Abstract
Background: Obesity is increasing in prevalence worldwide and has potential implications on chemotherapy dosing and selection of patients for therapy. Auto HCT improves outcomes for patients with MM, but optimal chemotherapy dosing for obese patients is poorly defined.
Methods: We identified 1087 patients reported to the CIBMTR between 1995 and 2003 who underwent auto HCT for MM as part of initial therapy, defined as within 18 months of diagnosis, and received high-dose melphalan conditioning, with or without total body irradiation (TBI). We categorized patients by body mass index (BMI) as normal (18.5– 24.9), overweight (25–29.9), obese (30–34.9), or severely obese (≥35). Underweight patients (BMI <18.5, N=9) were excluded from analysis. We analyzed overall survival (OS) and progression-free survival (PFS) from date of transplant, using Kaplan-Meier curves and the log-rank test for univariate analyses and using Cox proportional hazards models for multivariate analyses.
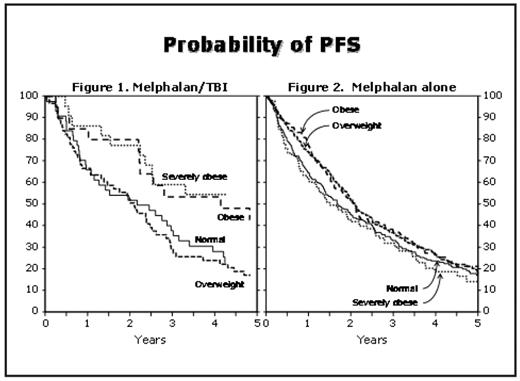
Results: Cases were reported from 114 centers in 10 countries. There were 292 patients of normal weight (27%); 472 were overweight (43%), 198 were obese (18%), and 125 severely obese (11%). Median follow-up of survivors was 63, 61, 60 and 59 months, respectively. Significant baseline differences among BMI groups indicate that obese patients selected for transplant were younger (median age 58, 58, 56, and 55 years, respectively, p=0.005) and had less severe disease at diagnosis, with lower bone marrow plasmacytosis and less frequent renal failure, hypercalcemia, and severe anemia. Obese patients received higher total melphalan doses but lower doses per square meter of body surface area (calculated based on actual body weight). Univariate analyses show no significant effect of BMI category on either OS or PFS. However, among patients who received TBI as part of conditioning, multivariable analyses show a significant effect of BMI on PFS (p-value for interaction 0.006). In this subgroup, a higher BMI was associated with longer PFS (p=0.006, Figure 1). Among patients who received melphalan alone, no effect of BMI was apparent (Figure 2). The difference in PFS for patients receiving melphalan/TBI was due to a decreased risk of relapse among obese patients. Pairwise comparisons of conditioning regimen (TBI vs. no TBI) within BMI categories showed significant reduction in risk of treatment failure for obese (HR=0.54, p=0.04) and severely obese (HR=0.32, p=0.001) patients who received TBI. No differences in OS were apparent in multivariate analyses.
Relative risks (RR) for PFS from a multivariable model adjusting for possible confounders are shown below:
| . | TBI . | No TBI . | ||||
|---|---|---|---|---|---|---|
| . | n . | RR (95% CI) . | P . | n . | RR (95% CI) . | P . |
| Normal | 44 | 1.00 | Poverall=0.006 | 248 | 1.00 | Poverall=0.18 |
| Overweight | 62 | 0.92 (0.60–1.40) | 0.69 | 405 | 0.90 (0.75–1.08) | 0.24 |
| Obese | 21 | 0.49 (0.27–0.90) | 0.021 | 177 | 0.85 (0.68–1.07) | 0.16 |
| Severely obese | 22 | 0.39 (0.20–0.76) | 0.005 | 100 | 1.12 (0.86–1.45) | 0.42 |
| . | TBI . | No TBI . | ||||
|---|---|---|---|---|---|---|
| . | n . | RR (95% CI) . | P . | n . | RR (95% CI) . | P . |
| Normal | 44 | 1.00 | Poverall=0.006 | 248 | 1.00 | Poverall=0.18 |
| Overweight | 62 | 0.92 (0.60–1.40) | 0.69 | 405 | 0.90 (0.75–1.08) | 0.24 |
| Obese | 21 | 0.49 (0.27–0.90) | 0.021 | 177 | 0.85 (0.68–1.07) | 0.16 |
| Severely obese | 22 | 0.39 (0.20–0.76) | 0.005 | 100 | 1.12 (0.86–1.45) | 0.42 |
Conclusion: Obesity, when measured by BMI, has no statistically significant effect on OS among patients with myeloma receiving high-dose melphalan. Among patients receiving melphalan with TBI, a higher BMI is associated with improved PFS. The reason for the restriction of this effect to TBI-containing conditioning regimens requires further investigation. The current common strategy of reducing melphalan doses (i.e. calculating based on ideal or adjusted body weight) does not appear to impair outcomes for obese patients. Obesity should not exclude patients from consideration of autologous transplantation.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal