with Cy3 (red)- and FITC (green)-fluorescence, and viewed by CLSM. (A) Three-dimensional images of thrombi, representative of 5 independent flow experiments, and the corresponding fluorescence intensity (mean ± SD of 5 areas randomly selected in a single perfusion) corrected for background value (negative control IgGs), indicate that VWF cleavage by ADAMTS13 (red color) within thrombi is significantly (*; P < .01) reduced in the presence of anti-ADAMTS13 antibody as compared with control thrombi (original magnification; ×600). (B) Merged 3-dimensional images and (C) the corresponding longitudinal views of thrombi; in the merged images, portions stained with both green and red fluorescence basically show the color of the higher pixel value, whereas a yellowish color is seen when both pixel values are nearly equal. Thus, the predominantly reddish appearance of the surface portions of control thrombi suggests that ADAMTS13 is more active on the surface of thrombi forming under very high shear rate conditions, while this tendency is barely visible in the presence of anti-ADAMTS13 antibody. (D) Longitudinal views of thrombi, generated at 3 minutes' perfusion without an anti-ADAMTS13 antibody under various shear rates and double-stained, are representative of 5 separate experiments. Note the prominent red color, especially at the surface portions of thrombi, indicating higher ADAMTS13 activity under higher shear rates.
While the preceding observations were made under a much higher shear rate than the high shear rate typically used in platelet functional studies (ie, 1500 s−1 in our laboratory14-16 ), similar observations, although less pronounced, were confirmed under lower shear rates (Figure 2D). In addition, longitudinal views of thrombi revealed the preferential VWF-cleavage activity of ADAMTS13 at the surface portions of forming thrombi during thrombogenesis (Figure 2B,C). The thrombus surface is thought to directly encounter blood flow with the highest shear rate under such flow circumstances, where the wall shear rate can increase as the flow path narrows in parallel with thrombus growth.15 Together, these observations strongly suggest a shear rate–dependent property of ADAMTS13 function.
Shear forces are thought to transform the globular conformation of the immobilized VWF multimer observed under static conditions to a shape resembling a spreading bird wing, consistent with the shear rate–dependent acceleration of the VWF-glycoprotein Ib interaction under high shear.18 By analogy, a stretching of the VWF multimeric structure by shear forces may also be critical for the action of ADAMTS13 in exposing the latent reactive site on the VWF molecule. In this regard, increased tensile strength of the VWF multimeric structure on binding to platelets might augment the stretching effects of shearing forces, resulting in up-regulated ADAMTS13 activity.19,20 This possibility seems compatible with recent findings indicating that even under low shear rate conditions, ADAMTS13 can cleave ULVWF released from endothelial cells,9,10 because a greater number of platelets can bind spontaneously to ULVWF as compared with normal-sized VWF without shearing forces.
The mechanisms described here represent an exquisite orchestration by platelets, VWF, and ADAMTS13 under high shear to properly regulate the final size of mural thrombi in vivo and prevent excessive thrombogenesis from occluding the vessel lumen. Because ADAMTS13 activity appears to be triggered in response to the increased local shear rate associated with the development of thrombi, our results may provide a novel avenue toward strategies that prevent arterial thrombosis without bleeding complications.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Marina Hoffman for editorial assistance.
This work was supported in part by Ministry of Education, Culture, Sports, Science and Technology of Japan grants 15390305 and 19591129 (M.S.), 19592095 (K.N.), and 17390304 (A.Y.), and by the Ministry of Health and Welfare of Japan (M.S.) for Clinical Research of Myocardial Infarction, Stroke and Diabetes Mellitus.
National Institutes of Health
Authorship
Contribution: Y.S. performed most of the flow experiments, data analysis, and the manuscript preparation; T.M. and M.H. helped perform the flow experiments and data analysis; S.K., M.M., and Y.F. produced and characterized monoclonal antibodies; A.Y. and K.O. provided direction throughout the work and helped prepare the manuscript; and M.S. and K.N. provided the overall experimental designs and direction of this work, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mitsuhiko Sugimoto, MD, or Kenji Nishio Department of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; e-mail: sugi-ped@naramed-u.ac.jp. or knishio@naramed-u.ac.jp.