Megakaryocytes and platelets express the Gs-coupled VPAC1 receptor, for which the pituitary adenylyl cyclase–activating peptide (PACAP) and the vasointestinal peptide (VIP) are agonists. We here demonstrate a regulatory role for VPAC1 signaling during megakaryopoiesis. A total of 2 patients with trisomy 18p with PACAP overexpression and transgenic mice overexpressing PACAP in megakaryocytes have thrombopathy, a mild thrombocytopenia, and a reduced number of mature megakaryocytes in their bone marrow. In vitro differentiation of hematopoietic stem cells from the patient and transgenic mice shows a reduced number of megakaryocyte colonies compared with controls. The addition of PACAP, VIP, or the adenylyl cyclase activator forskolin to CD34+ cells inhibits megakaryocyte differentiation. In contrast, neutralizing monoclonal anti-PACAP (PP1A4) or anti-VPAC1 (23A11) antibodies inhibit cAMP formation and stimulate megakaryopoiesis in a thrombopoietin-independent manner. Moreover, wild-type mice obtain an increased platelet count after subcutaneous injection of PP1A4 or 23A11. These antibodies also elevate platelet numbers in animal models of myelosuppressive therapy and in GATA1-deficient mice with congenital thrombocytopenia. Furthermore, 23A11 stimulates the in vitro megakaryocyte differentiation of both normal and GATA1-deficient human CD34+ cells. Together, our data strongly suggest that VPAC1 signaling tempers normal megakaryopoiesis, and that inhibition of this pathway stimulates megakaryocyte differentiation, enhancing platelet recovery after myelosuppressive therapy and in GATA1 deficiency.
Introduction
Vasointestinal peptide (VIP) and pituitary adenylyl cyclase–activating peptide (PACAP) belong to the glucagon hormone superfamily, which in humans includes secretin, growth hormone–releasing hormone (GHRH), glucagon, glucagon-like peptides 1 and 2 (GLP-1 and GLP-2), peptide histidine methionine (PHM), and glucose-dependent insulinotropic polypeptide (GIP).1 These peptides have been grouped into a superfamily because the precursor molecules share a strikingly similar structural organization and similar amino acid sequences; in general, the hormones are related in terms of distribution and function. PACAP and VIP effects have been described in the digestive tract, cardiovascular system, airways, reproductive system, immune system, endocrine glands, and brain.1,2 These pleiotropic neuropeptides share 2 common G protein–coupled receptors, VPAC1 (VIPR1) and VPAC2 (VIPR2), while PACAP has an additional specific receptor, PAC1 (ADCYAP1R1).1,3 The human PACAP gene (ADCYAP1) is located on chromosome 18p32,4 and is mainly expressed in testis and brain, but this peptide crosses the blood-brain barrier.5
We previously found an important role for PACAP and its receptor VPAC1 in platelet function.6 Studies in 2 related patients with a partial trisomy 18p and monosomy 20p revealed 3 copies of the PACAP gene and elevated PACAP concentrations in plasma. The patients suffer from mental retardation, have a bleeding tendency with thrombopathy and a mild thrombocytopenia, and their fibroblasts show increased PACAP mRNA levels. The VPAC1 receptor in platelets and fibroblasts is coupled to adenylyl cyclase activation. Accordingly, we found highly increased basal cAMP levels in the patients' fibroblasts and platelets, providing an explanation for the reduced platelet aggregation in these patients. We phenocopied the platelet defect described in the patients in a transgenic mouse model with PACAP overexpression specifically in the megakaryocyte lineage by cloning the PACAP gene after the GPIIb promoter.6 The opposite phenotype (ie, enhanced platelet reactivity) was observed in mice treated with the neutralizing monoclonal PACAP antibody PP1A4, with a polyclonal VPAC1 antibody or with a specific PACAP inhibitor PACAP6-38.6
Since these patients with trisomy 18p also have a moderate thrombocytopenia, we have now investigated whether the VPAC1 signaling cascade modulates megakaryopoiesis and platelet production, complex processes still poorly understood. Megakaryopoiesis and thrombopoiesis are critically orchestrated by various thrombopoietic cytokines, including interleukin-3 (IL-3), stem cell factor (SCF), IL-6, IL-11, thrombopoietin (TPO), and many other regulators.7,,,–11 Lineage-specific transcription factors as GATA1 and its cofactor FOG1 regulate the differentiation of hematopoietic stem cells into megakaryocytes.12,13 The stromal-derived factor 1 (SDF1) and its Gi protein–coupled chemokine receptor CXCR4 stimulate the homing of megakaryocytic progenitors.14 Whether VIP/PACAP and their common Gs protein–coupled VPAC1 receptor modulate megakaryopoiesis is unknown. A previous study showed that megakaryocytes and platelets express the VIP-specific receptor VPAC1, pointing to a potential direct effect of VIP on megakaryopoiesis.15 VIP was also found to inhibit the proliferation of bone marrow progenitors through this receptor.16
To address the contribution of VPAC1 signaling to megakaryopoiesis, we studied the maturation and polyploidy of megakaryocytes from a patient with elevated PACAP plasma levels and from transgenic mice overexpressing PACAP. Megakaryocyte differentiation of normal hematopoietic stem cells was also studied in the presence of PACAP, VIP, and the direct activator of adenylyl cyclase forskolin. Furthermore, the effects of inhibiting the VPAC1 receptor or blocking its ligands on the differentiation of hematopoietic stem cells into megakaryocytes and platelets were examined in vitro and in vivo. Our results indicate that mice treated with neutralizing anti-PACAP (PP1A4) or anti-VPAC1 (23A11) monoclonal antibodies have increased platelet numbers without obvious side effects. If VPAC1 inhibition potentiates megakaryopoiesis directly or indirectly, such inhibition may become of therapeutic use during myelosuppressive and myeloablative therapy to prevent or treat thrombocytopenia. It could also be used for the treatment of congenital thrombocytopenia due to defective megakaryocyte differentiation. Such therapeutic applicability may therefore equally be studied.
Methods
Approval was obtained from the KU Leuven Ethical Commission for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Patients
The clinical and biological features of the patients with trisomy 18p with 3 copies of the PACAP gene have been described previously.6 A patient with a severe congenital thrombocytopenia due to a D218Y GATA1 mutation has also been reported.17 Peripheral blood and bone marrow samples from these patients and healthy unrelated controls were obtained after informed consent. The legal representative of the patients was the mother.
Animals
Megakaryocyte-specific PACAP-overexpressing mice (PACAP-Tg9) were developed as previously described into the Friend leukemia virus, strain B (FVB) background.6 PACAP-Tg9 and wild-type littermates were bred and maintained under specific pathogen–free conditions. Male C57BL-6/GATA1 mice (ΔneoΔHS) have been described previously,18,19 and were kindly provided by Dr Paresh Vyas (Department of Hematology and Medical Research Council [MRC] Molecular Hematology Unit, Weatherall Institute of Molecular Medicine, Oxford, United Kingdom). Mice were tested between 6 and 12 weeks of age. Animal procedures and experiments were approved by the Animal Ethical Committee of the University of Leuven (Leuven, Belgium).
Antibodies
The following antibodies were used for flow cytometry: PE-conjugated anti-CD34 (8G12), FITC-conjugated anti-CD41a (HIP8), FITC-conjugated anti-CD61 (VI-PL2), PerCP-conjugated anti-CD61 (RUU-PL7F12), FITC-conjugated anti-Sca1 (Ly-6A/E; BD Biosciences Pharmingen, Heidelberg, Germany), and FITC-conjugated anti-CD41/61 (Leo-D2; Emfret Analysis, Wurzburg, Germany). The monoclonal IgM antibody PP1A4 and the monoclonal IgG1 antibody 23A11 were obtained in our laboratory from BALB/c mice, immunized with, respectively, human recombinant PACAP1-386 and human recombinant VPAC1, both coupled to glutathione S–transferase (GST). These recombinant fusion proteins were expressed in Escherichia coli and purified by affinity chromatography on immobilized glutathione (Amersham Biosciences, Freiburg, Germany). The primary antibodies were purified on protein A Sepharose beads (Amersham Biosciences) and controlled for their reactivity toward recombinant PACAP1-38 and VPAC1 by enzyme-linked immunosorbent assay (ELISA).
Hematologic investigations
Peripheral EDTA-anticoagulated blood samples were obtained from the patients, the retro-orbital sinus of anesthetized FVB mice (0.2 mL), or from the ears of New Zealand White (NZW) rabbits (1 mL). An aliquot of whole blood was analyzed on an automated blood cell counter (Cell-Dyn 1300; Abbott Laboratories, Abbott Park, IL) to determine leukocyte, erythrocyte, and platelet counts, hematocrit, and hemoglobin levels.
Electron microscopy of megakaryocytes
Bone marrow cells from PACAP-Tg9 and wild-type FVB mice were collected by flushing femurs with phosphate-buffered saline (PBS) containing 2% heat-inactivated fetal bovine serum and 2 mM EDTA using a 22-gauge needle. Patient, human control, and mouse bone marrow samples were immediately fixed in 2.5% glutaraldehyde and 0.1 M phosphate buffer at 4°C overnight. At 1 hour after fixation in 1% osmium tetroxide and 0.1 M phosphate buffer at 4°C, samples were dehydrated in graded series of alcohol and embedded in epoxy resin. Ultrathin sections were cut, stained with uranyl acetate-lead citrate, and examined using a Zeiss electron microscope (Jena, Germany).
cAMP measurements
MEG01 and Caco2 cells were grown in RPMI and Dulbecco modified Eagle medium (DMEM), respectively. The cAMP levels were measured using a cAMP enzyme immunoassay (GE Healthcare Life Sciences, Uppsala, Sweden) as previously described.6,20 The incubation times with PACAP1–38 (1 μM), VIP (1 μM), PP1A4 (10 μg/mL), or 23A11 (10 μg/mL) were 720, 60, and 10 minutes. Recombinant PACAP1-38 and VIP were from Bachem (Bubendorf, Switzerland).
Immunoblot analysis
Caco2 and MEG01 cells were lysed in ice-cold PBS containing 1% igepal CA-630 (Sigma-Aldrich, St Louis, MO), 2 mM Na3VO4, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 mM DTE, and 1 protease inhibitor cocktail tablet/50 mL (Roche Applied Science, Indianapolis, IN). The proteins were resolved by SDS-PAGE and transferred to Hybond ECL-nitrocellulose membrane (Amersham Biosciences). Blots were revealed with a monoclonal antiserum against VPAC1 (23A11) and stained with Western blotting electrochemiluminescence (ECL) detection reagent (Amersham Biosciences).
TPO measurement in plasma
TPO levels were determined in human EDTA and mouse citrated plasma using commercially available species-specific kits (R&D Systems, Minneapolis, MN).
Generation and quantification of megakaryocytic colonies
Human CD34+ cells were isolated from bone marrow or cord blood by magnetic cell sorting (Miltenyi Biotec, Auburn, CA). We purified a Sca1+ population from bone marrow cells pooled from mice using the EasySep Sca1-positive selection kit (StemCell Technologies, Vancouver, BC). Human CD34+ cells (5 × 103) and mice Sca1+ cells (5 × 104) were cultured in Megacult-C 04973 with cytokines (for human) and Megacult-C 04964 supplemented with recombinant cytokines according to the manufacturer's instructions (for mice; StemCell Technologies). The number of megakaryocytic colonies (colony-forming units–megakaryocyte [CFU-MK]) was determined in a semisolid culture system using a commercially available kit (StemCell Technologies). The total number of colonies was counted 12 days later using a light microscope (Leica DM RBE; Wetzlar, Germany) in cultures performed in duplicate. Slides were photographed at 40×/0.5 magnification with a Zeiss Axiocam MRc camera and captured with Zeiss Axiovision (Carl Zeiss, Jena, Germany). Images were analyzed with Java ImageJ 1.34g image processing software (National Institutes of Health, Bethesda, MD). Megakaryocytes were identified as large cells with lobulated nuclei and basophilic staining.
Quantification of megakaryocyte ploidy and proliferation
Isolated CD34+ cord blood cells (1.5 × 105) or Sca1+ murine bone marrow cells (5 × 105) were cultured in Iscove modified Dulbecco medium (IMDM) with stable glutamine (Invitrogen, Carlsbad, CA), which was supplemented with 0.5% bovine serum albumin, 200 μg/mL iron-saturated transferrin, 10 μg/mL human insulin, 50 μM β-mercaptoethanol (all from Sigma-Aldrich), 25 ng/mL TPO, 10 ng/mL IL6, 10 ng/mL IL3, and 25 ng/mL SCF (all from Peprotech, London, United Kingdom). To determine megakaryocyte ploidy, bone marrow cells or in vitro–differentiated megakaryocytes (day 12) were costained with FITC-conjugated CD61 (for human samples) or FITC-conjugated CD41/61 (for mice samples) and propidium iodide (Sigma-Aldrich), and the DNA content was determined by 2-color flow cytometry.21 PACAP1-38 (1 μM), VIP (1 μM), both (1 μM each) or forskolin (10 μM) were added to the cultures at days 0 and 8. Expanding FITC-conjugated CD41a+ and PerCP-conjugated CD61+ megakaryocytes were also quantified by flow cytometry on days 6 and 12. We used Cell Quest software for 2-color immunofluorescence acquisition on a FACSCalibur flow cytometer (both from BD Biosciences Pharmingen) and for data analysis.
VPAC1 inhibition in mice and rabbits
We administered 1 mg/kg PP1A4 or 23A11 antibody reconstituted in 40% polyethylene glycol 400 (PEG400) by subcutaneous injections on days 0, 3, and 7 for mice (n = 10 for PP1A4, n = 5 for 23A11, and n = 15 for PBS) and on days 0, 3, 7, and 10 for NZW rabbits (n = 3 for 23A11 or n = 3 for PBS). To induce myelosuppression, recipients were treated with a double dose of busulfan (20 mg/kg intraperitoneally; Orphan Medical, Minnetonka, MN) on days 7 and 10. This dose of busulfan preferentially suppresses the megakaryocytic lineage in mice and rabbits.22 For the second myelosuppressive model, mice subcutaneously injected with PP1A4 (1 mg/kg) or PBS on days 0, 3, and 7 (n = 5 for PP1A4 and n = 5 for PBS) were exposed to a sublethal irradiated dose of 8 Gy on day 6. At different time intervals, blood was removed for cell counts and TPO measurements as described in “Hematologic investigations” and “TPO measurement in plasma.” For the myeloablative model, mice subcutaneously injected with PP1A4 (1 mg/kg) or PBS on days 0, 3, and 7 (n = 5 for PP1A4 and n = 5 for PBS) were exposed to a lethal dose of 9 Gy at day 6, and survival was determined during the following 4 weeks.
Statistics
We used GraphPad InStat 3.01 software (GraphPad Software, San Diego, CA). Continuous variables with little to mild skewness were summarized as means plus or minus SD and compared by means of the Student t test for unpaired data. To determine differences in MK differentiation (percentage of CD41+ and CD61+ MKs), MK ploidy, and platelet counts after myelosuppressive therapy, a 1-way analysis of variance (ANOVA) test was used, complemented with the Bonferroni multiple comparison t test to identify statistically differences at each individual time point. We used GraphPad Prism4 software for the statistical analysis of survival times during myeloablative therapy with the Kaplan-Meier test followed by the log-rank test. Data were considered significant in all cases when the P value was less than .05.
Results
Phenotypic characterization of platelets and megakaryocytes from a trisomy 18p patients and PACAPTg9 mice
Patients with trisomy 18p who had 3 copies of the PACAP gene in all tissues and PACAPTg9 mice with PACAP overexpression specifically in their megakaryocytic lineage all have a prolonged bleeding time, high PACAP plasma levels, and a platelet functional defect.6 Their hematologic parameters are summarized in Table 1. The patients and PACAPTg9 mice have a mild thrombocytopenia, and the patients' platelets have a decreased mean platelet volume (MPV). Whereas the white blood cell count was normal in the patients, it was decreased in PACAPTg9 mice. Electron microscopy of the patients' platelets showed no structural abnormalities (data not shown). The PACAP-overexpressing patient and the PACAPTg9 mice had normal TPO levels (Table 1).
Hematologic parameters and TPO plasma levels
| . | Plt, × 106/L . | MPV, fL . | WBC, × 106/L . | RBC, × 109/L . | Hct, L/L . | Hgb, g/L . | TPO, pg/mL . |
|---|---|---|---|---|---|---|---|
| Normal human values, range | 150–450 | 9.0–13.0 | 4.0–10.0 | 4.5–6.0 | 0.4–0.54 | 140–180 | ND-196 |
| PACAP-overexpressing patient 1 | 68, 90 | 8.2, 8.0 | 8.0, 9.8 | 4.31, 4.37 | 0.37, 0.36 | 121, 126 | 54, 86 |
| PACAP-overexpressing patient 2 | 99 | 8.2 | 6.3 | 5.24 | 0.42 | 148 | NA |
| Normal FVB mice (n=20) | 785 ± 75 | NA | 4.3 ± 1.2 | 7.1 ± 0.4 | 0.3 ± 0.02 | 115 ± 9 | 106 ± 77 |
| PACAPTG9-FVB (n=20) | 636 ± 42* | NA | 3.3 ± 1.1† | 6.9 ± 0.3 | 0.3 ± 0.01 | 113 ± 6 | 136 ± 54 |
| . | Plt, × 106/L . | MPV, fL . | WBC, × 106/L . | RBC, × 109/L . | Hct, L/L . | Hgb, g/L . | TPO, pg/mL . |
|---|---|---|---|---|---|---|---|
| Normal human values, range | 150–450 | 9.0–13.0 | 4.0–10.0 | 4.5–6.0 | 0.4–0.54 | 140–180 | ND-196 |
| PACAP-overexpressing patient 1 | 68, 90 | 8.2, 8.0 | 8.0, 9.8 | 4.31, 4.37 | 0.37, 0.36 | 121, 126 | 54, 86 |
| PACAP-overexpressing patient 2 | 99 | 8.2 | 6.3 | 5.24 | 0.42 | 148 | NA |
| Normal FVB mice (n=20) | 785 ± 75 | NA | 4.3 ± 1.2 | 7.1 ± 0.4 | 0.3 ± 0.02 | 115 ± 9 | 106 ± 77 |
| PACAPTG9-FVB (n=20) | 636 ± 42* | NA | 3.3 ± 1.1† | 6.9 ± 0.3 | 0.3 ± 0.01 | 113 ± 6 | 136 ± 54 |
Values for patient 1 were measured on different occasions. Values are means plus or minus SD.
Plt indicates platelets; MPV, mean platelet volume; WBC, white blood cells; RBC, red blood cells; Hct, hematocrit; Hgb, hemoglobin; NA, not available; and ND, not detectable.
P < .001.
P = .003.
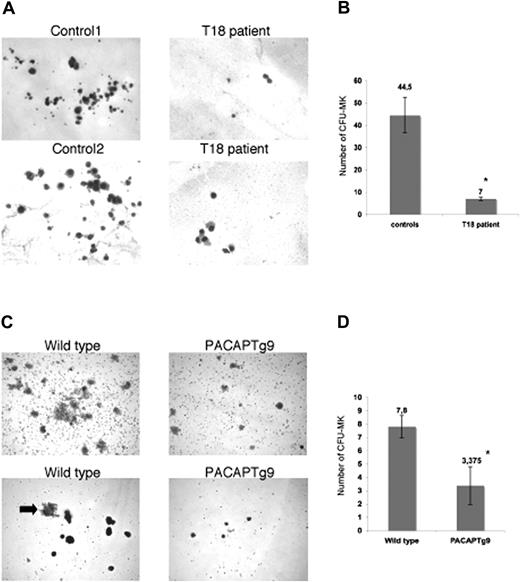
Multiple mechanisms could result in defective thrombopoiesis. To exclude a role for PACAP signaling in platelet turnover, we studied the survival of human platelets in a rabbit model.23,24 Patient and control platelets were intravenously injected in a thrombocytopenic rabbit that had been pretreated with busulfan and ethyl palmitate, and human platelets were counted after certain time points to determine the platelet survival. We observed no differences in platelet survival between wild-type and patient platelets (data not shown). Examination of the bone marrow from the patients with trisomy 18p and PACAPTg9 mice by electron microscopy showed the presence of a normal red blood cell lineage, normal myeloid and lymphoid lineage, and multiple megakaryocyte progenitors, but almost no mature megakaryocytes or proplatelet-forming megakaryocytes. The immature megakaryoblasts seemed to have a decreased amount of less-grouped small granules. In agreement, platelets from the patient have a reduced dense granule ATP secretion (0.39 and 0.43 μM on separate occasions; normal values, 2–7 μM after 10 μg/mL collagen stimulation) and a reduced alpha-granule beta-thromboglobuline secretion (4 and 5.5 mg/L; normal values, 6–20 mg/L). The defect in MK maturation was further studied via MK in vitro differentiation assays of hematopoietic stem cells. A reduced number of smaller megakaryocyte colonies (CFU-MK) were generated from bone marrow–derived CD34+ cells of the patient with trisomy 18 compared with 4 unrelated normal bone marrow donors (Figure 1A,B). Analogous observations were made when Sca1+ bone marrow cells from the PACAPTg9 mice were differentiated into MKs. The number and size of the MK colonies was significantly reduced in the PACAPTg9 mice compared with wild-type littermates (Figure 1C,D).
Megakaryocyte generation. (A) Megakaryocyte (MK) colonies on day 12 from bone marrow–derived CD34+ cells from a control donor or a patient with trisomy 18p with 3 copies of the PACAP gene. (B) Number of CFU-MKs from 5 × 103 bone marrow–derived CD34+ cells from 4 unrelated control donors or a patient with trisomy 18p. Bars represent the means plus or minus SD of the CFU-MK counts of 1 plate from the patient and 4 plates from 4 controls (*P < .001). (C) MK colonies on day 12 in wild-type and PACAPTg9 mice. Sca1+ bone marrow cells from wild-type mice develop into more and larger colonies compared with those of PACAPTg9 mice. MK are also larger (arrow) in wild-type mice. (D) Number of CFU-MKs from 5 × 104 bone marrow–derived Sca1+ cells from 8 PACAPTg9 mice or 5 wild-type littermate controls. Bars represent the means plus or minus SD of the CFU-MK counts of 1 plate for each PACAPTg9 and wild-type mouse (*P < .001).
Megakaryocyte generation. (A) Megakaryocyte (MK) colonies on day 12 from bone marrow–derived CD34+ cells from a control donor or a patient with trisomy 18p with 3 copies of the PACAP gene. (B) Number of CFU-MKs from 5 × 103 bone marrow–derived CD34+ cells from 4 unrelated control donors or a patient with trisomy 18p. Bars represent the means plus or minus SD of the CFU-MK counts of 1 plate from the patient and 4 plates from 4 controls (*P < .001). (C) MK colonies on day 12 in wild-type and PACAPTg9 mice. Sca1+ bone marrow cells from wild-type mice develop into more and larger colonies compared with those of PACAPTg9 mice. MK are also larger (arrow) in wild-type mice. (D) Number of CFU-MKs from 5 × 104 bone marrow–derived Sca1+ cells from 8 PACAPTg9 mice or 5 wild-type littermate controls. Bars represent the means plus or minus SD of the CFU-MK counts of 1 plate for each PACAPTg9 and wild-type mouse (*P < .001).
Inhibition of in vitro megakaryopoiesis by stimulation of VPAC1 signaling
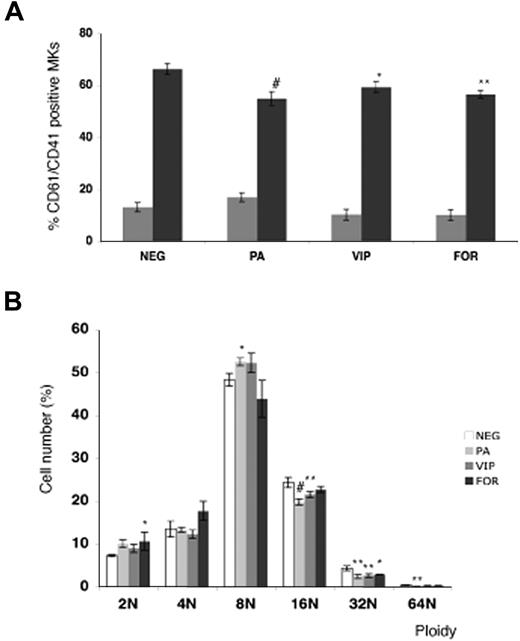
The role of VPAC1 signaling was further studied during the MK differentiation of normal cord blood–derived CD34+ cells in the presence of the VPAC1 agonists PACAP or VIP. The addition of PACAP and VIP significantly decreased the amount of double-stained CD41+ and CD61+ MKs at day 12 (Figure 2A). In addition, the DNA ploidy analysis of the CD61+ MKs at day 12 showed a reduced ploidy in the cultures with PACAP or VIP compared with the basal condition (Figure 2B). Since PACAP and VIP stimulate cAMP formation via binding to the Gs-coupled receptor VPAC1,1 in vitro MK differentiation was also studied in the presence of the direct adenylyl cyclase activator forskolin. Similar as found for PACAP and VIP, forskolin was also able to inhibit the MK differentiation and the MK DNA ploidy level (Figure 2A,B).
Effect of PACAP, VIP, and forskolin on in vitro megakaryopoiesis. (A) Flow cytometric analysis of day-6 (▩) and day-12 (■) differentiated MKs from cord blood–derived CD34+ cells in the presence of PACAP, VIP, or forskolin (FOR). Each point represents the mean amount of dual-stained CD41+ and CD61+ MKs plus or minus SD of 3 experiments. (B) The DNA ploidy distribution of CD61+ MKs in vitro–differentiated from CD34+ cells isolated from cord blood in the absence (NEG) or presence of PACAP, VIP, or FOR on day 12. Each point represents the mean number of CD61+ MKs of each ploidy level plus or minus SD of 3 experiments. *P < .05; **P < .01; #P < .001 by ANOVA.
Effect of PACAP, VIP, and forskolin on in vitro megakaryopoiesis. (A) Flow cytometric analysis of day-6 (▩) and day-12 (■) differentiated MKs from cord blood–derived CD34+ cells in the presence of PACAP, VIP, or forskolin (FOR). Each point represents the mean amount of dual-stained CD41+ and CD61+ MKs plus or minus SD of 3 experiments. (B) The DNA ploidy distribution of CD61+ MKs in vitro–differentiated from CD34+ cells isolated from cord blood in the absence (NEG) or presence of PACAP, VIP, or FOR on day 12. Each point represents the mean number of CD61+ MKs of each ploidy level plus or minus SD of 3 experiments. *P < .05; **P < .01; #P < .001 by ANOVA.
Stimulation of in vitro megakaryopoiesis by inhibition of VPAC1 signaling
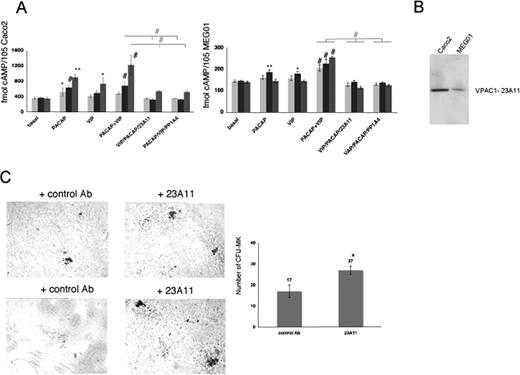
The monoclonal antibodies PP1A4 and 23A11 inhibit VPAC1 signaling, and their presence causes the opposite phenotype to that depicted in the patient with trisomy 18p or in PACAPTg9 mice. PP1A4 was raised against the active PACAP1-38 recombinant peptide,6 and also cointeracts with the highly homologous VIP peptide (68%) as determined by ELISA (data not shown). Therefore, this antibody probably blocks the effects of both ligands on the VPAC1 receptor. The epitope of 23A11 is located in a region within the second and third extracellular loop (amino acids 278-373) of the VPAC1 receptor, as determined by immunoblot analysis using different recombinant fragments of the VPAC1 receptor (data not shown). The second extracellular loop is known to be involved in the VPAC1 ligand-mediated cAMP response.25 The antibodies were further evaluated in the human enterocyte-like cell line Caco2, for which it is described that VPAC1 is expressed and PACAP and VIP can modulate the intracellular cAMP level.26 PACAP and VIP are able to rise cAMP levels in Caco2 cells, and this cAMP formation was significantly inhibited by the presence of 23A11 or PP1A4 during different incubation times of 12 hours, 1 hour, and 10 minutes (Figure 3A). A similar but less-pronounced observation was made in the human megakaryocytic cell line MEG01 (Figure 3A). This difference in cAMP response toward PACAP and VIP could be explained by the observation that Caco2 cells have higher expression levels of VPAC1 compared with MEG01 cells as shown via immunoblot analysis (Figure 3B). We also investigated whether inhibition of VPAC1 affected megakaryopoiesis by performing in vitro CFU-MK assays. More megakaryocytes were generated from Sca1+ cells derived from normal mouse bone marrow after 12 days of incubation with 23A11 (Figure 3C).
Effect of 23A11 on cAMP generation and in vitro megakaryopoiesis. (A) Measurements of cAMP levels in Caco2 (left panel) and MEG01 (right panel) cells under basal conditions and after stimulation with PACAP, VIP, and both agonists in the absence and presence of PP1A4 or 23A11 after 12 hours (light-gray bars), 1 hour (■), and 10 minutes ( ) incubation time. Bars represent the means plus or minus SD of 3 experiments. *P < .05; **P < .01; #P < .001 by ANOVA. (B) Immunoblot analysis of VPAC1 expression in Caco2 and MEG01 cells after loading equal amounts of total protein (50 μg). (C) Megakaryocyte colonies on day 12 from bone marrow–derived Sca1+ cells incubated with a control antibody or 23A11 (left panel). Sca1+ murine bone marrow cells incubated with 23A11 resulted in increased numbers of CFU-MKs after 12 days (right panel). Bars represent the means plus or minus SD (P < .01).
) incubation time. Bars represent the means plus or minus SD of 3 experiments. *P < .05; **P < .01; #P < .001 by ANOVA. (B) Immunoblot analysis of VPAC1 expression in Caco2 and MEG01 cells after loading equal amounts of total protein (50 μg). (C) Megakaryocyte colonies on day 12 from bone marrow–derived Sca1+ cells incubated with a control antibody or 23A11 (left panel). Sca1+ murine bone marrow cells incubated with 23A11 resulted in increased numbers of CFU-MKs after 12 days (right panel). Bars represent the means plus or minus SD (P < .01).
Effect of 23A11 on cAMP generation and in vitro megakaryopoiesis. (A) Measurements of cAMP levels in Caco2 (left panel) and MEG01 (right panel) cells under basal conditions and after stimulation with PACAP, VIP, and both agonists in the absence and presence of PP1A4 or 23A11 after 12 hours (light-gray bars), 1 hour (■), and 10 minutes ( ) incubation time. Bars represent the means plus or minus SD of 3 experiments. *P < .05; **P < .01; #P < .001 by ANOVA. (B) Immunoblot analysis of VPAC1 expression in Caco2 and MEG01 cells after loading equal amounts of total protein (50 μg). (C) Megakaryocyte colonies on day 12 from bone marrow–derived Sca1+ cells incubated with a control antibody or 23A11 (left panel). Sca1+ murine bone marrow cells incubated with 23A11 resulted in increased numbers of CFU-MKs after 12 days (right panel). Bars represent the means plus or minus SD (P < .01).
) incubation time. Bars represent the means plus or minus SD of 3 experiments. *P < .05; **P < .01; #P < .001 by ANOVA. (B) Immunoblot analysis of VPAC1 expression in Caco2 and MEG01 cells after loading equal amounts of total protein (50 μg). (C) Megakaryocyte colonies on day 12 from bone marrow–derived Sca1+ cells incubated with a control antibody or 23A11 (left panel). Sca1+ murine bone marrow cells incubated with 23A11 resulted in increased numbers of CFU-MKs after 12 days (right panel). Bars represent the means plus or minus SD (P < .01).
Stimulation of in vivo megakaryopoiesis by inhibition of VPAC1 signaling
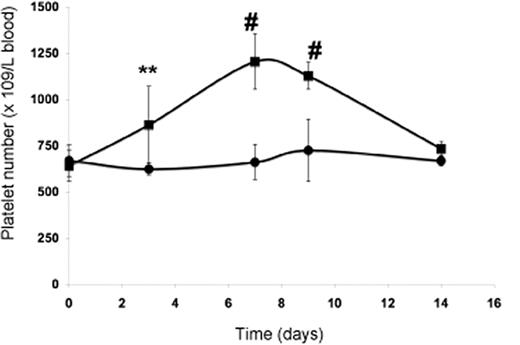
The subcutaneous injection of wild-type mice with PP1A4 on days 0, 3, and 7 resulted in a significantly increased platelet count compared with mice injected with a control monoclonal antibody (IgM) against human β2 glycoprotein I (Figure 4). Similar to what was previously found for mice subcutaneously injected with an adenovirus vector encoding TPO, the maximum platelet rise was seen on day 7 after starting treatment and returned to baseline by day 14.27 The administration of PP1A4 never caused thrombocytosis but a rather moderate increase in platelet count. Injection of PP1A4 stimulates platelet production without interfering with the TPO pathway, since TPO plasma levels at day 14 were comparable between FVB mice injected with PP1A4 (158 ± 111 pg/mL) or with the control antibody (134 ± 64 pg/mL). No obvious side effects were noted in mice treated with PP1A4.
In vivo evaluation of PP1A4-induced platelet production. Platelet count in blood following administration of PP1A4 (■) or a control antibody (●) on days 0, 3, and 7. Each point represents the mean platelet count for 5 mice plus or minus SD. **P < .01; #P < .001.
In vivo evaluation of PP1A4-induced platelet production. Platelet count in blood following administration of PP1A4 (■) or a control antibody (●) on days 0, 3, and 7. Each point represents the mean platelet count for 5 mice plus or minus SD. **P < .01; #P < .001.
Inhibition of VPAC1 signaling against thrombocytopenia following myelosuppressive and myeloablative therapy
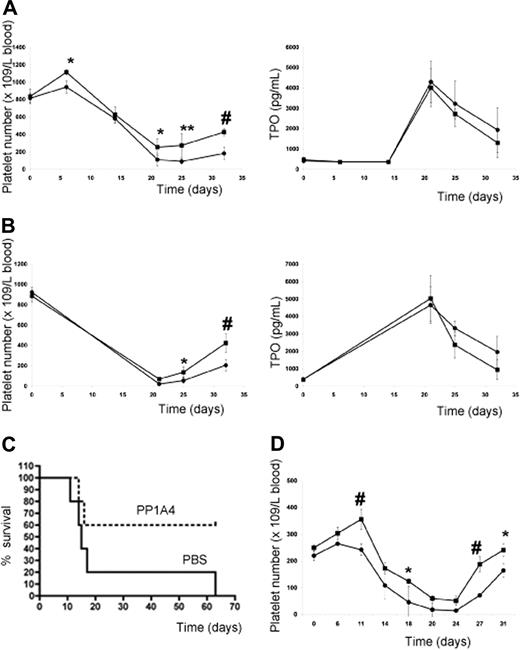
Since administration of PP1A4 only resulted in moderately increased platelet levels, the question arose whether VPAC1 inhibition would protect experimental animals from life-threatening thrombocytopenia following myelosuppressive therapy. The subcutaneous injection of PP1A4 (on days 0, 3, and 7) was indeed capable of stimulating platelet counts in mice after chemotherapy-induced thrombocytopenia (busulfan treatment on days 7 and 10) in a TPO-independent manner (Figure 5A). Similar observations were made for 23A11 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In the group treated with PBS, 5 of 15 mice died between 2 and 22 days after the last busulfan injection because of internal bleeding, whereas in the group treated with PP1A4 or 23A11 all mice survived chemotherapy. Similar to what was found with the adenoviral overexpression of TPO,27 the effectiveness of the therapy was fully dependent on the moment the antibody was given. Administration of antibody after the start of chemotherapy had no effect on the platelet recovery (data not shown). An additional advantage of blocking VPAC1 signaling during chemotherapy-induced thrombocytopenia is the fact that this also enhances the platelet reactivity.6 The therapeutic effect of PP1A4 on myelosuppression was also tested in another model of whole-body irradiation. The platelet recovery of mice subcutaneously injected with PP1A4 2 times before and 1 day after irradiation (8 Gy) was significantly increased compared with nontreated mice (Figure 5B). The difference in platelet recovery between the 2 groups was again not dependent on differences in TPO generation. Finally, the therapeutic effect of PP1A4 was studied in a severe model of myelosuppression, as mice were lethally irradiated (9 Gy) and their survival was determined. Mice treated with PP1A4 had an increased survival percentage compared with nontreated mice (Figure 5C).
VPAC1 inhibition during myelosuppressive therapy. (A) Platelet count in mice (n = 5 in each group) following administration of PP1A4 (■) or PBS (●) on days 0, 3, and 7 and busulfan treatment on days 7 and 10 (left panel). The plasma TPO levels from the same mice treated with PP1A4 (■) or PBS (●) at the indicated days (right panel). Each point represents the mean TPO value plus or minus SD. (B) Platelet count in mice (n = 5 in each group) following administration of PP1A4 (■) or PBS (●) on days 0, 3, and 7 and sublethal whole-body irradiation (8 Gy) on day 6 (left panel). The plasma TPO levels from the same mice treated with PP1A4 (■) or PBS (●) at the indicated days (right panel). Each point represents the mean TPO value plus or minus SD. (C) Mortality of mice (n = 5 in each group) injected with PP1A4 (■) or PBS (●) on days 0, 3, and 7 and lethal whole-body irradiation (9 Gy) on day 6. (D) Platelet count in rabbits (n = 3 in each group) following administration of 23A11 (■) or PBS (●) on days 0, 3, 7, and 10 and busulfan treatment on days 7 and 10. Each point represents the mean platelet count plus or minus SD. *P < .05; **P < .01; #P < .001 by ANOVA.
VPAC1 inhibition during myelosuppressive therapy. (A) Platelet count in mice (n = 5 in each group) following administration of PP1A4 (■) or PBS (●) on days 0, 3, and 7 and busulfan treatment on days 7 and 10 (left panel). The plasma TPO levels from the same mice treated with PP1A4 (■) or PBS (●) at the indicated days (right panel). Each point represents the mean TPO value plus or minus SD. (B) Platelet count in mice (n = 5 in each group) following administration of PP1A4 (■) or PBS (●) on days 0, 3, and 7 and sublethal whole-body irradiation (8 Gy) on day 6 (left panel). The plasma TPO levels from the same mice treated with PP1A4 (■) or PBS (●) at the indicated days (right panel). Each point represents the mean TPO value plus or minus SD. (C) Mortality of mice (n = 5 in each group) injected with PP1A4 (■) or PBS (●) on days 0, 3, and 7 and lethal whole-body irradiation (9 Gy) on day 6. (D) Platelet count in rabbits (n = 3 in each group) following administration of 23A11 (■) or PBS (●) on days 0, 3, 7, and 10 and busulfan treatment on days 7 and 10. Each point represents the mean platelet count plus or minus SD. *P < .05; **P < .01; #P < .001 by ANOVA.
The effect of 23A11 on in vitro megakaryocyte differentiation was more pronounced than that of PP1A4, and therefore the in vivo study in a larger animal model was only carried out with 23A11. The subcutaneous injection with 23A11 (on days 0, 3, 7, and 10) also reduced the deep drop in platelet count and accelerated the recovery of the platelet number in busulfan-treated rabbits (Figure 5D). Rabbits injected with 23A11 and treated with busulfan show increased platelet numbers (188 ± 27 × 109 platelets/L on day 27) compared with rabbits injected with PBS (72 ± 14 × 109 platelets/L on day 27; P < .001 by ANOVA). Except for a stimulatory role on the megakaryocytic lineage, we never observed any effect of the inhibition of VPAC1 signaling on the number of red blood cells or leukocytes.
Inhibition of VPAC1 signaling against GATA1 deficiency–induced thrombocytopenia
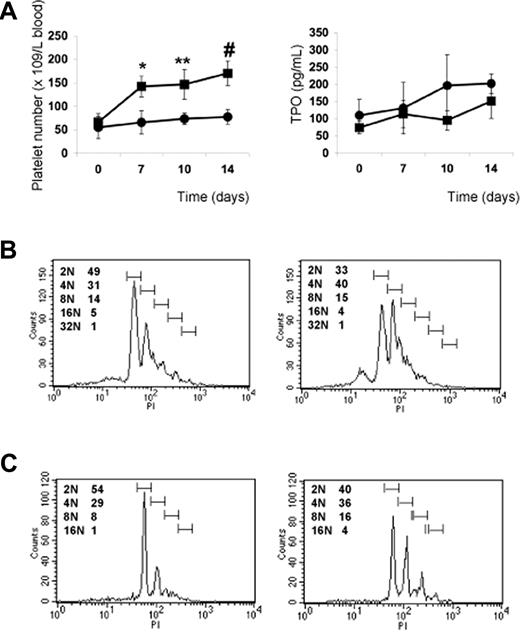
The previous results indicate that PP1A4 and 23A11 are potent stimulators of megakaryopoiesis in vitro, in vivo, and in a clinical setting of chemotherapy-induced thrombocytopenia. We next studied their use in a model of congenital thrombocytopenia, such as GATA1 deficiency. A defect in the hematopoietic transcription factor GATA1 leads to a variable degree of thrombocytopenia but also to an increased number of megakaryocytes characterized by marked ultrastructural abnormalities.17,–19 We hypothesized that VPAC1 signaling regulates primarily the later stages of megakaryopoiesis and platelet formation, and therefore that 23A11 would rescue the GATA1-defective phenotype characterized by a blockade in the megakaryoblast stage. Indeed, GATA1-deficient mice (ΔneoΔHS) are thrombocytopenic, with a platelet count of 50 plus or minus 15 × 109 platelets/L. This platelet number increases to 150 plus or minus 40 × 109 platelets/L after subcutaneous injection with 23A11, while injection with PBS had no influence on the platelet count (Figure 6A). TPO plasma levels in these injected GATA1-deficient mice showed no association between anti-VPAC1 treatment and stimulation of the TPO pathway (Figure 6A). The platelets produced after antibody treatment had a normal size and morphology. Fluorescence-activated cell sorter (FACS) analysis of bone marrow–derived Sca1+ cells from ΔneoΔHS mice cultured in vitro for 12 days in megakaryocyte differentiation medium showed an increased megakaryocyte DNA ploidy when 23A11 was added (Figure 6B). Isolated CD34+ cells from the bone marrow of a GATA1-deficient patient with severe thrombocytopenia17 were also differentiated in vitro into megakaryocytes in the absence or presence of 23A11. FACS analysis at day 12 of the differentiated GATA1-D218Y–deficient megakaryocytes showed a maturation defect with immature megakaryocytes (mostly 2N phase), while added 23A11 again increased the DNA ploidy of these megakaryocytes to 16N (Figure 6C).
Effect of VPAC1 inhibition in mouse and human thrombocytopenia due to GATA1 defect. (A) Platelet count in blood following administration of 23A11 (■) on days 0, 3, and 7 or PBS (●) in ΔneoΔHS GATA1-deficient mice (left panel). Each point represents the mean platelet count from 4 (■) or 3 (●) mice plus or minus SD. **P < .01; #P < .001 by ANOVA. The plasma TPO levels from the same GATA1-deficient mice treated with 23A11 (■) or PBS (●) at the indicated days (right panel). Each point represents the mean TPO value from 4 (■) or 3 (●) mice plus or minus SD. (B) Histograms demonstrate the DNA ploidy distribution of CD41+ megakaryocytes in vitro–differentiated from Sca1+ cells isolated from bone marrow from ΔneoΔHS mice in the absence (left) or presence (right) of 23A11 on day 12. (C) Histograms demonstrate the DNA ploidy distribution of CD41+ megakaryocytes differentiated from CD34+ cells from bone marrow of the GATA1-D218Y patient in the absence (left) or presence of 23A11 (right) on day 12. FACS results are representative of 2 separate analyses.
Effect of VPAC1 inhibition in mouse and human thrombocytopenia due to GATA1 defect. (A) Platelet count in blood following administration of 23A11 (■) on days 0, 3, and 7 or PBS (●) in ΔneoΔHS GATA1-deficient mice (left panel). Each point represents the mean platelet count from 4 (■) or 3 (●) mice plus or minus SD. **P < .01; #P < .001 by ANOVA. The plasma TPO levels from the same GATA1-deficient mice treated with 23A11 (■) or PBS (●) at the indicated days (right panel). Each point represents the mean TPO value from 4 (■) or 3 (●) mice plus or minus SD. (B) Histograms demonstrate the DNA ploidy distribution of CD41+ megakaryocytes in vitro–differentiated from Sca1+ cells isolated from bone marrow from ΔneoΔHS mice in the absence (left) or presence (right) of 23A11 on day 12. (C) Histograms demonstrate the DNA ploidy distribution of CD41+ megakaryocytes differentiated from CD34+ cells from bone marrow of the GATA1-D218Y patient in the absence (left) or presence of 23A11 (right) on day 12. FACS results are representative of 2 separate analyses.
Discussion
We here show that high levels of PACAP result in defective megakaryocyte maturation, as our patients with trisomy 18p with 3 copies of the PACAP gene and elevated PACAP plasma levels present with a severe bleeding phenoype and mild thrombocytopenia in addition to their neurologic and endocrinologic abnormalities.6 The defective platelet phenotype, having no other obvious abnormalities, could partially be mimicked in transgenic PACAPTg9 mice with overexpression of PACAP in the megakaryocyte lineage. The lineage-specific overexpression of PACAP in these mice is in contrast with the overall PACAP overexpression in the patients with trisomy 18p, which is reflected by a weaker bleeding phenotype, a milder thrombocytopenia, and a less-pronounced difference in CFU-MK number after in vitro hematopoietic stem-cell differentiation assays in the transgenic mice compared with the patients. This is suggestive of an additional role for PACAP expressed by other cell types, such as bone marrow stromal cells or endothelial cells. Yet, bone marrow examination in both the patients and PACAPTg9 mice revealed increased numbers of immature megakaryocytes and almost no mature or proplatelet-forming megakaryocytes. These observations suggest a role for PACAP during the late stages of megakaryopoiesis and platelet formation, but not at the time of bipotential erythro-megakaryocyte progenitor formation from hematopoietic stem cells. Indeed, the addition of PACAP but also of VIP to normal CD34+ cells inhibits MK differentiation and DNA ploidy (Figure 2). We never observed any abnormalities in erythrocytes or their progenitors and, except for a lower white blood cell count in the PACAPTg9 mice (but not in the patients), no other abnormalities were observed. TPO plasma levels from the patients with trisomy 18p and PACAPTg9 transgenic mice were within normal limits, suggestive of a TPO-independent interference of the PACAP/VPAC1 pathway during megakaryopoiesis and thrombopoiesis.
Induction of the opposite phenotype by using neutralizing anti-PACAP or anti-VPAC1 antibodies underscores that PACAP modulates normal megakaryopoiesis via its Gs-coupled receptor VPAC1. We could show that the antibodies are able to inhibit the cAMP formation induced by PACAP and/or VIP. Different experiments showed that in vitro and in vivo megakaryopoiesis is stimulated by administration of PP1A4 or 23A11. VPAC1 knockout mice are not available, and PACAP-defective mice have a platelet defect6 but a normal platelet count (Dr Hashimoto, oral communication, January 2004). The monoclonal anti-PACAP antibody PP1A4 cross-reacts with the highly homologous VIP neuropeptide and therefore blocks both ligands of the VPAC1 receptor.
Thrombocytopenia is an important complication of myeloablative therapy. The search for an efficient drug to reduce thrombocytopenia or accelerate platelet recovery without adverse effect on the bone marrow is still warranted. The most obvious candidate for such therapy appeared to be TPO, a major cytokine regulating megakaryopoiesis and thrombopoiesis.8 A total of 2 recombinant TPOs have undergone extensive clinical testing: the pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF), and recombinant human TPO (rHuTPO).28 PEG-rHuMGDF was effective in some clinical settings and was withdrawn from clinical trials in 1998 because of the development of neutralizing antibodies to TPO, followed by thrombocytopenia or pancytopenia.29 Studies on the use of rHuTPO in the treatment of chemotherapy- and nonchemotherapy-induced thrombocytopenia are ongoing, with close attention to potential side effects, especially since chronic overexpression of TPO following ex vivo transduction of bone marrow progenitors resulted in persistent elevation of platelets, myelofibrosis, and osteosclerosis.30 The effects of PP1A4 and 23A11 on in vitro and in vivo megakaryopoiesis cannot be compared with that of TPO, and even left circulating TPO levels unaffected in our different animal models. They proved to be effective in myelosuppressive therapy as demonstrated by a significant decrease in the degree and duration of thrombocytopenia in different experimental animal models. PP1A4 also seems to stimulate survival during myeloablative therapy in mice, but additional experiments using larger animal models as baboons injected with the humanized antibodies will have to substantiate the present initial observations. In contrast to the risk of persistent high platelet numbers during chronic TPO delivery, PP1A4 or 23A11 only raised platelet numbers temporarily but at the critical moment (ie, when platelet numbers drop below safe threshold levels). PP1A4 and 23A11 also enhance platelet reactivity6 in addition to their effect on megakaryopoiesis: only a small rise in platelet count, coinciding with improved aggregability, can improve hemostasis substantially.
Another important implication of this study is that VPAC1 inhibition may constitute an applicable treatment for GATA1 deficiency. Some GATA1-deficient patients have severe thrombocytopenia and elevated mortality at relatively young age, primarily due to uncontrolled, life-threatening hemorrhagic episodes.17 Frequent platelet transfusions offer a temporary solution for these patients, but eventually this treatment option becomes ineffective due to immune responses directed against allogeneic platelets. The lack of HLA-identical hematopoietic stem cell or bone marrow donors often precludes this form of treatment. The in vitro differentiation of Sca1+ cells from GATA1-deficient mice with a deletion of a hypersensitive region of the GATA1 promoter regulating GATA1 expression in megakaryocytes18,19 or of CD34+ cells from a patient with the D218Y GATA1 mutation17 showed a defective megakaryocyte maturation. Studies of the DNA ploidy by FACS showed the presence of immature megakaryocytes (mostly 2N) despite the simultaneous presence of TPO in the culture medium: the addition of 23A11 could increase the DNA ploidy of GATA1-deficient megakaryocytes to 16N in these cell cultures. These experiments strongly suggest that inhibition of VPAC1 signaling mainly interferes with the megakaryocyte differentiation, while TPO preferentially stimulates the megakaryocyte proliferation. VPAC1 inhibition never interfered with the TPO plasma concentration.
From these studies, we demonstrate that endogenous VPAC1 signaling tempers megakaryopoiesis and platelet production, but further studies are needed to identify the downstream players of the Gs-coupled signaling pathway(s). PACAP has been involved in a wide variety of physiologic responses and in development, and is therefore believed to act through as many intracellular pathways.1 PACAP signaling has been described through cAMP, calcium, phospholipase C, and MAPK (and it might also use the NFκB signaling pathway), and regulates the expression of many genes.31,32 We could exclude an interference of PACAP in the downstream signaling pathway of TPO with no influences of PACAP, PP1A4, or 23A11 on the TPO-mediated MAPK pathways (data not shown). Several studies in literature indicate that cAMP might be involved in megakaryopoiesis. Intracellular cAMP can interfere with the transcription of certain genes involved in thrombopoiesis under control of a promoter with CREB regulatory elements: mice with a mutation in the CREB-binding protein (CBP) paralog p300 have defects in megakaryocytes and platelets.33 In addition, the phosphodiesterase inhibitor anagrelide retards the intracellular degradation of cAMP and is used clinically to reduce the platelet count in patients with thrombocythemia.34,35 We could show that the adenylyl cyclase activator forskolin is able to reduce the MK differentiation and MK ploidy. In contrast to PACAP and VIP, forskolin significantly increased the number of 2N MKs, while PACAP and VIP seemed to have a more pronounced effect on the higher MK ploidy levels. Further studies are needed to define whether VPAC1 is up-regulated during megakaryopoiesis and therefore has a slower onset of MK differentiation inhibition.
By the genetic study of patients with a mild thrombocytopenia, we have identified PACAP as a new physiologic inhibitor of megakaryocyte maturation and platelet production, a conclusion experimentally validated via the use of a transgenic mouse model. We here provide the first evidence that VPAC1 inhibition by neutralizing antibodies stimulates in vitro and in vivo megakaryopoiesis, and furthermore illustrate that this strategy is applicable in chemotherapy-induced and congenital thrombocytopenia. This knowledge may lead to new modalities to prevent and treat thrombocytopenia.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank ThromboGenics for providing PP1A4 and 23A11 for this study.
This work was supported by the Excellentie financiering KULeuven (EF/05/013), by research grants G.0453.05 and G.0124.02 from the FWO-Vlaanderen (Belgium), and by GOA/2004/09 from the Research Council of the University of Leuven (Onderzoeksraad KU Leuven, Belgium). K.F. holds a postdoctoral research mandate, C.V.G. is holder of a clinical-fundamental research mandate, and K.P. is Research Assistant of the Fund for Scientific Research-Flanders (F.W.O.-Vlaanderen, Belgium).
Authorship
Contribution: K.F. participated in experimental design and analysis and manuscript preparation; K.P., C.T., and C.W. performed experiments and analyzed the data; R.D.V. performed bone marrow and platelet morphology studies; M.F.H. and J.V. participated in design and manuscript preparation; and C.V.G. studied the patients, analyzed the data, and participated in manuscript preparation. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kathleen Freson, Center for Molecular and Vascular Biology, University of Leuven, Herestraat 49, B-3000 Leuven, Belgium; e-mail: kathleen.freson@med.kuleuven.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal