Abstract
Background: High dose therapy and autologous transplant (HDT) clearly benefits many patients with myeloma, but the addition of other chemotherapeutics or TBI to high dose melphalan (HDM) does not improve outcomes. Bortezomib (B) is a proteasome inhibitor that synergizes with chemotherapy due to its effects on DNA repair enzymes. Recent data has shown that B upregulates the anti-apoptotic protein MCL-1, which would suggest that the sequence of administration may be critical to the combination of B and HDM. We hypothesize that B followed by M is inferior to M followed by B. To test this hypothesis, we designed a randomized phase I trial combining escalating doses of B and Melphalan 200 mg/ m2 (Mel200) in order to determine the toxicity, optimal dose and sequence of administration.
Methods: Patients were randomized to receive either B 24 hours before Mel 200 (ARM A) or B 24 hours after Mel 200 (ARM B). Doses for B escalated from 1.0mg/m2 up to 1.6mg/m2 as defined using the Escalation with Overdose Control (EWOC) method, a Bayesian phase I design. Patients eligible for the study were required to have achieved no better than a PR following induction therapy, and to have measurable disease in the bone marrow. Enrolled patients underwent BM aspirate on day -4 (before B) and day 0 (before PBSC infusion). Bone marrows were tested for annexin V staining, and myeloma cells were sorted for protein analysis. We compared the increase in annexin V and PI staining from the D-4 sample to D0 sample for each patient in order to determine which sequence was optimal for administration of B. Routine demographics, toxicity, and engraftment data were also collected.
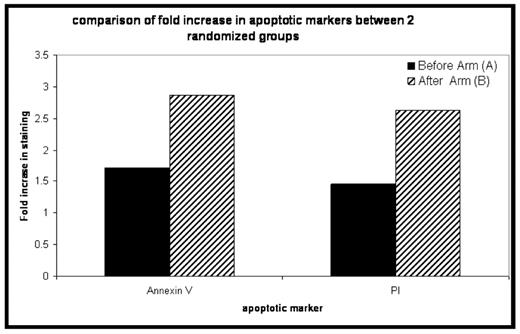
Results: 41 patients have been enrolled to date, with 34 evaluable for response assessment at day +100. B doses range from 1.0–1.6mg/m2 at both time points. Median age is 59 (44– 74). Median duration of ANC<500 and plts <20 was 11 and 5 days respectively, and was not different between the 2 bortezomib sequences. 20 patients have been randomized to ARM A of which 18 are evaluable for response, and 20 to ARM B of which 16 are evaluable for response. 18 of 34 (53%) evaluable patients achieved a VGPR or better at 100 days post transplant, with 32/34 (94%) achieving PR or better. The increase in annexin V and PI staining for the samples obtained from patients who were treated with the Arm B schedule of bortezomib was superior to the increase obtained with Arm A (see figure, p=ns). To date there is no difference in bone marrow IL6 or VEGF levels between the arms. There was no difference in mucositis or other toxicity between the 2 treatment arms.
Conclusion: The combination of B and MEL 200 is a safe with engraftment kinetics and toxicity similar to that seen in a historical cohort receiving MEL 200 alone. Efficacy data is favorable when compared to the Mayo clinic retrospective analysis (Kumar BMT 2008) for a group of patients with similar tumor burden at the time of transplant (VGPR 55% current study vs 30% historical). Preliminary lab data suggests that the administration of B following MEL 200 may be superior to B before MEL 200. Data on DNA repair and additional cytokine secretion in the marrow will be presented.
Disclosures: Lonial:Millennium: Consultancy, Research Funding; Celgene: Consultancy. Kaufman:Millennium: Speakers Bureau. Flowers:Millennium: Research Funding. Heffner:Millennium: Speakers Bureau. Off Label Use: use of bortezomib as part of transplant.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal