Modulating protein ubiquitination via proteasome inhibition represents a promising target for cancer therapy, because of the higher sensitivity of cancer cells to the cytotoxic effects of proteasome inhibition. Here we show that CEP-18770 is a novel orally-active inhibitor of the chymotrypsin-like activity of the proteasome that down-modulates the nuclear factor-κB (NF-κB) activity and the expression of several NF-κB downstream effectors. CEP-18770 induces apoptotic cell death in multiple myeloma (MM) cell lines and in primary purified CD138-positive explant cultures from untreated and bortezomib-treated MM patients. In vitro, CEP-18770 has a strong antiangiogenic activity and potently represses RANKL–induced osteoclastogenesis. Importantly, CEP-18770 exhibits a favorable cytotoxicity profile toward normal human epithelial cells, bone marrow progenitors, and bone marrow–derived stromal cells. Intravenous and oral administration of CEP-18770 resulted in a more sustained pharmacodynamic inhibition of proteasome activity in tumors relative to normal tissues, complete tumor regression of MM xenografts and improved overall median survival in a systemic model of human MM. Collectively, these findings provide evidence for the utility of CEP-18770 as a novel orally active proteasome inhibitor with a favorable tumor selectivity profile for the treatment of MM and other malignancies responsive to proteasome inhibition.
Introduction
The ubiquitin-proteasome pathway plays a key role in protein processing and degradation, regulating crucial transduction pathways for cell growth and survival, including cell-cycle control, transcriptional regulation, cellular stress responses, and antigen presentation.1,2 Numerous investigators have demonstrated that proteasome inhibition results in the disruption of normal homeostatic mechanisms, and that cancer cells are more likely to undergo apoptosis after proteasome inhibition compared with normal cells.3,,–6 It is known that proteasome inhibition can reverse the aberrant changes that perpetuate proliferation and block apoptosis pathways in cancer cells. In fact, treatment of cancer cells with proteasome inhibitors results in the stabilization of p21 and p27,7,8 as well as inhibition of nuclear factor-kappaB (NF-κB)–mediated transcription.9,10 The NF-κB pathway is activated in a variety of tumor types and often deregulated in chemotherapy-resistant cells, suggesting that NF-κB activation plays a role in cancer cell survival and chemoresistance, and that NF-κB inhibition via proteasome inhibition can sensitize transformed cells to apoptosis.11,–13
The prevalent sensitivity of transformed cells to proteasome inhibitors and the successful design of clinical protocols, with tolerable although relatively narrow therapeutic indices, have made the proteasome a novel and promising target for cancer treatment.2,6 Clinical validation for the application of proteasome inhibition as a therapeutic strategy was achieved with bortezomib (Velcade; Millennium Pharmaceuticals, Cambridge, MA), a modified dipeptidyl boronic acid, intravenously administered, which is a slowly reversible inhibitor of the chymotrypsin-like activity of the 26S mammalian proteasome.4,5,14 On the basis of pivotal clinical trials, bortezomib has proven efficacious as single agent for the treatment of multiple myeloma (MM), non-Hodgkin, and mantle cell lymphoma patients.15,–17 Although the toxicity profile of bortezomib is quite well controlled in clinical settings, its side effects include peripheral neuropathy, orthostatic hypotension, pyrexia, cardiac and pulmonary disorders, gastrointestinal adverse events, myelosuppression, thrombocytopenia, asthenia, and pain.18,19 Consequently, there is a need for the identification of proteasome inhibitors with enhanced tolerability and safety profiles. Salinosporamide A (NPI-0052) extracted from a marine actinomycete bacteria is a novel orally active proteasome inhibitor shown to effectively induce apoptosis in tumor cells of MM20 and chronic lymphocytic leukemia (CLL), while displaying a lower toxic activity to bone marrow-derived stromal cells (BMSC) compared with bortezomib.21 An irreversible epoxy-ketone peptidyl inhibitor of all 3 proteasome proteolytic sites (PR-171) is currently in clinical evaluation (Phase 1) for advanced solid tumors and refractory hematologic malignancies.22
In the present study, we describe the in vitro and in vivo biologic characterization of CEP-18770, a novel reversible P2 threonine boronic acid proteasome inhibitor (B. Dorsey et al, manuscript submitted, 2007). We demonstrate that CEP-18770 potently promotes apoptosis in human MM cell lines and patient-derived cells; inhibits endothelial cell survival, vasculogenesis, and osteoclastogenesis in vitro; and displays a favorable cytotoxicity profile toward normal cells. In addition, we show that intravenous or oral administration of CEP-18770 is well tolerated and results in sustained pharmacodynamic and antitumor activity and survival benefits in xenograft and systemic models of human MM.
Methods
Enzyme and cellular biochemical activity profiles against the mammalian proteasome
The proteasome inhibitory activity of CEP-18770 and bortezomib were evaluated in an isolated human erythrocyte proteasome fluorimetric kinetic assay of chymotrypsin-like proteasome activity.23 Experiments were performed in triplicate.
Cell lines and primary cell cultures and treatments
Human multiple myeloma (MM) cell lines CMA-03, JJN3, H929, KMM1, KMS11, KMS18, KMS27, SKMM1, U266, RPMI-8226, human chronic myelogenous leukemia cell line K562, T-cell leukemic line MOLT-4, human anaplastic lymphoma ALK positive TS cells were used.24,25 MM myeloid and T-cell lines were grown at 37°C in 5% CO2 humidified air in RPMI 1640 medium (Cambrex, Verviers, Belgium) or in Iscove modified Dulbecco medium (IMDM; Cambrex), respectively, all supplemented with 10% fetal calf serum (FCS), without growth factors or alternatively with 10 ng/mL IL-6 (CMA-03).26 Cells were seeded at 5 × 105 cells/mL for the proteasome inhibitor treatments. Normal human microvascular endothelial cells from derma (HMEC) were immortalized by infection with a replication-defective adeno-5/SV40 virus as previously described.27 The tumor-derived endothelial cells (TEC) have previously been described.28 HMEC and TEC cell lines were established and maintained in culture in endothelial cell basal medium (EBM) supplemented with epidermal growth factor (10 ng/mL), hydrocortisone (1 mg/mL), bovine brain extract (Cambrex Bioscience, Baltimore, MD), and 10% FCS.
Peripheral blood mononuclear cells (PBMC) were obtained from healthy individuals after Ficoll-Hypaque density separation. Bone marrow mononuclear cells (BMNCs) were used to establish long-term BMSC cultures from multiple myeloma patients (7) and from noncancenerous individuals (5). Briefly, BMNC (2 × 106 cells) were suspended in IMDM containing 20% FCS and plated onto 12-well plates. Nonadherent cells were subsequently removed after 24 hours of culture. Adherent BMSC showing fibroblast morphology were grown until they reached confluence and then expanded. BMSC were seeded at 30% confluence in 6-well plates and treated with proteasome inhibitors for 6 days.
CD138+ plasma cells (PCs) were isolated from BM aspirates of 15 patients with MM (PC > 10%) and enriched by magnetic bead based positive selection (CD138 MicroBeads; Miltenyi Biotech, Bergisch Gladbach, Germany). Purified PC were seeded (106 cells/mL) on a 50% confluent BMSC monolayer in 6-well plates and maintained in IMDM supplemented with 20% FCS and 10 ng/mL IL-6. PCs were then treated with proteasome inhibitors for 72 hours.
Flow cytometric analyses of apoptosis
Apoptosis was measured by flow cytometry with the mitochondrion-permeable, voltage sensitive dye tetrametylrodamine methyl ester (TMRM; Molecular Probes, Eugene, OR) as described.24
Proliferation and viability assays
The effect of proteasome inhibitors on mononuclear cell viability was measured using a methylthiazolyldiphenyl-tetrazolium bromide (MTT) based assay as detailed using the following formula: percentage cell viability = (OD of the experimental samples / mean OD of the control) × 100.29
Western blotting
The following primary antibodies were used for Western blotting as previously described30 : rabbit anti–phospho IKBα (Ser32), anti–cleaved caspase 3, anti–cleaved caspase 7, and anti–cleaved caspase 9 from Cell Signaling Technology (Beverly, MA); rabbit anti-PARP (H-250), anti-IKBα from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit anti-XIAP from R&D Systems (Minneapolis, MN); mouse anti-ubiquitin from Zymed Laboratories (South San Francisco, CA) or Invitrogen (Carlsbad, CA); and mouse anti–α-tubulin (B-5-1-1) from Sigma-Aldrich (St Louis, MO).
Electrophoretic mobility shift assay
Aliquots of total extracts (12 μg protein/sample) in 0.1% Triton X-100 lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Triton X-100, 5 mM EDTA, 1 mM Na3VO4, and 1 mM PMSF and protease inhibitors) were incubated with [32P]-labeled κB DNA probe in binding buffer (10 mM Tris-HCl, pH 7.5, 1mM EDTA, 5% glycerol) for 30 minutes at room temperature. Quantitative evaluation of NF-κB–DNA probe complex formation was determined as previously described.30
Reverse transcription–polymerase chain reaction
Total RNA from BMSC cultured in 10-cm plates (passage 4 to 6) and after treatment with proteasome inhibitors (60 hours) were extracted using the RNAeasy Mini Kit (Qiagen, Hilden, Germany). Reverse transcription–polymerase chain reactions (RT-PCRs) were performed (1 μg RNA) according to the manufacturer's protocols (Invitrogen). The oligonucleotide primer pairs and PCR conditions for RT-PCR are available upon request.
Culture assay for CFU-GM and BFU-E
BMMC derived from 16 patients with MM (PC < 10%) were seeded in IMDM supplemented with 10% FCS at 5 × 105 cells/mL and treated with proteasome inhibitors for 48 hours. Cells were then washed and cultured for CFU-GM and BFU-E assay. Briefly, 5 × 104 proteasome inhibitor-treated or control BMMC were cultured in 35-mm Petri dishes containing complete methylcellulose medium (Methocult GF H4434 with recombinant cytokines; Stem Cell Technologies; with: IMDM, FCS, methylcellulose, 2-mercaptoethanol, l-glutammine, IL-3, GM-CSF, “stem cell factor,” erythropoietin). Colony growth from early (day 14) CFU-GM and BFU-E was scored after 14 days of incubation at 37°C in a 5% CO2 atmosphere. The differences in colony growth from control and proteasome inhibitors-treated cells were analyzed by the Student t test.
Endothelial cell proliferation, apoptosis, and tubulogenesis assays
HMEC and TEC cells were seeded into 24-well plates at a density of 10 000 cells/well in DMEM supplemented with 5% FCS. After incubation with proteasome inhibitors (48 hours), cells were washed, air dried, and stained with crystal violet as described.28 Cell number was determined, with duplicate samples, on the basis of a standard curve obtained with known cell numbers. All experiments were performed in triplicate. In vitro formation of capillary-like structures was studied on cells (4 × 104 cells/well in 24-well plates) seeded onto Matrigel-coated wells in DMEM containing 0.25% BSA. HMEC and TEC cells (5 × 103 per well), suspended in 200 μL DMEM with 5% FCS (positive control), serum-free medium (negative control), were layered onto the Matrigel surface in the presence or absence of proteasome inhibitors. Cells were observed with a Nikon inverted microscope and experimental results were then recorded after a 6-hour incubation at 37°C. Data were analyzed, as the mean (± 1 SD) of total length of capillary-like structures, by the Micro-Image system (Casti Imaging, Mira, Italy) and expressed as mm/field by the computer analysis system in 5 different files at 100× magnification in duplicated wells of 4 different experiments.
Osteoclast formation
PBMC (2 × 106 cells/well) were cultured for 10 days in the presence of 25 ng/mL recombinant human M-CSF (R&D Systems, Abingdon, United Kingdom). Osteoclasts were then activated by 30 ng/mL RANKL (Alexix, San Diego, CA), and simultaneously treated with proteasome inhibitors for 5 days, to reach the maximal inhibition and to avoid possible irreversible effects of commitment osteoclastogenesis induced by RANKL signaling. Culture media were supplemented with fresh media every 3 to 4 days. At the end of the culture period, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP Kit; Sigma Diagnostics, St Louis, MO). Mature osteoclasts were identified as TRAP positive multinucleated cells, containing 3 or more nuclei and quantified.31
Animals
Female athymic nu/nu nude mice, severe combined immunodeficiency (SCID) mice, and nonobese diabetic (NOD)/SCID mice (6-8 weeks old; Charles River, Wilmington, MA) were maintained on standard sterilized microisolator units (Teklad Labchow, Madison, WI) under humidity and temperature controlled conditions. Experiments were approved by the Institutional Animal Care and Use Committee of Cephalon.
Subcutaneous tumor xenograft models
RPMI 8226 cell suspensions (6 × 106 cells) were injected subcutaneously into the flank of SCID mice. Treatment started after establishing a palpable subcutaneous tumor (100-140 mm3) usually 40 days after implantation after randomization. CEP-18770 was administered to tumor-bearing animals intravenously, twice a week for 4 weeks (2q7d×8 injections) at doses of 1.0 to 6.0 mg/kg, and orally in a solution of 3% DMSO, 10% Solutol, and 87% sterile NaCl 0.9% as multiple administrations, twice-a-week injections for 4 weeks at doses of 7.8, 10, 13 mg/kg in a volume of 20 mL/kg body weight of mouse. Bortezomib was given intravenously in sterile NaCl 0.9% at doses of 0.8, 1, 1.2 and 1.6 mg/kg, twice-a-week injections for 4 weeks in a volume of 10 mL/kg body weight of mouse.
Systemic human multiple myeloma model
ARP-1 human MM cells (gift from Joshua Epstein, University of Arkansas, Little Rock, AR) were intravenously injected (5 × 106) into NOD/SCID mice. Approximately 6 weeks after injections, mice were bled to detect positive human IgA levels as measured by enzyme-linked immunosorbent assay (ELISA; Bethyl Laboratories, Montgomery, TX), determining human myelomatous tumor burden. Randomization of animals into treatment groups was as follows: CEP-18770, 3.0 mg/kg intravenously, once weekly for 8 doses (1q7d×8 injections); CEP-18770, 3.0 mg/kg, intravenously, twice weekly for 8 doses (2q7d × 8 injections); CEP-18770, 4.0 mg/kg, intravenously, once weekly for 8 doses; CEP-18770, 4.0 mg/kg, intravenously, twice weekly for 8 doses; bortezomib, 1.2 mg/kg, intravenously, once weekly for 8 doses; bortezomib, 1.2 mg/kg, intravenously, twice weekly for 8 doses; high-dose dexamethasone, 1.5 mg/kg, intraperitoneally, once daily; melphalan, 10 mg/kg, intraperitoneally, every 3 weeks, and thalidomide, 100 mg/kg, intraperitoneally, once daily. The doses of CEP-18770 and bortezomib were chosen based on maximally tolerated dose (MTD) and 0.75 MTD with chronic administration in mice. The treatment efficacy of each protocol were determined by log-rank statistical analyses and plotted by the Kaplan-Meier method as required for datasets using SAS 8.2 (SAS Institute, Cary, NC). Additional statistical analyses were performed using Mann-Whitney rank sum test and ANOVA.
Results
Enzyme and cellular biochemical activity profiles against the ubiquitin-proteasome complex
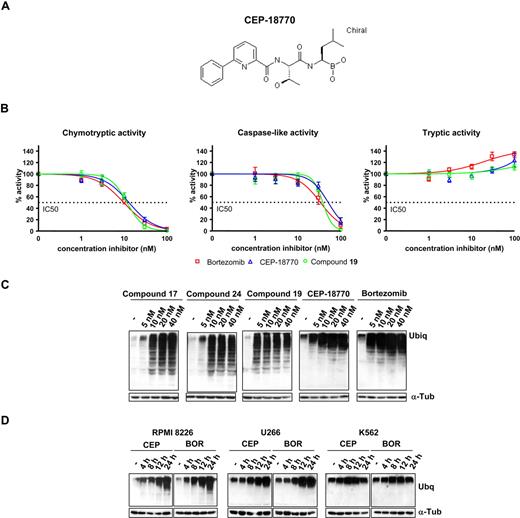
The proteasome inhibitory activity of CEP-18770 was first evaluated in comparison with bortezomib in the isolated human erythrocytes. CEP-18770 and bortezomib showed comparable potency against chymotrypsin-like proteasome activity, cellular inhibitory activity (IC50) values of 3.8 (± 1.0) nM and 3.8 (± 0.4) nM, respectively, but demonstrated marginal inhibition of the tryptic and peptidyl glutamyl activities of the proteosome (Figure 1). Analyses of the IC50 values of proteasome chymotryptic (β5) and caspase-like (β1) catalytic activities in MM and HeLa carcinoma cell lysates for CEP-18770 and bortezomib are shown in Figure 1B. The IC50 values of CEP-18770 were similar to those of bortezomib, with the chymotryptic and caspase-like activities being inhibited at low-nanomolar concentrations. CEP-18770 and several structurally related inhibitors not advancing into clinical development were also tested for their ability to inhibit the ubiquitin-proteasome pathway in several MM and in the chronic myelogenous leukemia cell line, K562 (and data not shown). Cells were treated with increasing concentrations (5, 10, 20, 40 nM) of the selected proteasome inhibitors, and the accumulation of ubiquitinated proteins measured by Western blotting. In this experimental setting, CEP-18770 induced an accumulation of ubiquitinated proteins over 4 to 8 hours with a profile similar to that observed after bortezomib treatment (Figure 1C,D).
Structure of CEP-18 770 and analyses of CEP boronic acid proteasome inhibitors in human multiple myeloma (MM) cells. (A) Structure of CEP-18770; (B) Cellular proteasome inhibitory profile in cell lysates obtained from RPMI-8226 MM incubated with increasing concentrations of bortezomib, CEP-18770, and a related analog, compound 19 (Dorsey et al, manuscript submitted). Inhibition was measured using fluorogenic substrates for the different catalytic activities of the proteasome. Results were plotted as percentage inhibition compared with nontreated lysates. Experiments were performed in triplicate. Error bars represent SD. (C) Ubiquitination assay: RPMI-8226 cells were treated for 24 hours with compounds 17, 24, 19 described previously (Dorsey et al, 2007) as well as CEP-18770 and bortezomib at the indicated concentrations; the accumulation of ubiquitinated proteins induced by proteasome inhibitors was assayed by Western blotting (WB) with antiubiquitin antibody. α-tubulin protein expression was included for protein loading normalization. (D) RPMI-8226, U266, and K562 cells were treated for different times with 20 nM CEP-18770 and 10 nM bortezomib (BOR); accumulation of ubiquitinated proteins was assayed by WB as described.
Structure of CEP-18 770 and analyses of CEP boronic acid proteasome inhibitors in human multiple myeloma (MM) cells. (A) Structure of CEP-18770; (B) Cellular proteasome inhibitory profile in cell lysates obtained from RPMI-8226 MM incubated with increasing concentrations of bortezomib, CEP-18770, and a related analog, compound 19 (Dorsey et al, manuscript submitted). Inhibition was measured using fluorogenic substrates for the different catalytic activities of the proteasome. Results were plotted as percentage inhibition compared with nontreated lysates. Experiments were performed in triplicate. Error bars represent SD. (C) Ubiquitination assay: RPMI-8226 cells were treated for 24 hours with compounds 17, 24, 19 described previously (Dorsey et al, 2007) as well as CEP-18770 and bortezomib at the indicated concentrations; the accumulation of ubiquitinated proteins induced by proteasome inhibitors was assayed by Western blotting (WB) with antiubiquitin antibody. α-tubulin protein expression was included for protein loading normalization. (D) RPMI-8226, U266, and K562 cells were treated for different times with 20 nM CEP-18770 and 10 nM bortezomib (BOR); accumulation of ubiquitinated proteins was assayed by WB as described.
CEP-18770 targets NF-κB activity
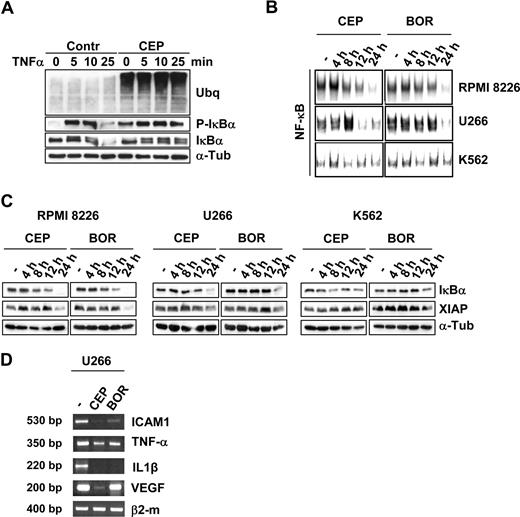
The most compelling rationale for the therapeutic use of proteasome inhibitors in oncology relies on their ability to inhibit the nuclear factor-κB (NF-κB) transcriptional activity resulting in the reduced expression of several target genes.11,–13 Because proteasome inhibitors modulate the activation of NF-κB primarily by blocking IκBα degradation,9 the ability of CEP-18770 to inhibit the TNFα-stimulated activation of NF-κB was examined. RPMI-8226 cells were pretreated with 20 nM CEP-18770 for 8 hours and subsequently stimulated by TNFα (10 ng/mL). IκBα serine 32 to 36 phosphorylation was rapidly induced (5 minutes) by TNFα, however, IκBα degradation was completely blocked by pretreatment with CEP-18770 (Figure 2A). The effect of CEP-18770 or bortezomib treatment on NF-κB DNA-binding activity revealed that both compounds significantly inhibited high levels of NF-κB activity in both RPMI-8226 and U266 cells. In contrast, low basal activity of NF-κB was not appreciably modified in K562 cells (Figure 2B). The time- and concentration-dependent inhibition of NF-kB DNA-binding activity in MM cell lines by CEP-18770 resulted in a reduction of several NF-κB-modulated genes mediating the growth and survival of tumor cells including IkBα itself, the X-chromosome-linked inhibitor-of-apoptosis protein (XIAP), the pro-inflammatory cytokines TNF-α and interleukin-1β (IL-1β), the intracellular adhesion molecule (ICAM1), and the pro-angiogeneic factor vascular endothelial growth factor (VEGF; Figure 2C,D). Recent studies indicate that the expression of these NF-κB–mediated genes and their modulation by bortezomib are associated with more favorable clinical responsiveness to this agent,32 highlighting their potential prognostic value in response to CEP-18770 exposure.
CEP-18 770 modulates the NF-κB signaling pathway in human MM cell lines. (A) RPMI-8226 cells were pretreated with control solvent (dimethyl sulfoxide; DMSO) or CEP-18770 (20 nM) for 6 hours before stimulation with TNF-α (10 ng/mL) for the indicated times. Whole cell extracts were immunoblotted with anti-IκBα and phosphorylated IκBα (P-IκBα) to assess IKK-mediated NF-κB activation. α-tubulin immunoblotting was included for protein loading normalization. (B) Whole-cell extracts were analyzed for NF-κB activation by electrophoretic mobility shift assay (EMSA). A section of the fluorogram is shown. (C) RPMI-8226, U266, and K562 cells were treated with CEP-18770 (20 nM) or BOR (10 nM) and immunoblotted with the specified antibodies to detect levels of total IκBα, P-IκBα and XIAP proteins. (D) U266 cells were treated with control diluent, CEP-18770 (20 nM), or BOR (10 nM) for 12 hours. Levels of ICAM1, TNF-α, IL1β, VEGF, and β2-microglobulin mRNA were analyzed by semiquantitative RT-PCR.
CEP-18 770 modulates the NF-κB signaling pathway in human MM cell lines. (A) RPMI-8226 cells were pretreated with control solvent (dimethyl sulfoxide; DMSO) or CEP-18770 (20 nM) for 6 hours before stimulation with TNF-α (10 ng/mL) for the indicated times. Whole cell extracts were immunoblotted with anti-IκBα and phosphorylated IκBα (P-IκBα) to assess IKK-mediated NF-κB activation. α-tubulin immunoblotting was included for protein loading normalization. (B) Whole-cell extracts were analyzed for NF-κB activation by electrophoretic mobility shift assay (EMSA). A section of the fluorogram is shown. (C) RPMI-8226, U266, and K562 cells were treated with CEP-18770 (20 nM) or BOR (10 nM) and immunoblotted with the specified antibodies to detect levels of total IκBα, P-IκBα and XIAP proteins. (D) U266 cells were treated with control diluent, CEP-18770 (20 nM), or BOR (10 nM) for 12 hours. Levels of ICAM1, TNF-α, IL1β, VEGF, and β2-microglobulin mRNA were analyzed by semiquantitative RT-PCR.
CEP-18770 induces apoptosis of human MM cell lines and primary human MM explant cultures
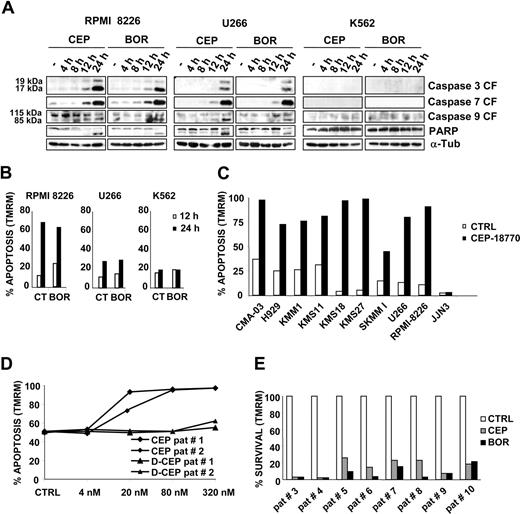
The pharmacologic evaluation of CEP-18 770 in vitro demonstrated that CEP-18 770 treatment resulted in a progressive appearance of cleaved caspases-3, -7, and -9 between 12 and 24 hours' exposure in the human MM cell lines, RPMI-8226, and U266, with a kinetic profile comparable with that of bortezomib. Activation of caspases was further confirmed by the cleavage of the poly(ADP-ribose) polymerase (PARP) protein (Figure 3A) and induction of apoptosis was further supported by TMRM staining. No activation of caspases or apoptosis was however observed in the K562 cell line after proteasome inhibitor treatment (Figure 3B). To further extend these findings, 8 additional MM cell lines were treated with CEP-18770 or control vehicle. Overall, 9 of 10 MM cell lines were sensitive to the apoptogenic action of CEP-18770 (Figure 3C), with the only exception of JJN3. The antiproliferative activity of CEP-18770 and bortezomib in vitro was then evaluated in a panel of human hematologic and solid tumor cell lines. CEP-18770 had a 2- to 11-fold lower cytotoxic potency compared with bortezomib against solid tumor cell lines, comparable potency against 2 hematologic tumor cell lines, and a similar spectrum of antiproliferative activity with IC50 values for both compounds of less than 35 nM (Table 1). To determine whether CEP-18770 similarly affected the viability of primary neoplastic PCs, the concentration-related proapoptotic activity of both inhibitors were evaluated against CD138+ MM cells obtained from the bone marrow aspirates of 10 MM patients, who had relapsed after multiple therapeutic protocols. These studies demonstrated comparable concentration-dependent proapoptotic effects of CEP-18770 and bortezomib ex vivo after 48 hours exposure as measured by mitochondrial depolarization assays, with complete MM cell death observed at 20 nM for both CEP-18770 and bortezomib (Figure 3D). Similar findings were observed for CEP-18770 with primary MM biopsies cultured ex vivo from 3 bortezomib-resistant MM patients (Figure 3E). These data indicate that the proapoptotic activity of CEP-18770 against MM is not limited solely to tumor-derived MM cell lines, but extends to primary MM explants from relapsed or refractory patients including those previously treated with bortezomib.
CEP-18770 induces apoptosis in human MM cell lines and in purified primary human MM explant cultures. (A) RPMI-8226, U266, and K562 cells were treated with CEP-18770 (20 nM) or BOR (10 nM) for the indicated times. Levels of cleaved fragments of caspase 3, caspase 7, and caspase 9 (CF) and of full-length (115 kDa) and cleaved fragment of PARP (85 kDa) were determined by immunoblotting on whole-cell lysates with the indicated antibodies; α-tubulin protein expression was included as loading control. (B) Apoptosis was measured by flow cytometry with the mitochondrion-permeable dye TMRM in RPMI-8226, U266, and K562 cells after 12 and 24 hours of CEP-18770 (20 nM) or BOR (10 nM) treatment. Histograms are representative of the percentage of apoptotic cells in one of 3 experiments (C) Human MM cell lines were treated with control diluent or CEP-18770 (20 nM) for 24 hours, percentages of apoptosis were measured after TMRM staining by flow cytometry. Histograms are representative of the percentage of apoptotic cells in one of 3 experiments (D) CD138+ PC, obtained from 2 patients with MM, were separated by positive selection and PC were seeded on a BMSC monolayer and treated with CEP-18770 or inactive deboronated CEP-18770 for 72 hours at different concentrations. The percentage of purified MM cells undergoing apoptosis as measured by staining with TMRM and flow cytometry is indicated. (E) CD138+ PC obtained from 8 patients with MM (PC > 10%) as described above, were treated with CEP-18870 (20 nM) or bortezomib (BOR) (10 nM) for 72 hours. Apoptosis was measured by flow cytometry after TMRM staining. Histograms represent the percentage of viable cells normalized versus control samples.
CEP-18770 induces apoptosis in human MM cell lines and in purified primary human MM explant cultures. (A) RPMI-8226, U266, and K562 cells were treated with CEP-18770 (20 nM) or BOR (10 nM) for the indicated times. Levels of cleaved fragments of caspase 3, caspase 7, and caspase 9 (CF) and of full-length (115 kDa) and cleaved fragment of PARP (85 kDa) were determined by immunoblotting on whole-cell lysates with the indicated antibodies; α-tubulin protein expression was included as loading control. (B) Apoptosis was measured by flow cytometry with the mitochondrion-permeable dye TMRM in RPMI-8226, U266, and K562 cells after 12 and 24 hours of CEP-18770 (20 nM) or BOR (10 nM) treatment. Histograms are representative of the percentage of apoptotic cells in one of 3 experiments (C) Human MM cell lines were treated with control diluent or CEP-18770 (20 nM) for 24 hours, percentages of apoptosis were measured after TMRM staining by flow cytometry. Histograms are representative of the percentage of apoptotic cells in one of 3 experiments (D) CD138+ PC, obtained from 2 patients with MM, were separated by positive selection and PC were seeded on a BMSC monolayer and treated with CEP-18770 or inactive deboronated CEP-18770 for 72 hours at different concentrations. The percentage of purified MM cells undergoing apoptosis as measured by staining with TMRM and flow cytometry is indicated. (E) CD138+ PC obtained from 8 patients with MM (PC > 10%) as described above, were treated with CEP-18870 (20 nM) or bortezomib (BOR) (10 nM) for 72 hours. Apoptosis was measured by flow cytometry after TMRM staining. Histograms represent the percentage of viable cells normalized versus control samples.
Cytotoxicity profile of CEP-18770 and bortezomib in representative human solid and hematological tumor cell lines
| Compound . | A2780 ovarian CA . | PC3 prostate CA . | H460 SC lung CA . | LoVo colon CA . | RPMI8226 multiple myeloma . | HS-Sultan anaplastic NHL . |
|---|---|---|---|---|---|---|
| CEP-18770 | 13.7 ± 2.3 | 22.2 ± 2.5 | 34.2 ± 5 | 11.3 ± 1.7 | 5.6 ± 3.2 | 8.2 ± 3.3 |
| Bortezomib | 1.7 ± 0.4 | 4.8 ± 1.2 | 4.8 ± 0.5 | 0.97 ± 0.2 | 2.7 ± 0.4 | 4.1 ± 0.4 |
| Compound . | A2780 ovarian CA . | PC3 prostate CA . | H460 SC lung CA . | LoVo colon CA . | RPMI8226 multiple myeloma . | HS-Sultan anaplastic NHL . |
|---|---|---|---|---|---|---|
| CEP-18770 | 13.7 ± 2.3 | 22.2 ± 2.5 | 34.2 ± 5 | 11.3 ± 1.7 | 5.6 ± 3.2 | 8.2 ± 3.3 |
| Bortezomib | 1.7 ± 0.4 | 4.8 ± 1.2 | 4.8 ± 0.5 | 0.97 ± 0.2 | 2.7 ± 0.4 | 4.1 ± 0.4 |
Data are mean IC50 values (nM) plus or minus SD.
CA indicate cancer; and NHL, non-Hodgkin lymphoma.
Cytotoxicity profile of CEP-18770 in normal versus tumor human cells
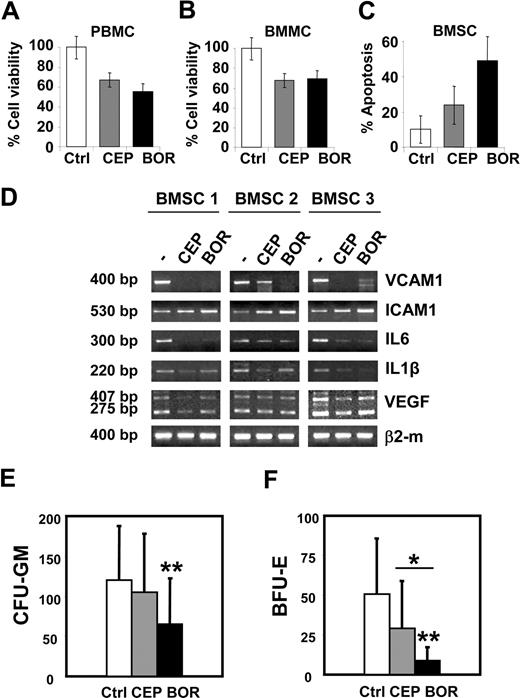
The potential tumor-selective cytotoxic activity of CEP-18770 against MM cells as compared with normal human cells was examined, studying the susceptibility to these compounds of normal BMMCs and PBMCs obtained from healthy volunteers or from MM ex vivo. CEP-18770 and bortezomib resulted in a significant and comparable decrease of cell viability after 48 hours' treatment in both type of mononuclear cells (Figure 4A,B).
Cytotoxicity profile of CEP-18870 against normal human cells. (A,B) BMMCs and PBMCs were treated with control diluent, CEP-18770 (20 nM) or BOR (10 nM) for 48 hours. PBMCs were derived from 5 nonmyelomatous donors; BMMCs from 2 nonmyelomatous donors and from 7 patients with MM; cell viability was measured by MTT assay. Cells were incubated in triplicate for each condition. Histograms represent means (± SD, error bars) of data derived from different patients, as indicated above in this paragraph. (C) BMMCs derived from 5 nonmyelomatous donors and from 7 patients with MM were used to establish long-term BMSC cultures. BMSCs were treated with control diluent, CEP-18770 (20 nM), or BOR (10 nM) for 6 days. Apoptosis was measured by flow cytometry after TMRM staining. Histograms represent the means (± SD, error bars). (D) CEP-18870 inhibits the expression of inflammatory cytokines and adhesion molecules in BMSCs. BMSC cultures, obtained as described above, were expanded for 4 to 6 passages and treated with control diluent, CEP-18870 (20 nM), or bortezomib (BOR) (10 nM) for 60 hours. Levels of VCAM1, ICAM1, IL6, IL1β, VEGF isoforms, and β2-microglobulin mRNA were analyzed by semiquantitative RT-PCR. Fragment length is indicated; 275 base pairs (bp) and 407 bp amplification fragments correspond to the known VEGF isoforms of 121 and of 165 amino acids, respectively. BMSCs were derived from 1 patient with MM (BMSC 1) and from 2 nonmyelomatous donors (BMSC 2 and 3). (E,F) Hematotoxicity of CEP-18770 and BOR on bone marrow progenitors. BMMC derived from 16 patients with MM (PC < 10%) were pretreated with control diluent, CEP-18870 (20 nM), or BOR (10 nM) for 48 hours. Cells were washed and cultured in cytokine-containing methylcellulose medium for granulocyte-macrophages (CFU-GM) or erythroid (BFU-E) colony formation. (E) Early CFU-GM and (F) BFU-E-derived colonies were scored after 14 days of incubation. Histograms represent the means (± SD; error bars). *P < .05; **P < .01 as analyzed by Student t test.
Cytotoxicity profile of CEP-18870 against normal human cells. (A,B) BMMCs and PBMCs were treated with control diluent, CEP-18770 (20 nM) or BOR (10 nM) for 48 hours. PBMCs were derived from 5 nonmyelomatous donors; BMMCs from 2 nonmyelomatous donors and from 7 patients with MM; cell viability was measured by MTT assay. Cells were incubated in triplicate for each condition. Histograms represent means (± SD, error bars) of data derived from different patients, as indicated above in this paragraph. (C) BMMCs derived from 5 nonmyelomatous donors and from 7 patients with MM were used to establish long-term BMSC cultures. BMSCs were treated with control diluent, CEP-18770 (20 nM), or BOR (10 nM) for 6 days. Apoptosis was measured by flow cytometry after TMRM staining. Histograms represent the means (± SD, error bars). (D) CEP-18870 inhibits the expression of inflammatory cytokines and adhesion molecules in BMSCs. BMSC cultures, obtained as described above, were expanded for 4 to 6 passages and treated with control diluent, CEP-18870 (20 nM), or bortezomib (BOR) (10 nM) for 60 hours. Levels of VCAM1, ICAM1, IL6, IL1β, VEGF isoforms, and β2-microglobulin mRNA were analyzed by semiquantitative RT-PCR. Fragment length is indicated; 275 base pairs (bp) and 407 bp amplification fragments correspond to the known VEGF isoforms of 121 and of 165 amino acids, respectively. BMSCs were derived from 1 patient with MM (BMSC 1) and from 2 nonmyelomatous donors (BMSC 2 and 3). (E,F) Hematotoxicity of CEP-18770 and BOR on bone marrow progenitors. BMMC derived from 16 patients with MM (PC < 10%) were pretreated with control diluent, CEP-18870 (20 nM), or BOR (10 nM) for 48 hours. Cells were washed and cultured in cytokine-containing methylcellulose medium for granulocyte-macrophages (CFU-GM) or erythroid (BFU-E) colony formation. (E) Early CFU-GM and (F) BFU-E-derived colonies were scored after 14 days of incubation. Histograms represent the means (± SD; error bars). *P < .05; **P < .01 as analyzed by Student t test.
Because the interaction of MM cells with the bone marrow microenvironment elicits complex signals sustaining the paracrine growth of tumor cells and protecting MM cells against drug-induced apoptosis,33,34 the effects of CEP-18770 on the viability of BMSCs was also examined. Treatment of BMSCs (from 5 healthy donors and 7 MM patients) with CEP-18770 (20 nM) was significantly less cyototoxic (P < .05) compared with bortezomib (Figure 4C). Importantly, CEP-18770 exposure decreased the expression of interleukins IL-6, IL-1β, vascular cell adhesion molecule (VCAM1), and VEGF comparably in these cells. ICAM levels remained largely unchanged (Figure 4D). To compare further the potential hematotoxicity of CEP-18770 and bortezomib on human bone marrow progenitors, proteasome treated (48 hours) BMMC derived from 16 MM patients were plated on methylcellulose in the presence of myelopoietic or erythtropoietic cytokines. These conditions enabled the assessment of the effects of proteasome inhibitor treatment on bone marrow progenitor cells of both myeloid and erythroid lineages, respectively, as assayed by granulocyte-macrophage (CFU-GM) and erythroid (BFU-E) colony formation. Suppression of the clonogenic potential of BMMC was observed after both treatments. However, the percentage inhibition of CFU-GM and CFU-E colony formation was significant only after bortezomib treatment (P < .008 and P < .001, respectively), versus control cultures, but not upon exposure to CEP-18770. Bortezomib was also 3 times more potent in inhibiting the growth of BFU-E colonies compared with CEP-18770 treatment (P < .01) (Figure 4F). Finally, CEP-18770 exhibited reduced cytotoxicity (P < .05) compared with bortezomib against normal human intestinal epithelium cells (CCD-18Co; data not shown).
Collectively these results indicate that CEP-18770 is less cytotoxic compared with bortezomib against normal human epithelial cells, bone marrow progenitors, and BMSCs at concentrations effective in inducing apoptosis in a panel of human MM cell lines and primary human MM explants.
CEP-18770 inhibits endothelial cell survival, proliferation, and morphogenesis
Bone marrow–derived angiogenesis is a hallmark of MM progression.35,36 Inhibition of NF-κB-mediated regulation of angiogenic cytokines and adhesion molecules (ICAM-1, VCAM-1, and VEGF) and modulation of tumor cell–endothelial cell interactions, are, in part, responsible for the antiangiogenic activity of bortezomib.19,35 The antiangiogenic effects of CEP-18770 were evaluated in normal human microvascular endothelial cells from derma (HMEC) and from renal carcinoma (TEC)37 treated with CEP-18770 and bortezomib. Apoptosis and cell growth were used as readouts. As shown in Figure 5A,B, 20 nM CEP-18770 inhibited proliferation and triggered levels of apoptosis comparable with 10 nM bortezomib, in both HMEC and TEC, as assessed by mitochondrial depolarization and crystal violet staining assays at 24 and 48 hours, respectively. Antiangiogenic activity was evaluated further by functional analyses of human endothelial cell capillary-tubule formation on a 2-dimensional Matrigel synthetic basement membrane matrix. Both compounds demonstrated comparable concentration-related inhibition of capillary-tubule formation of both HMEC and TEC (after a 6-hour exposure), with effective concentration for 50% response (EC50) values of approximately 10 nM (Figure 5C,D).
CEP-18770 inhibits EC survival, proliferation and tubular morphogenesis and M-CSF-RANKL–mediated osteoclastogenesis. (A-C) HMEC and TEC cells were treated with CEP-18770 and bortezomib (BOR) at the indicated concentrations. (A) Apoptosis was measured at 24 hours by flow cytometry after staining with TMRM. (B) Proliferation was assayed at 48 hours by crystal violet staining. Absorbance was read at 595 nm with an ELISA reader. Error bars represent SD. (C) Representative micrographs of capillary-like structure formation on Matrigel-coated wells in the presence or absence of proteasome inhibitors. Cells were observed with a Nikon inverted microscope and experimental results recorded after 6 hours from seeding. (D) Data show the mean (± 1 SD) of total length of capillary-like structures analyzed by the Micro-Image system (Casti Imaging) and expressed as mm/field by the computer analysis system in 5 different files at 100× magnification in duplicated wells of 4 different experiments. (E,F) To obtain fully differentiated osteoclasts (OC), PBMC were cultured for 10 days in the presence of recombinant human M-CSF. OC were activated by RANKL and simultaneously treated with CEP-18770 (CEP, 20 nM) or BOR (10 nM) for 5 days. Cells were incubated in duplicate for each condition. Mature OC were identified as TRAP+ multinucleated cells containing 3 or more nuclei. (E) Histograms represent means (± SD; error bars) of absolute numbers of OC from 6 nonmyelomatous donors. **P < .01 as analyzed by Student t test. (F) Three representative photographs of OC treated with control diluent (Control), CEP-18770 20 nM (CEP), and bortezomib (BOR) 10 nM. Arrows indicate TRAP+ cells (final magnification 400×).
CEP-18770 inhibits EC survival, proliferation and tubular morphogenesis and M-CSF-RANKL–mediated osteoclastogenesis. (A-C) HMEC and TEC cells were treated with CEP-18770 and bortezomib (BOR) at the indicated concentrations. (A) Apoptosis was measured at 24 hours by flow cytometry after staining with TMRM. (B) Proliferation was assayed at 48 hours by crystal violet staining. Absorbance was read at 595 nm with an ELISA reader. Error bars represent SD. (C) Representative micrographs of capillary-like structure formation on Matrigel-coated wells in the presence or absence of proteasome inhibitors. Cells were observed with a Nikon inverted microscope and experimental results recorded after 6 hours from seeding. (D) Data show the mean (± 1 SD) of total length of capillary-like structures analyzed by the Micro-Image system (Casti Imaging) and expressed as mm/field by the computer analysis system in 5 different files at 100× magnification in duplicated wells of 4 different experiments. (E,F) To obtain fully differentiated osteoclasts (OC), PBMC were cultured for 10 days in the presence of recombinant human M-CSF. OC were activated by RANKL and simultaneously treated with CEP-18770 (CEP, 20 nM) or BOR (10 nM) for 5 days. Cells were incubated in duplicate for each condition. Mature OC were identified as TRAP+ multinucleated cells containing 3 or more nuclei. (E) Histograms represent means (± SD; error bars) of absolute numbers of OC from 6 nonmyelomatous donors. **P < .01 as analyzed by Student t test. (F) Three representative photographs of OC treated with control diluent (Control), CEP-18770 20 nM (CEP), and bortezomib (BOR) 10 nM. Arrows indicate TRAP+ cells (final magnification 400×).
CEP-18770 inhibits M-CSF-RANKL–mediated osteoclastogenesis
A unique feature of MM patients is a sustained bone destruction; a major source of morbidity (severe bone pain and pathologic fractures) due primarily to an increased osteoclast activity.31,38 Because NF-κB plays a central role in osteoclast formation, blocking NF-κB activity is a potential strategy for inhibition of osteoclast formation. To investigate whether CEP-18770 and bortezomib could similarly affect the osteoclastogenic potential, PBMCs from normal donors were induced to differentiate into osteoclasts for 10 days in the presence of recombinant human M-CSF (25 ng/mL), followed by RANKL (30 ng/mL) activation, for additional 5 days. Numerous, large tartrate-resistant acid phosphatase (TRAP)–positive osteoclasts were reproducibly generated (osteoclasts averaged 195 ± 127 per well). Consistent with earlier studies,39,40 both CEP-18770 or bortezomib added simultaneously with RANKL dramatically suppressed osteoclastogenesis (Figure 5E,F). Importantly, CEP-18770 resulted in a more pronounced inhibition of osteoclast formation (13 ± 8; P < .003 vs bortezomib) compared with that of bortezomib (83 ± 100; P < .06 vs controls). These findings indicate that CEP-18770 is a potent inhibitor of RANKL-mediated osteoclastogenesis in vitro.
Efficacy profile of CEP-18770 in human MM models in mice
The in vivo antitumor efficacy of CEP-18770 compared with bortezomib was examined first in a series of studies using the human MM RPMI 8226 subcutaneous xenograft model in SCID mice after repeated intravenous or oral administration. CEP-18770 exhibited sustained and dose-related (from 1.5 to 4 mg/kg intravenously, 2q7d×8 injections) relative tumor weight inhibition (RTWI = 100% at all tested doses, P < .001 vs untreated groups). Bortezomib at doses of 0.8, 1.0 and 1.2 mg/kg (2q7d×8 injections) was similarly efficacious in inhibiting MM growth (RTWI from 93% to 98%, P < .001 vs untreated group) with a comparable tumor growth delay profile. CEP-18770 administration, however, resulted in dose-related induction of complete tumor regressions (CR from 78% to 100%), as compared with bortezomib treatment, which resulted in a 50% incidence of CR at its MTD of 1.2 mg/kg intravenously (Figure 6A, and data not shown). In contrast to bortezomib, CEP-18770 exhibited dose-related increases in the incidence of tumor-free mice by the completion of these studies (120 days after tumor transplantation); within the 3- and 4-mg/kg intravenous treatment groups, 89% and 80%, respectively, of CEP-18770–treated mice were tumor-free and treatment was well tolerated. Oral administration of CEP-18770 resulted in a significant reduction of tumor weight (P < .001) and notable dose-related incidence of complete tumor regression (75% incidence of CR and 25% tumor-free mice at 10 mg/kg orally; 100% incidence of CR and tumor-free mice at 13 mg/kg orally) with minimal changes in animal body weight over the course of 120 day studies (Figure 6B).
Antitumor efficacy and pharmacodynamic profile of proteasome inhibition of CEP-18770 and bortezomib in MM tumor xenografts. RPMI 8226 human MM xenografts were implanted subcutaneously in SCID mice. Treatment started after establishing a palpable subcutaneous tumor (100-140 mm3). Representative intravenous and oral efficacy data are shown. (A) CEP-18770 and bortezomib were administered intravenously, twice a week for 4 weeks (2q7d×8 injections) at the doses indicated as a solution of 10% Solutol HS15/87% buffered saline/3% DMSO in a volume of 10 mL/kg body weight mouse. (B) CEP-18770 was administered orally (p.o.) in a solution of 3% DMSO, 10% Solutol, and 87% sterile NaCl 0.9% twice a week for 4 weeks at the indicated doses in a volume of 20 mL/kg body weight mouse. Tumor parameters were measured and analyzed as detailed in “Subcutaneous tumor xenograft models.” (C,D) Pharmacodynamic profile of proteasome inhibition in RPMI 8226 xenografts and normal peripheral tissues of mice at the maximum tolerated doses (MTD) of CEP-18770 and bortezomib administered intravenously in SCID mice. Time-course proteasome inhibition after a single intravenous administration was measured by an ex vivo fluorimetric kinetic assay of chymotrypsin-like activity. Proteasome percentage inhibition (PI %) in sample tissue lysates from treated versus vehicle-treated control mice was calculated as: (Slope of control tissues − slope of treated tissues) / (Slope of control tissues × 100) after normalization for protein content of tissue and hemoglobin content of whole blood. Results shown are means (± standard deviation) of 3 independent experiments (3 mice per time point).
Antitumor efficacy and pharmacodynamic profile of proteasome inhibition of CEP-18770 and bortezomib in MM tumor xenografts. RPMI 8226 human MM xenografts were implanted subcutaneously in SCID mice. Treatment started after establishing a palpable subcutaneous tumor (100-140 mm3). Representative intravenous and oral efficacy data are shown. (A) CEP-18770 and bortezomib were administered intravenously, twice a week for 4 weeks (2q7d×8 injections) at the doses indicated as a solution of 10% Solutol HS15/87% buffered saline/3% DMSO in a volume of 10 mL/kg body weight mouse. (B) CEP-18770 was administered orally (p.o.) in a solution of 3% DMSO, 10% Solutol, and 87% sterile NaCl 0.9% twice a week for 4 weeks at the indicated doses in a volume of 20 mL/kg body weight mouse. Tumor parameters were measured and analyzed as detailed in “Subcutaneous tumor xenograft models.” (C,D) Pharmacodynamic profile of proteasome inhibition in RPMI 8226 xenografts and normal peripheral tissues of mice at the maximum tolerated doses (MTD) of CEP-18770 and bortezomib administered intravenously in SCID mice. Time-course proteasome inhibition after a single intravenous administration was measured by an ex vivo fluorimetric kinetic assay of chymotrypsin-like activity. Proteasome percentage inhibition (PI %) in sample tissue lysates from treated versus vehicle-treated control mice was calculated as: (Slope of control tissues − slope of treated tissues) / (Slope of control tissues × 100) after normalization for protein content of tissue and hemoglobin content of whole blood. Results shown are means (± standard deviation) of 3 independent experiments (3 mice per time point).
To compare CEP-18770 and bortezomib inhibition of proteasome activity in tumors and peripheral normal host tissues, their ex vivo proteasome pharmacodynamic (PD) inhibition profiles were characterized in RPMI 8266 MM tumor-bearing SCID mice. Relative to bortezomib, equiactive doses of CEP-18770 demonstrated a greater and more sustained dose-related inhibition of tumor proteasome activity, corresponding temporally with maximum induction of caspase-3 and 7 activity (Figure 6C,D, and data not shown). The maximum apoptotic signal was 2.5 fold greater for CEP-18770 versus bortezomib. In contrast, proteasome inhibition profiles of CEP-18870 and bortezomib were comparable in the normal peripheral mouse tissues examined (liver, lungs, whole blood, and brain [no activity]), in both their magnitude and their duration. No proteasome inhibition was detected in brain tissue at any time point for either compound.
The antitumor efficacy profile of CEP-18770 relative to bortezomib was further evaluated in a ARP-1 systemic model of human MM. Female NOD/SCID mice were implanted intravenously with ARP-1 human MM cells and myelomatous mice (confirmed based upon plasma human IgA levels) were randomized and administered CEP-18770 or bortezomib (2q7d×8 injections) using dosing regimens comparable with those described in subcutaneous MM xenograft studies. Analyses of median survival data obtained with CEP-18770, bortezomib, and standard-of-care therapeutic agents relative to vehicle-treated mice are summarized in Table 2. These data indicate comparable and similarly robust median survival benefits for twice-weekly administration of CEP-18770 (at 3 and 4 mg/kg intravenously) and bortezomib (at 1.2 mg/kg intravenously), as well as high-dose dexamethasone administration. Melphalan treatment was associated with the greatest survival benefit relative to vehicle control mice (P < .001).
Effects of CEP-18770, bortezomib (BOR), high-dose dexamethasone (HD Dex), and melphalan (MLP) on survival in the systemic ARP-1 human multiple myeloma model
| Survival . | Vehicle group . | CEP-18770 3 mg/kg intravenously once weekly . | CEP-18770 3 mg/kg intravenously twice weekly . | CEP-18770 4 mg/kg intravenously once weekly . | CEP-18770 4 mg/kg intravenously twice weekly . | BOR 1.2 mg/kg intravenously once weekly . | BOR 1.2 mg/kg intravenously twice weekly . | HD Dex 1.5 mg/kg intraperitoneally once daily . | MLP 10 mg/kg intraperitoneally once daily . |
|---|---|---|---|---|---|---|---|---|---|
| Mean, d | 34.8 | 60.4 | 75.8 | 54.5 | 63.6 | 58.3 | 58.7 | 56.2 | 90.5 |
| Median, d | 34 | 51 | 61.5 | 50 | 65 | 47 | 59 | 57 | 100 |
| SEM of median survival, d | 5.5 | 10.5 | 13.7 | 10.4 | 11.3 | 11.2 | 6.7 | 5.7 | 9.3 |
| P values versus vehicle | — | .06 | .02 | .14 | .05 | .09 | .02 | .02 | <.001 |
| Survival . | Vehicle group . | CEP-18770 3 mg/kg intravenously once weekly . | CEP-18770 3 mg/kg intravenously twice weekly . | CEP-18770 4 mg/kg intravenously once weekly . | CEP-18770 4 mg/kg intravenously twice weekly . | BOR 1.2 mg/kg intravenously once weekly . | BOR 1.2 mg/kg intravenously twice weekly . | HD Dex 1.5 mg/kg intraperitoneally once daily . | MLP 10 mg/kg intraperitoneally once daily . |
|---|---|---|---|---|---|---|---|---|---|
| Mean, d | 34.8 | 60.4 | 75.8 | 54.5 | 63.6 | 58.3 | 58.7 | 56.2 | 90.5 |
| Median, d | 34 | 51 | 61.5 | 50 | 65 | 47 | 59 | 57 | 100 |
| SEM of median survival, d | 5.5 | 10.5 | 13.7 | 10.4 | 11.3 | 11.2 | 6.7 | 5.7 | 9.3 |
| P values versus vehicle | — | .06 | .02 | .14 | .05 | .09 | .02 | .02 | <.001 |
Survival data from Kaplan-Meier analyses of different dosing schedules of CEP-18770 and bortezomib relative to that achieved with first- line standard-of-care therapies used for the treatment of MM. Details and statistical analyses of data are described in “Systemic human multiple myeloma model.”
— indicates not applicable.
Discussion
The ubiquitin proteasome pathway represents a promising target for cancer therapy, because of the higher sensitivity of cancer cells to cytotoxic effects of proteasome inhibition compared with normal cells.6,19 To date, bortezomib is the only proteasome inhibitor currently approved for human use, despite relevant side effects, including peripheral neuropathy, orthostatic hypotension, pyrexia, gastrointestinal symptoms, thrombocytopenia, asthenia, and pain.14,15 Because targeting the ubiquitin-proteasome system is anticipated as a powerful strategy for the treatment of a large spectrum of human malignancies, the discovery of new antiproteasome drugs with a more favorable profile and enhanced tolerability is recommended.20,22,41
A primary rationale for the therapeutic use of proteasome inhibitors in oncology relies on their ability to inhibit NF-κB activity. The inhibition of NF-κB activity reduces the expression of several target genes regulating cell proliferation and survival of cancer cells.11,–13 Therefore, the modulation of NF-κB activity offers significant therapeutic results in numerous malignancies, including MM, lymphomas, and several solid tumors. Notably, NF-κB also mediates important cellular functions in normal stromal and lymphoid cells, key players regulating the growth, survival, and apoptosis of tumor cells, as well as in initiating specific antitumor immune responses in MM patients. This report describes the pharmacologic and activity profile of the novel P2 threonine boronic acid proteasome inhibitor, CEP-18770, a potent inhibitor of both constitutive and TNF-α triggered NF-κB activation. Of note in these studies was the observation that CEP-18770 and bortezomib exhibit a comparable cytotoxic profile against a panel of human MM cell lines in vitro and in explant cultures of purified CD138 positive cells derived from bone marrow aspirates of MM subjects.
Given that protein degradation and proteasome activity are required for normal cell survival and proliferation, it was of interest to compare the effects of CEP-18770 in normal human cells. CEP-18770 demonstrated a marked reduction in toxicity toward human bone marrow progenitors, bone marrow stromal cells, and normal human intestinal cells compared with bortezomib despite the ability of CEP-18770 to efficiently inhibit the secretion of BMSC-derived growth factors, cytokines, and adhesion molecules (IL-6, IL1-β, and VCAM1). The down-modulation of these molecules by CEP-18770 may affect the interaction of tumor cells with their microenvironment, impacting tumor growth, survival, and immune surveillance. Similarly, CEP-18770 abrogates VEGF production in both MM and BMSC cells, suggesting an inhibitory effect on both MM cell migration and vasculogenesis from endothelial progenitors. Inhibition of angiogenesis is also supported by a direct effect of CEP-18770 on endothelial cell proliferation, survival, and capillary tubular morphogenesis. Collectively, these data support the view that CEP-18770 exerts both direct and an indirect antiangiogenic activity on endothelial cells, defining a supplementary mechanism that may contribute to its anti-MM activity.
A hallmark in the clinical presentation of MM patients is lytic bone disease, a consequence of enhanced differentiation and activity of osteoclasts. Osteoclastogenesis can be driven directly by cell–cell contact with tumor cells, or by a paracrine stimulation induced by circulating factors such as IL-1, IL-6, PTHrP, MIP-1α, and RANKL, produced by tumor and/or surrounding stromal cells.42,43 Remarkably, CEP-18770 was significantly more effective than bortezomib in inhibiting RANKL-induced osteoclast differentiation in vitro. These findings could indicate a potential benefit of CEP-18770 in managing the osteolytic-related morbidity and mortality associated with the progression of MM.
The proteasome pharmacodynamic profile demonstrated that in MM tumor xenografts CEP-18770 achieved a superior and persistent dose-related inhibition of proteasome activity after intravenous administration, compared with normal peripheral murine tissues. Moreover, the tumor proteasome inhibition profile and induction of tumor apoptosis achieved by the intravenous MTD of CEP-18770 was comparable with that achieved after single-dose oral administration of CEP-18770 at 10 and 13 mg/kg (oral MTD in mice). These PD effects achieved with intravenous or oral administration of CEP-18770 were directly related to significant and sustained antitumor efficacy and median survival benefits in subcutaneous and systemic models of human MM, respectively, upon chronic administration.
In summary our data show that the novel, water-soluble and orally bioavailable proteasome inhibitor CEP-18770 exhibits (1) low-nanomolar inhibition of chymotrypsin-like proteasome activity in silico and in vitro; (2) suppression of NF-κB–mediated signaling pathways and transcriptional targets of NF-κB; (3) concentration-related induction of apoptotic cell death in human MM and tumor-derived cell lines and in primary purified CD138 positive MM cells in the absence of intrinsic DNA intercalating or topoisomerase inhibitory activities; (4) a favorable cytotoxicity profile toward normal human endothelial cells, bone marrow progenitors, and BMSCs relative to bortezomib; (5) inhibition of endothelial cell survival and morphogenesis in vitro; (6) potent inhibition of RANKL-induced osteoclastogenesis compared with bortezomib; and (7) significant and sustained tumor proteasome inhibition and antitumor efficacy and survival benefits in preclinical models of human MM. The in vitro and in vivo antitumor and anticlastogenic pharmacologic profile of CEP-18870 and its diminished cytotoxicity against a variety normal human cell lineages compared with tumor cells provide the rationale for further studies evaluating its preclinical and clinical efficacy in MM and other hematologic malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The discovery and development of CEP-18770 was supported by Cephalon, West Chester, PA, in collaboration with Cell Therapeutics Europe, Bresso, Italy. Additional support for the studies described was provided by Ministero dell'Università e Ricerca Scientifica (MIUR), Regione Piemonte, Compagnia di San Paolo, Torino (Progetto Oncologia), and Associazione Italiana per la Ricerca sul Cancro (AIRC). R.P. is supported by the MIUR program “Incentivazione alla mobilità di studiosi residenti all'estero.”
Authorship
Contribution: R.P., G.C., I.T., D.F., V.G., M. Coscia, S.P., M.M., G.P., C.A., N.P., M. Cassin, S.d.G., P.N., P.d.F., I.S., I.R., R.F., B.B., G.C., S.J.-B., K.H., H.Z., A.N., A.P., C.B., H.O., and A.B. designed, performed, and analyzed experimental data; and R.P., B.R., M.W., and G.I. oversaw the direction of all experimental studies and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests. Cephalon, Sede Secondaria Della Cell Therapeutics, and RBM have no commercial products that are discussed in the text of this manuscript. The compound CEP-18770 is in clinical development as an oncology agent but is not a commercial or marketed product now.
Correspondences: Giorgio Inghirami, Center for Experimental Research and Medical Studies (CeRMS), Department of Biomedical Sciences and Human Oncology, University of Torino, Via Santena 5, 10126 Torino, Italy; e-mail: giorgio.inghirami@unito.it.
References
Author notes
R.P. and B.R. contributed equally to this manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal