Abstract
A plethora of myeloma growth factors (MGFs) has been identified, but their relative importance and cooperation have not been determined. We investigated 5 MGFs (interleukin-6 [IL-6], insulin-like growth factor type 1 [IGF-1], hepatocyte growth factor [HGF], HB–epidermal growth factor [HB-EGF], and a proliferation-inducing ligand [APRIL]) in serum-free cultures of human myeloma cell lines (HMCLs). In CD45− HMCLs, an autocrine IGF-1 loop promoted autonomous survival whereas CD45+ HMCLs could not survive without addition of MGFs, mainly IGF-1 and IL-6. IGF-1 was the major one: its activity was abrogated by an IGF-1R inhibitor only, whereas IL-6, HGF, or HB-EGF activity was inhibited by both IGF-1R– and receptor-specific inhibition. APRIL activity was inhibited by its specific inhibitor only. Of the investigated MGFs and their receptors, only expressions of IGF-1R and IL-6R in multiple myeloma cells (MMCs) of patients delineate a group with adverse prognosis. This is mainly explained by a strong association of IGF-1R and IL-6R expression and t(4;14) translocation, but IGF-1R expression without t(4;14) can also have a poor prognosis. Thus, IGF-1–targeted therapy, eventually in combination with anti–IL-6 therapy, could be promising in a subset of patients with MMCs expressing IGF-1R.
Introduction
Multiple myeloma (MM) is a clonal plasma cell disorder. MM cells (MMCs) from almost all patients harbor chromosomal abnormalities by fluorescence in situ hybridization (FISH)1,2 and aberrant gene expression3 at diagnosis in symptomatic disease. These abnormalities are not sufficient to promote MMC growth ex vivo, and the tumor microenvironment expresses adhesion molecules and produces myeloma growth factors (MGFs) that are critical to trigger MMC survival.4,5 A plethora of MGFs have been identified: interleukin-6 (IL-6),6 soluble IL-6 receptor,7 the IL-6 family,8 insulin-like growth factor type 1 (IGF-1),9,10 BAFF/APRIL B-cell growth factors,11,12 the epidermal growth factor (EGF) family,13 hepatocyte growth factor (HGF),14 tumor necrosis factor,15 the Wnt family,16 IL-10,17 IL-21,18 and the NOTCH ligand family.19 Some MGFs can be produced by the tumor environment (IL-6, BAFF/APRIL, IGF-1, EGF family, Wnt family, HGF) or by MMCs themselves (IL-6, IGF-1, HGF, EGF family, Wnt family, Notch ligand family).20,21 These MGFs activate their specific receptors, which in turn results in the activation of several signal transduction pathways,22 including the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), PI-3 kinase/AKT, Ras/mitogen-activated protein kinase (MAPK), nuclear factor–kappa B, and the β-catenin pathway. In a minority of patients with extramedullary proliferation, MMCs may grow without the support of the bone marrow microenvironment, and human myeloma cell lines (HMCLs) can be obtained. The numerous MGFs make it difficult to understand their respective role in the natural history of the disease and whether they are necessary and sufficient or redundant. This is especially relevant because MGFs can cooperate to enhance MMC growth as for IL-6 and EGF family members,23 IL-6 and FGF,24 and IL-6 and IGF-1.25 This is complicated by the fact that some growth factors are autocrinely produced or present in the culture medium, thus masking their contribution. Regarding IL-6 and IGF-1, various data were reported using different techniques and focusing on different aspects, which could yield to challenging conclusions. Descamps et al have reported that IGF-1 MGF activity was restricted to CD45− HMCLs, unlike the CD45+ HMCLs.26 IL-6 could efficiently trigger the growth of CD45+ HMCLs, and the IL-6 activity was unaffected by an IGF-1 inhibitor. An explanation was that the phosphastase activity of CD45 down-regulates the kinase activity of IGF-1R, making the CD45+ HMCLs insensitive to IGF-1. Abroun et al have shown that IL-6 can trigger membrane IL-6R binding to IGF-1R and induce IGF-1R phosphorylation independently of the addition of IGF-1.25 In this study, IL-6 is a major MGF, making it possible to trigger both gp130 and IGF-1R phosphorylation in case of high IL-6R expression. Mitsiades et al have shown the importance of serum IGF-1 to support the IL-6–dependent growth of the ANBL6 cell line.10 Regarding the other MGF, in particular HGF, EGF family, or BAFF/APRIL, their respective role was not studied comparatively yet.
Another major question is the in vivo relevance of these MGFs. Serum levels of IL-6 and soluble IL-6R and of IGF-1 were linked with bad prognosis.27,28 Divergent data exist regarding the prognostic value of IGF-1R on MMCs. With a small cohort of 37 newly diagnosed patients, Bataille et al have shown that IGF-1R expression on MMCs, detected by fluorescence-activated cell sorter (FACS) analysis, had poor prognosis value.29 Using a cohort of 72 newly diagnosed patients and IGF-1R expression detected by Affymetrix microarray (Affymetrix, Santa Clara, CA), Chng et al30 failed to find a prognosis value of IGF-1R expression, whereas IGF-1R expression was increased in poor prognosis groups. The prognostic value of the other MGF receptors was not documented yet.
To look for a possible ranking of 5 well-documented MGFs, we used a defined serum-free culture medium able to sustain growth of all our HMCLs to avoid unidentified components present in serum, in particular IGF-1, which might confound interpretation of the results. We also look for the prognostic value of MGF receptor gene expression on MMCs using 2 independent large patient cohorts.
Methods
Cell samples
The 9 HMCLs were obtained in our laboratory31 or purchased from ATCC (Manassas, VA). They were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA), 10% fetal bovine serum (PAA Laboratories, Linz, Austria), and, for the IL-6–dependent cell lines, with 2 ng/mL IL-6 (Abcys, Paris, France). Normal bone marrow plasma cells (BMPCs) were obtained from healthy donors after informed consent was given. Plasma cells, CD27+ memory B cells (MBCs), and polyclonal plasmablasts (PPCs, CD38++, CD20−) were obtained as previously described.32
MMCs of 171 patients with previously untreated MM were included after written informed consent was obtained, in accordance with the Declaration of Helsinki, at the university hospitals of Heidelberg (Germany) or Montpellier (France). These 171 patients were treated with high-dose therapy and autologous stem cell transplantation and were termed in the following Heidelberg-Montpellier (HM) series. We also used Affymetrix data of a cohort of 345 purified MMCs from previously untreated patients from the Arkansas research group (Little Rock, AR). The patients were treated with total therapy 233 and termed in the following LR-TT2 series. These data are publicly available via the online Gene Expression Omnibus.34 The study was approved by the ethics boards of Heidelberg University and Montpellier University.
Reagents
Human recombinant (r) IL-6 (rIL-6), rIGF-1, and rHGF were purchased from Abcys, and human rHB-EGF, rAPRIL, anti–human HGF monoclonal antibody (mAb), and B-cell maturation antigen (BCMA)–Fc from R&D Systems (Minneapolis, MN). The B-E8 anti–IL-6 mAb was a generous gift from Dr Wijdenes (Diaclone, Besancon, France),35 the NVP-AEW541 IGF-1R inhibitor from Novartis (Basel, Switzerland),36 and PD169540 pan-ErbB kinase inhibitor from Pfizer Global Research and Development (Ann Arbor, MI). We used Syn H, an Iscove-based fully defined culture medium containing human albumin without insulin (ABCell-Bio, Montpellier, France).
Interphase FISH, microarray hybridization, real-time reverse-transcribed polymerase chain reaction
Interphase FISH analysis was performed according to our previously reported standard protocol.2 RNA was extracted and hybridized to human Affymetrix microarrays as previously described.37 IGF-1R expression was evaluated by real-time reverse-transcribed polymerase chain reaction (RT-PCR) using the assays-on-demand primers and probes and the TaqMan Universal Master Mix (Applied Biosystems, Courtaboeuf, France) as reported.37
Flow cytometric analysis
The expression of CD45 isoforms and IGF-1R on HMCLs was evaluated by incubating 5 × 105 cells with phycoerythrin-conjugated anti-CD45RO, anti-CD45RA (Immunotech, Marseille, France), anti-CD45RB (BD Biosciences, San Jose, CA), anti–IGF-1R (Santa Cruz Biotechnology, Santa Cruz, CA), or an isotype-matched control antibody in phosphate-buffered saline, and flow cytometric analysis was performed on a FACScan (BD Biosciences).
Growth assay for myeloma cells
HMCLs were IL-6– and serum-starved for 2 hours and cultured for 4 days in 96-well flat-bottom microtiter plates in serum-free culture medium without cytokine (control) or with rIL-6 (200 pg/mL), rIGF-1 (100 ng/mL), rHB-EGF (1 μg/mL), rHGF (200 ng/mL), or rAPRIL (200 ng/mL), without or with the B-E8 anti–IL-6 mAb (10 μg/mL), the IGF-1R inhibitor NVP-AEW541 (1 μM), the anti-HGF mAb (25 μg/mL), the PD169540 pan-ErbB kinase inhibitor (1 μM), or BCMA-Fc (10 μg/mL). In some experiments, myeloma cells were grown with graded IGF-1 concentrations. The growth of myeloma cells was evaluated by quantifying intracellular ATP amount with a Cell Titer Glo Luminescent Assay (Promega, Madison, WI) with a Centro LB 960 luminometer (Berthold Technologies, Bad Wildbad, Germany).
Signal transduction, IGF-1 production, and immunoblot analysis
To look for signal transduction, myeloma cell lines were starved for 18 hours, washed, and then incubated with the various prewarmed MGF with or without inhibitors for 20 minutes. Cells were lysed and transferred to a nitrocellulose membrane (Whatman Schleicher and Schuell, Dassel, Germany), as previously described.37 Membranes were immunoblotted with a rabbit anti–IGF-1 (Abcam, Cambridge, United Kingdom), antiphospho-Akt, antiphospho-MAPK, antiphospho-Stat3, anti-Akt, anti-MAPK antibodies (Cell Signaling Technology, Danvers, MA), and with a mouse anti-Stat3 antibody (Cell Signaling Technology). As a control for protein loading, we used a mouse monoclonal anti–β-actin antibody (Sigma-Aldrich, St Louis, MO). The primary antibodies were visualized with goat anti–rabbit (Sigma-Aldrich) or goat anti–mouse (Bio-Rad, Hercules, CA) peroxidase-conjugated antibodies by an enhanced chemiluminescence detection system.
Measurement of cytokine concentration by using enzyme-linked immunosorbent assay
HMCLs were cultured for 2 days in Syn H serum-free culture medium without cytokine and IL-6 and IGF-1 in the culture supernatant were measured using enzyme-linked immunosorbent assay (ELISA) kits with a detection level of 3 pg/mL and 45 pg/mL, respectively (R&D Systems).
Spiked MMSET expression surrogating t(4;14)
The t(4;14) translocation results in aberrant FGFR3 expression in 70% of patients and MMSET spiked expression in 100% of patients,38 and spiked MMSET expression has been taken as surrogate for the presence of t(4;14).3 In our series of 94 patients with FISH analysis, 20 of 94 patients had t(4;14) resulting in aberrant FGFR3 expression in 16 of 20 and spiked MMSET expression (range of Affymetrix signal, 500-2500) in 19 of 20 using the 222777_s_at MMSET probe set with the highest variation coefficient among MMC samples. In the 74 patients lacking t(4;14), no FGFR3 and a low MMSET expression (Affymetrix signal, 1-300) were found. We defined a spiked MMSET gene if MMSET signal ≥ Q3 + 3 (Q3-Q1) with Q3 and Q1 being the MMSET signals of the first and third quartile of MMC samples. Using this definition, 19 of 20 (95%) of patients with t(4;14) had spiked MMSET and only 2 of 74 lacking documented t(4;14) had spiked MMSET.
Statistical analysis
A difference in the mean values of 2 paired groups was evaluated with a paired Student t test using the SPSS10 software. Gene expression profiles were analyzed with our bioinformatics platform (RAGE: http://rage.montp.inserm.fr)39 and with the Amazonia website.40 The prognostic value of a probe set was evaluated combining Affymetrix data obtained with human genome U133 set or U133 Plus 2.0 microarrays. We used the Affymetrix call (“present” or “absent”) determined by the Affymetrix GCOS software as indicating whether a gene is expressed or not. When a probe set was absent in MMCs of a fraction of patients (IGF-1R and c-Met), the survival of patients with MMC with a present or absent call was compared. When a probe set was present in MMCs of all patients (IL-6R, gp130, TACI, BCMA), the survival of patients with a signal below or above the median signal was compared. The statistical significance of differences in survival between groups of patients was calculated by the log-rank test. An event was defined as relapse or death (for event-free survival [EFS]) or as death (for overall survival [OS]). Multivariate analysis was performed using the Cox proportional hazards model. Survival curves were plotted using the Kaplan-Meier method.
Results
Autocrine IGF-1 is a critical survival factor of autonomously surviving CD45− HMCLs in serum-free culture medium
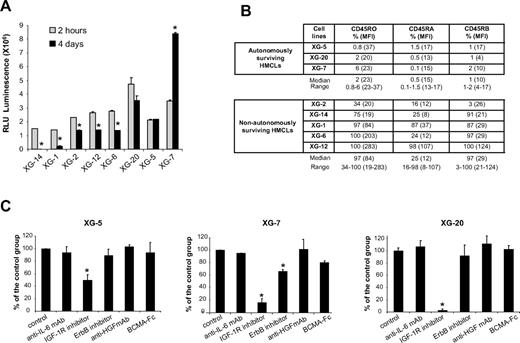
Without adding exogenous MGF and in serum-free culture medium, the number of viable cells in 5 HMCLs (XG-1, XG-2, XG-6, XG-12, XG-14) was decreased at day 4 of culture (Figure 1A; P ≤ .05). This effect was observed with cell concentrations ranging from 4 × 103 to 4 × 105 cells/mL. The survival of 3 HMCLs (XG-5, XG-20, XG-7) was not affected, and XG-7 showed even an increased growth (P ≤ .05; Figure 1A). A difference between autonomously and nonautonomously surviving HMCL is CD45 expression. The 3 autonomously surviving HMCLs expressed the 3 CD45 isoforms either at a low level or not at all (≤ 6%, Figure 1B), and the 5 nonautonomously surviving HMCLs expressed at least 2 isoforms, mainly CD45RO and CD45RB. CD45RA+ HMCLs are less frequent, and their behavior may not always be representative of the wider spectrum of HMCLs (Figure 1B). Looking for the simultaneous expression of genes coding for a growth factor and its receptors using Affymetrix microarrays, a possible autocrine IL-6/IL-6R/gp130 loop is found in 6 of 8 HMCLs, an IGF-1/IGF-1R loop in 7 of 8, an HGF/c-met loop in 2 of 8, BAFF-APRIL/BAFFR-BCMA-TACI loops in 3 of 8, and EGF family/ErbB family loops in 8 of 8 (results not shown). To investigate IGF-1R expression, we used the 203627_at probe set, which is the only 1 of 8 IGF-1R probe sets that correlated with IGF-1R expression assayed by reverse-transcribed polymerase chain reaction (r = 0.85, P = .002) and FACS analysis using HMCLs (r = 0.67, P = .03).
Survival of myeloma cell lines in serum-free culture medium. (A) HMCLs were starved for 2 hours and cultured for 4 days without cytokine in the Syn H serum-free culture medium. The cell concentration at the start of the culture was 2 × 105 cells/mL for all HMCLs. Results are the mean luminescent signals of a luciferase assay in 3 independent experiments 2 hours and 4 days after culture start. The mean value is significantly different from that obtained at 2 hours using a Student t test for pairs (*P ≤ .05). (B) CD45 protein expression was determined by flow cytometry using murine anti-CD45RO, anti-CD45RA, and anti-CD45RB mAbs in the 3 autonomously surviving HMCLs and 5 nonautonomously surviving ones. The fluorescence intensity was set up to get a mean fluorescence intensity between 3 and 5 with isotype-matched control antibodies. Results are the percentage of positive cells and in parentheses, the mean fluorescence intensity of positive cells. These data are from 1 experiment representative of 3. (C) XG-5, XG-7, and XG-20 HMCLs were starved for 2 hours and cultured for 4 days without inhibitor (control) or with the anti–IL-6 mAb (10 μg/mL) or the IGF-1R inhibitor (1 μM) or the pan-ErbB kinase inhibitor (1 μM) or the anti-HGF mAb (25 μg/mL) or BCMA-Fc (10 μg/mL) in the Syn H culture medium. The cell concentration at the start of the culture was 105 cells/mL. Results are the mean percentages (± SD) of the luminescent signal of each group compared with that of the control group in 3 independent experiments. The mean value was significantly different from that obtained with the control group using a Student t test for pairs (*P ≤ .05).
Survival of myeloma cell lines in serum-free culture medium. (A) HMCLs were starved for 2 hours and cultured for 4 days without cytokine in the Syn H serum-free culture medium. The cell concentration at the start of the culture was 2 × 105 cells/mL for all HMCLs. Results are the mean luminescent signals of a luciferase assay in 3 independent experiments 2 hours and 4 days after culture start. The mean value is significantly different from that obtained at 2 hours using a Student t test for pairs (*P ≤ .05). (B) CD45 protein expression was determined by flow cytometry using murine anti-CD45RO, anti-CD45RA, and anti-CD45RB mAbs in the 3 autonomously surviving HMCLs and 5 nonautonomously surviving ones. The fluorescence intensity was set up to get a mean fluorescence intensity between 3 and 5 with isotype-matched control antibodies. Results are the percentage of positive cells and in parentheses, the mean fluorescence intensity of positive cells. These data are from 1 experiment representative of 3. (C) XG-5, XG-7, and XG-20 HMCLs were starved for 2 hours and cultured for 4 days without inhibitor (control) or with the anti–IL-6 mAb (10 μg/mL) or the IGF-1R inhibitor (1 μM) or the pan-ErbB kinase inhibitor (1 μM) or the anti-HGF mAb (25 μg/mL) or BCMA-Fc (10 μg/mL) in the Syn H culture medium. The cell concentration at the start of the culture was 105 cells/mL. Results are the mean percentages (± SD) of the luminescent signal of each group compared with that of the control group in 3 independent experiments. The mean value was significantly different from that obtained with the control group using a Student t test for pairs (*P ≤ .05).
The survival of the 3 autonomously surviving CD45− HMCLs was strongly inhibited (50%-95%, P ≤ .05) by the NVP-AEW541 IGF-1R kinase inhibitor (Figure 1C). The ErbB inhibitor partially affected XG-7 survival (40% reduction, P ≤ .05). The inhibitors to IL-6, HGF, or BAFF/APRIL did not affect these 3 HMCLs. The specificity of NVP-AEW541 IGF-1R kinase36 has been confirmed in our hands, ie, by the lack of inhibition of the IGF-1R− XG-12 HMCL, the inhibition of the transduction pathways activated by IGF-1 and the lack of inhibition of those induced by IL-6 and HGF (Figure 2A). We also looked for the production of autocrine IGF-1 or IL-6. IL-6 could be detected in the culture supernatants of some cell lines (XG-1, XG-2, and XG-6) in agreement with previous reports.41 IGF-1 protein was detected by Western blot in the HMCLs that expressed IGF-1 gene by Affymetrix microarrays (Figure 2B), but IGF-1 concentration in HMCL culture supernatants was below the ELISA detection limit (≥ 45 pg/mL).
Specificity of MGF inhibitors and IGF-1 production by HMCLs. (A) XG-2 cells were starved at 37°C for 18 hours in Syn H serum-free culture medium. Cells were then cultured without cytokine (control) or with either IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) or HGF (20 ng/mL) and without inhibitor or with anti–IL-6 mAb (10 μg/mL) or IGF-1R inhibitor (1 μM) or anti-HGF mAb (25 μg/mL) for 20 minutes at 37°C in the Syn H culture medium. The receptor kinase inhibitors were added to the cells for 4 hours at the end of starvation culture and during exposure to rMGF. The anti-MGF antibodies were preincubated with the rMGF for 1 hour before to be added to cells. Cell lysates were immunoblotted with anti–phospho-Akt antibody and then reprobed with anti-akt antibody, anti–phospho-MAPK antibody, and then reprobed with anti-MAPK, anti–phospho-Stat3 antibody, and then reprobed with anti-Stat3 antibody. Anti–β actin was used as a loading control. (B) HMCLs were cultured for 2 days without cytokine in the Syn H serum-free culture medium. Cell lysates were immunoblotted with an anti–IGF-1 antibody. Anti–β actin was used as a loading control and the U266 HMCL as a negative control for IGF-1 production (no expression of IGF-1 gene using Affymetrix microarrays). (C) XG-2 cells were starved for 2 hours in Syn H serum-free culture medium and then cultured without cytokine (control) or with increased concentrations of rIGF-1 for 4 days. Results are the mean luminescent signals ± SD determined in sextuplicate culture wells and are those of 1 experiment representative of 3. Data are expressed as percentage of the signal obtained without cytokine. The mean value was significantly different from that obtained in the control group using a Student t test (*P ≤ .05).
Specificity of MGF inhibitors and IGF-1 production by HMCLs. (A) XG-2 cells were starved at 37°C for 18 hours in Syn H serum-free culture medium. Cells were then cultured without cytokine (control) or with either IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) or HGF (20 ng/mL) and without inhibitor or with anti–IL-6 mAb (10 μg/mL) or IGF-1R inhibitor (1 μM) or anti-HGF mAb (25 μg/mL) for 20 minutes at 37°C in the Syn H culture medium. The receptor kinase inhibitors were added to the cells for 4 hours at the end of starvation culture and during exposure to rMGF. The anti-MGF antibodies were preincubated with the rMGF for 1 hour before to be added to cells. Cell lysates were immunoblotted with anti–phospho-Akt antibody and then reprobed with anti-akt antibody, anti–phospho-MAPK antibody, and then reprobed with anti-MAPK, anti–phospho-Stat3 antibody, and then reprobed with anti-Stat3 antibody. Anti–β actin was used as a loading control. (B) HMCLs were cultured for 2 days without cytokine in the Syn H serum-free culture medium. Cell lysates were immunoblotted with an anti–IGF-1 antibody. Anti–β actin was used as a loading control and the U266 HMCL as a negative control for IGF-1 production (no expression of IGF-1 gene using Affymetrix microarrays). (C) XG-2 cells were starved for 2 hours in Syn H serum-free culture medium and then cultured without cytokine (control) or with increased concentrations of rIGF-1 for 4 days. Results are the mean luminescent signals ± SD determined in sextuplicate culture wells and are those of 1 experiment representative of 3. Data are expressed as percentage of the signal obtained without cytokine. The mean value was significantly different from that obtained in the control group using a Student t test (*P ≤ .05).
IL-6 and IGF-1 are the major MGFs, and rIL-6-, HGF-, and HB-EGF-mediated survival of HMCLs is dependent on the presence of an autocrine IGF-1 loop, whereas the activity of recombinant APRIL is not
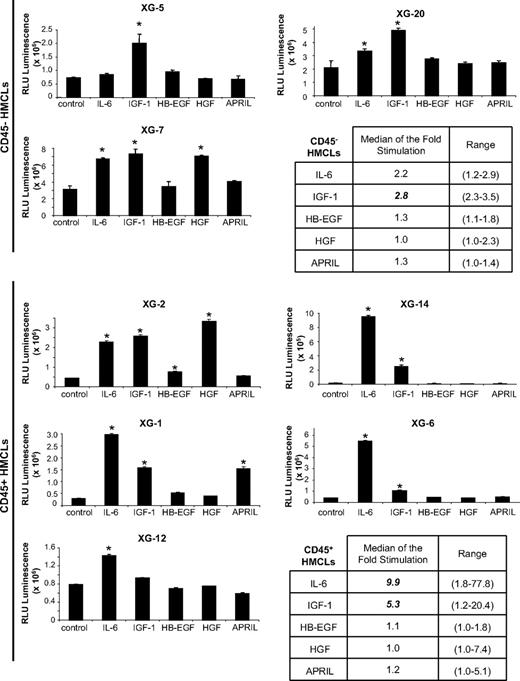
rIL-6 significantly stimulated (P ≤ .05) the growth of 2 of 3 CD45− HMCLs and of 5 of 5 CD45+ HMCLs and IGF-1 of 3 of 3 CD45− and 4 of 5 CD45+ HMCLs (Figure 3). The CD45+ IGF-1R− XG-12 HMCLs was not stimulated by rIGF-1. The lowest concentration of rIGF-1 stimulating these HMCLs was 27 pg/mL (Figure 2C), below ELISA detection limit (≤ 45 pg/mL), and that of rIL-6 was 2 pg/mL.41 The median rate of stimulation by IL-6 and IGF-1 was 2.2- and 2.8-fold for the CD45− HMCLs (P ≤ .05) and 9.9- and 5.3-fold for the CD45+ HMCLs (P ≤ .05; Figure 3), respectively. The other 3 MGFs stimulated significantly 1 or 2 of the 8 HMCLs (Figure 3). These data indicated that, of the factors investigated, IL-6 and IGF-1 are the most important MGF. APRIL, HB-EGF, or HGF stimulated only 1 or 2 of the 8 HMCLs. Regarding transduction pathways, IGF-1 and HGF activated AKT and MAPK phosphorylations, unlike STAT3, IL-6–induced STAT3 and MAPK phosphorylations, unlike AKT and their activity was blocked by their specific inhibitors unlike other MGF inhibitors (Figure 2A). The nuclear factor-κB pathway (p65 phosphorylation) could not be switched off by MGF starvation in the investigated HMCLs, suggesting a constitutive activation (results not shown).
Growth activity of 5 factors in 8 HMCLs. HMCLs were starved for 2 hours and cultured without growth factor (group) or with either IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) or HB-EGF (1 μg/mL) or HGF (200 ng/mL) or APRIL (200 ng/mL) for 4 days in the Syn H culture medium. The cell concentration were 2 × 105 cells/mL for XG-1, XG-2, XG-6, XG-12, and XG-14 HMCLs and 105 cells/mL for XG-5, XG-7, and XG-20 HMCLs. Results are the mean luminescent signal determined in 6 replicate culture wells and are those from 1 of 3 representative experiments. The mean value was significantly different from that obtained in the control group using a Student t test (*P ≤ .05).
Growth activity of 5 factors in 8 HMCLs. HMCLs were starved for 2 hours and cultured without growth factor (group) or with either IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) or HB-EGF (1 μg/mL) or HGF (200 ng/mL) or APRIL (200 ng/mL) for 4 days in the Syn H culture medium. The cell concentration were 2 × 105 cells/mL for XG-1, XG-2, XG-6, XG-12, and XG-14 HMCLs and 105 cells/mL for XG-5, XG-7, and XG-20 HMCLs. Results are the mean luminescent signal determined in 6 replicate culture wells and are those from 1 of 3 representative experiments. The mean value was significantly different from that obtained in the control group using a Student t test (*P ≤ .05).
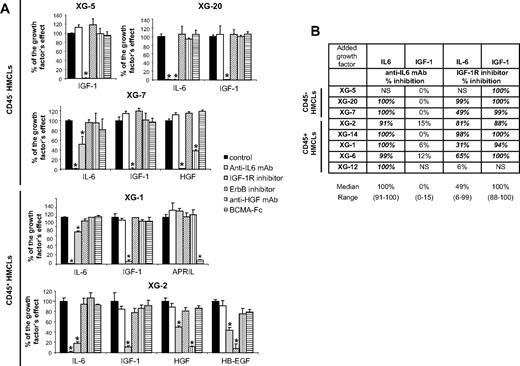
To investigate a possible cooperation of exogenous and autocrinely active MGF, the 8 HMCLs were coincubated with each of the 5 recombinant MGFs and each of the inhibitors of the 5 MGF. Detailed results for 5 HMCLs (XG-1, XG-2, XG-5, XG-7, XG-20) are shown in Figure 4A, and data for the 8 HMCLs summed up in Figure 4B. The IGF-1–induced stimulation of the 7 IGF-1-sensitive HMCLs was inhibited by IGF-1R inhibitor only (median inhibition, 100%; range, 88%-100%; P ≤ .05). The IL-6-induced stimulation of the 7 IL-6–sensitive HMCLs was inhibited by the B-E8 anti–IL-6 mAb (median inhibition, 100%; range, 91%-100%, P ≤ .05). IL-6–induced stimulation was also inhibited by IGF-1R inhibitor for 6 of 7 HMCLs (median inhibition, 73%; range, 31%-99%; P ≤ .05), unlike the XG-12 HMCL that did not express IGF-1R (Figure 4B). It was unaffected by the other 3 inhibitors (pan-ErbB kinase inhibitor, anti-HGF mAb, and BCMA-Fc BAFF/APRIL inhibitor). Two HMCLs are stimulated by HGF (XG-2 and XG-7), and the HGF effect was blocked by the anti-HGF mAb and the IGF-1R inhibitor (Figure 4A; P ≤ .05) and unaffected by IL-6, ErbB, and BAFF/APRIL inhibitors. The same is true for HB-EGF stimulation in XG-2 cells. It is inhibited by the ErbB kinase inhibitor and by IGF-1R inhibitor also. The XG-1 HMCL was stimulated by APRIL, and this effect was blocked by the BCMA-Fc APRIL inhibitor but not influenced by the other 4 inhibitors (Figure 4A; P ≤ .05).
IGF-1R inhibitor inhibited the effect of IL-6, HGF, and HB-EGF, unlike that of APRIL. (A) HMCLs were starved for 2 hours and cultured without cytokine or with either IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) or APRIL (200 ng/mL) or HGF (20 ng/mL) or HB-EGF (1 μg/mL) and without inhibitor or with anti–IL-6 mAb (10 μg/mL) or IGF-1R inhibitor (1 μM) or pan-ErbB kinase inhibitor (1 μM) or anti-HGF mAb (25 μg/mL) or BCMA-Fc (10 μg/mL) for 4 days in the Syn H culture medium. The cell concentrations were 2 × 105 cells/mL for XG-1 and XG-2 HMCLs and 105 cells/mL for XG-5, XG-7, and XG-20 HMCLs. Results are the mean luminescent signals ± SD determined in sextuplicate culture wells and are those of 1 experiment representative of 3. Data are expressed as percentage of the signal obtained with the growth factor. *The mean value was significantly different from that obtained in the control group using a Student t test (P ≤ .05). XG-5 HMCL was only stimulated by IGF-1 (2.8-fold), XG-20 HMCL by IL-6 (2.9-fold), or IGF-1 (3.5-fold), XG-7 HMCL by IL-6 (2.2-fold), IGF-1 (2.3-fold), or HGF (2.7-fold), XG-1 HMCL by IL-6 (11-fold), IGF-1 (5-fold), or APRIL (5-fold), and XG-2 by IL-6 (11-fold), IGF-1 (17-fold), HGF (17-fold), or HB-EGF (2-fold). (B) HMCLs were starved for 2 hours and cultured without cytokine or with IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) and without inhibitor or with an anti–IL-6 mAb (10 μg/mL) or an IGF-1R inhibitor (1 μM) for 4 days in the Syn H culture medium. Data are expressed as the mean percentage of the inhibition of the cytokine stimulation by the inhibitor in 3 independent experiments. When the percentages were different with a Student t test for pairs (P ≤ .05), data are shown in bold and italic. NS indicates not stimulated.
IGF-1R inhibitor inhibited the effect of IL-6, HGF, and HB-EGF, unlike that of APRIL. (A) HMCLs were starved for 2 hours and cultured without cytokine or with either IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) or APRIL (200 ng/mL) or HGF (20 ng/mL) or HB-EGF (1 μg/mL) and without inhibitor or with anti–IL-6 mAb (10 μg/mL) or IGF-1R inhibitor (1 μM) or pan-ErbB kinase inhibitor (1 μM) or anti-HGF mAb (25 μg/mL) or BCMA-Fc (10 μg/mL) for 4 days in the Syn H culture medium. The cell concentrations were 2 × 105 cells/mL for XG-1 and XG-2 HMCLs and 105 cells/mL for XG-5, XG-7, and XG-20 HMCLs. Results are the mean luminescent signals ± SD determined in sextuplicate culture wells and are those of 1 experiment representative of 3. Data are expressed as percentage of the signal obtained with the growth factor. *The mean value was significantly different from that obtained in the control group using a Student t test (P ≤ .05). XG-5 HMCL was only stimulated by IGF-1 (2.8-fold), XG-20 HMCL by IL-6 (2.9-fold), or IGF-1 (3.5-fold), XG-7 HMCL by IL-6 (2.2-fold), IGF-1 (2.3-fold), or HGF (2.7-fold), XG-1 HMCL by IL-6 (11-fold), IGF-1 (5-fold), or APRIL (5-fold), and XG-2 by IL-6 (11-fold), IGF-1 (17-fold), HGF (17-fold), or HB-EGF (2-fold). (B) HMCLs were starved for 2 hours and cultured without cytokine or with IL-6 (200 pg/mL) or IGF-1 (100 ng/mL) and without inhibitor or with an anti–IL-6 mAb (10 μg/mL) or an IGF-1R inhibitor (1 μM) for 4 days in the Syn H culture medium. Data are expressed as the mean percentage of the inhibition of the cytokine stimulation by the inhibitor in 3 independent experiments. When the percentages were different with a Student t test for pairs (P ≤ .05), data are shown in bold and italic. NS indicates not stimulated.
Expression of MGF receptors
The expression of 6 genes coding for receptor complexes of 4 of the 5 MGF (IGF-1R, IL-6R, gp130, c-Met, TACI, BCMA) could be evaluated with Affymetrix U133 2.0 Plus microarrays. IL-6R, gp130, and BCMA expressions are up-regulated throughout B-cell to plasma cell differentiation (P ≤ .05), unlike TACI. IGF-1R is not expressed in MBCs, PPCs, or BMPCs, but expressed in MMCs in a fraction of the 123 patients (Figure 5).
Gene expression profile of MGF receptors. Expression of IGF-1R, IL-6R, gp130, c-met, TACI, and BCMA genes were determined with Affymetrix human U133 Plus 2.0 in 6 memory B cells (MBCs), 7 normal PPCs, 7 normal BMPCs, MMCs of 123 patients with previously untreated MM, and 20 HMCLs. □ indicates that the gene had an absent Affymetrix call in the sample;  , the MBC samples;
, the MBC samples;  , the PPC samples; ▤, the BMPC samples; ■, the MMC samples; and
, the PPC samples; ▤, the BMPC samples; ■, the MMC samples; and  , the HMCLs.
, the HMCLs.
Gene expression profile of MGF receptors. Expression of IGF-1R, IL-6R, gp130, c-met, TACI, and BCMA genes were determined with Affymetrix human U133 Plus 2.0 in 6 memory B cells (MBCs), 7 normal PPCs, 7 normal BMPCs, MMCs of 123 patients with previously untreated MM, and 20 HMCLs. □ indicates that the gene had an absent Affymetrix call in the sample;  , the MBC samples;
, the MBC samples;  , the PPC samples; ▤, the BMPC samples; ■, the MMC samples; and
, the PPC samples; ▤, the BMPC samples; ■, the MMC samples; and  , the HMCLs.
, the HMCLs.
Prognostic value of MGF receptor expression
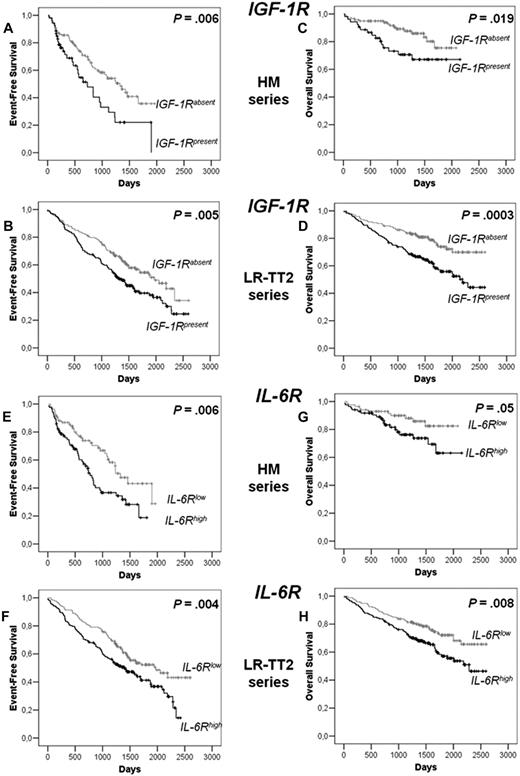
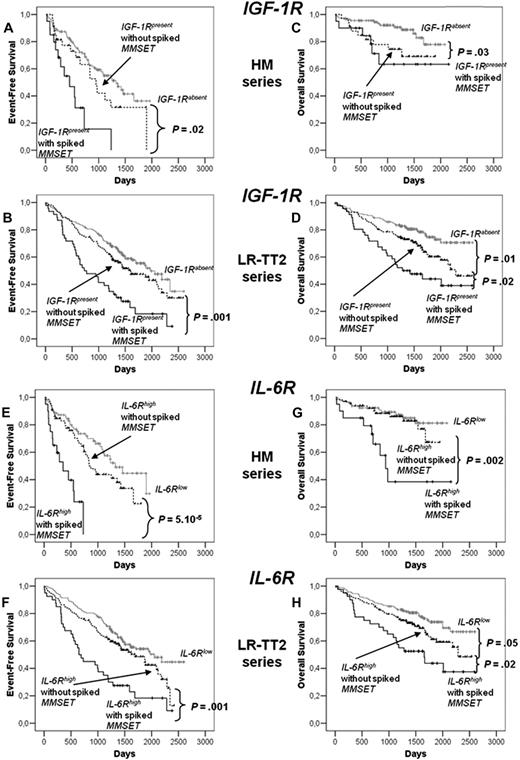
Among the 6 receptors, only IGF-1R (probe set 203627_at) and IL-6R (probe set 205945_at) expression had prognostic value in the 2 independent series of newly diagnosed patients, the HM series of 171 patients and LR-TT2 series of 345 patients. The IGF-1R probe set had a present call in the MMCs of 31% and 50% of patients in the HM and LR-TT2 series, respectively. Gp130, BCMA, and TACI were present in all MMC samples and c-Met in MMCs of 56% and 39% of patients of the 2 series, respectively. Patients with IGF-1Rabsent MMCs had a longer median event-free survival than patients with IGF-1Rpresent MMCs (P = .006 and .005, Figure 6A,B). The same holds true for patients with IL-6Rlow MMCs and IL-6Rhigh MMCs (P = .006 and .004; Figure 6E,F). The median EFS of the whole cohort in the 2 series were 1077 and 1604 days, respectively. Patients with IGF-1Rabsent MMCs had a longer OS than patients with IGF-1Rpresent in the 2 patient series (P = .02 and 3 × 10−4, Figure 6C,D). Patients with IL-6Rlow MMCs had also a longer OS than patients with IL-6Rhigh MMCs (P = .005 and .008; Figure 6G,H).
EFS and OS of patients with previously untreated MM with IGF-1Rabsent or IGF-1Rpresent MMCs and with IL-6Rlow and IL-6Rhigh MMCs.IGF-1R and IL-6R expression was assayed in purified MMCs with Affymetrix U133 microarrays. Patients from Heidelberg or Montpellier hospitals (HM series) were treated with high-dose chemotherapy and autologous stem cell transplantation. A total of 118 patients had IGF-1Rabsent MMCs and 53 IGF-1Rpresent MMCs, and 85 patients had IL-6Rlow MMCs and 85 IL-6Rhigh MMCs. We used also patient data from the Arkansas-Little Rock group (GEO accession number GSE2658). These patients from the Arkansas group were treated with total therapy 2 and termed for convenience LR-TT2 series. A total of 114 patients had IGF-1Rabsent MMCs and 136 IGF-1Rpresent MMCs, and 172 patients had IL-6Rlow MMCs and 172 IL-6Rhigh MMCs. EFS of IGF-1Rabsent MMCs and IGF-1Rpresent MMCs patients from HM series (A) and from LR-TT2 series (B). OS of IGF-1Rabsent MMC and IGF-1Rpresent MMC patients from the HM series (C) and from the LR-TT2 series (D). The P value was determined with a log-rank test. EFS of IL-6Rlow MMC and IL-6Rhigh MMC patients from the HM series (E) and from the LR-TT2 series (F). OS IL-6Rlow MMC and IL-6Rhigh MMC patients from the HM series (G) and from the LR-TT2 series (H). The P value was determined with a log-rank test.
EFS and OS of patients with previously untreated MM with IGF-1Rabsent or IGF-1Rpresent MMCs and with IL-6Rlow and IL-6Rhigh MMCs.IGF-1R and IL-6R expression was assayed in purified MMCs with Affymetrix U133 microarrays. Patients from Heidelberg or Montpellier hospitals (HM series) were treated with high-dose chemotherapy and autologous stem cell transplantation. A total of 118 patients had IGF-1Rabsent MMCs and 53 IGF-1Rpresent MMCs, and 85 patients had IL-6Rlow MMCs and 85 IL-6Rhigh MMCs. We used also patient data from the Arkansas-Little Rock group (GEO accession number GSE2658). These patients from the Arkansas group were treated with total therapy 2 and termed for convenience LR-TT2 series. A total of 114 patients had IGF-1Rabsent MMCs and 136 IGF-1Rpresent MMCs, and 172 patients had IL-6Rlow MMCs and 172 IL-6Rhigh MMCs. EFS of IGF-1Rabsent MMCs and IGF-1Rpresent MMCs patients from HM series (A) and from LR-TT2 series (B). OS of IGF-1Rabsent MMC and IGF-1Rpresent MMC patients from the HM series (C) and from the LR-TT2 series (D). The P value was determined with a log-rank test. EFS of IL-6Rlow MMC and IL-6Rhigh MMC patients from the HM series (E) and from the LR-TT2 series (F). OS IL-6Rlow MMC and IL-6Rhigh MMC patients from the HM series (G) and from the LR-TT2 series (H). The P value was determined with a log-rank test.
We found a link between clinical data and presence or absence of IGF-1R in MMCs: IgA subtype and serum level of lactate dehydrogenase and between IL-6Rhigh and IL-6Rlow MMCs (IgA subtype; P ≤ .05; Table 1). Of note, the frequency of patients with high LDH levels (an adverse prognostic factor) is increased in patients with IGF-1Rabsent MMCs (with a better prognosis). Other clinical data (age, light or heavy chain isotype, occurrence of bone lesions, serum levels of β2-microglobulin, albumin, hemoglobin, C-reactive protein, or ISS stage) were not significantly different between IGF-1Rpresent and IGF-1Rabsent or IL-6Rhigh and IL-6Rlow groups (Tables 1,2). Genetic abnormalities were assayed in 79 to 129 (depending on the abnormality) patients of the 171 HM patients' series (Table 3). Patient groups with MMCs showing a presence of t(4;14), 1q21, or del13 had a significantly increased frequency of IGF-1Rpresent MMCs or IL-6Rhigh MMCs, respectively, with del17 an increased frequency of patients with IGF-1Rpresent MMCs, with t(11;14) a decreased frequency of patients with IL-6Rhigh MMCs (P ≤ .05; Table 3). Thus, a possible explanation for the adverse prognosis of IGF-1Rpresent MMCs or IL-6Rhigh MMC groups is the high fraction of patients with the poor prognosis t(4;14) translocation in these groups. As documented, t(4;14) was not available for all our HM patients and for LR-TT2 patients, we used spiked MMSET expression to further analysis a link between spiked MMSET and IGF-1Rpresent MMCs or IL-6Rhigh MMCs; 15% (26 of 171) and 14% (49 of 345) of patients had spiked MMSET in HM and LR-TT2 series, respectively. Among the patients with spiked MMSET, 77% and 73% had also spiked FGFR3 expression in agreement with reported data.1,3,38 In the HM and LR-TT2 series, patients with spiked MMSET have an increased frequency of patients with IGF-1Rpresent MMCs compared with patients with unspiked MMSET (77% vs 23%, P = 2 × 10−14 for HM series and 94% vs 43%, P = 8 × 10−15 for LR-TT2 series) or with IL-6Rhigh MMCs (77% vs 45% P = 6 × 10−4 for HM series and 82% vs 45% P = 5 × 10−8 for LR-TT2 series). Considering the 8 patients subgroups defined by Zhan et al using GEP,3 the frequency of patients with IGF-1Rpresent MMCs is increased in proliferation (PR) and MMSET (MS) groups and decreased in hyperdiploid (HY) and CCND1/CCND3 (CD1) groups (P ≤ .05). The frequency of patients with IL-6Rhigh MMCs is increased in PR, LB, MS, and MF groups and decreased in CD1 and CD2 groups (P ≤ .05; Table 4). Of note, patients with IGF-1Rpresent MMCs and lacking spiked MMSET had decreased OS compared with patients with IGF-1Rabsent MMCs in the HM and LR-TT2 series but increased EFS (in the 2 series) and OS (in the LR-TT2 series) compared with patients with spiked MMSET and IGF-1Rpresent MMCs (Figure 7A-D). Because of the low number of patients with spiked MMSET and IGF-1Rabsent MMCs (6 and 3, respectively, in the 2 series) and of patients with spiked MMSET and IL-6Rlow (6 and 9, respectively, in the 2 series), their survival could not be evaluated. High IL-6R expression without spiked MMSET had prognostic value for OS compared with patients with IL-6Rlow MMCs in the LR-TT2 series only. Patients with spiked MMSET and IL-6Rhigh MMCs had decreased EFS and OS compared with patients with IL-6Rhigh MMCs and without spiked MMSET in the 2 series (Figure 7E-H). Using univariate Cox analysis, IGF-1Rabsent MMCs or spiked MMSET and IGF-1Rpresence MMCs had prognostic value for EFS and OS in the 2 patient series. MMCs with IGF-1Rpresence and lacking spiked MMSET had no prognostic value. Using multivariate Cox analysis, none of the 3 parameters had prognostic value for either EFS or OS in the 2 series (results not shown).
Clinical data of patients with IGF-1Rabsent and IGF-1Rpresent MMCs and of patients with IL-6Rlow and IL-6Rhigh MMCs
| Category . | IGF-1R, % of patients in each group . | IL-6R, % of patients in each group . | ||
|---|---|---|---|---|
| IGF-1Rabsent (n = 118), % . | IGF-1Rpresent (n = 53), % . | IL-6Rlow (n = 85), % . | IL-6Rhigh (n = 85), % . | |
| Age ≥ 65 y | 26 | 21 | 26 | 24 |
| Kappa light chain | 64 | 64 | 63 | 65 |
| Lambda light chain | 33 | 34 | 36 | 32 |
| Nonsecreting | 3 | 2 | 1 | 3 |
| B2M ≤ 3.5 mg/mL | 60 | 58 | 63 | 56 |
| B2M > 5.5 mg/mL | 17 | 17 | 19 | 15 |
| IgA subtype | 20* | 33* | 15* | 33* |
| LDH ≥ 240 IU/L | 26* | 12* | 24 | 19 |
| Albumin < 35 g/L | 37 | 36 | 44 | 31 |
| Hemoglobin < 10 g/dL | 31 | 32 | 29 | 34 |
| C-reactive protein ≥ 5 mg/L | 40 | 40 | 43 | 38 |
| Bone lesions | ||||
| 0, normal bone structure | 18 | 27 | 21 | 22 |
| 1, osteopenia/osteoporosis | 28 | 25 | 22 | 33 |
| 2, osteolyse (1–3) | 8 | 10 | 9 | 9 |
| 3, major structural damage (>3) | 46 | 38 | 48 | 36 |
| Category . | IGF-1R, % of patients in each group . | IL-6R, % of patients in each group . | ||
|---|---|---|---|---|
| IGF-1Rabsent (n = 118), % . | IGF-1Rpresent (n = 53), % . | IL-6Rlow (n = 85), % . | IL-6Rhigh (n = 85), % . | |
| Age ≥ 65 y | 26 | 21 | 26 | 24 |
| Kappa light chain | 64 | 64 | 63 | 65 |
| Lambda light chain | 33 | 34 | 36 | 32 |
| Nonsecreting | 3 | 2 | 1 | 3 |
| B2M ≤ 3.5 mg/mL | 60 | 58 | 63 | 56 |
| B2M > 5.5 mg/mL | 17 | 17 | 19 | 15 |
| IgA subtype | 20* | 33* | 15* | 33* |
| LDH ≥ 240 IU/L | 26* | 12* | 24 | 19 |
| Albumin < 35 g/L | 37 | 36 | 44 | 31 |
| Hemoglobin < 10 g/dL | 31 | 32 | 29 | 34 |
| C-reactive protein ≥ 5 mg/L | 40 | 40 | 43 | 38 |
| Bone lesions | ||||
| 0, normal bone structure | 18 | 27 | 21 | 22 |
| 1, osteopenia/osteoporosis | 28 | 25 | 22 | 33 |
| 2, osteolyse (1–3) | 8 | 10 | 9 | 9 |
| 3, major structural damage (>3) | 46 | 38 | 48 | 36 |
The 171 previously untreated patients with MM were treated at the university hospitals of Heidelberg or Montpellier. Patients were separated into 2 groups: patients with IGF-1Rabsent MMCs and patients with IGF-1Rpresent MMCs or patients with IL-6Rlow MMCs and IL6Rhigh MMCs, as assayed with Affymetrix microarrays. Data are the percentages of patients within these 2 groups with the indicated clinical or biologic parameters.
B2M indicates β2-microglobulin; and LDH, lactate dehydrogenase.
The percentages were different with a χ2 test (P ≤ .05).
ISS staging data
| . | IGF-1Rabsent (n = 118) . | IGF-1Rpresent (n = 53) . | IL-6Rlow (n = 85) . | IL-6Rhigh (n = 85) . |
|---|---|---|---|---|
| Stage I | 44 | 42 | 44 | 42 |
| Stage II | 36 | 42 | 35 | 41 |
| Stage III | 20 | 16 | 21 | 17 |
| . | IGF-1Rabsent (n = 118) . | IGF-1Rpresent (n = 53) . | IL-6Rlow (n = 85) . | IL-6Rhigh (n = 85) . |
|---|---|---|---|---|
| Stage I | 44 | 42 | 44 | 42 |
| Stage II | 36 | 42 | 35 | 41 |
| Stage III | 20 | 16 | 21 | 17 |
Values are stated as percentages.
Genetic abnormalities of patients with IGF-1Rabsent and IGF-1Rpresent MMCs and of patients with IL-6Rlow and IL-6Rhigh MMCs
| IGF-1R . | t(4;14)+ (n = 20), % . | t(4;14)− (n = 74), % . | IL-6R . | t(4;14)+ (n = 20), % . | t(4;14)− (n = 74), % . |
|---|---|---|---|---|---|
| IGF-1Rpresent | 70* | 26* | IL-6Rhigh | 85* | 53* |
| IGF-1Rabsent | 30* | 74* | IL-6Rlow | 15* | 47* |
| del13+ (n = 71), % | del13− (n = 59), % | del13+ (n = 71), % | del13− (n = 59), % | ||
| IGF-1Rpresent | 45* | 19* | IL-6Rhigh | 66* | 40* |
| IGF-1Rabsent | 55* | 81* | IL-6Rlow | 34* | 60* |
| del17+ (n = 24), % | del17− (n = 97), % | del17+ (n = 24), % | del17− (n = 97), % | ||
| IGF-1Rpresent | 58* | 27* | IL-6Rhigh | 46 | 58 |
| IGF-1Rabsent | 42* | 73* | IL-6Rlow | 54 | 42 |
| 1q21+ (n = 48), % | 1q21− (n = 66), % | 1q21+ (n = 48), % | 1q21− (n = 66), % | ||
| IGF-1Rpresent | 42* | 27* | IL-6Rhigh | 67* | 47* |
| IGF-1Rabsent | 58* | 73* | IL-6Rlow | 33* | 53* |
| t(11;14)+ (n = 15), % | t(11;14)− (n = 104), % | t(11;14)+ (n = 15), % | t(11;14)− (n = 104), % | ||
| IGF-1Rpresent | 33 | 33 | IL-6Rhigh | 40* | 57* |
| IGF-1Rabsent | 67 | 67 | IL-6Rlow | 60* | 43* |
| IGF-1R . | t(4;14)+ (n = 20), % . | t(4;14)− (n = 74), % . | IL-6R . | t(4;14)+ (n = 20), % . | t(4;14)− (n = 74), % . |
|---|---|---|---|---|---|
| IGF-1Rpresent | 70* | 26* | IL-6Rhigh | 85* | 53* |
| IGF-1Rabsent | 30* | 74* | IL-6Rlow | 15* | 47* |
| del13+ (n = 71), % | del13− (n = 59), % | del13+ (n = 71), % | del13− (n = 59), % | ||
| IGF-1Rpresent | 45* | 19* | IL-6Rhigh | 66* | 40* |
| IGF-1Rabsent | 55* | 81* | IL-6Rlow | 34* | 60* |
| del17+ (n = 24), % | del17− (n = 97), % | del17+ (n = 24), % | del17− (n = 97), % | ||
| IGF-1Rpresent | 58* | 27* | IL-6Rhigh | 46 | 58 |
| IGF-1Rabsent | 42* | 73* | IL-6Rlow | 54 | 42 |
| 1q21+ (n = 48), % | 1q21− (n = 66), % | 1q21+ (n = 48), % | 1q21− (n = 66), % | ||
| IGF-1Rpresent | 42* | 27* | IL-6Rhigh | 67* | 47* |
| IGF-1Rabsent | 58* | 73* | IL-6Rlow | 33* | 53* |
| t(11;14)+ (n = 15), % | t(11;14)− (n = 104), % | t(11;14)+ (n = 15), % | t(11;14)− (n = 104), % | ||
| IGF-1Rpresent | 33 | 33 | IL-6Rhigh | 40* | 57* |
| IGF-1Rabsent | 67 | 67 | IL-6Rlow | 60* | 43* |
Interphase FISH analysis was performed on CD138-purified plasma cells for 79 to 129 patients of the HM series. Patients were separated into 2 groups: patients with IGF-1Rabsent MMCs and patients with IGF-1Rpresent MMCs or patients with IL-6Rlow MMCs and IL-6Rhigh MMCs, as assayed with Affymetrix microarrays. Data are the percentages of patients within these 2 groups with the biologic parameters.
The percentages were different with a χ2 test (P ≤ .05).
IGF-1R and IL-6R expressions in MMCs according to the molecular classification of multiple myeloma
| . | All patients . | PR . | LB . | MS . | HY . | CD1 . | CD2 . | MF . | MY . |
|---|---|---|---|---|---|---|---|---|---|
| Patient subgroups | 100 | 8.4 | 9.0 | 12.2 | 18.8 | 6.4 | 11.9 | 6 | 27.5 |
| Frequency of patients with IGF-1Rpresent | 49.9 | 75.9* | 61.3 | 92.9* | 33.8* | 22.7* | 43.9 | 55 | 37.9 |
| Frequency of patients with IL-6Rhigh | 50 | 82.8* | 93.5* | 78.6* | 38.5 | 9.1* | 17.1* | 75.0* | 39.4 |
| . | All patients . | PR . | LB . | MS . | HY . | CD1 . | CD2 . | MF . | MY . |
|---|---|---|---|---|---|---|---|---|---|
| Patient subgroups | 100 | 8.4 | 9.0 | 12.2 | 18.8 | 6.4 | 11.9 | 6 | 27.5 |
| Frequency of patients with IGF-1Rpresent | 49.9 | 75.9* | 61.3 | 92.9* | 33.8* | 22.7* | 43.9 | 55 | 37.9 |
| Frequency of patients with IL-6Rhigh | 50 | 82.8* | 93.5* | 78.6* | 38.5 | 9.1* | 17.1* | 75.0* | 39.4 |
Values are percentages. The percentage of patients with IGF-1Rpresent and IL-6Rhigh MMC was determined in the 8 patient subgroups defined by Zhan et al.3
PR indicates proliferation; LB, low bone disease; MS, MMSET; HY, hyperdiploid; CD-1 and CD-2, CCND1/CCND3; MF, MAF/MAFB; and MY, myeloid group.
The percentages of patients IGF-1Rpresent or IL-6Rhigh MMCs in a subgroup were significantly different from that in all patients with a χ2 test (P ≤ .05).
EFS and OS of patients with previously untreated MM with IGF-1Rabsent or IGF-1Rpresent with or without spiked MMSET MMCs and with IL-6Rlow and IL-6Rhigh with or without spiked MMSET MMCs. Spiked MMSET was determined with Affymetrix U133 microarrays in the patients from HM series and LR-TT2 series (Spiked MMSET expression surrogating t(4;14)). In HM series, 112 patients had IGF-1Rabsent MMCs, 33 IGF-1Rpresent without spiked MMSET MMCs and 20 IGF-1Rpresent with spiked MMSET MMCs. A total of 79 patients had IL-6Rlow MMCs, 65 IL-6Rhigh without spiked MMSET MMCs, and 20 IL-6Rhigh with spiked MMSET MMCs. In the LR-TT2 series, 170 patients had IGF-1Rabsent MMCs, 126 IGF-1Rpresent without spiked MMSET MMCs, and 46 IGF-1Rpresent with spiked MMSET MMCs. A total of 163 patients had IL-6Rlow MMCs, 132 IL-6Rhigh without spiked MMSET MMCs, and 40 IL-6Rhigh with spiked MMSET MMCs. EFS of IGF-1Rabsent MMCs and IGF-1Rpresent with or without spiked MMSET MMCs patients from the HM series (A) and from the LR-TT2 series (B). OS of IGF-1Rabsent MMCs and IGF-1Rpresent with or without spiked MMSET MMC patients from the HM series (C) and from the LR-TT2 series (D). The P value was determined with a log-rank test. EFS of IL-6Rlow MMC and IL-6Rhigh with or without spiked MMSET MMC patients from the HM series (E) and from the LR-TT2 series (F). OS IL-6Rlow MMC and IL-6Rhigh MMC with or without spiked MMSET patients from HM series (G) and from LR-TT2 series (H). The P value was determined with a log-rank test.
EFS and OS of patients with previously untreated MM with IGF-1Rabsent or IGF-1Rpresent with or without spiked MMSET MMCs and with IL-6Rlow and IL-6Rhigh with or without spiked MMSET MMCs. Spiked MMSET was determined with Affymetrix U133 microarrays in the patients from HM series and LR-TT2 series (Spiked MMSET expression surrogating t(4;14)). In HM series, 112 patients had IGF-1Rabsent MMCs, 33 IGF-1Rpresent without spiked MMSET MMCs and 20 IGF-1Rpresent with spiked MMSET MMCs. A total of 79 patients had IL-6Rlow MMCs, 65 IL-6Rhigh without spiked MMSET MMCs, and 20 IL-6Rhigh with spiked MMSET MMCs. In the LR-TT2 series, 170 patients had IGF-1Rabsent MMCs, 126 IGF-1Rpresent without spiked MMSET MMCs, and 46 IGF-1Rpresent with spiked MMSET MMCs. A total of 163 patients had IL-6Rlow MMCs, 132 IL-6Rhigh without spiked MMSET MMCs, and 40 IL-6Rhigh with spiked MMSET MMCs. EFS of IGF-1Rabsent MMCs and IGF-1Rpresent with or without spiked MMSET MMCs patients from the HM series (A) and from the LR-TT2 series (B). OS of IGF-1Rabsent MMCs and IGF-1Rpresent with or without spiked MMSET MMC patients from the HM series (C) and from the LR-TT2 series (D). The P value was determined with a log-rank test. EFS of IL-6Rlow MMC and IL-6Rhigh with or without spiked MMSET MMC patients from the HM series (E) and from the LR-TT2 series (F). OS IL-6Rlow MMC and IL-6Rhigh MMC with or without spiked MMSET patients from HM series (G) and from LR-TT2 series (H). The P value was determined with a log-rank test.
Discussion
We selected 5 documented MGFs for which recombinant MGFs and inhibitors are commercially available to define a hierarchy of their biologic action on HMCLs. We have found that IGF-1 is the major MGF in agreement with several studies,9,42 IL-6 an important one, and that HGF, EGF family, and BAFF/APRIL act on a subset of HMCLs only. In serum-free cultures, only the 3 CD45− HMCLs could survive within 4 to 6 days of culture through an autocrine IGF-1/IGF-1R loop. These cells coexpressed IGF-1R and IGF-1 genes and IGF-1R and IGF-1 proteins, and the NVP-AEW541 IGF-1R inhibitor, unlike other MGF inhibitors, abrogated their survival. Regarding CD45+ HMCLs, although an autocrine IGF-1/IGF-1R loop was present in 4 of 5 HMCLs, it was not sufficient to promote survival. But this autocrine IGF-1/IGF-1R loop was necessary for the growth activity of IL-6, HB-EGF, or HGF when MMCs expressed IGF-1R. Adding a high concentration of IL-6 (up to 30 ng/mL) could not rescue from apoptosis because of IGF-1 pathway inhibition (data not shown). The specificity of NVP-AEW541 for IGF-1R targeting was previously reported36 and is emphasized here by its lack of effect on the IGF-1R− XG-12 HMCL and its lack of inhibition of IL-6 or HGF-induced transduction signals. IL-6 increases proliferation of 7 of 8 HMCLs tested, but interestingly its effect is dependent on the presence of an autocrine IGF-1/IGF-1R loop when MMCs expressed IGF-1R. IGF-1 is detected by Western blot in myeloma cells but could not be detected in HMCL culture supernatant. This does not preclude a bioactive role of autocrine IGF-1 because the bioactive concentration of rIGF-1 on HMCLs (27 pg/mL) is below the detection limit of commercially available IGF-1 ELISA (≥ 45 pg/mL). In addition, the survival of the CD45− HMCLs and the IL-6–induced stimulation of CD45+ HMCLs in serum-free medium are also blocked by recombinant IGF-binding protein 3 (IGFBP-3), another IGF-1 inhibitor (results not shown). To study the cooperation between IL-6 and IGF-1, different techniques have been used focusing on different aspects that may yield to challenging conclusions.10,25,26 Our current data did not confirm a previous study showing that the IL-6–induced growth of CD45+ HMCLs was not inhibited by an IGF-1R inhibitor.26 An explanation may be the use of fetal calf serum containing medium, which comprises IGF-1 but also insulin that stimulates MMC growth.43 We used here a serum-free culture medium, devoid of insulin, making it possible to unravel this major role of autocrine IGF-1. This matter is of great importance in view of anti–IGF-1 therapy. Indeed, the report by Descamps et al suggest that an anti–IGF-1R mAb therapy will be unable to target CD45+ MMCs, which include the proliferating MMCs.26 On the contrary, our data suggest that an IGF-1R inhibitor therapy could be useful in patients with IGF-1Rpresent MMCs, independently of CD45 expression. Only 2 of 8 HMCLs were stimulated by HGF, although c-Met is expressed by 7 of 8 HMCLs. Another HMCL is stimulated by HB-EGF, whereas 8 of 8 HMCLs expressed at least 1 of the 4 ErbB receptors.44 These effects were abrogated by the specific inhibitor of HGF or HB-EGF and also by the IGF-1R inhibitor, but not the anti–IL-6 mAb, BCMA-FC, and pan-ErbB kinase inhibitor (for HGF effect) or anti-HGF mAb (for HB-EGF effect). Thus, targeting IGF-1R could also help to block their activity. Only APRIL activity is not affected by IGF-1R inhibition. Of the 3 BAFF/APRIL receptors (BAFF-R, TACI, BCMA), MMCs expressed always BCMA, TACI in one-third of HMCLs, and rarely BAFF-R.45
These in vitro data fit well with the prognostic value of receptor expression of these 5 MGFs on MMCs because only IGF-1R and IL-6R expression has prognostic value using 2 independent patient series. IGF-1R gene is not expressed by normal B and plasma cells, including plasmablastic cells. Thus, IGF-1R is aberrantly expressed by 31% to 50% of MMCs of previously untreated patients. Of note, 90% of HMCLs expressed IGF-1R. HMCLs are mainly obtained from patients with extramedullary proliferation,31,46 and this increased frequency of IGF-1Rpresence in HMCLs compared with that in primary MMCs may reflect an increase frequency of IGF-1Rpresent MMCs in patients with extramedullary proliferation. Alternatively, it might be the result of the way of obtaining HMCLs using culture medium and serum that contain large amount of circulating IGF-1, thus favoring the growth of IGF-1Rpresent MMCs.
Presently, no conclusive data have been published regarding the prognostic value of IGF-1R on MMCs.29,30 We have shown here that IGF-1R expression is prognostically significant in 2 independent large sets of patients obtained in 2 centers, using different methods for the Affymetrix probe preparation (single or double in vitro transcription amplification) and 2 different Affymetrix platforms.3,47 The poor prognosis of patients with IGF-1Rpresent MMCs is not only explained by a strong association of IGF-1Rpresent MMCs and poor prognosis t(4;14) translocation and spiked MMSET expression.1 Indeed, patients with IGF-1Rpresent MMCs and unspiked MMSET had also a significantly shorter survival than patients with IGF-1Rabsent MMCs. This might be explained by the increased proportion of patients with IGF-1Rpresent MMCs in the poor prognosis proliferation group (75.9% vs 49.9%)3 and in patients with del17, another poor prognosis abnormality1 that occurs independently of t(4;14). Of note, we show here that IGF-1 is a major factor driving the proliferation of MMCs, which could account for the proliferation signature. Patients with both IGF-1Rpresent MMCs and t(4;14) had the shortest survival. A possible explanation is that patients with t(4;14) need to acquire additional aberrations (eg, aberrant expression of IGF-1R) for the outbreak of overt MM.
MMCs are “bathed” in high levels of IGF-1 in the tumor milieu in vivo. First, IGF-1 is directly produced in the bone marrow, by MMCs and by osteoclasts. In addition, high levels of IGF-1, bound to IGFBP-3 and ALS protein, circulate in patients with MM and healthy persons,48 and serum levels of IGF-1 correlated with poor prognosis in patients with MM.28 These circulating IGF-1-IGFBP-3-ALS complexes can be captured by MMCs that expressed highly syndecan-1, which bind IGFBP-3.48 IGFBP-3 binding to heparan sulfate chains weakens its affinity with IGF-1, which is thus able to bind membrane IGF-1R and exert its biologic activity. In addition, MMCs produce soluble syndecan-1, in particular though a heparanase-controlled process,45,49,50 providing an extracellular matrix able to bind circulating IGF-1-IGFBP complexes and to release IGF-1 close to MMCs.
IL-6R is variably expressed in MMCs of all patients with MM. Dividing MM patients within 2 groups using IL-6R median expression, we found that patients with IL-6Rhigh MMCs had a shorter survival. This might be explained by the increased proportion of patients of poor prognosis groups (proliferation, MAF, and MMSET groups)3 in the IL-6Rhigh group. Patients with both IL-6Rhigh MMCs and t(4;14) had a worse survival.
A message of this study is not that IGF-1R expression can be useful to define new prognostic classification, as the adverse prognosis value of IGF-1R expression is explained mainly by their expression in already identified poor prognosis groups, ie, t(4;14), del17 and proliferation groups. But a message is that the adverse prognosis value of IGF-1R expression in MMCs together with its major MGF activity emphasize that targeting IGF-1 could be promising for the treatment of patients with MM. A phase 1 study of anti–IGF-1R antibody therapy in patients with refractory MM was recently reported.51 This trial showed no toxicity and disease stabilization in approximately half of the patients. Because IGF-1R is present on MMC of 30% to 50% of the newly diagnosed patients, IGF-1R expression on MMCs should be evaluated in patients treated with anti–IGF-1 therapy. Anti–IL-6 mAb treatment was also shown to block MMC proliferation with temporary disease stabilization.52 Thus, anti–IL-6 therapy could be a useful combination with an IGF-1 inhibitor.
In conclusion, this study makes it possible to define a hierarchy of the biologic action of 5 well-documented MGFs on HMCLs, with IGF-1 being the major one, IL-6 an important one, and HGF, EGF family, and BAFF/APRIL acting only on a subset of HMCLs. Of interest, this hierarchy of biologic activity of these 5 MGFs using HMCLs fully paralleled with the prognostic value of the expression of the genes of the receptors of these MGFs in MMCs because IGF-1R and IL-6R expression in MMCs had prognostic value. Thus, gene expression profiles of MMCs and of the tumor environment are highly recommended for a better understanding and anticipation of the efficacy of growth factor-targeted therapy in patients with MM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Ligue Nationale Contre le Cancer (Équipe Labellisée), Paris, France; Instituto Nacional de Cancer (R07001FN), European Myeloma Stem Cell Network European Strep (E06005FF), the Hopp-Foundation, Germany; the University of Heidelberg, Heidelberg, Germany; the National Center for Tumor Diseases, Heidelberg, Germany; and the Tumorzentrum Heidelberg/Mannheim, Heidelberg, Mannheim, Germany. A.C.S. is supported by a grant from Guillaume Espoir (St Genis-Laval, France).
Authorship
Contribution: A.C.S. designed research, performed the experiments, and wrote the paper; D.H., A.S., J.M., M.H., M.J., T.M., A.J., K.M., U.B., J.F.R., and H.G. collected bone marrow samples and clinical data; L.C. provided some new reagents; T.R. and A.K. participated in the analyzing of the data; J.S. and B.B. provided GEP and patient data and participated in the writing of the paper; D.H. and H.G. participated in the writing of the paper; and B.K. is the senior investigator who designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernard Klein, Inserm U847, Institute for Research in Biotherapy, Centre Hospitalier Universitaire Montpellier, Hospital St Eloi, Av Augustin Fliche, 34295 Montpellier, France; e-mail: bernard.klein@inserm.fr.