Abstract
Serglycin (SG), the hematopoietic cell secretory granule proteoglycan, is crucial for storage of specific secretory proteins in mast cells, neutrophils, and cytotoxic T lymphocytes. We addressed the role of SG in platelets using SG−/− mice. Wild-type (WT) but not SG−/− platelets contained chondroitin sulfate proteoglycans. Electron microscopy revealed normal α-granule structure in SG−/− platelets. However, SG−/− platelets and megakaryocytes contained unusual scroll-like membranous inclusions, and SG−/− megakaryocytes showed extensive emperipolesis of neutrophils. SG−/− platelets had reduced ability to aggregate in response to low concentrations of collagen or PAR4 thrombin receptor agonist AYPGKF, and reduced fibrinogen binding after AYPGKF, but aggregated normally to ADP. 3H-serotonin and ATP secretion were greatly reduced in SG−/− platelets. The α-granule proteins platelet factor 4, β-thromboglobulin, and platelet-derived growth factor were profoundly reduced in SG−/− platelets. Exposure of P-selectin and αIIb after thrombin treatment was similar in WT and SG−/− platelets. SG−/− mice exhibited reduced carotid artery thrombus formation after exposure to FeCl3. This study demonstrates that SG is crucial for platelet function and thrombus formation. We propose that SG−/− platelet function deficiencies are related to inadequate packaging and secretion of selected α-granule proteins and reduced secretion of dense granule contents critical for platelet activation.
Introduction
Proteoglycans (PGs) consist of protein “cores” to which negatively charged glycosaminoglycan (GAG) chains (eg, heparin, heparan sulfate, or chondroitin sulfate) are attached.1 PGs are abundant in the extracellular matrix (ECM) and are also found on the cell surface of most cell types. A number of different PGs have been identified in ECM (eg, aggrecan, perlecan, decorin) and on cell surfaces (eg, glypicans, syndecans). In addition, PGs are major components of secretory granules, in particular in hematopoietic cells. The secretory granules of hematopoietic cells appear to contain one single class of PG, serglycin (SG).2 The SG core protein cDNA was originally cloned from rat L2 yolk sac cells,3,4 and subsequently from a murine mastocytoma.5 Serglycin was later cloned from cells of human origin (eg, large granular lymphocyte tumor cells,6 and human promyelocytic [HL60]7 and erythroleukemia [HEL] cells8 ). SG mRNA expression and core protein have been detected in a multitude of cell types of hematopoietic origin, including mast cells,9 macrophages,10 T lymphocytes,11 cytotoxic T lymphocytes (CTLs),12,13 natural killer cells,14 neutrophils,15 and platelets.8,16 In addition, SG is expressed by certain cells of nonhematopoietic origin (eg, parietal endoderm,17,18 endothelial cells,19 and murine uterine decidua17 ).
The name “serglycin” is derived from the long Ser-Gly repeat found in the central region of the core protein (ie, the GAG attachment region). The resultant dense clustering of the attached GAG chains is unique and likely explains the known protease resistance of SG.20 The type and size of GAG chains decorating the SG core protein are cell specific, and include heparin, heparan sulfate, and chondroitin sulfates.20-28
SG PGs are thought, by virtue of their negatively charged GAG chains, to be important for secretory granule homeostasis by binding to and promoting storage of basically charged granule components in a cell-specific, protein-specific, and GAG chain–specific manner.29 Mice deficient in heparin synthesis had abnormal mast cell granules.22,30 Granule abnormalities occur in blood cells of mice in which the SG gene was targeted for deletion.31 Mast cells lacking SG expression displayed severely distorted granule morphology and were essentially devoid of certain, but not all, stored proteases.31,32 SG is crucial for storage of granzyme B, but not granzyme A or perforin in CTLs.13 SG is essential for storage of neutrophil elastase, but not of cathepsin G.33 Furthermore, SG transcription is regulated in a highly cell-specific fashion,34 allowing for coordination of SG synthesis with granulogenesis.
Many years ago, platelets were shown to contain chondroitin sulfate in the form of a PG that was described as a “proteoglycan carrier for platelet factor 4” (PF4).21 N-terminal sequencing of the platelet PG revealed its identity with SG.16 Although it is reasonable to assume that platelet SG regulates storage of α-granule proteins, it has previously not been possible to determine the specific role of SG in platelets. In this study, we addressed this question by taking advantage of the SG−/− mouse strain. We show that SG deficiency is associated with alterations in platelet morphology; defects in PF4, β-thromboglobulin, and platelet-derived growth factor (PDGF) storage in platelets; defective platelet aggregation and secretion in vitro; and defective thrombus formation in vivo. Our results implicate, for the first time, SG as a major player in the regulation of thrombosis.
Methods
Animals
Serglycin knockout mice were generated by Abrink et al31 and wild-type C57BL/6 mice were from the same stock into which the knockouts had been bred. All experimental work in this study was approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University.
Platelet isolation
For biochemical studies, mice were anesthetized by inhalation of 3% isoflurane. Blood was collected by closed-chest cardiac puncture into 1-cc syringes containing 0.15 mL acid-citrate-dextrose (ACD) anticoagulant. Apyrase was added to 0.05 U/mL. Blood was diluted with 2 volumes of Tyrode solution and centrifuged at 250g for 5 minutes. Platelet-rich plasma (PRP) was removed and diluted to 14 mL with HEN buffer (10 mM HEPES, 1 mM Na2EDTA, 150 mM NaCl), platelets were pelleted at 750g for 10 minutes, the pellet was resuspended in HEN, platelets were counted, and platelets were pelleted again. Pellets were resuspended in Laemmli treatment buffer for protein analysis or in 8 M urea/50 mM Tris HCl/0.1 M NaCl/0.2% Triton X-100 for PG analysis.
Preparation of plasma and serum
Plasma was obtained from ACD-anticoagulated blood centrifuged at 750g for 10 minutes to remove all cellular elements. Serum was obtained from supernatants of blood collected without anticoagulant and allowed to clot.
Preparation of bone marrow
Mice were anesthetized with pentobarbital 100 mg/kg intraperitoneally. Femora and tibiae were removed, and marrow was flushed from the bones with phosphate-buffered saline. Marrow was resuspended in Trizol (Invitrogen Life Technologies, Carlsbad, CA) for RNA isolation or in 8 M urea/50 mM Tris HCl/0.1 M NaCl/0.2% Triton X-100 for PG analysis.
Western blotting
Proteins were electrophoresed on 4% to 20% gradient gels (Criterion system; BioRad Laboratories, Richmond, CA). Gels were stained with Coomassie blue. Proteins were electroblotted to nitrocellulose for Western blot analysis. Antibodies were rabbit polyclonal antimouse PF4, antihuman β-thromboglobulin (Peprotech, Rocky Hill, NJ), and anti-PDGF (Santa Cruz Biotechnology, Santa Cruz, CA). For PF4, the secondary antibody was biotinylated goat anti–rabbit IgG (Vector Laboratories, Burlingame CA). Blots were incubated with streptavidin and developed with TMB Membrane Peroxidase Substrate (KPL, Gaithersburg, MD). For β-thromboglobulin and PDGF, goat antirabbit antibody was from Jackson Immunoresearch (West Grove, PA), and detection was by ECL Western Blotting Detection Reagents (Amersham/GE Healthcare, Little Chalfont, United Kingdom). PF4 in plasma and serum was quantitated by enzyme-linked immunosorbent assay (ELISA, mPF4 Elisa kit; R&D Systems, Minneapolis, MN).
Proteoglycan analysis
Platelets and bone marrow were disrupted in 8 M urea/50 mM Tris HCl/0.1 M NaCl/0.2% Triton X-100. Lysates were applied to DEAE-Sephacel columns (0.5 mL of gel) pre-equilibrated with 8 M urea/50 mM Tris HCl/0.1M NaCl. Proteins were eluted with 0.1 and then 0.3 M NaCl in 8 M urea/50 mM Tris HCl, and PGs with 1 M NaCl in 8 M urea/50 mM Tris HCl. A wash with 8 M urea/50 mM Tris HCl/1 M NaCl/0.2% Triton X-100 was used to recover putative membrane proteoglycans.35,36 PGs were concentrated and dialyzed using Centricon 30 filters (Millipore, Billerica, MA). PGs were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using 4% to 20% gels (Criterion; BioRad). Gels were stained with 0.25% Alcian blue in 2% acetic acid and destained with 5% acetic acid. Chondroitinase and heparitinase digestion were performed as described.28,36 Chondroitinase ABC (Seikagaku, Tokyo, Japan) was from Associates of Cape Cod (East Falmouth, MA) and heparitinase II from Sigma Chemical (St Louis, MO). Core protein was detected by Western blotting using a chick anti-SG antibody.17,37
RNA isolation and analysis
RNA was extracted with Trizol (Invitrogen Life Technologies). Reverse-transcription–polymerase chain reaction (RT-PCR) was performed using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Fisher BioReagents, Fair Lawn, NJ) and Taq polymerase (Qiagen, Valencia, CA). Primers were as follows: SG forward 5′-GCTGCAAACCGAATGGCTTT, reverse 5′-GTCTTCTGGTTGGTCTTGGT; actin forward 5′-ACCAACTGGGACGACATGGAGAAA, reverse 5′-TTAATGTCACGCACGATTTCCCGC; and mouse PF4 forward 5′-CGCGCCGCGGATCCTCGCTGCGGTGTTTCGAG, reverse 5′-CGGCCGGAATTCAGGCAACTCACTATGTTGAG. Conditions included denaturation at 94°C for 3 minutes, then 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute, followed by 10 minutes at 72°C in a Thermo Hybaid PCR Express thermal cycler (Thermo Scientific Waltham, MA). Samples were analyzed on 1% agarose gels containing ethidium bromide.
Morphology
Whole blood and bone marrow smears were prepared on glass slides and stained with Wright-Giemsa stain on a clinical automated stainer. Slides were viewed in a Nikon microscope (Tokyo, Japan) and pictures were taken electronically. For electron microscopy, PRP was prepared as described in “Platelet isolation,” diluted with an equal volume of White saline containing 0.2% glutaraldehyde, incubated for 20 minutes, pelleted, and overlayered with 3% glutaraldehyde in White saline.38 Marrow was flushed from femora and tibiae with 3% glutaraldehyde in White saline. Samples were shipped to Minneapolis by overnight courier. The cells were osmicated and embedded in plastic in preparation for analysis. Thin sections cut from the plastic blocks on an ultramicrotome were evaluated unstained or after staining with uranyl acetate and lead citrate to enhance contrast. Examination was carried out in a Philips (FEI, Hillsboro, OR) 301 electron microscope. Pictures were taken using an Olympus BX40 microscope (Olympus America, Center Valley, PA) equipped with a 100×/1.3 NA objective and a Polaroid PDMC-3 camera. Figures were prepared using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Platelet aggregation studies
For platelet aggregation studies, animals were sedated with pentobarbital 100 mg/kg, and blood was collected by vena cava puncture into a syringe containing 150 U/mL heparin (1:9 dilution with blood).39 Blood was diluted with one-half volume of Tyrode solution, and PRP was prepared by centrifugation. Platelets were counted on a Coulter counter (Z1; Beckman-Coulter, Hialeah, FL) and platelet counts were adjusted with mouse plasma to 2.5 × 108/mL. Aggregation studies were performed in a dual-channel aggregometer (Chronolog, Havertown, PA). Samples from SG null mice were run concurrently with samples from wild type mice at the same platelet concentration and identical concentrations of aggregation agents. Data were recorded using AGGROLINK software (Chronolog). Reagents used were adenosine diphosphate (ADP, 1-10 μM; Chronolog); PAR4 peptide AYPGKF (0.5-1.2 mM; New England Peptide, Gardner, MA); and collagen (2.5-10 μg/mL; Chronolog).
Measurement of serotonin secretion from dense granules
PRP from 2 mice of each genotype per experiment was prepared from ACD-anticoagulated blood, pooled, and gel-filtered to obtain platelets. Platelets were then incubated for 30 minutes at 37°C with 3H-serotonin (2 μCi [0.074 MBg]/mL), washed once with HEN buffer, then resuspended in HEPES-Tyrode buffer containing 1 μM imipramine and 1 mM CaCl2. Platelets (0.5 × 108) were stimulated with the indicated concentrations of AYPGKF for 10 minutes at 37°C. Reactions were stopped with an equal volume of 0.1 M EDTA/2% formaldehyde and centrifuged for 5 minutes at 10 000g. 3H in supernatants and pellets was counted and the percentage of 5-HT secretion was defined as the agonist-related increase in extracellular 3H divided by the total intracellular 3H at the start of the experiment.
FeCl3-induced carotid artery thrombosis
Mice (8-10 weeks old) were anesthetized by administration of pentobarbital (100 mg/kg) and secured supine under a dissecting microscope. The right common carotid artery was exposed by blunt dissection. A miniature Doppler flow probe was placed on the surface of the artery, and flow was measured to ensure proper placement of the probe. A 2.5-mm strip of filter paper was saturated with 10% FeCl3 and applied to the adventitial surface of the exposed artery for either 2.25 or 2.50 minutes, removed, and flushed with saline. Arterial flow rate was monitored for 30 minutes. Stable occlusive thrombi were scored as complete cessation of blood flow that remained for the 30-minute duration of the assay. Thrombi were scored unstable if flow resumed before the end of the 30-minute time period or decreased by at least 80% from the initial flow rate, but remained incomplete. The animal was scored as having no occlusive thrombus if the flow rate never decreased by 80% of the initial flow rate during the term of the assay.39
Flow cytometry
To measure fibrinogen binding, washed mouse platelets (108/mL) were incubated simultaneously with Alexafluor488-labeled mouse fibrinogen (30 μg/mL; Molecular Probes, Eugene, OR) and indicated concentrations of agonist peptide AYPGKF at 37°C for 10 minutes, then fixed in 1% formalin containing Tyrode buffer for 10 minutes at room temperature, diluted 5 times with Tyrode buffer, and analyzed by flow cytometry.39 For P-selectin and αIIb binding, mouse PRP and washed platelets were prepared using blood collected from the inferior vena cava in ACD (1:5 vol/vol), immediately diluted 1:3 (vol/vol) in modified Tyrode buffer containing PGE1 (final concentration, 1 μg/mL), and centrifuged at 200g for 4 minutes at room temperature (RT). PRP was centrifuged at 800g for 10 minutes at RT, and the pellet was washed and resuspended in modified Tyrode buffer. Platelets were activated by thrombin 1 U/mL. Monoclonal antibodies were anti–mouse CD41 (αIIb) PE (BD Pharmingen, San Diego, CA) and anti–mouse P-selectin FITC (BD Pharmingen). Washed platelets were incubated with anti-bodies for 15 minutes at RT, and binding of antibodies to the platelet was identified using a Becton Dickinson FACSscan (San Jose, CA). Data for forward-angle scatter (FSC), side-angle scatter (SSC), and fluorescence were obtained with gain settings in logarithmic mode. Platelets were identified and gated according to the SSC and immunofluorescence with anti-CD41a mAb.
Results
Evaluation of blood and bone marrow:
Whole-blood platelet counts were similar (∼ 4-7 × 108/mL) in SG−/− and WT animals. Transmission electron microscopic (TEM) analysis (Figure 1) revealed no gross defects in SG−/− platelet α-granule structure. Counting α-granules of 200 platelets in low-power fields showed a similar range of α- and dense granules per platelet from WT and SG−/− platelets. Electron-dense nucleoids were visible in most α-granules of both phenotypes, suggesting that absence of SG did not affect aggregation of the nucleoid proteins. However, a striking abnormality in platelets and megakaryocytes from SG−/− mice was the appearance of unusual scroll-like membranous inclusions, decorated with glycogen particles (Figure 1Ai-iii,vi). These inclusions were seen in SG−/− platelets of various sizes and degrees of granulation. Such structures were described previously as “cigars” in platelets from patients suffering from the Medich syndrome, a gray plateletlike syndrome,40 and in Wistar Furth heritable macrothrombocytopenic rat platelets40 ; the latter disorder is characterized by abnormal platelet PG GAG structure and a bleeding diathesis.41 Interestingly, emperipolesis of neutrophils was observed within the cytoplasm of megakaryocytes of all stages of maturation from SG−/− (Figure 1Aiv-vii) but not WT mice. As many as 8 neutrophils were observed within a single large megakaryocyte. This phenomenon also has been observed in megakaryocytes from gray platelet syndrome42-44 and myelofibrosis.43,44
Megakaryocytes (MKs) and platelets from SG−/− mice. (A) Electron micrographs of SG−/− platelets and megakaryocytes. (i) Two average-size platelets with scroll-like membranes called “cigars,” elongated intracellular membranous structures decorated with glycogen particles. (ii) Large hypogranulated platelet and small platelet with cigars. (iii) Large heavily granulated platelet with cigar. No cigars were seen in normal platelets. (iv) Immature MKs with neutrophil. (v) Mature MKs with several neutrophils. “Cigar” is just to left of *. (vi) Detail showing “cigar” from panel v; neutrophil is the one directly left of * in panel v. (vii) Neutrophil surrounded by demarcation membrane system in mature MKs. α-Granules had nucleoids, but many granules were elongated (Figure 1A center; Figure 1C platelet on right). Magnification (at microscope objective): Platelets: (i) ×4200; (ii) ×5500; (iii) ×4200. MKs: (iv,v) ×1000; (vii) ×1600. Additional image information available in “Morphology.” (B) Wright-Giemsa–stained blood smear. Photographs were taken electronically and are presented in their original form. Magnification was ×1000. Note identical appearance of red blood cells in the WT and KO smears.
Megakaryocytes (MKs) and platelets from SG−/− mice. (A) Electron micrographs of SG−/− platelets and megakaryocytes. (i) Two average-size platelets with scroll-like membranes called “cigars,” elongated intracellular membranous structures decorated with glycogen particles. (ii) Large hypogranulated platelet and small platelet with cigars. (iii) Large heavily granulated platelet with cigar. No cigars were seen in normal platelets. (iv) Immature MKs with neutrophil. (v) Mature MKs with several neutrophils. “Cigar” is just to left of *. (vi) Detail showing “cigar” from panel v; neutrophil is the one directly left of * in panel v. (vii) Neutrophil surrounded by demarcation membrane system in mature MKs. α-Granules had nucleoids, but many granules were elongated (Figure 1A center; Figure 1C platelet on right). Magnification (at microscope objective): Platelets: (i) ×4200; (ii) ×5500; (iii) ×4200. MKs: (iv,v) ×1000; (vii) ×1600. Additional image information available in “Morphology.” (B) Wright-Giemsa–stained blood smear. Photographs were taken electronically and are presented in their original form. Magnification was ×1000. Note identical appearance of red blood cells in the WT and KO smears.
Wright-Giemsa staining showed intense staining and granulation of WT platelets (Figure 1B), whereas SG−/− platelets were more spread out and much less bright without a granular appearance (Figure 1B), reminiscent of human gray platelets.45-47 Giant platelets were observed in smears from both WT and SG−/− animals. Giant platelets from SG−/− mice were occasionally larger than the red blood cells (RBCs).
Proteoglycans in WT and SG−/− platelets
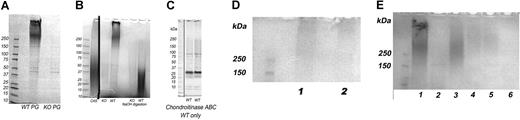
The intact PGs from the WT platelets migrated at the top of the gel with apparent MW greater than 250 kDa, as determined by SDS-PAGE analysis and Alcian blue staining (Figure 2A,B,E), similar to the size of SG in human platelets, and had 2 major components. No intact PGs were detected in platelets from the SG−/− mice (Figure 2 A,B). GAG chains released from the protein core by treatment with 0.2 M NaOH are shown in Figure 2B. The WT platelet GAGs ranged in size from 15 to 45 kDa. A faint smear of free GAGs was seen at the same size range in some WT and KO samples (Figure 2B) before NaOH digestion. This smear likely represents residual plasma GAGs trapped in the platelet pellet, since the intensity increased greatly in the NaOH digest of WT but was unchanged after NaOH digestion of the SG−/− PGs. Alternatively, they could be derived from a non-SG PG that is rapidly degraded and undetectable as an intact PG.
Proteoglycans, GAGs, and serglycin core proteins of platelets. Samples were analyzed on 4% to 20% gradient SDS-PAGE gels. Eluates of 4 × 108 platelets were applied to each lane. Proteoglycans and GAGs are stained with Alcian blue and appear as smears on the gels. No samples were applied to lanes that are not marked. (A) The 0.8 M NaCl DEAE-Sephacel eluates from WT and KO PGs are shown. Gel also stained with Coomassie blue revealed several sharp protein bands. A high Mr proteoglycan with 2 components was present in the WT platelets. No proteoglycans were visible on the gel of KO platelets. (B) Intact and 0.2 M NaOH-digested proteoglycans. C4S is shark chondroitin 4-sulfate. Lanes left to right: KO PGs, WT PGs, blank lane, KO GAGs, WT GAGs after NaOH digestion. A vertical line has been inserted to indicate a repositioned lane containing the C4S standard. (C) Immunoblot of SG core protein from 2.3 × 108 WT platelets per lane, one mouse per lane. (D) Lanes 1 and 2: PGs from SG+/− mice. (E) Lane 1: Intact PGs from 0.8 M NaCl eluate from WT platelets. Lane 2: 0.8 M NaCl eluate from KO platelets. Lane 3: 0.8 M/0.2% TX-100 eluate, WT platelets. Lanes 4–5: 0.8 M NaCl/0.2% TX-100 eluate from SG+/− platelets. Lane 6: 0.8 M NaCl/0.2% TX-100 eluate from KO platelets.
Proteoglycans, GAGs, and serglycin core proteins of platelets. Samples were analyzed on 4% to 20% gradient SDS-PAGE gels. Eluates of 4 × 108 platelets were applied to each lane. Proteoglycans and GAGs are stained with Alcian blue and appear as smears on the gels. No samples were applied to lanes that are not marked. (A) The 0.8 M NaCl DEAE-Sephacel eluates from WT and KO PGs are shown. Gel also stained with Coomassie blue revealed several sharp protein bands. A high Mr proteoglycan with 2 components was present in the WT platelets. No proteoglycans were visible on the gel of KO platelets. (B) Intact and 0.2 M NaOH-digested proteoglycans. C4S is shark chondroitin 4-sulfate. Lanes left to right: KO PGs, WT PGs, blank lane, KO GAGs, WT GAGs after NaOH digestion. A vertical line has been inserted to indicate a repositioned lane containing the C4S standard. (C) Immunoblot of SG core protein from 2.3 × 108 WT platelets per lane, one mouse per lane. (D) Lanes 1 and 2: PGs from SG+/− mice. (E) Lane 1: Intact PGs from 0.8 M NaCl eluate from WT platelets. Lane 2: 0.8 M NaCl eluate from KO platelets. Lane 3: 0.8 M/0.2% TX-100 eluate, WT platelets. Lanes 4–5: 0.8 M NaCl/0.2% TX-100 eluate from SG+/− platelets. Lane 6: 0.8 M NaCl/0.2% TX-100 eluate from KO platelets.
The core protein of the WT platelet chondroitinase ABC-digested PGs is shown in Figure 2C. The core protein appeared, as expected, at approximately 28 kDa, along with an additional band at approximately 20 to 25 kDa and 2 minor bands of less than 10 kDa, with the latter likely being SG degradation products. Figure 2D shows intact proteoglycans from 2 SG+/− mice. A uniformly stained smear extended from the top of the gel to just more than 250 kDa. The biphasic character of the smear in the WT PGs was not present. Figure 2E shows an experiment designed to assess whether a membrane PG was present. This was done because it is thought that platelet surface PGs are involved in platelet activation.48-50 Proteoglycans were observed in the TX-100 wash in WT and SG+/− but not the SG−/− eluates. The size of the TX-eluted PG of the SG+/− platelets appears larger than that of the WT platelets.
SG deficiency causes defective platelet aggregation
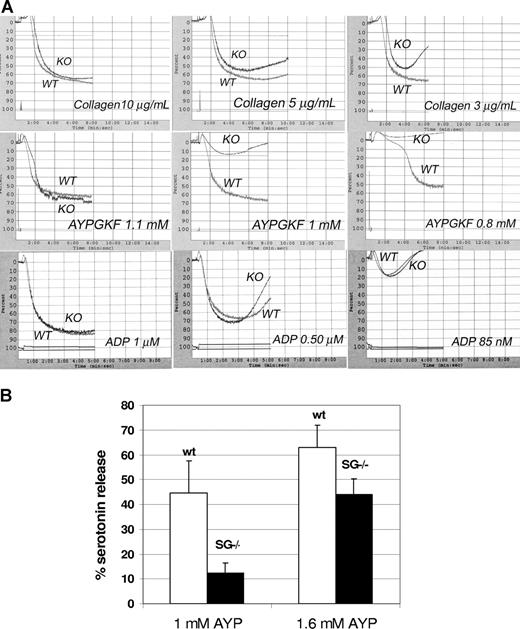
To determine whether serglycin deficiency adversely affects platelet function, platelet aggregation was compared in platelet-rich plasma prepared from WT and SG−/− animals. Aggregations of WT and SG−/− platelets were compared simultaneously in a 2-channel aggregometer. SG−/− platelets showed a markedly defective aggregation response to low concentrations of collagen type 1 and the PAR4 agonist peptide AYPGKF compared with WT platelets (Figure 3). It is notable that the SG−/− platelets showed a greater tendency than WT platelets to disaggregate at concentrations of collagen less than 10 μg/mL. In contrast, WT and SG−/− platelets aggregated identically in response to all concentrations of ADP tested, including very low concentrations that induced only 10% to 20% aggregation (Figure 3A). Aggregation defects at low concentrations of agonists for thrombin or collagen receptors are frequently indicative of a defect in secretion or secretory granule content.39
Aggregation and secretion. (A) Aggregation of WT and SG KO platelets: WT and KO platelets were run simultaneously in a dual-channel aggregometer with the concentrations of agents indicated on each panel. Results shown are representative of 2 to 3 independent experiments with very similar results. (B) Serotonin release: PAR4 agonist-induced release of serotonin from WT and SG−/− platelets was measured after loading platelets with 3H-serotonin. The mean percentage releases (± SD) from 3 separate experiments are shown.
Aggregation and secretion. (A) Aggregation of WT and SG KO platelets: WT and KO platelets were run simultaneously in a dual-channel aggregometer with the concentrations of agents indicated on each panel. Results shown are representative of 2 to 3 independent experiments with very similar results. (B) Serotonin release: PAR4 agonist-induced release of serotonin from WT and SG−/− platelets was measured after loading platelets with 3H-serotonin. The mean percentage releases (± SD) from 3 separate experiments are shown.
SG deficiency causes defective platelet secretion
Serotonin uptake was normal but platelet serotonin release was markedly reduced in platelets from SG−/− mice in response to AYPGKF (Figure 3B). In 2 experiments, ATP release was approximately 50% reduced in SG−/− platelets (not shown), consistent with the serotonin secretion results.
Flow cytometric evaluations of platelet activation
Platelet agonists induce signaling that results in an activation-dependent conformational change of the major platelet membrane receptor, αIIbβ3. Fibrinogen binding to activated αIIbβ3 is a critical step in platelet aggregation. We assessed whether the defects in aggregation in SG−/− platelets were associated with reduced fibrinogen binding and therefore activation of αIIbβ3. Incubation of platelets with increasing concentrations of AYPGKF caused a proportional increase in fibrinogen binding to WT platelets. In comparison with WT platelets, fibrinogen binding was reduced in SG−/− platelets at low and intermediate concentrations of agonist (0.4 and 0.8 mM AYPGKF). Even at 1.2 mM AYPGKF, the WT platelets were completely activated, whereas fewer than 50% of the SG−/− platelets were positive (Figure 4A). Thus the absence of SG results in reduced αIIbβ3 activation at low peptide concentrations. At 1.5 mM AYPGKF, however, SG−/− platelets bound amounts of fibrinogen equivalent to those of WT platelets (not shown).
Surface labeling of αIIbβ3 and P-selectin. (A) Fibrinogen binding to platelets activated with AYPGKF: platelets from WT and SG−/− mice were treated with the PAR4 agonist peptide AYPGKF at the concentrations indicated, followed by incubation with FITC-labeled fibrinogen as described in “Flow cytometry.” Binding was quantitated by flow cytometry. Each assay represents 50 000 platelets. Results shown are representative of 2 independent experiments with very similar results. (B) Exposure of P-selectin. Platelets were treated with 1 U/mL thrombin and stained with anti–P-selectin antibody. Each point represents platelets from a single mouse.
Surface labeling of αIIbβ3 and P-selectin. (A) Fibrinogen binding to platelets activated with AYPGKF: platelets from WT and SG−/− mice were treated with the PAR4 agonist peptide AYPGKF at the concentrations indicated, followed by incubation with FITC-labeled fibrinogen as described in “Flow cytometry.” Binding was quantitated by flow cytometry. Each assay represents 50 000 platelets. Results shown are representative of 2 independent experiments with very similar results. (B) Exposure of P-selectin. Platelets were treated with 1 U/mL thrombin and stained with anti–P-selectin antibody. Each point represents platelets from a single mouse.
Surface exposure of αIIb determined by anti-CD41 treatment was increased after platelets were treated with either thrombin or AYPGKF. Exposure of αIIb was similar in both WT and SG−/− platelets. Therefore the defect in fibrinogen binding appears to be attributable to a defect in activation of integrin αIIbβ3, rather than a defect in agonist-induced surface exposure of the integrin. In addition, P-selectin exposure was similar in WT and SG−/− platelets treated with 0.2 to 1 U/mL thrombin (shown for 1 U/mL, Figure 4B), as well as 1 mM AYPGKF. Since all of platelet P-selectin and a large amount of αIIb are localized to the α-granule membrane,51,52 serglycin deficiency does not appear to alter activation-dependent α-granule inner membrane exposure.
Defective FeCl3-induced carotid artery thrombosis in SG−/− mice
To assess whether the absence of SG affects blood clotting in vivo, mice were subjected to FeCl3-induced carotid artery thrombosis,39,53 a widely used model for studying the role of endothelial injury in platelet-induced thrombosis. Figure 5A summarizes the results of studies with 7 WT and 9 SG−/− male mice after treatment of the carotid artery with FeCl3 for 2 minutes 15 seconds. Most WT mice (4/7) developed stable occlusive thrombi 2 to 4 minutes after FeCl3 treatment. In contrast, only 1 of 9 SG−/− mice formed stable occlusive thrombi under the same conditions. Only 1 of 7 WT mice failed to form thrombi after injury, whereas 5 of 9 SG−/− mice failed to form thrombi. Tracings of the individual flow rates are shown in Figure 5B. Results were very similar in 8 WT and 8 SG−/− mice treated with FeCl3 for 2 minutes 30 seconds. One of 8 WT mice failed to form occlusive thrombi, whereas 5 of 8 SG−/− mice failed to form occlusive thrombi (data not shown).
Thrombus formation in the carotid artery after FeCl3 treatment. Experiments were carried out as described in the text. (A) The histogram shows the distribution of responses to FeCl3 treatment. Black indicates stable thrombi; gray, unstable thrombi; and white, no thrombi. (B) Tracings for all mice tested using 2-minute 15-second exposure to FeCl3 are shown. Similar results were found for mice exposed to FeCl3 for 2 minutes 30 seconds. Normal flow rate is approximately 1 mL/min. A flow rate of 0 indicates complete occlusion of the artery by the thrombus.
Thrombus formation in the carotid artery after FeCl3 treatment. Experiments were carried out as described in the text. (A) The histogram shows the distribution of responses to FeCl3 treatment. Black indicates stable thrombi; gray, unstable thrombi; and white, no thrombi. (B) Tracings for all mice tested using 2-minute 15-second exposure to FeCl3 are shown. Similar results were found for mice exposed to FeCl3 for 2 minutes 30 seconds. Normal flow rate is approximately 1 mL/min. A flow rate of 0 indicates complete occlusion of the artery by the thrombus.
Reduced content of platelet factor 4, β-thromboglobulin, and PDGF in SG−/− platelets
Platelet factor 4 (PF4), a major component of the platelet α-granule, has long been thought to associate with the platelet secretory granule PG.21 However, the in vivo functional significance of this interaction has not been determined previously. To address the role of SG in PF4 storage, we analyzed SG−/− platelets for the presence of PF4 by immunoblot analysis. As shown in Figure 6A, the levels of PF4 were dramatically reduced in platelets from SG−/− animals. The protein control was the actin band of a Coomassie-stained gel run with identical aliquots of the samples on the blot. The content of β-thromboglobulin and PDGF were similarly reduced in SG−/− platelets (Figure 6B,C). To determine whether the low SG−/− platelet content of PF4 might be due to secretion to blood, we measured PF4 in plasma and serum. However, PF4 was profoundly reduced in both plasma and serum from SG−/− animals (Figure 6D). During preparation of blood serum, platelets are activated and release their α-granule contents. The approximately 15-fold higher level of PF4 in serum is consistent with the observed content of PF4 of WT versus SG−/− platelet lysates.
α-Granule proteins of platelets, plasma, and serum in WT and SG−/− mice. (A) Immunoblot of platelet PF4. Proteins from 4 × 107 platelets per sample (each sample represents a single mouse) were subjected to SDS-PAGE, followed by immunoblot analysis for PF4. The top panel in panel A shows Coomassie staining of the same samples as in the lower panel; note the actin band. (B) Immunoblot of NAP-2. Proteins from equal numbers of WT and KO platelets were applied to the gel. NAP-2 was identified by immunoblotting (bottom panel). The same blot was probed with antiactin (top panel). Detection was by ECL. (C) Immunoblot of PDGF. Aliquots of same samples shown in panel B were applied to the gels. Labeling of PDGF was detected by ECL. (D) ELISA for quantitation of PF4 in plasma and serum. Half of the blood sample from each mouse was anticoagulated (plasma) and half was allowed to clot (serum). Two WT and 2 SG−/− mice were tested.
α-Granule proteins of platelets, plasma, and serum in WT and SG−/− mice. (A) Immunoblot of platelet PF4. Proteins from 4 × 107 platelets per sample (each sample represents a single mouse) were subjected to SDS-PAGE, followed by immunoblot analysis for PF4. The top panel in panel A shows Coomassie staining of the same samples as in the lower panel; note the actin band. (B) Immunoblot of NAP-2. Proteins from equal numbers of WT and KO platelets were applied to the gel. NAP-2 was identified by immunoblotting (bottom panel). The same blot was probed with antiactin (top panel). Detection was by ECL. (C) Immunoblot of PDGF. Aliquots of same samples shown in panel B were applied to the gels. Labeling of PDGF was detected by ECL. (D) ELISA for quantitation of PF4 in plasma and serum. Half of the blood sample from each mouse was anticoagulated (plasma) and half was allowed to clot (serum). Two WT and 2 SG−/− mice were tested.
Normal content of PF4 RNA
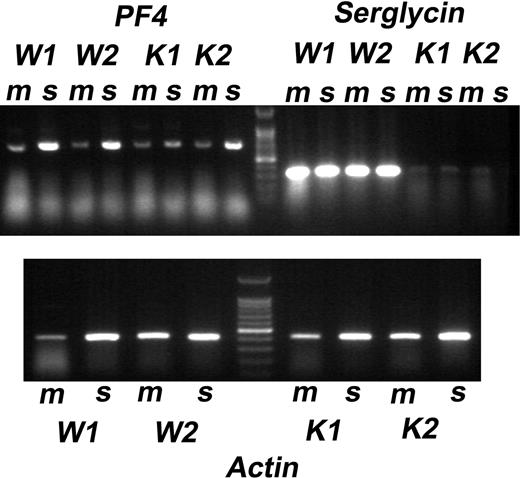
To determine whether the reduction of PF4 in platelets and plasma could be explained by reduced transcription of PF4, we evaluated mRNA expression in spleens and bone marrow cells of WT and SG−/− mice. Aliquots of the same RNA extract were subjected to RT-PCR for PF4 and SG expression, using actin as a control. PF4 is synthesized only by megakaryocytes, but not by mature platelets, whereas SG may be expressed by a multitude of cell types found within both tissues. As shown in Figure 7, similar levels of PF4 mRNA were found in samples from WT and SG−/− mice, both from bone marrow and from spleen. SG expression was detected both in bone marrow and spleen from WT animals but, as expected, not in samples from SG−/− animals. These results indicate that PF4 mRNA is expressed to the same extent in WT and SG−/− animals. Presumably the protein is synthesized but not incorporated into the platelets. It is not accumulated in plasma in these mice (Figure 6B), unlike the gray platelet and other α-granule defects in humans in which several platelet chemokines are elevated in plasma.46 A likely explanation is that megakaryocytes synthesize PF4 but either secrete it into the matrix or degrade it, or it is degraded in the matrix. The reduced level of PF4 protein in SG−/− platelets is most likely due to defective storage capacity in the α-granules rather than a lower rate of PF4 synthesis.
mRNA expression of PF4, serglycin, and actin in bone marrow and spleen of WT and SG−/− cells. mRNA was extracted from bone marrow (m) and spleen (s) of 2 WT (W1 and W2) and 2 KO (K1 and K2) mice. The gel shows the RT-PCR products. Top panel: mRNA for PF4 and SG; marker is the 100-bp ladder. Bottom panel: actin mRNA.
mRNA expression of PF4, serglycin, and actin in bone marrow and spleen of WT and SG−/− cells. mRNA was extracted from bone marrow (m) and spleen (s) of 2 WT (W1 and W2) and 2 KO (K1 and K2) mice. The gel shows the RT-PCR products. Top panel: mRNA for PF4 and SG; marker is the 100-bp ladder. Bottom panel: actin mRNA.
Discussion
Here we demonstrate, for the first time, that the serglycin PG has a crucial role in normal platelet function. Although previous studies have suggested that SG is found within platelet α-granules,21,36,54 it has previously not been possible to determine the relative contribution of SG to the total pool of platelet PGs. Our study shows that the knockout of SG causes an essentially complete loss of platelet PGs, indicating that SG is by far the dominant, and most likely the only, PG found within platelets. Thus, any PG-dependent platelet functions can most likely be specifically related to SG. The lack of PGs in the TX-100 eluate from the DEAE column of SG−/− platelets suggests that SG is the membrane PG in normal platelets. This is unexpected, but it was reported that SG is present on the surface of rat L2 yolk sac tumor cells.55
Ultrastructural examination revealed essentially normal structure of SG−/− α-granules, including electron-dense nucleoids. Thus SG does not appear to be necessary to aggregate proteins into the nucleoid. SG−/− platelets and megakaryocytes displayed unusual scroll-like membranous inclusions, reminiscent of those found in Medich platelet syndrome in humans,40 a disease characterized by excess bleeding, thrombocytopenia, and giant platelets with a gray plateletlike appearance. Similar membrane inclusions are also found in Wistar Furth hereditary macrothrombocytopenic rat platelets,40 which we have shown to have abnormally small PG GAG chains.41 These rats have a bleeding defect. We therefore suggest that the scroll-like membranous inclusions are related to defects in SG synthesis. The defect arises in the megakaryocyte (Figure 1Avi). Another unusual feature of the SG−/− megakaryocytes was the large degree of neutrophil emperipolesis. Interestingly, this phenomenon has been observed also in the gray platelet syndrome,42 a disease in which the α-granules are severely defective and in which patients suffer from myelofibrosis, as well as in patients with myelofibrosis from other causes.43 It has been suggested that emperipolesis of neutrophils by megakaryocytes is related to premature secretion of chemokines and growth factors from α-granules forming in the megakaryocytes, as well as to the development of marrow myelofibrosis.43 It will therefore be of interest in the future to determine whether myelofibrotic disorders can be related to defects in SG expression or GAG structure.
This study showed that the lack of SG resulted in notable defects in platelet aggregation in response to both collagen and PAR4 agonist peptide, thus demonstrating that platelets are functionally compromised when lacking SG. In contrast, platelet aggregation was normal in response to ADP. Interestingly, this pattern has not been shown in any other mouse platelet protein knockouts,56 thus providing additional support for a link between the gray platelet syndrome and defects in SG structure. In addition to the defects in aggregation, we also noted that platelets from SG−/− mice showed a stronger tendency than WT platelets to disaggregate. Stabilization of the platelet plug is critical for hemostasis, and reduced stability of platelet aggregates may therefore lead to impaired prevention of bleeding in response to an injury. Clearly, our results indicate that SG has an important role in both the platelet aggregation phase as well as in the subsequent stabilization process. We also observed reduced serotonin secretion and ATP release in SG−/− platelets, suggesting that SG affects platelet aggregation also through an effect on dense granule function. Our findings would not be relevant to Quebec syndrome, a disorder involving both α-granules and dense granules, in which the α-granule defect arises by proteolysis of granule proteins.47
A number of mice in which genes for platelet proteins have been deleted show defects in thrombus formation in response to FeCl3 or in other models of thrombosis (reviewed in Sachs and Nieswandt57 ). We show here that the in vivo thrombosis response toward FeCl3 is defective in mice lacking SG, as indicated by a greatly reduced tendency to form occlusive thrombi. In most animals, either no thrombus formed or thrombus instability was evidenced by resumption of blood flow following initial partial vessel occlusion. These results thus are consistent with the effects seen on platelet aggregation and the increased tendency of the SG−/− platelets to disaggregate at low agonist concentrations. The effect may also result from altered interactions of platelets with endothelium or other blood cells. Mice lacking PF4 display similar defects in in vivo thrombosis following FeCl3-induced injury.58 It appears plausible that the defects seen in SG−/− mice can, at least partly, be attributed to the secondary lack of stored PF4, and that the SG-PF4 complex may be a functional entity. We also found that SG−/− platelets were deficient in β-thromboglobulin, a basic protein, and in PDGF. In contrast, the PF4 knockout mice contain normal β-thromboglobulin. The relative contributions to the thrombotic and functional defects of these proteins are not known.
What might be the mechanism behind the aggregation and stabilization defects? A major component of the aggregation process is the induction of a conformational change and thereby activation of αIIbβ3, a major platelet membrane receptor,59 by inside-out signaling. The activated αIIbβ3 is then able to bind fibrinogen and initiate outside-in signaling to amplify the platelet aggregation response.60 The absence of SG is associated with reduced activation of the integrin as evidenced by a large right shift in the concentration curve for fibrinogen binding in response to AYPGKF. These results suggest defects in the series of events that lead to activation of αIIbβ3 on the platelet surface. We propose that the defects are related to the absence of α-granule proteins such as PF4 and a reduction in secretion of dense granule contents. The absence of PF4 has previously been shown to result in reduced thrombosis, and this chemokine likely binds to membrane PG.61 Additional proteins likely to be missing from SG−/− platelets are α-granule chemokines such as RANTES or MIP-1α, which have been shown to bind to serglycin.20,62,63 Clemetson et al have proposed that surface GAGs promote chemokine activation of platelets.48 Other proteins thought to be released from α-granules to support aggregation, possibly by binding to the platelet surface (eg, fibrinogen, fibronectin, and platelet factor V), might also be reduced in SG−/− platelets.
Since SG is a dominating proteoglycan species of hematopoietic cells and is a “committed” intracellular proteoglycan located mainly in secretory granules,29 a major issue has been to outline its role in the granulogenesis of hematopoietic cell types. Accordingly, previous studies have shown that SG is essential for storage of various secretory granule enzymes in mast cells, CTLs, and neutrophils (“Introduction”). In some cells (mast cells, CTLs) granules are affected ultrastructurally by the lack of SG, whereas in others (macrophages, neutrophils) the granule morphology is retained. In this study, we extend the repertoire of secretory granule compounds that are dependent on SG for storage to include PF4, β-thromboglobulin, and PDGF. Most likely, the interaction of these proteins with SG is likely based on electrostatic interactions between their basically charged surface regions with the strongly anionic SG GAG chains, and it reasonable to assume this interaction is essential for bringing and/or retaining proteins into the α-granule. Studies on various mast cell chymases have shown that the degree of SG dependence for storage is closely correlated with the net basic charge of the respective chymase.32 PF4 binds to serglycin with Kd of approximately 300 nM (B.P.S., unpublished observations, March 2002), compared with 840 nM for granzyme B.60 PDGF has been found to bind to PG core proteins64,65 and GAGs.66
In summary, our results thus point to an important role for SG in regulating platelet aggregation and thrombus formation in vivo. Our data are clearly compatible with a model where activation of αIIbβ3 at low agonist concentrations is defective in the absence of SG, likely due to impairment of the amplifying effects of the release of α-granule and dense granule contents. The inability of SG−/− platelets to store PF4 and the absence of PF4 from plasma likely contribute to the impaired platelet function in vivo and in vitro.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Mortimer Poncz (Children's Hospital of the University of Pennsylvania, Philadelphia, PA) for helpful discussions and for contribution of reagents for this study. We thank Dongjun Li for expert technical assistance.
This work was supported by National Institutes of Health grants R21 GM070630 (B.P.S.), K01 DK66218, and RO1 HL081241 (D.S.W.) and the Swedish Research Council (G.P.).
National Institutes of Health
Authorship
Contribution: B.P.S. designed the overall project, performed research, and wrote the paper; D.S.W. designed and performed research and wrote the paper; S.A., J.K.L., L.R., and M.A.K. performed research; J.G.W. performed and interpreted the electron microscopy experiments and contributed to writing the paper; G.P. and M.A. developed the SG knockout mouse; and G.P. contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara P. Schick, Cardeza Foundation for Hematologic Research, Thomas Jefferson University, 1015 Walnut St, Philadelphia, PA 19107; e-mail: barbara.schick@jefferson.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal