Abstract
Waldenström macroglobulinemia (WM) is an incurable low-grade B-cell lymphoma characterized by high protein turnover. We dissected the biologic role of the proteasome in WM using 2 proteasome inhibitors, NPI-0052 and bortezomib. We found that NPI-0052 inhibited proliferation and induced apoptosis in WM cells, and that the combination of NPI-0052 and bortezomib induced synergistic cytotoxicity in WM cells, leading to inhibition of nuclear translocation of p65NF-κB and synergistic induction of caspases-3, -8, and -9 and PARP cleavage. These 2 agents inhibited the canonical and noncanonical NF-κB pathways and acted synergistically through their differential effect on Akt activity and on chymotrypsin-like, caspaselike, and trypsinlike activities of the proteasome. We demonstrated that NPI-0052–induced cytotoxicity was completely abrogated in an Akt knockdown cell line, indicating that its major activity is mediated through the Akt pathway. Moreover, we demonstrated that NPI-0052 and bortezomib inhibited migration and adhesion in vitro and homing of WM cells in vivo, and overcame resistance induced by mesenchymal cells or by the addition of interleukin-6 in a coculture in vitro system. Theses studies enhance our understanding of the biologic role of the proteasome pathway in WM, and provide the preclinical basis for clinical trials of combinations of proteasome inhibitors in WM.
Introduction
Although considered a rare disease, Waldenström macroglobulinemia (WM) is becoming a model of low-grade lymphoma to test and validate therapeutic compounds that are specifically active in this biologically unique malignancy. WM is characterized by the presence of lymphoplasmacytic cells in the bone marrow (BM) and the secretion of IgM monoclonal protein in the serum, indicating that WM cells present a high protein turnover.1-3 Protein metabolism is a tightly regulated process, and inhibition of its turnover may lead to apoptosis in malignant cells, such as with proteasome inhibitors.4,5 The major activity of proteasome inhibitors is through targeting the interleukin (IL)–6 and nuclear factor (NF)–κB signaling pathways. Both these pathways are critical regulators of survival and proliferation in B-cell malignancies including WM.6-8 Previous studies have also demonstrated that adhesion of multiple myeloma cells to stromal cells induces NF-κB activation, which in turn regulates IL-6 excretion by stromal cells.9,10
The multicatalytic ubiquitin-proteasome pathway is responsible for the degradation of eukaryotic cellular proteins. This pathway also controls the activation of NF-κB by regulating degradation of NF-[κ]B inhibitor-α (IκBα). NF-κB plays a critical role in regulating many cellular responses including immunity, inflammation, proliferation, survival, and angiogenesis.11 Inactive NF-κB complexes with its inhibitor, IκBα, and remains sequestered in the cytosol. A variety of stimuli triggers the phosphorylation of IκB by IκB kinase (IKK).12 Phosphorylated IκB is then a target for ubiquitination and proteasome-mediated degradation, which in turn releases NF-κB to translocate from the cytosol to the nucleus. Once in the nucleus, NF-κB stimulates transcription of numerous cytokines, chemokines, and cell adhesion molecules. NF-κB is constitutively activated in numerous hematologic malignancies, including plasma cell dyscrasias such as multiple myeloma.10 This pathway also interacts with the PI3K/Akt pathway, a critical regulator of survival in WM cells based on our previous studies.13 Akt indirectly activates NF-κB through direct phosphorylation and activation of IκB kinase alpha (IKKα), thereby inducing degradation of IκBα by the ubiquitin-proteasome pathway.10
One of the most extensively studied proteasome inhibitors is bortezomib (Millennium Pharmaceuticals, Cambridge, MA). Bortezomib inhibits the ubiquitin-26S proteasome pathway, which regulates the turnover of a vast number of intracellular proteins, and has become an exciting target in a variety of malignancies, most notably multiple myeloma.14 The proper functioning of this system is crucial for cell-cycle regulation, gene transcription, and signal transduction. Inhibition of the proteasome effectively increases the presence of IκBα and prevents NF-κB release to the nucleus. Based on its activity in multiple myeloma, single-agent bortezomib was tested in WM in phase 2 trials and achieved 40% to 80% responses.15 .
These striking clinical responses indicate that proteasome activity is critical for the survival of WM cells. Similarly, other proteasome inhibitors have recently been developed including NPI-0052 (Salinosporamide A; Nereus Pharmaceuticals, San Diego, CA).16 NPI-0052 has a different chemical structure, toxicity profile, and mechanism of action than bortezomib. It regulates all 3 activities of the proteasome, and apoptosis mediated by this agent appears to be predominately through the caspase-8 cell death cascade.17
In this study, we sought to determine the activity of the new proteasome inhibitor NPI-0052 in WM on the canonical and noncanonical NF-κB pathways in WM, and to determine its cytotoxic activity in combination with bortezomib. In addition, we investigated mechanisms of synergistic activity of these 2 agents on WM cells and in the presence of the BM milieu, including their activity on the different catalytic activities of the proteasome, on the PI3K/Akt pathway, and on caspase cleavage. Finally, we determined the effect of these 2 agents alone and in combination on homing and adhesion of WM cells to the BM in vitro and in vivo. These studies enhance our understanding of the biologic role of the proteasome pathway in WM, and provide the preclinical basis for clinical trials of combinations of proteasome inhibitors in WM and other low-grade lymphomas.
Methods
Cells
The WM cell lines (BCWM.1, WM-WSU) and IgM-secreting low-grade lymphoma cell lines (MEC-1, Namalwa) were used in this study. The BCWM.1 is a recently described WM cell line that has been developed from a patient with untreated WM.18 WSU-WM was kindly provided by Dr Al Katib (Wayne State University, Detroit, MI). MEC-1 was a gift from Dr Kay (Mayo Clinic, Rochester, MN). RL cell line was purchased from ATCC (Manassas, VA). All cell lines were cultured at 37°C in RPMI-1640 containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO, Grand Island, NY).
Primary WM cells were obtained from bone marrow (BM) samples from previously treated WM patients using CD19+ microbead selection (Miltenyi Biotec, Auburn, CA) with more than 90% purity, as confirmed by flow cytometric analysis with monoclonal antibody reactive to human CD20-PE (BD Biosciences, San Jose, CA). Peripheral blood mononuclear cells (PBMCs) were obtained from healthy subjects by Ficoll-Hypaque density sedimentation. Cells were cultured at 37°C in RPMI-1640 containing 10% fetal bovine serum (FBS; Sigma-Aldrich), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO). Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients and healthy volunteers in accordance with the Declaration of Helsinki.
Reagents
NPI-0052 was provided by Nereus Pharmaceuticals. Bortezomib was obtained from Millennium Pharmaceuticals. Both bortezomib and NPI-0052 were diluted in dimethyl sulfoxide (DMSO) and stored at −20°C until use; they were then diluted in culture medium immediately before use. The maximum final concentration of DMSO (< 0.1%) did not affect cell proliferation and did not induce cytotoxicity on all the cell lines and primary cells tested (data not shown).
Fluorogenic substrates suc-LLVY-amc and z-LLE-amc were obtained from Calbiochem (San Diego, CA).
Growth inhibition assay
The inhibitory effect of NPI-0052, alone or in combination with bortezomib, on WM cell growth was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) dye absorbance, as previously described.19
DNA synthesis
WM cell lines and CD19+ primary WM cells were incubated in the presence of RPMI (10% FBS) with NPI-0052 (2.5-40 nM) for 48 hours at 37°C. DNA synthesis was measured by [3H]-thymidine ([3H]-TdR; Perkin Elmer, Boston, MA) uptake, as previously described.19
Detection of apoptosis
Annexin V–FITC and PI staining were used to detect and quantify apoptosis by flow cytometry, as previously described.13
Immunoblotting
BCWM.1 cells were harvested and lysed using lysis buffer (Cell Signaling Technology, Beverly, MA) reconstituted with 5 mM NaF, 2 mM Na3VO4, 1 mM PMSF (polymethylsulfonyl fluoride), 5 μg/mL leupeptine, and 5 μg/mL aprotinin. Whole-cell lysates (50 μg/lane) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyldene fluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA). The antibodies used for immunoblotting included anti–phospho (p)-Akt (Ser473), -Akt, -AIF, –p-GSK3α-β (Ser21/9), –p-ERK1/2 (Thr202/Tyr204), –caspase-3, –caspase-8, –caspase-9, -PARP, -mcl1, -survivin, –p-S6 ribosomal, Smac, c-IAP1, XIAP, -CHOP, –p-eIF2α, p-HSP-27, HSP-27, HSP-70, HSP-90, p-STAT3, p-IkB, -IkB, p-FAK, -ILK (Cell Signaling Technology), and –α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA). Nuclear extracts of the cells were prepared using the Nuclear extraction kit (Panomics, Redwood City, CA) and subjected to immunoblotting with anti–p-p65, -p50/p105, -p52/p100, -RelB, and antinucleolin antibodies (Santa Cruz Biotechnology).
In vitro Akt kinase assay
In vitro Akt kinase assay (Cell Signaling Technology) was performed as previously described.19 Following Akt immunoprecipitation, the cell lysate was then resuspended with ATP and GSK-3 fusion protein. Kinase activity was detected by immunoblotting with anti–phospho-GSK-3α/β (Ser21/9) antibody (Cell Signaling Technology).
Lentivirus shRNA vector construction and Akt gene transduction
To further determine the role of NPI-0052 in the regulation of the Akt pathway, we established an Akt knockout BCWM.1 cell line using a lentivirus transfection system, as previously described.20-22 The sense and antisense oligonucleotide sequence for construction of Akt shRNA were as follows: clone no. 10162: target sequence GGACAAGGACGGGCACATTAA; no. 10163: target sequence CGAGTTTGAGTACCTGAAGCT.
NF-κB activity
NF-κB activity was investigated using the Active Motif TransAM kits, a DNA-binding enzyme-linked immunosorbent assay (ELISA)–based assay (Active Motif North America, Carlsbad, CA). Briefly, BCWM.1 cells were treated with NPI-0052 (10 nM) or bortezomib (10 nM) alone or in combination for 4 hours, and stimulated with TNF-α (10 ng/mL) during the last 20 minutes of culture. NFκBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts, following the manufacturer's procedure.
Immunofluorescence
The effect of NPI-0052 in combination with bortezomib on tumor necrosis factor-α (TNF-α)–induced nuclear translocation of p65 was examined by an immunocytochemical method. Briefly, BCWM.1 cells were cultured in presence or absence of NPI-0052 (10 nM) and bortezomib (10 nM) for 4 hours, and then stimulated with TNF-α (10 ng/mL) during the last 20 minutes of culture. Immunocytochemical analysis was performed using an epifluorescence microscope (Nikon Eclipse E800; Nikon, Avon, MA) and a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX), as previously described.23
20S proteasome activity
The chymotrypsin-like, trypsinlike, and caspaselike activity of the 20S proteasome was determined by measurement of fluorescence generated from cleavage of the fluorogenic substrates suc-LLVY-amc, boc-LRR-amc, and z-LLE-amc, respectively.24 Cells were incubated for 4 hours in the presence of diluent or NPI-0052 10 nM, bortezomib 10 nM, or bortezomib + NPI-0052, washed with phosphate-buffered saline (PBS) and resuspended in 300 μL of a solution containing 20 mM Tris (Tris(hydroxymethyl)aminomethane), pH 7.5, 0.1 mM EDTA (ethylenediaminetetraacetic acid), pH 8.0, 20% glycerol, 0.05% Nonidet-P40, 1 mM 2-β mercaptoethanol, and 1 mM adenosine triphosphate (ATP), and lysed by freezing and thawing 3 times on dry ice. After centrifugation, supernatants were combined with substrate buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5, 5 mM EGTA [ethylene glycol tetraacetic] acid, pH 7) and the specific fluorogenic substrate in a 96-well plate and analyzed on a spectrofluorometer Mithras LB940 (Berthold Technologies, Oak Ridge, TN), using an excitation of 380 nm and an emission of 460 nm.
Effect of NPI-0052 and bortezomib on paracrine WM cell growth in the BM
To evaluate growth stimulation and signaling in WM cells adherent to bone marrow stromal cells (BMSCs), 3 × 104 BCWM.1 cells were cultured in BMSC-coated 96-well plates for 48 hours in the presence or absence of NPI-0052 alone or combined with bortezomib. DNA synthesis was measured as described.19
Transwell migration assay
We performed transwell migration assay (Costar Corning, Acton, MA) using BCWM.1 cells in the presence or absence of 30 nM SDF-1.13 In brief, cells were suspended in 1% FCS media and were placed (2 × 105 cells) in the upper chambers of the transwell plates, with serial concentrations of SDF-1 in the lower chambers in 1 mL 1% FCS media. After 4 hours at 37°C, cells that migrated to the lower chambers were counted. Triplicates of each concentration were performed, and the means and standard deviations were calculated.
Adhesion assay
BCWM.1 cells and primary CD19+ cells were pretreated with NPI-0052 (10 nM) alone or in combination with bortezomib (10 nM) for 4 hours, prior to an in vitro adhesion assay to fibronectin, a ligand of VLA-4, following the manufacturer's recommendations (EMD Biosciences, San Diego, CA). Calcein AM was used to measure adherent cells, and the degree of fluorescence was measured using a spectrophotometer (485-520 nm). BSA-coated wells served as a negative control.
In vivo flow cytometry
The effects of NPI-0052 alone or in combination with bortezomib on homing in vivo were tested using BALB/c mice with in vivo flow cytometry, as previously described.25,26 Briefly, BCWM.1 cells were treated with each agent either alone or in combination, or control PBS, for 4 hours, and then injected into the mice. Treated cells and untreated cells were fluorescently labeled by incubation with carbocyanine membrane dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) and DiR (1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indotricarbocyanine iodide) 5 μM, respectively, for 30 minutes (Molecular Probes, Carlsbad, CA). Each mouse received both DiI-labeled and DiR-labeled cells. Fluorescence signal was detected on an appropriate artery in the ear and digitized for analysis with Matlab software (MathWorks, Natick, MA) developed in house, as described.25,26
Statistical analysis
Statistical significance of differences in drug-treated versus control cultures was determined using Student t test. The minimal level of significance was P less than .05. The interaction between NPI-0052 and bortezomib was analyzed by isobologram analysis using the CalcuSyn software program (Biosoft, Ferguson, MO) to determine whether the combinations were additive or synergistic. This program is based on the Chou-Talalay method, which calculates a combination index (CI) to indicate additive or synergistic effects. When CI is equal to 1, effects are additive; when CI is less than 1.0, effects are synergistic. Results from viability assay (MTT) were expressed as fraction of cells killed by the single drug or the combination in drug-treated versus untreated cells.19
Results
NPI-0052 inhibits DNA synthesis and induces cytotoxicity of WM cells
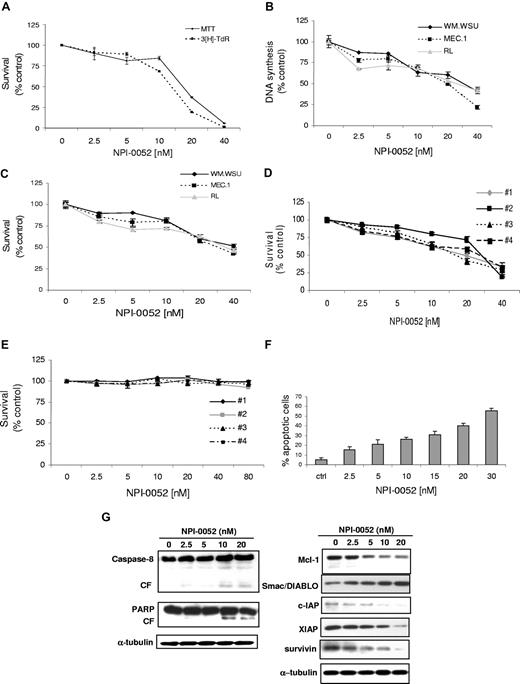
WM and IgM-secreting cell lines next were cultured for 48 hours in the presence of NPI-0052 (2.5-40 nM). As shown in Figure 1A, NPI-0052 inhibited BCWM.1 proliferation, as measured by [3H]-thymidine uptake assay, with an IC50 of 15 nM. NPI-0052 demonstrated similar activity on all cell lines tested, with IC50 between 20 and 30 nM at 48 hours (Figure 1B). We next studied the cytotoxic effect of NPI-0052 (2.5-40 nM) on cell lines and WM patient cells by MTT assay. NPI-0052 decreased survival of BCWM.1 cells (IC50, 18 nM; Figure 1A) and other IgM-secreting cell lines (IC50 30-40 nM; Figure 1C). Similarly, NPI-0052 induced cytotoxicity in primary CD19+ cells isolated from 3 patients with WM (IC50 20-30 nM; Figure 1D). In contrast, NPI-0052 had no cytotoxic effect on PBMCs from 4 healthy volunteers (Figure 1E; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results demonstrate that NPI-0052 triggers significant cytotoxicity in tumor cell lines and patient WM cells, without toxicity in normal PBMCs.
NPI-0052 induces decrease in DNA synthesis, triggers cytotoxicity, and induces apoptosis in WM cells. (A) Thymidine uptake assay and cytotoxicity assessed by MTT. BCWM.1 cells were cultured with NPI-0052 (2.5-40 nM) for 48 hours. (B) Thymidine uptake assay. Several IgM-secreting cell lines, WM-WSU (♦), MEC-1 (■), and Namalwa, were cultured with NPI-0052 (2.5-40 nM) for 48 hours. (C) Several IgM-secreting cell lines, WM-WSU, MEC-1, and RL, were cultured with NPI-0052 for 48 hours. Cytotoxicity was assessed by MTT assay. (D) Freshly isolated bone marrow CD19+ tumor cells from 4 patients with WM were cultured with NPI-0052 (2.5-40 nM) for 48 hours. Cytotoxicity was assessed by MTT assay. (E) Freshly isolated PBMCs from 4 healthy donors were cultured with NPI-0052 (2.5-40 nM) for 48 hours. Cytotoxicity was assessed by MTT assay. (F) BCWM.1 cells were cultured with NPI-0052 for 48 hours at doses that range from 2 to 30 nM, and the percentage of cells undergoing apoptosis was studied by Apo2.7 staining. (G) BCWM.1 cells were cultured with NPI-0052 (2.5-20 nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-8, -PARP, –Mcl-1, -Smac/DIABLO, -cIAP1, -XIAP, -survivin, and –α-tubulin antibodies.
NPI-0052 induces decrease in DNA synthesis, triggers cytotoxicity, and induces apoptosis in WM cells. (A) Thymidine uptake assay and cytotoxicity assessed by MTT. BCWM.1 cells were cultured with NPI-0052 (2.5-40 nM) for 48 hours. (B) Thymidine uptake assay. Several IgM-secreting cell lines, WM-WSU (♦), MEC-1 (■), and Namalwa, were cultured with NPI-0052 (2.5-40 nM) for 48 hours. (C) Several IgM-secreting cell lines, WM-WSU, MEC-1, and RL, were cultured with NPI-0052 for 48 hours. Cytotoxicity was assessed by MTT assay. (D) Freshly isolated bone marrow CD19+ tumor cells from 4 patients with WM were cultured with NPI-0052 (2.5-40 nM) for 48 hours. Cytotoxicity was assessed by MTT assay. (E) Freshly isolated PBMCs from 4 healthy donors were cultured with NPI-0052 (2.5-40 nM) for 48 hours. Cytotoxicity was assessed by MTT assay. (F) BCWM.1 cells were cultured with NPI-0052 for 48 hours at doses that range from 2 to 30 nM, and the percentage of cells undergoing apoptosis was studied by Apo2.7 staining. (G) BCWM.1 cells were cultured with NPI-0052 (2.5-20 nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–caspase-8, -PARP, –Mcl-1, -Smac/DIABLO, -cIAP1, -XIAP, -survivin, and –α-tubulin antibodies.
NPI-0052 induces apoptosis in WM cells
We next examined the molecular mechanisms whereby NPI-0052 induces cytotoxicity in WM cells. We demonstrated that NPI-0052 induced dose-dependent apoptosis, as evidenced by Apo2.7 staining in flow cytometric analysis. The percentage of apoptotic BCWM.1 cells increased from 5% (untreated) to 21.2% and 40% after 48 hours of treatment with NPI-0052 5 nM and 20 nM, respectively (Figure 1F). Similar data were obtained on other IgM-secreting cell lines (data not shown).
We next defined mechanisms whereby NPI-0052 induces apoptosis in WM, and demonstrated that NPI-0052 induced caspase-8 and PARP cleavage in a dose-dependent manner (Figure 1G) without affecting caspases-3 and -9 (data not shown). Moreover, NPI-0052 induced downmodulation of the antiapoptotic protein Mcl-1, with an increased release of the second mitochondria-derived activator of caspases (Smac/DIABLO) from the mitochondria to the cytosol (Figure 1G). It has been reported that Smac/DIABLO can abrogate the protective effects of inhibitors of apoptosis proteins (IAPs), such as X-linked inhibitor of apoptosis (XIAP).27 We therefore treated BCWM.1 cells with NPI-0052 (2.5-20 nM) for 12 hours and demonstrated that NPI-0052 down-regulated the expression of XIAP in a dose-dependent manner, accompanied by an inhibition of other IAPs members, such as c-IAP1 and survivin (Figure 1G). The viability of BCWM.1 cells assessed by MTT was not affected by NPI-0052 treatment at 12 hours (data not shown).
NPI-0052 and bortezomib synergistically induce cytotoxicity of WM cells
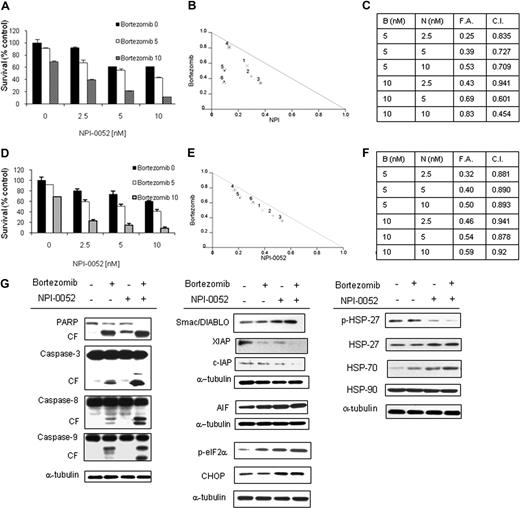
Previous studies have shown that the novel proteasome inhibitor NPI-0052 induces apoptosis in MM cells with mechanism distinct from bortezomib.17 We therefore investigated whether the combination of 2 proteasome inhibitors, NPI-0052 and bortezomib, could be synergistic in inducing cytotoxicity in WM cells. BCWM.1 cells were cultured with NPI-0052 (2.5, 5, and 10 nM) for 48 hours, in the presence or absence of bortezomib (5-10 nM). NPI-0052 showed significant cytotoxic effects when combined with bortezomib in BCWM.1 cells, as demonstrated using MTT assays at 48 hours (Figure 2A). NPI-0052 (5 nM) induced cytotoxicity in 12.4% of BCWM.1 cells, which was increased to 39.8% and 69.4% in the presence of bortezomib at 5 nM (combination index, CI: 0.72) and 10 nM (CI: 0.6), respectively, indicating synergism. Isobologram analysis, fractions affected, and the combination indexes for each of these combinations are summarized in Figure 2B,C. Similar data were observed on primary CD19+ cells (Figure 2D-F) and IgM-secreting cell lines (Figure S1B-D).
NPI-0052–induced cytotoxicity is enhanced in combination with bortezomib. (A) BCWM.1 cells were cultured with NPI-0052 (2.5, 5, and 10 nM) for 48 hours, in the presence or absence of bortezomib (5 and 10 nM). Cytotoxicity was assessed by MTT assay. (B) Representative isobologram of NPI-0052 associated to bortezomib with the CalcuSyn software demonstrating synergy for the combination. (C) Combination indexes (CIs) and fractions affected (FAs) of the combinations of NPI-0052 and bortezomib. All experiments were repeated in triplicate. (D) CD19+ primary WM cells were cultured with NPI-0052 (2.5, 5, and 10 nM) for 48 hours, in the presence or absence of bortezomib (5 and 10 nM). Cytotoxicity was assessed by MTT assay. (E) Representative isobologram of NPI-0052 associated to bortezomib with the CalcuSyn software demonstrating synergy for the combination. (F) Combination indexes (CIs) and fractions affected (FAs) of the combinations of NPI-0052 and bortezomib. All experiments were repeated in triplicate. (G) BCWM.1 cells were cultured with NPI-0052 (10 nM) in the presence or absence of bortezomib (10 nM) for 12 hours. Whole cell lysates were subjected to Western blotting using anti–caspase-8, –caspase-9, –caspase-3, -PARP, -Smac/DIABLO, -cIAP1, -XIAP, -survivin, -AIF, –p-eIF2α, -CHOP, –p-HSP27, -HSP27, -HSP70, -HSP90 and –α-tubulin antibodies.
NPI-0052–induced cytotoxicity is enhanced in combination with bortezomib. (A) BCWM.1 cells were cultured with NPI-0052 (2.5, 5, and 10 nM) for 48 hours, in the presence or absence of bortezomib (5 and 10 nM). Cytotoxicity was assessed by MTT assay. (B) Representative isobologram of NPI-0052 associated to bortezomib with the CalcuSyn software demonstrating synergy for the combination. (C) Combination indexes (CIs) and fractions affected (FAs) of the combinations of NPI-0052 and bortezomib. All experiments were repeated in triplicate. (D) CD19+ primary WM cells were cultured with NPI-0052 (2.5, 5, and 10 nM) for 48 hours, in the presence or absence of bortezomib (5 and 10 nM). Cytotoxicity was assessed by MTT assay. (E) Representative isobologram of NPI-0052 associated to bortezomib with the CalcuSyn software demonstrating synergy for the combination. (F) Combination indexes (CIs) and fractions affected (FAs) of the combinations of NPI-0052 and bortezomib. All experiments were repeated in triplicate. (G) BCWM.1 cells were cultured with NPI-0052 (10 nM) in the presence or absence of bortezomib (10 nM) for 12 hours. Whole cell lysates were subjected to Western blotting using anti–caspase-8, –caspase-9, –caspase-3, -PARP, -Smac/DIABLO, -cIAP1, -XIAP, -survivin, -AIF, –p-eIF2α, -CHOP, –p-HSP27, -HSP27, -HSP70, -HSP90 and –α-tubulin antibodies.
To better define the mechanisms of NPI-0052/bortezomib-induced cytotoxicity, we investigated the effect of NPI-0052 (10 nM), either alone or in combination with bortezomib 10 nM, on BCWM.1 cells using immunoblotting after 12-hour treatment. Interestingly, we demonstrated that PARP cleavage was significantly higher using the combination compared with the effect of each agent alone. To further dissect whether apoptosis is mediated through the intrinsic or extrinsic pathways, we investigated the effect of NPI-0052, bortezomib, and the combination on caspases-3, -8, and -9. As shown in Figure 2D, we demonstrated that single-agent NPI-0052 induced mild capsase-8 cleavage without affecting caspases-3 and -9 cleavage, while the combination of NPI-0052 and bortezomib induced significant caspases-3, -8, and -9 cleavage. In addition, previous studies have shown that the release of Smac/DIABLO from the mitochondria to the cytoplasm results in activation of caspase-9–induced apoptotic cascade, and that XIAP inhibits apoptosis through binding to caspases-3 and -9.27 We therefore investigated the effect of bortezomib and NPI-0052 on Smac/DIABLO and XIAP. As shown in Figure 2G, the combination of the 2 proteasome inhibitors induced more Smac/DIABLO release and decrease of XIAP than either agent alone. Similarly, we demonstrated that the IAP member, c-IAP1, was strongly down-regulated by the combination versus single-agent treatment (Figure 2G). Moreover, it has been recently reported that proteasome inhibition triggers apoptosis by up-regulating the expression of the apoptosis-inducing factor (AIF) and by inducing terminal unfolded protein response (UPR).28-30 We therefore wondering whether NPI-0052, either alone or in combination with bortezomib, could modulate AIF protein level expression, as well as the terminal UPR in WM. We found that AIF is up-regulated by the 2 proteasome inhibitors either as single agent or in combination. Similarly the combination of NPI-0052 and bortezomib induced an increase of p-eIF2-α, as well as its target protein CHOP, suggesting that proteasome inhibition could also result in induction of caspase-independent apoptosis in WM (Figure 2G).
In addition, recent studies have shown that HSP-27 functions as an inhibitor of caspase activation and also inhibits release of Smac/DIABLO from the mitochondria.31 We showed that NPI-0052 down-regulates HSP-27 phosphorylation, which in turn leads to increase in the release of Smac/DIABLO from the mitochondria and induction of caspase-9 cleavage. In parallel, we showed an up-regulation of HSP70, while HSP-90 expression was not modulated (Figure 2D).
NPI-0052 and bortezomib synergistically inhibit NF-κB activation in WM cells
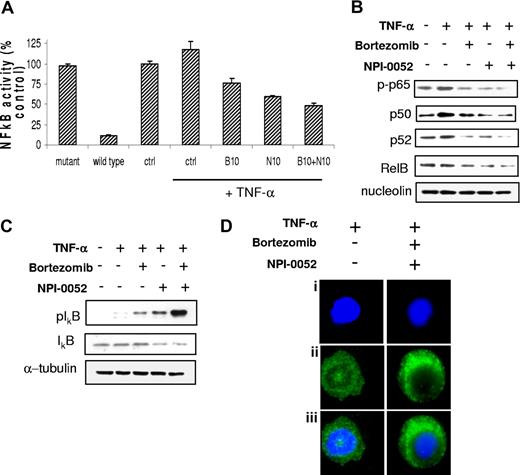
NF-κB pathway plays a pivotal role in regulating growth and survival of plasma cell malignancies.10 We therefore sought to investigate whether the combination of the 2 proteasome inhibitors would lead to synergistic modulation of this pathway. We first investigated the effect of NPI-0052, either alone or in combination with bortezomib, on the NF-κBp65 DNA-binding activity, studying nuclear extracts from treated cells using the Active Motif assay. We showed that TNF-α treatment induced NF-κB recruitment to the nucleus in BCWM.1 cells, which was inhibited by NPI-0052 more than bortezomib, and more significantly by the combination of the 2 proteasome inhibitors (Figure 3A). Similarly, proteasome inhibitors affected also basal NF-κBp65 activity (Figure S1E). Moreover, immunoblotting from nuclear extracts demonstrated that p65 phosphorylation and p50NF-κB expression were inhibited by NPI-0052, either alone or in combination with bortezomib, more than bortezomib used as single agent (Figure 3B). We further confirmed that phospho-p65 translocation from the cytoplasmic compartment to the nucleus was inhibited by the combination of bortezomib and NPI-0052, resulting in a significant increase in p-p65 expression in the cytoplasmic compartment as shown by immunofluorescence (Figure 3D). We next examined whether the combination of NPI-0052 and bortezomib altered the noncanonical NF-κB pathway. Immunoblotting from nuclear extracts showed that these 2 proteasome inhibitors used in combination inhibited the expression of p52 and RelB, which are activated mostly through the noncanonical pathway (Figure 3B).32 Moreover, each agent alone, and more significantly their combination, up-regulated the phosphorylation of the inhibitor protein IκB, as shown in Figure 3C. Taken together, these data demonstrate that the combination of the 2 proteasome inhibitors regulates both canonical and noncanonical pathways of NF-κB in WM.
NPI-0052 (N) and bortezomib (B) inhibit NF-κB function in WM cells. (A) BCWM.1 cells were cultured with either NPI-0052 (10 nM), bortezomib (10 nM), or the combination for 4 hours, and then TNF-α (10 ng/mL) was added for the last 20 minutes. NF-κBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. Wild-type and mutant are wild-type and mutated consensus competitor oligonucleotides, respectively. All results represent means (± SD) of triplicate experiments. (B,C) BCWM.1 cells were cultured with either NPI-0052 (10 nM), bortezomib (10 nM), or the combination for 4 hours, and TNF-α (10 ng/mL) was added for the last 20 minutes. Cytoplasmic and nuclear extracts were subjected to Western blotting using anti–p-NF-κBp65, –NF-κBp50, –NF-κBp52 IkBα, -RelB, –p-IκB, -IκB, -nucleolin, and –α-tubulin antibodies. (D) BCWM.1 cells were cultured with NPI-0052 (10 nM) and bortezomib (10 nM) for 4 hours, or control medium, and TNF-α (10 ng/mL) was added for the last 20 minutes. Immunocytochemical analysis was assessed using anti–p-NF-κBp65 antibody. DAPI was used to stain nuclei.
NPI-0052 (N) and bortezomib (B) inhibit NF-κB function in WM cells. (A) BCWM.1 cells were cultured with either NPI-0052 (10 nM), bortezomib (10 nM), or the combination for 4 hours, and then TNF-α (10 ng/mL) was added for the last 20 minutes. NF-κBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. Wild-type and mutant are wild-type and mutated consensus competitor oligonucleotides, respectively. All results represent means (± SD) of triplicate experiments. (B,C) BCWM.1 cells were cultured with either NPI-0052 (10 nM), bortezomib (10 nM), or the combination for 4 hours, and TNF-α (10 ng/mL) was added for the last 20 minutes. Cytoplasmic and nuclear extracts were subjected to Western blotting using anti–p-NF-κBp65, –NF-κBp50, –NF-κBp52 IkBα, -RelB, –p-IκB, -IκB, -nucleolin, and –α-tubulin antibodies. (D) BCWM.1 cells were cultured with NPI-0052 (10 nM) and bortezomib (10 nM) for 4 hours, or control medium, and TNF-α (10 ng/mL) was added for the last 20 minutes. Immunocytochemical analysis was assessed using anti–p-NF-κBp65 antibody. DAPI was used to stain nuclei.
NPI-0052 and bortezomib synergistically inhibit PI3K/Akt pathway in WM cells
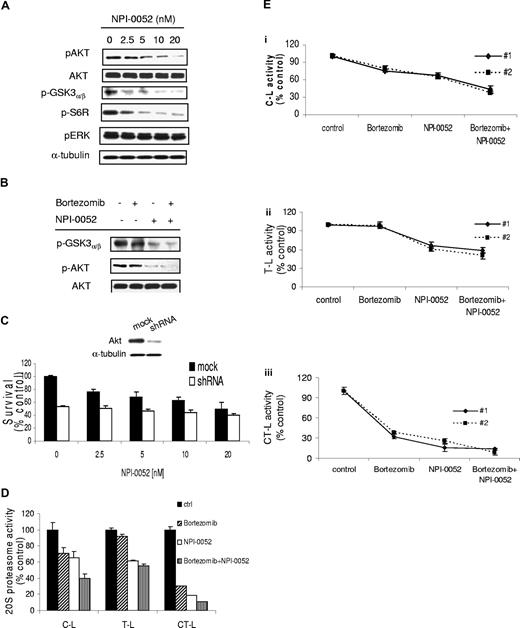
To further investigate other mechanisms of synergy between NPI-0052 and bortezomib, we sought to determine the effect of these agents on the PI3K/Akt pathway. Previous studies in MM have demonstrated that bortezomib up-regulates Akt, which may be a potential mechanism of resistance to this agent.19 We therefore examined the effect of NPI-0052 and the combination on Akt activation. The PI3K/Akt pathway is implicated in promoting growth and survival of tumor B cells.33 We first investigated whether NPI-0052 could affect PI3K/Akt signaling pathway in WM cells. BCWM.1 cells were treated with increasing doses of NPI-0052 (2.5-20 nM) for 6 hours. As shown in Figure 4A, NPI-0052 inhibited phosphorylation of Akt (Ser473), and downstream GSK3α/β and ribosomal protein S6 in a dose-dependent manner, with no activity on the phosphorylation of the MAP kinase ERK1/2 (Thr202/Tyr204). We next investigated the effect of NPI-0052 (10 nM), alone or combined with bortezomib (10 nM), on Akt kinase activity, using an in vitro Akt kinase assay. We showed that NPI-0052 decreased phosphorylation of GSK3α/β fusion protein, while bortezomib did not modulate Akt phosphorylation. The combination of NPI-0052 and bortezomib showed significant inhibition of Akt activity, indicating a possible mechanism of synergy where NPI-0052 overcomes Akt-dependent bortezomib resistance (Figure 4B). To further validate the role of the Akt pathway in NPI-0052–dependent cytotoxicity, we used an Akt knockdown BCWM.1 cell line established using lentivirus infection20-22 and demonstrated that the cytotoxic effect of NPI-0052 was abrogated in the absence of Akt (Figure 4C), indicating that Akt plays an essential role in the cytotoxic activity of NPI-0052, and that this could be an important differential effect between the 2 proteasome inhibitors and a mechanism of synergy between them in WM.
NPI-0052 inhibits Akt pathway and synergizes with bortezomib in inhibiting Akt and 20S proteasome activities. (A) BCWM.1 cells were cultured with NPI-0052 (2.5-20 nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, –p-GSK3α/β, –p-S6R, –p-ERK, and –α-tubulin antibodies. (B) In vitro Akt kinase assay. BCWM.1 cells were cultured with control media or NPI-0052 (2.5-20 nM) for 6 hours. Whole-cell lysates were immunoprecipitated with anti-Akt antibody. Then the immunoprecipitate was washed and subjected to in vitro kinase assay according to the manufacturer's protocol. Western blotting used anti–p-GSK3α/β and anti-Akt antibodies. (C) BCWM.1 cells were transduced with Akt shRNA for 48 hours. Mock indicates control plasmid. BCWM.1 transfected cells or BCWM.1 control cells were treated with NPI-0052 (2.5-20 nM) for 48 hours. Cytotoxicity was assessed by MTT assay. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, and –α-tubulin antibodies (insert in C). (D) BCWM.1 cells or primary CD19+ tumor cells from 2 patients with WM (Ei-iii) were incubated for 4 hours in the presence of diluent or 10 nM NPI-0052, bortezomib 10 nM, or bortezomib + NPI-0052. The chymotrypsin-like (CT-L), trypsinlike (T-L), and caspaselike (C-L) activity of the 20S proteasome of BCWM.1 was determined by measurement of fluorescence generated from the cleavage of the fluorogenic substrates suc-LLVY-amc, boc-LRR-amc, and z-LLE-amc, respectively.
NPI-0052 inhibits Akt pathway and synergizes with bortezomib in inhibiting Akt and 20S proteasome activities. (A) BCWM.1 cells were cultured with NPI-0052 (2.5-20 nM) for 6 hours. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, –p-GSK3α/β, –p-S6R, –p-ERK, and –α-tubulin antibodies. (B) In vitro Akt kinase assay. BCWM.1 cells were cultured with control media or NPI-0052 (2.5-20 nM) for 6 hours. Whole-cell lysates were immunoprecipitated with anti-Akt antibody. Then the immunoprecipitate was washed and subjected to in vitro kinase assay according to the manufacturer's protocol. Western blotting used anti–p-GSK3α/β and anti-Akt antibodies. (C) BCWM.1 cells were transduced with Akt shRNA for 48 hours. Mock indicates control plasmid. BCWM.1 transfected cells or BCWM.1 control cells were treated with NPI-0052 (2.5-20 nM) for 48 hours. Cytotoxicity was assessed by MTT assay. Whole-cell lysates were subjected to Western blotting using anti–p-Akt, -Akt, and –α-tubulin antibodies (insert in C). (D) BCWM.1 cells or primary CD19+ tumor cells from 2 patients with WM (Ei-iii) were incubated for 4 hours in the presence of diluent or 10 nM NPI-0052, bortezomib 10 nM, or bortezomib + NPI-0052. The chymotrypsin-like (CT-L), trypsinlike (T-L), and caspaselike (C-L) activity of the 20S proteasome of BCWM.1 was determined by measurement of fluorescence generated from the cleavage of the fluorogenic substrates suc-LLVY-amc, boc-LRR-amc, and z-LLE-amc, respectively.
NPI-0052 inhibits the 3 20S proteolytic activities within the proteasome
We further examined the effect of NPI-0052 and bortezomib, as single agents or in combination, on proteasome activities in BCWM.1 and CD19+ WM cells. Cells were treated with NPI-0052 (10 nM) either alone or in combination with bortezomib (10 nM) for 4 hours, and the chymotrypsin-like (CT-L), caspaselike (C-L), and trypsinlike (T-L) activities were measured using distinct fluorogenic peptides specific for each enzymatic activity.24 As shown in Figure 4D, bortezomib induced 29%, 5%, and 69% reduction of the C-L, T-L, and CT-L activities, respectively, whereas NPI-0052 induced 34.3%, 38.7%, and 81% reduction of the C-L, T-L, and CT-L activities, respectively. Interestingly, the inhibition of the C-L activity significantly increased to 60% when NPI-0052 and bortezomib were used in combination (Figure 4D). Similar data were obtained on CD19+ primary cells (Figure 4Ei-iii).
The combination of NPI-0052 and bortezomib overcomes resistance induced by the bone marrow microenvironment and IL-6
Since the BM microenvironment confers growth and induces drug resistance in malignant cells,34 we next investigated whether NPI-0052, alone or in combination with bortezomib, inhibits WM cell growth in the context of the BM milieu. BCWM.1 cells were cultured with NPI-0052 (2.5-20 nM) and/or bortezomib (10 nM) in the presence or absence of BMSCs for 48 hours. The viability of BMSCs assessed by MTT was not affected by NPI-0052 treatment (data not shown). Using [3H]-TdR uptake assay, adherence of BCWM.1 cells to BMSCs triggered an increase of 55% in proliferation, which was inhibited by NPI-0052 in a dose-dependent manner. This effect was significantly enhanced by the combination with bortezomib (Figure 5A), confirming that the combination of the 2 proteasome inhibitors enhanced the antitumor activity of each drug used as a single agent, even in the presence of BMSCs.
Neither growth factors nor adherence to BMSCs protect against NPI-0052–induced cytotoxicity. (A) BCWM.1 cells were cultured with control media, and with NPI-0052 (N) (2.5-20 nM), with and without bortezomib (B) (10 nM) for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (± SD) of triplicate experiment. (B) BCWM.1 cells were cultured with control media or NPI-0052 (2.5-20 nM), with and without bortezomib (10 nM) for 48 hours, in the presence or absence of IL-6 (25 ng/mL) (10 μM). Proliferation was assessed by thymidine uptake assay. (C) BCWM.1 cells were cultured with control media or NPI-0052 (10 nM) with and without bortezomib (10 nM), for 8 hours. Cells were then stimulated with IL-6 (25 ng/mL) for 10 minutes. Whole-cell lysates were subjected to Western blotting using anti–p-AKT, anti-AKT, anti–p-STAT3, and anti–α-tubulin. (D) Colony-forming cell assay. Negative fraction after CD19+ selection of bone marrow mononuclear cells was cultured using methylcellulose semisolid technique in absence or presence of NPI-0052 (10 nM, 20 nM) either alone or in combination with bortezomib 10 nM. Erythroid burst-forming units (BFU-Es), granulocyte-macrophage colony-forming units (CFU-GMs), macrophage colony-forming units (CFU-Ms), and granulocyte, erythrocyte, monocyte, macrophage colony-forming units (CFU-GEMMs) were counted at day 14. All experiments have been done in triplicate.
Neither growth factors nor adherence to BMSCs protect against NPI-0052–induced cytotoxicity. (A) BCWM.1 cells were cultured with control media, and with NPI-0052 (N) (2.5-20 nM), with and without bortezomib (B) (10 nM) for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (± SD) of triplicate experiment. (B) BCWM.1 cells were cultured with control media or NPI-0052 (2.5-20 nM), with and without bortezomib (10 nM) for 48 hours, in the presence or absence of IL-6 (25 ng/mL) (10 μM). Proliferation was assessed by thymidine uptake assay. (C) BCWM.1 cells were cultured with control media or NPI-0052 (10 nM) with and without bortezomib (10 nM), for 8 hours. Cells were then stimulated with IL-6 (25 ng/mL) for 10 minutes. Whole-cell lysates were subjected to Western blotting using anti–p-AKT, anti-AKT, anti–p-STAT3, and anti–α-tubulin. (D) Colony-forming cell assay. Negative fraction after CD19+ selection of bone marrow mononuclear cells was cultured using methylcellulose semisolid technique in absence or presence of NPI-0052 (10 nM, 20 nM) either alone or in combination with bortezomib 10 nM. Erythroid burst-forming units (BFU-Es), granulocyte-macrophage colony-forming units (CFU-GMs), macrophage colony-forming units (CFU-Ms), and granulocyte, erythrocyte, monocyte, macrophage colony-forming units (CFU-GEMMs) were counted at day 14. All experiments have been done in triplicate.
Since IL-6 and NF-kB induction by adhesion represents the 2 major pathways regulated by the proteasome, we further investigated the effect of the 2 proteasome inhibitors on cytotoxicity of WM cells in the presence of IL-6.10,35 Previous studies using gene expression analysis in WM have demonstrated an up-regulation in IL-6 signaling.36 IL-6 also promotes plasmacytoid lymphocyte growth in WM, and serum IL-6 levels reflect tumor burden and disease severity.37 We therefore tested whether the addition of recombinant human IL-6 (25 ng/mL) can overcome the cytotoxic effect of NPI-0052 and bortezomib on WM cells. As shown in Figure 5B, IL-6 induced proliferation of BCWM.1 cells, and the addition of NPI-0052 (2.5-20 nM), bortezomib (10 nM), or the combination inhibited IL-6–induced proliferation of BCWM.1 cells, indicating that NPI-0052, alone or more significantly in combination with bortezomib, can overcome resistance induced by IL-6. In addition, IL-6 induces phosphorylation of Akt and STAT-319,38 ; conversely, NPI-0052 and bortezomib inhibited IL-6–triggered Akt and STAT-3 phosphorylation, which were more significantly down-regulated by the combination of NPI-0052 and bortezomib (Figure 5C). We next investigated whether the 2 proteasome inhibitors, used as single agents or in combination, could also target nonmalignant hematopoietic cells. We found that NPI-0052 (10 nM, 20 nM), bortezomib (10 nM), and the combination did not affect the growth of BM hematopoietic progenitor cells, as shown using colony-formation assay (Figure 5D).
NPI-0052 and bortezomib inhibit migration and adhesion of WM cells in vitro and homing in vivo
Previous studies have demonstrated that the PI3K/Akt pathway regulates migration and adhesion in B cells,13 and that adhesion induces activation of the NF-κB pathway.35 We therefore sought to investigate the effect of NPI-0052 and bortezomib on the migration and adhesion of WM cells. We first demonstrated that 30 nM stromal derived factor-1 (SDF-1), an important regulator of migration in B cells,25 induced migration in BCWM.1 cells and primary CD19+ cells. To study the effect of NPI-0052 on the migration of WM cells, BCWM.1 cells and CD19+ cells were incubated with NPI-0052 (10 nM), either alone or in combination with bortezomib (10 nM), for 4 hours. These doses and duration of incubation did not induce apoptosis in WM cells as confirmed by trypan blue and Apo2.7 staining by flow cytometry (data not shown). Cells were then examined for the migration assay, as previously described.25 NPI-0052 slightly inhibited WM cell line and primary tumor cell migration toward SDF-1, which was further inhibited by NPI-0052 used in combination with bortezomib (Figure 6A).
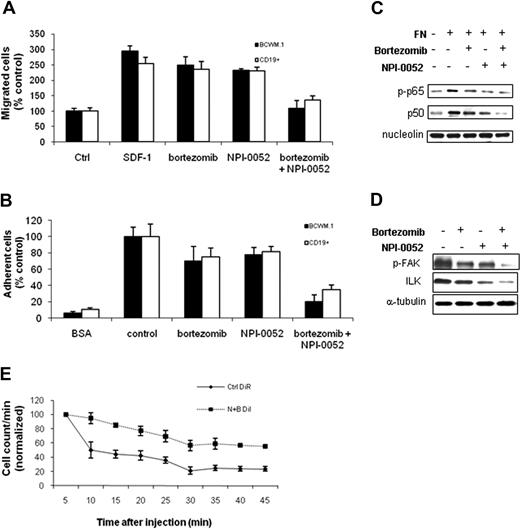
NPI-0052 inhibited migration and adhesion of BCWM.1 cells in vitro and homing in vivo. (A) Transwell migration assay showing inhibition of migration of BCWM.1 cells and primary CD19+ cells in the presence of NPI-0052 (2.5-20 nM), bortezomib (10 nM), or NPI-0052 (10 nM) in combination with bortezomib (10 nM). SDF-1 30 nM was placed in the lower chambers and induced migration compared with control with no SDF-1 (Ctrl, control). SDF-1 was placed in the lower chambers of the NPI-0052/bortezomib–treated wells. (B) Adhesion assay with BCWM.1 cells and primary CD19+ cells in the presence or absence of NPI-0052 (10 nM), either alone or in combination with bortezomib (10 nM). BCWM.1 cells demonstrated increased adhesion in fibronectin-coated wells (control) compared with BSA-coated wells (BSA, bovine serum albumin). All data represent mean (± SD) of triplicate experiments. (C) BCWM.1 cells were cultured with control media or NPI-0052 (10 nM) with and without bortezomib (10 nM) for 4 hours, in the presence or absence of fibronectin (FN). Nuclear extracts were subjected to Western blotting using anti–p-p65, -p50, and -nucleolin antibodies. (D) BCWM.1 cells were cultured with control media or NPI-0052 (10 nM), with and without bortezomib (10 nM) for 4 hours, in the presence of fibronectin. Whole-cell lysates were subjected to Western blotting using anti–p-FAK, anti-ILK, and anti–α-tubulin. (E) In vivo flow cytometry. DiI-labeled cells treated with bortezomib (B) and NPI-0052 (N) and DiR-labeled untreated cells were injected in the tail vein of 2 BALB/c mice. Cells were counted every 5 minutes for 45 minutes, as described in “Methods.”
NPI-0052 inhibited migration and adhesion of BCWM.1 cells in vitro and homing in vivo. (A) Transwell migration assay showing inhibition of migration of BCWM.1 cells and primary CD19+ cells in the presence of NPI-0052 (2.5-20 nM), bortezomib (10 nM), or NPI-0052 (10 nM) in combination with bortezomib (10 nM). SDF-1 30 nM was placed in the lower chambers and induced migration compared with control with no SDF-1 (Ctrl, control). SDF-1 was placed in the lower chambers of the NPI-0052/bortezomib–treated wells. (B) Adhesion assay with BCWM.1 cells and primary CD19+ cells in the presence or absence of NPI-0052 (10 nM), either alone or in combination with bortezomib (10 nM). BCWM.1 cells demonstrated increased adhesion in fibronectin-coated wells (control) compared with BSA-coated wells (BSA, bovine serum albumin). All data represent mean (± SD) of triplicate experiments. (C) BCWM.1 cells were cultured with control media or NPI-0052 (10 nM) with and without bortezomib (10 nM) for 4 hours, in the presence or absence of fibronectin (FN). Nuclear extracts were subjected to Western blotting using anti–p-p65, -p50, and -nucleolin antibodies. (D) BCWM.1 cells were cultured with control media or NPI-0052 (10 nM), with and without bortezomib (10 nM) for 4 hours, in the presence of fibronectin. Whole-cell lysates were subjected to Western blotting using anti–p-FAK, anti-ILK, and anti–α-tubulin. (E) In vivo flow cytometry. DiI-labeled cells treated with bortezomib (B) and NPI-0052 (N) and DiR-labeled untreated cells were injected in the tail vein of 2 BALB/c mice. Cells were counted every 5 minutes for 45 minutes, as described in “Methods.”
We also tested the effect of NPI-0052 and bortezomib on adhesion of BCWM.1 cell line and primary CD19+ cells in vitro. We found that NPI-0052 induced significant inhibition of adhesion to fibronectin (FN), when used in combination with bortezomib (Figure 6B). Previous studies have shown that FN induces members of NF-κB family transcription factors.35 We therefore next examined the effect of NPI-0052 and bortezomib on NF-κB activity, in the presence or absence of FN. As shown in Figure 6C, FN induced a significant increase in p65 phosphorylation and p50NF-κB expression in BCWM.1 cells, which were inhibited by NPI-0052, bortezomib, and more significantly by the 2 drugs in combination. It has been reported that integrin-linked kinase (ILK) and focal adhesion kinase (FAK), whose expression is NF-κB mediated, regulate cell adhesion.39,40 Therefore, we investigated the effect of proteasome inhibition on the expression of ILK and FAK. We found that both p-FAK and ILK are down-regulated using NPI-0052, bortezomib, and more significantly by the combination of the 2 drugs (Figure 6C), suggesting a possible role of these proteins in the proteasome-dependent inhibited adhesion. Moreover, it has been shown that ILK phosphorylates Akt.39 Therefore, reduction of ILK protein level may also contribute to NPI-0052–induced Akt downmodulation.
We have previously shown that adhesion of neoplastic cells to the BM microenvironment confers resistance to apoptosis.41 Therefore, we sought to investigate the effect of NPI-0052, either alone or in combination with bortezomib, on homing of WM cells in vivo. DiI-labeled BCWM.1 cells treated with NPI-0052 (10 nM, 4 hours), alone or in combination with bortezomib (5 nM), or DiR-labeled untreated BCWM.1 control cells were injected in the tail vein of BALB/c mice, followed by in vivo flow cytometry every 5 minutes for 45 minutes after injection. Neither NPI-0052 nor bortezomib as single agents significantly inhibited homing of WM cells to the bone marrow, as evidenced by a rapid decrease of circulating BCWM.1 cells that was observed in the untreated cells as well as on cells treated with NPI-0052 or bortezomib (data not shown). Interestingly, pretreatment of BCWM.1 cells with NPI-0052 in combination with bortezomib resulted in a significant inhibition of homing, with 45% decreased untreated cells in the circulation at 45 minutes versus 77% decreased untreated cells, suggesting that the 2 proteasome inhibitors together inhibit homing of WM cells to the BM (Figure 6D).
Discussion
WM is a biologically unique low-grade B-cell lymphoma characterized by high protein turnover. Little is known about the signaling pathways regulating survival and proliferation in this disease. Clinical studies have demonstrated that bortezomib induces significant cytotoxicity in WM cells,15 indicating an important role of the proteasome in WM. In this study, we dissect the biologic role of the proteasome in WM using 2 proteasome inhibitors, NPI-0052 and bortezomib.17 We first demonstrated that the novel proteasome inhibitor NPI-0052 inhibits proliferation and induces apoptosis in WM cell lines and CD19+ primary WM cells at doses consistent with previous studies and achievable in vivo.23,42 We then demonstrated that the combination of NPI-0052 and bortezomib leads to synergistic cytotoxicity on WM cell lines, IgM-secreting cell lines, and patient cells. These 2 agents lead to inhibition of nuclear translocation of p65 NF-κB, targeting both the canonical and noncanonical NF-κB pathway, with synergistic induction of caspase-dependent and -independent apoptosis as shown by caspases-3, -8, and -9 as well as PARP cleavage, release of Smac/DIABLO and AIF from the mitochondria, and activation of terminal UPR. This study therefore begins to delineate the role of the canonical and noncanonical NF-κB pathways in WM.
We further dissected the mechanism of synergy of these 2 agents and demonstrated that they have differential activity on Akt pathway, as well as on chymotrypsin-like, caspaselike, and trypsinlike activities of the 20S proteasome. We demonstrated that NPI-0052–induced cytotoxicity was completely abrogated in an Akt knockdown cell line, indicating that its major activity is mediated through the Akt pathway, while bortezomib modestly activated Akt activity. Previous studies have demonstrated that Akt pathway is up-regulated in WM cells compared with normal control43 and that the activation of the Akt survival pathway may be one of the mechanisms of resistance of malignant B cells to bortezomib.19 In this study, we demonstrate that the major activity of NPI-0052 is mediated through inhibition and not activation of Akt; therefore, its use in combination with bortezomib may overcome resistance to bortezomib in vivo.
Similarly, it has been shown that NF-κB pathway plays a pivotal role in supporting growth and survival of B-cell malignancies, including WM.10,43 Specifically, proteomic studies have indicated that NF-κB pathway is active in this disease.42 Based on these observations and on our data showing that NPI-0052 inhibits both PI3/AKT and NF-κB pathways, as well as the related in vitro activities, we can therefore hypothesize that NPI-0052–induced downmodulation of those up-regulated pathways could result in a more selective activity of NPI-0052 against WM cells rather than normal cells. Indeed, we demonstrated that NPI-0052, either alone or in combination with bortezomib, does not induce cytotoxicity in normal PBMCs.
We then showed that NPI-0052 and bortezomib inhibit migration and adhesion of WM cells to cytokines or fibronectin in the BM microenvironment. Adhesion of WM cells to fibronectin led to NF-κB stimulation, which was abrogated by NPI-0052 and bortezomib, by inhibiting FAK and ILK protein expression. In addition, the combination of NPI-0052 and bortezomib led to inhibition of homing of WM cells in our homing model in vivo. Since IL-6 and NF-κB induction by adhesion are 2 major pathways regulated by the proteasome,10,35 we demonstrated that NPI-0052 and bortezomib overcome resistance induced by mesenchymal cells and the addition of IL-6 in a coculture in vitro system. Finally, we demonstrated that the combination of these 2 agents does not induce cytotoxicity on hematopoietic stem cells using colony-formation assays. These studies demonstrate that the combination of NPI-0052 and bortezomib is active even in the BM microenvironment. Little is known about the role of the BM microenvironment in WM. Here, we demonstrate that adhesion of WM cells to the BM milieu induces NF-κB activation and IL-6 induces Akt activation, which are both down-regulated in presence of NPI-0052 alone, and more significantly inhibited in combination with bortezomib. The combination of the 2 agents therefore overcomes the protective effect of the BM niches, without affecting the growth and differentiation of normal hematopoietic components. In addition, homing is a complex process that is regulated by migration and adhesion of malignant cells to their specific BM niches. In this study, we therefore demonstrate that NPI-0052 and bortezomib inhibit migration and adhesion of WM cells, as well as their homing in vivo. Together, theses studies enhance our understanding of the biologic role of the proteasome pathway in WM, and provide the preclinical framework for clinical trials of combined NPI-0052 and bortezomib to improve patient outcome in WM and other low-grade lymphomas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by R21 1R21CA126119–01A1 from the National Cancer Institute, International Waldenström Macroglobulinemia Foundation (IWMF), the Leukemia and Lymphoma Research Foundation, the Lymphoma Research Foundation, Italian Association for Cancer Research (A.M.R.), and Berlucchi Foundation (A.M.R.).
National Institutes of Health
Authorship
Contribution: A.M.R., X.L., T.H., S.P.T., D.C., K.C.A., M.A.P., and I.M.G. designed the research, performed research, analyzed the data, and wrote the paper; A.M.R., A.S., X.J., A.-S.M., H.T.N., and M.F. performed in vitro research; A.M.R., M.M., J.R., A.A., F.A., and N.B. analyzed the data.
Conflict-of-interest disclosure: M.P. is a cofounder, shareholder, and Chief Technology Officer of Nereus Pharmaceuticals. I.M.G. and K.C.A. declare grant support by Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Mayer 548A, Boston, MA 02115; e-mail: irene_ghobrial@dfci.harvard.edu.

![Figure 5. Neither growth factors nor adherence to BMSCs protect against NPI-0052–induced cytotoxicity. (A) BCWM.1 cells were cultured with control media, and with NPI-0052 (N) (2.5-20 nM), with and without bortezomib (B) (10 nM) for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (± SD) of triplicate experiment. (B) BCWM.1 cells were cultured with control media or NPI-0052 (2.5-20 nM), with and without bortezomib (10 nM) for 48 hours, in the presence or absence of IL-6 (25 ng/mL) (10 μM). Proliferation was assessed by thymidine uptake assay. (C) BCWM.1 cells were cultured with control media or NPI-0052 (10 nM) with and without bortezomib (10 nM), for 8 hours. Cells were then stimulated with IL-6 (25 ng/mL) for 10 minutes. Whole-cell lysates were subjected to Western blotting using anti–p-AKT, anti-AKT, anti–p-STAT3, and anti–α-tubulin. (D) Colony-forming cell assay. Negative fraction after CD19+ selection of bone marrow mononuclear cells was cultured using methylcellulose semisolid technique in absence or presence of NPI-0052 (10 nM, 20 nM) either alone or in combination with bortezomib 10 nM. Erythroid burst-forming units (BFU-Es), granulocyte-macrophage colony-forming units (CFU-GMs), macrophage colony-forming units (CFU-Ms), and granulocyte, erythrocyte, monocyte, macrophage colony-forming units (CFU-GEMMs) were counted at day 14. All experiments have been done in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/9/10.1182_blood-2007-11-120972/4/m_zh80100819160005.jpeg?Expires=1767946267&Signature=NuBG6UbDeSDOkdej~cGzhT1uwzOnCtcQj~toGE7l8TOxmLmMosxMutG260rE515DVjhNxC7JfbLYPOqOnhpYNmRR5L4YcrJhdqL48wKeuglkrP2charf2e-mymKEeL2KqUl7Pa~9reGwYyZ~YI7s1YWhn6HOv-zVL-fEyV9mg0kxG5QHqk2CURDdfHY7yUh-4NDonohHxUtGAyxsW7f3zXYcPh0RVYOefQ3kSmoR2KPgHfNtIFu4PLNepKXA9vMWMlr1POrZVsJuFci5-5BlquCxil1-gzBGAglXC5odPfuk9V2pWd3yNAytusDlR0q9BGMmkQtAUY1xa4cgL4g8jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal