Abstract
Background: High dose chemotherapy and autologous hematopoietic cell transplantation (AHCT) is the most effective treatment for recurrent and refractory Hodgkin lymphoma (HL). Disease recurrence is still a major cause of treatment failure. Augmented BCNU-regimens are reported to have good outcomes but greater pulmonary toxicity. A novel transplant regimen incorporating gemcitabine and vinorelbine, active drugs in HL different from alkylating agents, was tested to establish the maximum tolerated dose (MTD) of gemcitabine and to assess safety and efficacy at the MTD.
Methods: In this phase I/II study, dose escalation was performed with gemcitabine in combination with vinorelbine followed by BCNU at reduced dose, etoposide (VP-16), cyclophosphamide (CY) and AHCT. The first 7 patients had dose escalation of gemcitabine before determining the MTD at 1250 mg/m2 on the basis of elevated liver transaminases and a symptom complex of fever, headache, and skin toxicity. For phase II, a total of 92 patients with recurrent or refractory HL have been treated at the MTD to establish safety and efficacy. The regimen consists of gemcitabine 1250 mg/m2 IV at 10 mg/min and vinorelbine 30 mg/m2 on day-13 and day-8, BCNU 10 mg/kg on day-6, VP-16 60 mg/kg on day-4, CY 100 mg/kg on day-2. Regimen-related toxicity, freedom from progression (FFP) and overall survival (OS) were endpoints of the trial.
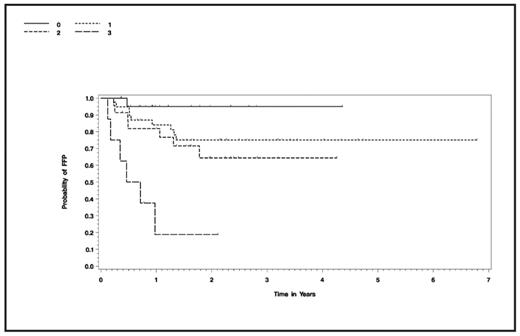
Results: 70 patients were high risk (based on stage IV disease at relapse, failure to achieve minimal disease, or B symptoms at relapse) and 22 patients were low risk (having no risk features). Median age was 33 years. Median follow-up is 2 years (.26–6.8 years). Median time to neutrophil engraftment was 10 days. Regimen-related toxicities of grade 3 skin rash, fever, headache and liver transaminase elevations were transient. Remarkable was the reduction in incidence of systemic steroid-requiring pulmonary toxicity <100 days post-transplant to 15.2% (14 of 92 patients treated at the MTD), as compared to our standard regimen incidence of 35%. Also encouraging is the OS at 2 years for the entire group of 81% (+/−9%) and FFP at 2 years of 71%(+−11%). By risk factors, FFP at 2 years was 95%(+−10%), 75%(+−14%), 64%(+−22%), and 19%(+−31%) for patients with 0, 1, 2, or 3 risk factors. These results compare favorably to the historical FFP of 41% for patients with one or more of these same risk factors (Horning 1997). In the current study, having 1 or 2 risk factors resulted in similar FFP with the new regimen (p=.46) and significantly improved FFP as compared to patients with 3 risk factors (1 vs 3, p<.001; 2 vs 3, p=.004)- see figure.
Conclusions: This novel transplant regimen for HL has decreased incidence of pulmonary toxicity compared with augmented BCNU-regimens and is associated with encouraging OS and FFP, even in the high risk groups.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal