Abstract
Activated protein C (APC) reduces mortality in severe sepsis patients. APC exerts anticoagulant activities via inactivation of factors Va and VIIIa and cytoprotective activities via endothelial protein C receptor and protease-activated receptor-1. APC mutants with selectively altered and opposite activity profiles, that is, greatly reduced anticoagulant activity or greatly reduced cytoprotective activities, are compared here. Glu149Ala-APC exhibited enhanced in vitro anticoagulant and in vivo antithrombotic activity, but greatly diminished in vitro cytoprotective effects and in vivo reduction of endotoxin-induced murine mortality. Thus, residue Glu149 and the C-terminal region of APC's light chain are identified as functionally important for expression of multiple APC activities. In contrast to Glu149Ala-APC, 5A-APC (Lys191-193Ala + Arg229/230Ala) with protease domain mutations lacked in vivo antithrombotic activity, although it was potent in reducing endotoxin-induced mortality, as previously shown. These data imply that APC molecular species with potent antithrombotic activity, but without robust cytoprotective activity, are not sufficient to reduce mortality in endotoxemia, emphasizing the need for APC's cytoprotective actions, but not anticoagulant actions, to reduce endotoxin-induced mortality. Protein engineering can provide APC mutants that permit definitive mechanism of action studies for APC's multiple activities, and may also provide safer and more effective second-generation APC mutants with reduced bleeding risk.
Introduction
Human plasma proteins have been therapeutically useful since the 1940s, and their recombinant engineered analogs, such as coagulations factors VIII, IX, VIIa, and activated protein C (APC), are clinically used.1-3 Major challenges and opportunities for translational bench-to-bedside research to improve therapy are centered on improving nature's design in second-generation biologic agents. Notable success has been achieved for engineered second- and third-generation thrombolytic proteins for acute myocardial infarction and ischemic stroke that have prolonged half-life and gain of function for greater specific activity.4 For plasma proteins that have multiple distinct activities, such as thrombin5 or APC,6 there is an opportunity to engineer selectively one or another biologic activities, thereby retaining or enhancing a desired property while diminishing adverse side effects. Such a strategy is particularly relevant for APC because it is approved by the Food and Drug Administration for mortality reduction in severe sepsis patients, but its application is associated with serious bleeding risk.3,7
APC's multiple distinct activities encompass anticoagulant activities involving the irreversible proteolytic inactivation of factors Va and VIIIa as well as nonanticoagulant effects of APC acting directly on cells, collectively referred to as the protein C cytoprotective pathway.6,8-10 In reactions mediated by the endothelial protein C receptor (EPCR) and protease-activated receptor-1 (PAR-1), APC acts directly on cells to exert multiple cytoprotective effects including the following: (a) alteration of gene expression profiles; (b) anti-inflammatory activities; (c) antiapoptotic activity; and (d) protection of endothelial barrier function.11-15
Because APC manifests both potent anticoagulant activity and multiple cytoprotective activities,6 we previously made an engineered APC mutant with markedly reduced anticoagulant activity, but unaltered cytoprotective activities, designated 5A-APC, and showed that it was as effective as wild-type (wt) APC in reducing mortality in murine sepsis models.16,17 However, because murine (m) 5A-APC retains low, but significant anticoagulant activity, questions pertaining to the roles and contributions of APC's anticoagulant activity for reduction of mortality in severe sepsis remain to be answered. For instance, is the anticoagulant activity of APC both responsible for success in the Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial and also for the increased risk for serious bleeding in severe sepsis patients, or is anticoagulant activity dispensable without loss of APC efficacy for mortality reduction?3,18 Levi has summarized arguments in favor of a prominent role for the anticoagulant properties of APC in its beneficial effect.19 In this study, we show that protein engineering of APC helps to address these questions and permits in vitro and in vivo studies of APC's various activities in a manner that may decipher the importance or significance of APC's various activities in the course of in vitro and in vivo investigations.
Methods
Recombinant APCs
Mutagenesis, expression, purification of recombinant protein Cs, and generation of APC.
The E149A mutation was introduced using Quickchange mutagenesis (Stratagene, La Jolla, CA) using human or murine wt protein C in pcDNA3.1+neo (Invitrogen, Carlsbad, CA).20 Sequencing of the protein C coding region confirmed accuracy of the mutagenesis. Human and murine wt-, 5A-, and E149A-APC were prepared, purified, and converted to APC, as previously described (for more details see supplemental methods, available on the Blood website; see the Supplemental Materials link at the top of the online article).17,20-23 All protein C mutants showed typical expression levels of 2 to 5 mg/L with approximately 35% recovery of APC after purification and activation. APC concentrations were determined by active site titration according to Chase and Shaw, with minor modifications.20,24 Kinetic data for activation of purified zymogen E149A-human (h) PC by thrombin or by thrombin-thrombomodulin were indistinguishable from wt-hPC (data not shown). Furthermore, inhibition of E149A-hAPC by plasma protease inhibitors, as determined by the half-life of APC's amidolytic activity in plasma,25 was indistinguishable from inhibition of wt-hAPC (half-lives 22 minutes vs 20 minutes, respectively), indicating that the mutation did not have any detectable global effect on conformation and/or folding of the region around the APC active site.
APC in vitro assays
Coagulation assays.
Amidolytic assays, activated partial thromboplastin time (APTT) clotting assays, and endogenous thrombin generation (ETP) assays were performed using normal pooled plasma (George King) or protein S-depleted plasma (Enzyme Research Labs, South Bend, IN) supplemented with protein S (Enzyme Research Labs).17,26 The time course of APC-mediated inactivation of factor Va (fVa; Hematology Technologies, Essex Junction, VT) was determined by following the fVa cofactor function using prothrombinase assays.27
Cytoprotective activity assays.
APC anti-inflammatory activity was determined as the inhibition of cytokine release (interleukin-6 [IL-6]) by APC of lipopolysaccharide (LPS)–stimulated U937 cells.17 APC antiapoptotic activity was determined as the inhibition of staurosporine-induced EA.hy926 endothelial cell apoptosis, as described.12,17
EPCR-binding assays.
EPCR was cloned from EA.hy926 endothelial cells (see supplemental methods for details). Because the EA.hy926 endothelial cells were found to encode for the A3 EPCR haplotype, residue Gly219 was mutated to Ser found in the more common A1 haplotype using Quickchange mutagenesis. The sequence of the resulting wt-EPCR was verified and transfected into HEK-293 cells. Binding of wt-hAPC, 5A-hAPC, or E149A-hAPC to wt-EPCR-transfected cells was done as described using EDTA (ethylenediaminetetraacetic acid) elution and appropriate APC standard curves.28 Soluble EPCR (sEPCR) was generated by replacing the start of the transmembrane domain at Leu211 with a 6×His tag and produced in HEK-293 cells (see supplemental methods for details). APC binding to sEPCR was determined by surface plasmon resonance (SPR) using a Biacore 3000 biosensor system (BIAcore). An anti-His tag antibody (Immunology Consultants Laboratory, Newberg, OR) or a nonreactive control rabbit IgG was immobilized on a CM5 sensor chip (BIAcore; GE Healthcare, Piscataway, NJ) using NH2-coupling chemistry, according to the manufacturer's instructions. sEPCR (120 μg/mL) was injected at a flow rate of 5 μL/min with 12 minutes contact time generating a response ≈3500 RU. Increasing concentrations of wt-hAPC or E149A-hAPC were injected for 1.5 minutes at a flow rate of 5 μL/min. The association and dissociation phases of all sensorgrams were fitted globally using the biaevaluation software 3.0 (BIAcore). Kinetics of sEPCR binding to wt-hAPC/E149A-hAPC were determined using a 1:1 Langmuir-binding model. After each set of experiments, the anti-His tag antibody surface was regenerated with 10 mM glycine/HCl, pH 2.5. Any influence of mass transport effects was discounted from results of binding and dissociation at different flow rates.
PAR-1 cleavage assays.
Cleavage of the PAR-1 synthetic peptide representing residues 33-62 of the PAR-1 N-terminal tail by APC was performed as described.26 A PAR-1 cleavage reporter construct was made in which a secreted alkaline phosphatase (SEAP) was fused to the N terminus of PAR-1, which is released by proteolysis by APC or thrombin at residue Arg41 (see supplemental methods for details), similar to a truncated PAR-1 construct.29 SEAP-PAR1, either in the presence or absence of wt-EPCR, was transfected into HEK-293 cells to obtain stable cells expressing SEAP-PAR1 or wt-EPCR/SEAP-PAR1. E149A-APC or wt-APC was incubated with SEAP-PAR1 cells and wt-EPCR/SEAP-PAR1 cells in Hanks' balanced salt solution supplemented with 1.3 mM CaCl2, 0.6 mM MgCl2, and 0.1% BSA in 48-well plates. After 60 minutes, SEAP release was determined using 1-step p-nitrophenyl phosphate (Pierce). Values were corrected for background activity derived from the same cells incubated in the absence of APC. Endothelial cell surface PAR-1 was quantified as described.30
APC in vivo assays
Thrombosis model.
Antithrombotic potential of wt-mAPC, E149A-mAPC, and 5A-mAPC was tested in an acute carotid artery thrombosis model.31 Carotid blood flow was monitored for 30 minutes after a 10% FeCl3 (3-minute)-induced injury, and occlusion was defined as flow less than 0.2 mL/min. APC was administered in the jugular vein (t = 0). To determine the specificity of the administered APC for the observed antithrombotic effects, an inhibitory rat anti–murine protein C monoclonal antibody was used that blocked all APC activities (TVM1; J. Fernández and J.H.G., unpublished data, June 2008).
Endotoxemia model.
Survival was measured in mice given a 90% lethal dose (LD90) of endotoxin via intraperitoneal injection, followed by immediate treatment with 10 μg (0.33 mg/kg), 2 μg (0.067 mg/kg), or 0.2 μg (0.0067 mg/kg) of mAPC.16 Significance was determined using the log-rank test. All animal studies were approved by the Scripps Research Institute Institutional Review Board.
Statistical analysis
Survival analysis was performed using Kaplan-Meier plots using the log-rank test to determine whether the difference between survival curves was significant. A P value of less than .05 was considered significant. Statistical computations were performed using GraphPad Prism software, version 4.03 (GraphPad, San Diego, CA).
Results
Identification and characterization of APC mutants with specifically altered activity profiles of anticoagulant versus cytoprotective activities
Positive charged sequences on APC's surface that are remote from the active site, termed exosites, facilitate APC's interactions with cofactors and substrates to promote proteolysis. Because the substrate for APC's anticoagulant activity (fVa) is different from the substrate for APC's cytoprotective activity (PAR-1), exosite residues on APC that mediate the interaction with these substrates are most likely different, at least in part. Our previous studies focused on identifying APC exosite residues that affected anticoagulant activity of APC, but not cytoprotective activity of APC. Mutation of 5 basic residues (Lys191, Lys192, Lys193, Arg229, and Arg230) to Ala (designated 5A-APC) in 2 exposed surface loops of APC comprising part of the fVa exosite on APC was found to attenuate greatly anticoagulant activity, but not to alter normal antiapoptotic activity and normal ability to reduce mortality in murine sepsis models, which still required PAR-1 and EPCR.16,17,26
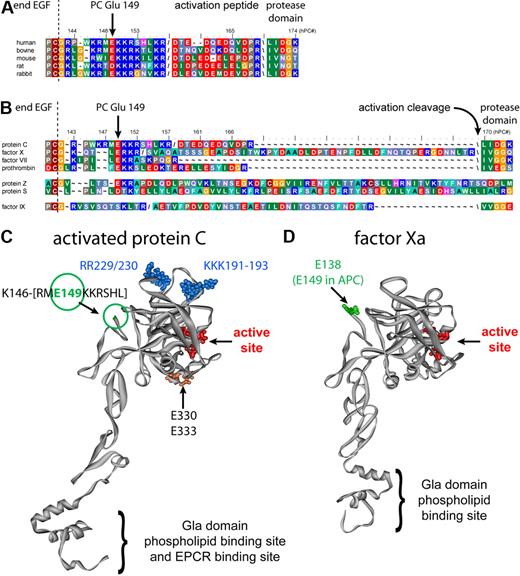
Screening additional surface-exposed regions in hAPC for potential differential contribution to anticoagulant versus cytoprotective activities yielded unexpected results for the C-terminal region of the protein C light chain. Normal processing of single chain protein C involves removal of Lys156-Arg157 giving a 2-chain molecule. The C terminus of the protein C light chain is a highly charged region that comprises residues Gly142-Leu155 on the opposite side of the active site in the protease domain (Figure 1C) that is well conserved among protein C from different species (Figure 1A). This sequence seems to be a recurrent motif in vitamin K–dependent coagulation factors, with the exception of factor IX (Figure 1B). Previously, a protein C complementary peptide corresponding to residues 142 to 155 implicated this region to be important for anticoagulant activity.32 Alanine-scanning mutagenesis of the individual charged residues in this region indicated that substitution of the basic residues by Ala generally resulted in moderate effects on anticoagulant and cytoprotective activities (L.D.M., J.H.G., unpublished data, June 2008). Surprisingly, replacement of negatively charged Glu149 by Ala (E149A-APC) resulted in markedly enhanced anticoagulant activity in combination with defective cytoprotective activities.
Analysis of highly conserved residue Glu149 in Protein C. (A) Conservation of the C-terminal light chain residues among various protein C species. Forward slash indicates the furin-like cleavage site for posttranslational processing of single-chain protein C into 2 chain; backward slash indicates the activation cleavage site to generate APC. Numbering is for human protein C. (B) The residue corresponding to Glu149 in protein C is conserved in all vitamin K-dependent coagulation factors, with the exception of factor IX. Numbering is for human protein C. (C) Molecular model of APC based on the crystal structure of the protease domain. The C terminus of the protein C light chain was not defined in the x-ray crystallographic structure beyond residue Lys146; hence, the green circle indicates the approximate location of Glu149. Other key residues indicated are the active site catalytic triad in red, 5A-APC substituted residues (Arg229 and Arg230 in the Ca2+ binding loop and Lys191, Lys192, and Lys193 in loop 37) in blue, and Glu330 and Glu333, which are apparently required for PAR-1 cleavage by APC, in orange.29 (D) Location of factor Xa residue Glu138 (green), corresponding to protein C Glu149, in a factor Xa model based on its crystallographic structure. The catalytic triad is indicated in red.
Analysis of highly conserved residue Glu149 in Protein C. (A) Conservation of the C-terminal light chain residues among various protein C species. Forward slash indicates the furin-like cleavage site for posttranslational processing of single-chain protein C into 2 chain; backward slash indicates the activation cleavage site to generate APC. Numbering is for human protein C. (B) The residue corresponding to Glu149 in protein C is conserved in all vitamin K-dependent coagulation factors, with the exception of factor IX. Numbering is for human protein C. (C) Molecular model of APC based on the crystal structure of the protease domain. The C terminus of the protein C light chain was not defined in the x-ray crystallographic structure beyond residue Lys146; hence, the green circle indicates the approximate location of Glu149. Other key residues indicated are the active site catalytic triad in red, 5A-APC substituted residues (Arg229 and Arg230 in the Ca2+ binding loop and Lys191, Lys192, and Lys193 in loop 37) in blue, and Glu330 and Glu333, which are apparently required for PAR-1 cleavage by APC, in orange.29 (D) Location of factor Xa residue Glu138 (green), corresponding to protein C Glu149, in a factor Xa model based on its crystallographic structure. The catalytic triad is indicated in red.
Anticoagulant activity of E149A-APC
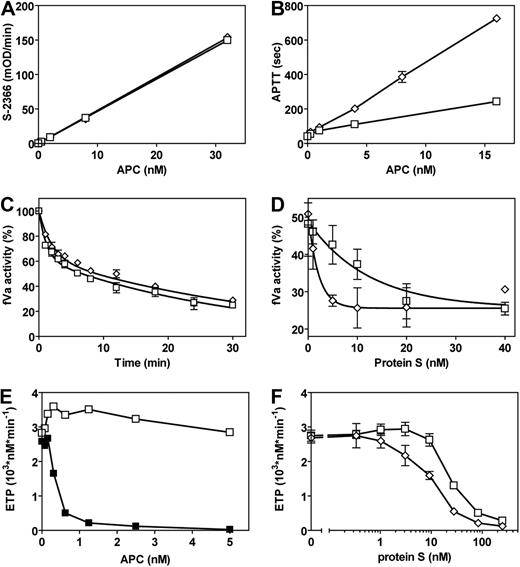
After purification, activation, and active enzyme concentration determination by active site titration, the amidolytic activities of E149A-hAPC and wt-hAPC for small substrates S-2366 (Figure 2A), Pefachrome PCa, and Spectrozyme aPC (Table S1) were assayed and were indistinguishable. Compared with wt-hAPC, E149A-hAPC had more than 3-fold increased anticoagulant activity in APTT clotting assays (Figure 2B).
Amidolytic and anticoagulant activities of E149A-APC. (A) Amidolytic activity of wt-hAPC (□) and E149A-hAPC (◇) for a small chromogenic substrate (S-2366). (B) APC anticoagulant activity of E149A-hAPC (◇) compared with wt-hAPC (□) in APTT clotting assays. (C) Time course of fVa inactivation by wt-hAPC (□) and E149A-hAPC (◇). After fVa incubation with APC for the times indicated, remaining fVa activity was determined in a prothrombinase assay. (D) The effect of protein S on the inactivation of fVa by wt-hAPC (□) and E149A-hAPC (◇). (E) wt-hAPC anticoagulant activity in protein S-depleted plasma reconstituted with (■) or without (□) protein S measured by the ETP method. (F) Anticoagulant effects of wt-hAPC (□) and E149A-hAPC (◇) in protein S-depleted plasma reconstituted with protein S. Each point represents the mean plus or minus SEM of at least 3 independent experiments.
Amidolytic and anticoagulant activities of E149A-APC. (A) Amidolytic activity of wt-hAPC (□) and E149A-hAPC (◇) for a small chromogenic substrate (S-2366). (B) APC anticoagulant activity of E149A-hAPC (◇) compared with wt-hAPC (□) in APTT clotting assays. (C) Time course of fVa inactivation by wt-hAPC (□) and E149A-hAPC (◇). After fVa incubation with APC for the times indicated, remaining fVa activity was determined in a prothrombinase assay. (D) The effect of protein S on the inactivation of fVa by wt-hAPC (□) and E149A-hAPC (◇). (E) wt-hAPC anticoagulant activity in protein S-depleted plasma reconstituted with (■) or without (□) protein S measured by the ETP method. (F) Anticoagulant effects of wt-hAPC (□) and E149A-hAPC (◇) in protein S-depleted plasma reconstituted with protein S. Each point represents the mean plus or minus SEM of at least 3 independent experiments.
Inactivation of fVa was analyzed to determine whether the increased anticoagulant activity of E149A-hAPC was derived from enhanced proteolysis of fVa. APC-mediated fVa inactivation was monitored by the loss of fVa cofactor activity in the prothrombinase complex using a system consisting of purified components. Remarkably, the time course for inactivation of fVa by E149A-hAPC was similar to that of wt-hAPC (Figure 2C). One important difference between the APTT assay and the purified fVa inactivation assay was the presence and absence of APC's cofactor, protein S. Addition of purified protein S differentially impacted inactivation of fVa by E149A-hAPC compared with wt-hAPC (Figure 2D). E149A-hAPC required approximately a 6-fold lower concentration of protein S to achieve half-maximal stimulation of fVa inactivation compared with wt-hAPC (50% effective concentration (EC50) = 1.3 nM vs 8.6 nM protein S for E149A-hAPC and wt-hAPC, respectively). A similar difference was observed for maximal stimulation of fVa inactivation by protein S (6 nM vs 40 nM protein S for E149A-hAPC and wt-hAPC, respectively).
To confirm that different sensitivity toward protein S cofactor activity could explain the observed increased anticoagulant activity of E149A-hAPC in plasma, tissue factor–induced thrombin formation during and after clot formation was monitored using the ETP method.33 The ETP method reflects not only the small amounts of thrombin that are sufficient for fibrin clot formation (ie, primary-initial thrombin formation measured by traditional clotting assays), but also the secondary-extended thrombin generation after initial clot formation. As reported, wt-hAPC readily inhibited thrombin generation in normal plasma (50% inhibitory concentration = 0.5 nM).17 However, in protein S–depleted plasma in ETP studies, wt-hAPC at 1.25 nM had no significant effect on inhibition of thrombin formation in the absence of protein S, but when deficient plasma was reconstituted with 250 nM protein S, 1.25 nM wt-hAPC completely inhibited thrombin formation (Figure 2E). In reconstitution studies, E149A-hAPC required less protein S than wt-hAPC to achieve half-maximal inhibition of thrombin generation compared with wt-hAPC (EC50 = 11.7 nM vs 31.3 nM protein S for E149A-hAPC and wt-hAPC, respectively). In the absence of protein S, no differences were observed between E149A-hAPC and wt-hAPC in protein S–depleted plasma, indicating equivalent anticoagulant activities for wt-hAPC and E149A-hAPC in plasma without protein S (Figure 2F). These results show that E149A-hAPC exhibited increased anticoagulant activity in plasma associated with an enhanced sensitivity for protein S cofactor activity.
In vivo antithrombotic activity of E149A-APC
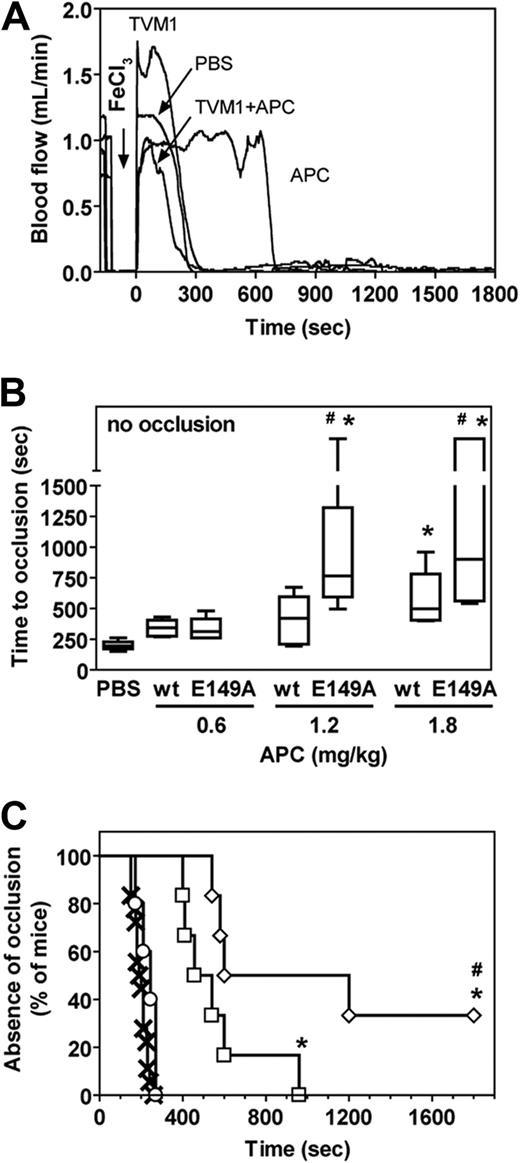
The wt-mAPC and murine heterologous APC mutants E149A-mAPC and 5A-mAPC (Lys192-194Ala + Arg230/231Ala) were used to determine in vivo antithrombotic potency in an acute carotid artery thrombosis model in mice after FeCl3-induced injury.31 The E149A-mAPC (Figure S1) and 5A-mAPC16 showed characteristics for anticoagulant activity and cytoprotective activity that were indistinguishable from their human counterparts. Under the conditions used, first occlusion occurred within 4 minutes in the absence of mAPC treatment (Figure 3), indicating the severity of the thrombotic injury. Time to first occlusion was prolonged 3-fold by a prior bolus injection of 1.8 mg/kg wt-mAPC. An APC activity-blocking rat anti–mouse protein C antibody (TVM1) that blocks all APC activities (amidolytic, anticoagulant, and cytoprotective) inhibited the antithrombotic effects of wt-mAPC (Figure 3A), indicating that the antithrombotic effects conveyed by wt-mAPC in this system were dependent on APC-mediated proteolysis. The time to first occlusion was effectively delayed by wt-mAPC in a dose-dependent manner (Figure 3B). The E149A-mAPC variant that exhibited potent in vivo antithrombotic activity was superior to wt-mAPC based on the delayed time to first occlusion (Figure 3C). Absence of appreciable occlusion in 2 of 6 E149A-mAPC–treated mice (1.8 mg/kg) versus 0 of 6 wt-mAPC–treated animals further illustrates its improved antithrombotic potential in vivo (Figure 3B). Thus, E149A-APC showed hyperactive anticoagulant activity in plasma-clotting assays as well as hyperactive antithrombotic potency in vivo. In contrast, 5A-mAPC failed to delay time to first occlusion even when tested at 4-fold higher concentrations (8.0 mg/kg), confirming that 5A-mAPC conveys no appreciable antithrombotic effects in vivo in this model (Figure 3C).
In vivo antithrombotic activity of E149A-APC. Antithrombotic effects of wt-mAPC, E149A-mAPC, and 5A-mAPC in a murine model of carotid artery thrombosis induced by FeCl3 injury were based on the time to first occlusion. (A) Doppler tracing of carotid artery blood flow after FeCl3 injury. PBS buffer control or wt-mAPC (1.8 mg/kg) in the presence and absence of a blocking rat monoclonal anti-mAPC antibody (TVM1) was administered in the jugular vein just before the thrombotic insult. Carotid artery blood flow was monitored for 30 minutes after application of FeCl3. Occlusion was defined as a flow less than 0.2 mL/min. (B) Time to first occlusion in mice treated with increasing doses of wt-mAPC or E149A-mAPC (0.6 mg/kg, 1.2 mg/kg, or 1.8 mg/kg) shown as a box-and-whiskers graph with median, 25th percentile, 75th percentile, and range. Points on discontinuous part of the y-axis above 1500 seconds indicate no occlusion within the 30 minutes experimental period. Statistical analysis was performed using analysis of variance with Bonferroni correction: * P < .05 APC versus PBS; # P < .05 E149A-APC versus wt-APC. (C) Occurrence of first occlusion versus time in mice treated with PBS (×), wt-mAPC (□) or E149A-mAPC (◇; both 1.8 mg/kg), or 5A-mAPC (○; 8.0 mg/kg) shown as a modified Kaplan-Meier plot. Statistical analysis was performed using the log-rank test: * P < .05 APC versus PBS; # P < .05 E149A-APC versus wt-APC. (B,C) Animals per group: PBS, n = 18; wt-mAPC, n = 6-7; E149A-mAPC, n = 4-6; and 5A-mAPC, n = 5.
In vivo antithrombotic activity of E149A-APC. Antithrombotic effects of wt-mAPC, E149A-mAPC, and 5A-mAPC in a murine model of carotid artery thrombosis induced by FeCl3 injury were based on the time to first occlusion. (A) Doppler tracing of carotid artery blood flow after FeCl3 injury. PBS buffer control or wt-mAPC (1.8 mg/kg) in the presence and absence of a blocking rat monoclonal anti-mAPC antibody (TVM1) was administered in the jugular vein just before the thrombotic insult. Carotid artery blood flow was monitored for 30 minutes after application of FeCl3. Occlusion was defined as a flow less than 0.2 mL/min. (B) Time to first occlusion in mice treated with increasing doses of wt-mAPC or E149A-mAPC (0.6 mg/kg, 1.2 mg/kg, or 1.8 mg/kg) shown as a box-and-whiskers graph with median, 25th percentile, 75th percentile, and range. Points on discontinuous part of the y-axis above 1500 seconds indicate no occlusion within the 30 minutes experimental period. Statistical analysis was performed using analysis of variance with Bonferroni correction: * P < .05 APC versus PBS; # P < .05 E149A-APC versus wt-APC. (C) Occurrence of first occlusion versus time in mice treated with PBS (×), wt-mAPC (□) or E149A-mAPC (◇; both 1.8 mg/kg), or 5A-mAPC (○; 8.0 mg/kg) shown as a modified Kaplan-Meier plot. Statistical analysis was performed using the log-rank test: * P < .05 APC versus PBS; # P < .05 E149A-APC versus wt-APC. (B,C) Animals per group: PBS, n = 18; wt-mAPC, n = 6-7; E149A-mAPC, n = 4-6; and 5A-mAPC, n = 5.
Cytoprotective effects of E149A-APC
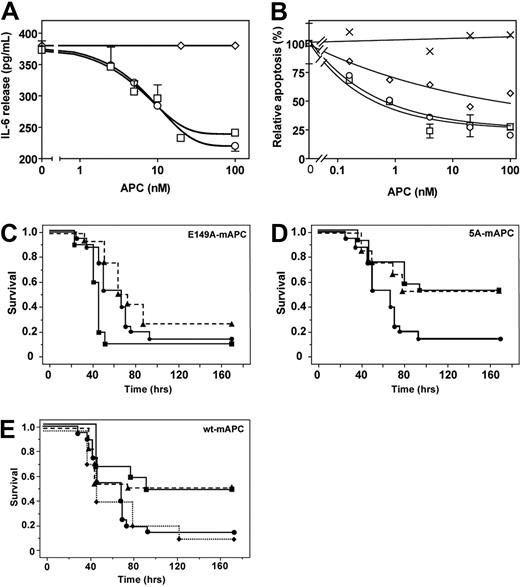
In contrast to its enhanced anticoagulant effects, E149A-hAPC was severely deficient in conveying direct cytoprotective effects on cells. In a model of LPS-stimulated IL-6 release from U937 cells, E149A-hAPC failed to down-regulate IL-6 release, whereas wt-hAPC and 5A-hAPC each similarly inhibited LPS-induced IL-6 release (Figure 4A). The 5A-hAPC and wt-hAPC, but not E149A-hAPC, inhibited staurosporine-induced EAhy926 endothelial cell apoptosis. Concentrations of E149A-hAPC required to achieve half-maximal inhibition of apoptosis were 18-fold higher compared with wt-hAPC (Figure 4B), indicating that E149A-hAPC retained less than 6% residual antiapoptotic activity.
Antiinflammatory, antiapoptotic, and mortality reduction activities of E149A-APC. (A) APC antiinflammatory activity determined as inhibition of IL-6 secretion of LPS-challenged U937 cells by wt-hAPC (□), E149A-hAPC (◇), and 5A-hAPC (○). (B) Inhibition of staurosporine-induced EAhy926 endothelial cell apoptosis by wt-hAPC (□), E149A-hAPC (◇), 5A-hAPC (○), and catalytically inactive S360A-hAPC (×). Apoptosis is expressed relative to that observed in the absence of added APC. Each point represents the mean plus or minus SEM of at least 3 independent experiments. (C) Survival of mice given an LD90 dose of endotoxin via intraperitoneal (IP) injection, followed by immediate treatment with E149A-mAPC (10 μg (■, solid line), 2 μg (▴, hatched line), or PBS control ●). Animals treated with 10 μg of E149A-mAPC showed significantly increased mortality (P < .05 by log-rank test) compared with PBS-treated mice. (D) Survival of mice given an LD90 dose of endotoxin via IP injection, followed by immediate treatment with 5A-mAPC (10 μg (■, solid line), 2 μg (▴, hatched line), or PBS control ●). Animals treated with 2 μg or 10 μg of 5A-mAPC showed significantly increased survival (P < .05 by log-rank test) compared with PBS-treated mice. (E) Survival of mice given an LD90 dose of endotoxin and treated with wt-mAPC (10 μg (■, solid line), 2 μg (▴, hatched line), or 0.2 μg (♦, dotted line) compared with PBS (●) alone treatment. Animals treated with 2 μg or 10 μg of wt-mAPC showed significant increased survival (P < .05 by log-rank test) compared with PBS-treated mice. (C-E) Animals per group: PBS, n = 20; each APC dose, n = 12.
Antiinflammatory, antiapoptotic, and mortality reduction activities of E149A-APC. (A) APC antiinflammatory activity determined as inhibition of IL-6 secretion of LPS-challenged U937 cells by wt-hAPC (□), E149A-hAPC (◇), and 5A-hAPC (○). (B) Inhibition of staurosporine-induced EAhy926 endothelial cell apoptosis by wt-hAPC (□), E149A-hAPC (◇), 5A-hAPC (○), and catalytically inactive S360A-hAPC (×). Apoptosis is expressed relative to that observed in the absence of added APC. Each point represents the mean plus or minus SEM of at least 3 independent experiments. (C) Survival of mice given an LD90 dose of endotoxin via intraperitoneal (IP) injection, followed by immediate treatment with E149A-mAPC (10 μg (■, solid line), 2 μg (▴, hatched line), or PBS control ●). Animals treated with 10 μg of E149A-mAPC showed significantly increased mortality (P < .05 by log-rank test) compared with PBS-treated mice. (D) Survival of mice given an LD90 dose of endotoxin via IP injection, followed by immediate treatment with 5A-mAPC (10 μg (■, solid line), 2 μg (▴, hatched line), or PBS control ●). Animals treated with 2 μg or 10 μg of 5A-mAPC showed significantly increased survival (P < .05 by log-rank test) compared with PBS-treated mice. (E) Survival of mice given an LD90 dose of endotoxin and treated with wt-mAPC (10 μg (■, solid line), 2 μg (▴, hatched line), or 0.2 μg (♦, dotted line) compared with PBS (●) alone treatment. Animals treated with 2 μg or 10 μg of wt-mAPC showed significant increased survival (P < .05 by log-rank test) compared with PBS-treated mice. (C-E) Animals per group: PBS, n = 20; each APC dose, n = 12.
Mortality reduction by APC was studied using a murine model. In vivo, E149A-mAPC failed to reduce 7-day mortality in mice given a dose of LPS causing 90% mortality (LD90) (Figure 4C). In contrast, a single i.v. bolus injection of wt-mAPC at 10 μg and 2 μg doses (Figure 4E) or 5A-mAPC at 10 μg and 2 μg doses (Figure 4D) effectively increased survival by converting 90% mortality to approximately 50% mortality (P < .05 compared with PBS, log-rank test), whereas wt-mAPC at 0.2 μg showed no effect compared with PBS vehicle alone (Figure 4E). In contrast to a 2-μg 5A-mAPC bolus that reduced mortality similar to 2-μg wt-mAPC bolus, a 2-μg E149A-mAPC bolus did not show any survival benefit. Remarkably, a 10 μg E149A-mAPC bolus showed a trend toward accelerated death (P < .05 compared with PBS, log-rank test; Figure 4C).
Interactions of E149A-APC with EPCR and PAR-1
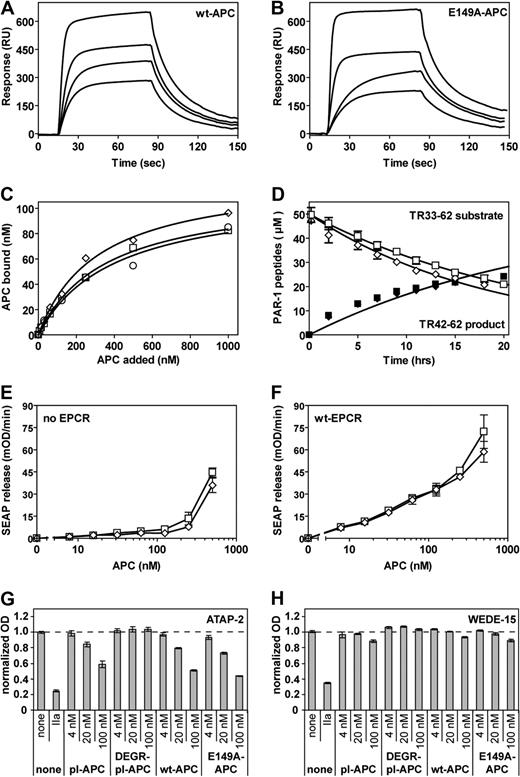
Both EPCR and PAR-1 are required for beneficial effects of APC in vivo, that is, for mortality reduction in experimental sepsis as well as for neuroprotection in ischemic stroke.16,34,35 E149A-hAPC binding to sEPCR was indistinguishable from wt-hAPC binding to sEPCR (Figure 5A,B). Binding of E149A-hAPC to K293 cells transfected with wt-EPCR confirmed that on cells the interaction of E149A-hAPC with EPCR was similar as that for wt-hAPC (Figure 5C), as anticipated, because binding of APC to EPCR is mediated by the Gla domain of APC.36,37
Interactions of E149A-APC with EPCR and PAR-1. (A,B) Binding of wt-hAPC (A) or E149A-hAPC (B) to immobilized sEPCR was determined by SPR analysis at various APC concentrations (480, 240, 140, and 65 nM). Derived kinetic-binding parameters for association rate constants (kon), dissociation rate constants (koff), and equilibrium-binding constants (KD) were indistinguishable (kon, 2.3 × 105 vs 2.0 × 105 M−1s−1; koff, 4.3 × 10−4 vs 3.6 × 10−4s−1; KD, 183 vs 180 nM for wt-hAPC vs E149A-hAPC, respectively). No binding of wt-hAPC to E86A-sEPCR was observed (data not shown). (C) Binding of wt-hAPC (□), E149A-hAPC (◇), and 5A-hAPC (○) to K293 cells transfected with wt-EPCR. (D) Cleavage of the TR33-62 PAR-1 peptide by E149A-hAPC was monitored over time as disappearance of the TR33-62 substrate peptide peak (open symbols) and as appearance of the TR42-62 product peptide peak (solid symbols). Symbols denote effects of wt-hAPC (■, □) and E149A-hAPC (♦, ◇). (E,F) Cleavage of a SEAP-PAR1 reporter construct by wt-hAPC (□) and E149A-hAPC (◇) on transfected K293 cells that lack (E) or contain EPCR (F). (G,H) Endogenous PAR-1 cleavage by APC on EA.hy 926 endothelial cells was monitored using cleavage-sensitive PAR-1 antibodies ATAP-2 (G) or H111 (data not shown). Cell surface PAR-1 antigen was detected using cleavage-insensitive PAR-1 antibodies WEDE15 (H) or S19 (data not shown). Antibody-staining results were corrected for residual nonspecific staining and normalized to non–agonist-treated cells (100%). Controls include plasma-derived APC (pl-APC) and DEGR-inhibited APC (DEGR-pl-APC) that is unable to cleave PAR-1. Each point represents the mean plus or minus SEM (n ≥ 3).
Interactions of E149A-APC with EPCR and PAR-1. (A,B) Binding of wt-hAPC (A) or E149A-hAPC (B) to immobilized sEPCR was determined by SPR analysis at various APC concentrations (480, 240, 140, and 65 nM). Derived kinetic-binding parameters for association rate constants (kon), dissociation rate constants (koff), and equilibrium-binding constants (KD) were indistinguishable (kon, 2.3 × 105 vs 2.0 × 105 M−1s−1; koff, 4.3 × 10−4 vs 3.6 × 10−4s−1; KD, 183 vs 180 nM for wt-hAPC vs E149A-hAPC, respectively). No binding of wt-hAPC to E86A-sEPCR was observed (data not shown). (C) Binding of wt-hAPC (□), E149A-hAPC (◇), and 5A-hAPC (○) to K293 cells transfected with wt-EPCR. (D) Cleavage of the TR33-62 PAR-1 peptide by E149A-hAPC was monitored over time as disappearance of the TR33-62 substrate peptide peak (open symbols) and as appearance of the TR42-62 product peptide peak (solid symbols). Symbols denote effects of wt-hAPC (■, □) and E149A-hAPC (♦, ◇). (E,F) Cleavage of a SEAP-PAR1 reporter construct by wt-hAPC (□) and E149A-hAPC (◇) on transfected K293 cells that lack (E) or contain EPCR (F). (G,H) Endogenous PAR-1 cleavage by APC on EA.hy 926 endothelial cells was monitored using cleavage-sensitive PAR-1 antibodies ATAP-2 (G) or H111 (data not shown). Cell surface PAR-1 antigen was detected using cleavage-insensitive PAR-1 antibodies WEDE15 (H) or S19 (data not shown). Antibody-staining results were corrected for residual nonspecific staining and normalized to non–agonist-treated cells (100%). Controls include plasma-derived APC (pl-APC) and DEGR-inhibited APC (DEGR-pl-APC) that is unable to cleave PAR-1. Each point represents the mean plus or minus SEM (n ≥ 3).
To determine the interaction between E149A-hAPC and PAR-1 and its ability to cleave the receptor, several different approaches were used. Initially, cleavage at Arg41 of a synthetic PAR-1 peptide, representing residues 33-62 of the PAR-1 extracellular domain, by APC was followed over time. The specificity constants (s = kcat/Km) for the cleavage of TR33-62 by wt-hAPC and E149A-hAPC (0.095 μM vs 0.10 μM/hour−1, respectively) were indistinguishable (Figure 5D). Similarly, extracellular N-terminal cleavage of a full-length PAR-1 reporter construct transfected into K293 cells showed no significant difference between wt-hAPC and E149A-hAPC (Figure 5E). Cotransfection of the PAR-1 reporter construct with EPCR into K293 cells promoted APC-mediated PAR-1 cleavage, but the cleavage of PAR-1 in the presence of EPCR was similar for wt-hAPC and E149A-hAPC (Figure 5F). Finally, cleavage of endogenous endothelial cell PAR-1 by wt-hAPC and E149A-hAPC was determined using a panel of cleavage-sensitive and cleavage-insensitive antibodies, as described.30 Both wt-hAPC and E149A-hAPC, like plasma-derived APC, cleaved PAR-1 similarly, as apparent from the disappearance of the cleavage-sensitive ATAP-2 epitope upon incubation of cells with APC (Figure 5G). In contrast to thrombin-cleaved PAR-1, which disappears from the cell surface most likely due to internalization, APC-cleaved PAR-1 is retained on the cell surface.30 PAR-1 cleaved by either wt-hAPC or E149A-hAPC was retained at the cell surface, as evident from the retention of the cleavage-insensitive WEDE-15 epitope on the cell surface (Figure 5H). In summary, these experiments demonstrate that E149A-APC has reduced cytoprotective and mortality reduction activities, despite normal binding to EPCR as well as normal cleavage of PAR-1.
Discussion
Reciprocal amplifications of inflammation and coagulation fuel a cytokine storm and disseminated intravascular coagulation that are found in severe sepsis patients with a deteriorating prognosis.38-41 The potent anticoagulant effects of APC prompted initial studies to curb the out-of-control coagulation-inflammation spiral associated with severe sepsis.39,42 As shown in the PROWESS and Extended Evaluation of Recombinant Human Activated Protein C United States (ENHANCE US) trials, treatment of severe sepsis patients with recombinant APC (Xigris) caused a significant reduction of 28-day all-cause mortality.3,43 Despite the survival benefit, administration of APC in the PROWESS trial was associated with an increase in serious bleeding events, most likely exacerbated by the potent anticoagulant activity of APC.3,7,18 The 2 failed similar phase III severe sepsis clinical trials for potent anticoagulant proteins, tissue factor pathway inhibitor44 and antithrombin III,45 raised questions pertaining to the relevance of APC's anticoagulant activity to the observed survival benefit in the PROWESS trial, and implied that other activities of APC could be responsible for its clinical success in severe sepsis. However, arguments for the importance of APC's anticoagulant action have been recently reiterated.19
The sepsis clinical trial results prompted us to undertake a molecular engineering effort to separate anticoagulant activity from cytoprotective activities in APC to determine the relative requirements for anticoagulant and cytoprotective activities for survival benefits in sepsis and other murine injury models. Alanine-scanning mutagenesis of charged surface patches in APC that could comprise potential exosites for either interactions with anticoagulant or cytoprotective substrates identified Glu149 in the C-terminal end of the protein C light chain to be of particular interest (Figure 1). As shown in this study, E149A-hAPC showed a 3-fold enhanced in vitro anticoagulant activity compared with wt-hAPC, whereas its cytoprotective effects in in vitro assays were severely diminished. Thus, E149A-APC has an anticoagulant-selective activity profile. Moreover, these data emphasize that the C-terminal region of APC's light chain is important for this enzyme's multiple anticoagulant and cytoprotective activities.
On the other side of the selectivity spectrum, cytoprotective-selective 5A-APC,16,17 with the inverse activity profile of E149A-APC, has less than 3% anticoagulant activity (< 10% for 5A-mAPC), yet full normal cytoprotective activities compared with wt-APC. Recent work showed that this mutant reduced endotoxin-induced mortality in mice.16 Several other APC mutants with selective alterations in their anticoagulant of cytoprotective functions have been recently described by other laboratories.29,46-48
Proof that APC's anticoagulant activity in plasma-clotting assays is predictive for in vivo antithrombotic potential of various APC mutants was obtained for the first time using a murine thrombosis model in which E149A-mAPC showed superior antithrombotic potency compared with wt-mAPC in a standard acute arterial thrombosis injury model. The 5A-mAPC, even when tested at a very high dose, did not significantly delay vascular occlusion, showing that its in vivo antithrombotic activity is also severely impaired.
If APC's anticoagulant activity were the major driving force behind APC's survival benefit in severe sepsis patients as was initially postulated, then the hyperanticoagulant E149A-mAPC would be expected to match or surpass the mortality reduction by wt-mAPC. Instead, E149A-mAPC failed to convey any survival benefit even when tested at a 5-fold higher dose than wt-mAPC, and this mutant actually showed a trend toward increased mortality. Thus, APC's anticoagulant activity alone is clearly not sufficient to reduce mortality in murine endotoxemia, emphasizing that APC's cytoprotective activities are critically responsible for the observed beneficial effects of wt-mAPC. Accordingly, cytoprotective-selective 5A-mAPC reduced LPS-induced mortality indistinguishably from wt-mAPC. Therefore, APC's anticoagulant activity is neither sufficient nor required to reduce mortality in LPS-induced lethal endotoxemia. Although the cause of mortality in high dose (10 μg) E149A-mAPC-treated animals remains undetermined, possible contributory mechanisms might include competition of nonprotective E149A-mAPC with endogenous mAPC for cytoprotective receptors (eg, EPCR) or excessive bleeding promoted by the increased antithrombotic activity of E149A-mAPC.
Whether extrapolation of endotoxin-induced sepsis results from murine model systems to severe sepsis patients is relevant or useful remains to be determined. Nonetheless, the extent to which murine endotoxemia data might be extrapolated helps to provide a rationale for why the antithrombin III and the tissue factor pathway inhibitor trials failed in humans. Murine endotoxemia data may also provide an avenue for further development of safer second-generation APC mutants. Such safer second-generation, cytoprotective-selective APC mutants could presumably diminish adverse bleeding events due to impaired anticoagulant activity, while at the same time could enable optimal dosing regimens to increase survival benefits due to APC's cytoprotective activities.
In contrast to the mutations in 5A-APC disrupting a well-defined exosite for fVa, the C-terminal part of the protein C light chain has not been previously targeted by mutagenesis for functional proteomics analysis. In plasma-clotting assays in vitro, the E149A-APC gain-of-anticoagulant function was dependent on the presence of APC's nonenzymatic anticoagulant cofactor protein S, suggesting that direct interactions with protein S might benefit from the elimination of a negative charge in this very positively charged region of the light chain (Figure 1). Alternatively, elimination of Glu149 might alter domain-domain intramolecular interactions and promote conformational changes in the protease domain, thereby facilitating APC's interactions with fVa, similar to the effects achieved by protein S.49 Clues toward the structural implications of the Glu149 to Ala mutation might be derived from the crystal structure of thrombin and from Ala scanning mutagenesis of the thrombin A-chain.50,51 Glu8 in thrombin (chymotrypsin numbering), homologous to Glu149 in the C-terminal end of the protein C light chain, contributes to an ion quartet implicated in stabilizing the position of A-chain residues to form numerous interactions with the thrombin B-chain. An ionic interaction of Glu8 with Lys202, which is only 7 residues away from the active site Ser195, provides a rationale how Ala substitution of Glu8 in thrombin modulates specificity of the active site more than 20 Å away.51 Whether E149 in protein C provides similar intramolecular interactions with APC's protease domain as does Glu8 in thrombin remains unclear. There is a lack of electron density in the APC crystal structure for E149, which might suggest a less rigid conformation of this region in APC compared with thrombin.50-52
The loss of cytoprotective functions in E149A-APC provides a unique opportunity to probe the molecular mechanisms involved in APC cytoprotective activities. According to the current paradigm for APC's cytoprotective activities, binding of APC to EPCR facilitates PAR-1 cleavage and thereby causes intracellular signaling. Analysis of APC binding to sEPCR or APC binding to EPCR expressed on cells revealed no differences for E149A-APC compared with wt-APC, and cleavage of PAR-1 by E149A-APC and wt-APC was indistinguishable as determined by 3 different methods. The C-terminal end of the protein C light chain (including Glu149) was not defined in the x-ray crystallographic 3-dimensional structure of APC. But based on the homology with other coagulation enzymes (Figure 1), it seems unlikely to be part of a recently reported exosite on APC for PAR-1 involving Glu330 and Glu333.29 At present, the functional defect of E149A-APC cannot be explained by the current APC-signaling paradigm. Hence, we speculate that there exist currently unknown molecular mechanisms for APC signaling and cytoprotective activities for which E149A-APC is defective.
In summary, we demonstrate that it is possible to engineer selectively the anticoagulant and cytoprotective properties of APC and thereby provide unique reagents to dissect in vitro and in vivo mechanisms of action. Our data imply that APC's anticoagulant activity is neither sufficient nor necessary for mortality reduction in sepsis. Protein engineering of APC may provide therapeutic mutants with activities tailored to suit potential targeted indications and may help the development of second-generation nonanticoagulant APC mutants that we speculate could reduce mortality without increasing risk for adverse serious bleeding associated with current wt-APC therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr C. J. S. Edgell (University of North Carolina, Chapel Hill, NC) for the EA.hy926 endothelial cell line; Dr S. L. Brass (University of Pennsylvania, Philadelphia, PA) and W. Ruf (The Scripps Research Institute) for anti–PAR-1 antibodies; and Sarah Coit for excellent technical assistance.
This work was supported in part by National Heart, Lung, and Blood Institute grants HL087618 (to L.O.M.), HL073318 (to M.R.), HL060655 and HL093388 (to H.W.), HL031950 (to Z.M.R.), and HL31950 and HL52246 (to J.H.G.).
National Institutes of Health
Authorship
Contribution: L.O.M., A.Z., E.J.K., R.A.S., Y.B., J.A.F., and X.V.Y. performed experiments; L.O.M., M.R., H.W., Z.M.R., and J.H.G. designed the research and analyzed results; and L.O.M. and J.H.G. wrote the paper.
Conflict-of-interest disclosure: L.O.M., H.W., and J.H.G. are inventors on pending patents concerning protein C that are the property of their employers. The remaining authors declare no competing financial interests.
Correspondence: Dr John H. Griffin, Department of Molecular and Experimental Medicine (MEM-180), The Scripps Research Institute, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: jgriffin@scripps.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal