Abstract
KW-2449, a multikinase inhibitor of FLT3, ABL, ABL-T315I, and Aurora kinase, is under investigation to treat leukemia patients. In this study, we examined its possible modes of action for antileukemic effects on FLT3-activated, FLT3 wild-type, or imatinib-resistant leukemia cells. KW-2449 showed the potent growth inhibitory effects on leukemia cells with FLT3 mutations by inhibition of the FLT3 kinase, resulting in the down-regulation of phosphorylated-FLT3/STAT5, G1 arrest, and apoptosis. Oral administration of KW-2449 showed dose-dependent and significant tumor growth inhibition in FLT3-mutated xenograft model with minimum bone marrow suppression. In FLT3 wild-type human leukemia, it induced the reduction of phosphorylated histone H3, G2/M arrest, and apoptosis. In imatinib-resistant leukemia, KW-2449 contributed to release of the resistance by the simultaneous down-regulation of BCR/ABL and Aurora kinases. Furthermore, the antiproliferative activity of KW-2449 was confirmed in primary samples from AML and imatinib-resistant patients. The inhibitory activity of KW-2449 is not affected by the presence of human plasma protein, such as α1-acid glycoprotein. These results indicate KW-2449 has potent growth inhibitory activity against various types of leukemia by several mechanisms of action. Our studies indicate KW-2449 has significant activity and warrants clinical study in leukemia patients with FLT3 mutations as well as imatinib-resistant mutations.
Introduction
Overexpression and activating mutations of protein tyrosine kinases (PTK) are frequently observed in several kinds of hematologic malignancies.1,2 Abnormally activated PTK-mediated signal transduction pathways are involved in their pathogenesis, such as autonomous proliferation, antiapoptosis, and differentiation block. The remarkable clinical success of the ABL kinase inhibitor, imatinib mesylate (IM), in the treatment of BCR/ABL-positive chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) has proved the principle of molecularly targeted therapy.3,4 Therapeutic intervention targeting PTKs is therefore highly expected to improve prognosis of patients with hematologic malignancy. FMS-like receptor tyrosine kinase (FLT3) is a class III receptor tyrosine kinase together with cKIT, FMS, and PDGFR.5,6 FLT3 mutations were first reported as internal tandem duplication (FLT3/ITD) of the juxtamembrane domain-coding sequence; subsequently, a missense point mutation at the Asp835 residue and point mutations, deletions, and insertions in the codons surrounding Asp835 within a tyrosine kinase domain of FLT3 (FLT3/KDM) have been found.7,8 FLT3 mutation is the most frequent genetic alteration in acute myeloid leukemia (AML) and involved in the signaling pathway of proliferation and survival in leukemia cells.5,6 Several large-scale studies have confirmed that FLT3/ITD is strongly associated with leukocytosis and a poor prognosis.9 In addition to FLT3 mutation, overexpression of FLT3 is an unfavorable prognostic factor for overall survival in AML, and it has been revealed that overexpressed FLT3 had the same sensitivity to the FLT3 inhibitor as FLT3/ITD.10 Because high-dose chemotherapy and stem cell transplantation cannot conquer the adverse effects of FLT3 mutations, it is expected that the development of FLT3 kinase inhibitors will make more efficacious therapeutic strategy for leukemia therapy.11,12 To date, several small-molecule tyrosine kinase inhibitors have been shown to have a potency to inhibit the FLT3 kinase, and several of them, such as CEP-701, PKC412, MLN-518, and SU11248, have been subjected to clinical trials.13-16 However, the clinical efficacy of these FLT3 inhibitors for AML with FLT3 mutations is limited to the transient clearance of leukemia blast cells as a single agent; thus, the therapeutic strategy of some FLT3 inhibitors moves toward a combination with conventional chemotherapy.17 This move is a logical step based on the in vitro evidence of the synergy with conventional cytotoxic agents,18,19 although it should be considered that several problems regarding adverse effects and pharmacokinetics have been apparent from clinical trials of monotherapy.20 Furthermore, because acute leukemia is a complex multigenetic disorder,21-23 a simultaneous inhibition of multiple protein kinases is thought to be advantageous over the increasing potency against the selective kinases. Recent high-throughput resequencing of TK in AML samples revealed new somatic mutations of JAK1, DDR1, and NRTK1 in addition to previously well-known FLT3, cKit, JAK2, and FGFR mutations.24,25 These observations collectively indicate that FLT3 inhibitors in the next generation should have an adequately balanced potency against key oncogenic kinases, which are responsible for the disease progression and/or the resistance to standard therapeutics. Here we describe efficacy of a novel small-molecule protein kinase inhibitor, KW-2449, which has a potent and unique kinase inhibition profile against FLT3, ABL, T315I-mutant ABL (ABL-T315I) tyrosine kinases as well as Aurora kinase.
Methods
Kinase inhibition profile
The in vitro kinase assays were performed according to the KinaseProfiler Assay Protocols of Upstate Biotechnology.
Growth inhibition profile cell-cycle analysis
FLT3/ITD-, FLT3/D835Y-expressing, wt-FLT3/FL-coexpressing, and FLT3/ITD-green fluorescent protein (GFP)–expressing murine myeloid-progenitor 32D cells were previously reported.26 Human leukemia cell line MOLM-13 was obtained from DSMZ (German Resource Center for Biological Material); MV4;11, RS4;11, K562, and HL60 from ATCC. Wt-BCR/ABL-positive human ALL cell line TCC-Y and its IM-resistant clones, TCC-Y/sr cells, which has the T315I-mutated BCR/ABL, were reported previously.27 Cell viability was determined by the sodium 3′-[1-(phenylaminocarbonyl)-3, 4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate assay after incubation with or without KW-2449 for 72 hours at 37°C. The number of viable cells was determined using the Cell Proliferation Kit II (Roche Diagnostics). For cell-cycle analysis, MOLM-13 and RS4;11 cells were treated with KW-2449. After 24, 48, and 72 hours of incubation at 37°C, DNA contents were analyzed as previously described.28 Cell cycle distribution of K562, TCC-Y, and TCC/Ysr was analyzed 24 hours after treatment with KW-2449 or imatinib.
Effects of hAGP on growth inhibitory activity by FLT3 inhibitors
MOLM-13 cells were incubated with various concentrations of KW-2449, PKC-412, and CEP-701 in the presence of 0.1% of human α1-acid glycoprotein (hAGP; Sigma-Aldrich). Cell viability was determined by sodium 3′-[1-(phenylaminocarbonyl)-3, 4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate assay after incubation for 72 hours at 37°C.
Western blot
MOLM-13 cells were treated with KW-2449 for 24 hours, and cell pellets were suspended with lysis buffer. FLT3 proteins were immunoprecipitated with anti-FLT3 antibody (S18; Santa Cruz Biotechnology). The precipitated samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and electroblotted onto Immobilon polyvinylidene difluoride membranes (Millipore). Immunoblotting was performed with antiphosphotyrosine antibody (4G10; Upstate Biotechnology). The membranes were incubated with the stripping buffer and then reprobed with anti-FLT3 antibody (C20; Santa Cruz Biotechnology). Signals were developed using an enhanced chemiluminescence system (GE Healthcare). To examine the phosphorylation level of STAT5, whole cell lysates were subjected to immunoblotting with antiphospho-STAT5 antibody (Kyowa Hakko Kogyo). The membranes were incubated with the stripping buffer and then reprobed with anti-STAT5 antibody (Santa Cruz Biotechnology).
RS4;11 cells were suspended in culture medium containing nocodazole with or without KW-2449. After a 30-minute incubation, cells were harvested and cell pellets were suspended in lysis buffer. Whole cell lysates were subjected to immunoblotting with antiphospho-HH3 (Ser10) antibody (Upstate Biotechnology). The membranes were incubated with the stripping buffer and then reprobed with anti-HH3 antibody (Cell Signaling).
Concentration of KW-2449 in plasma and tumors
Severe combined immunodeficiency (SCID) mice (Fox CHASE C.B-17/Icr-scidJcl, male, 5 weeks old) were purchased from CLEA Japan. Mice were treated with an intraperitoneal injection of antiasialo GM1 antibody (0.3 mg/mouse, Wako Pure Chemical Industries). The day after antiasialo GM1 antibody treatment, all mice were subcutaneously inoculated in the shaved area with 107 of MOLM-13 cells. Ten days after inoculation, KW-2449 at 20 mg/kg was orally administered to mice twice. Blood and tumor samples were collected 4, 8, 12, and 24 hours after the second administration. The plasma and tumor samples were analyzed to measure KW-2449 concentration with liquid chromatography–mass spectrometry–mass spectrometry (LC/MS/MS).
In vivo antileukemia effects on xenograft transplantation
SCID mice were subcutaneously inoculated with MOLM-13 cells. Five days after inoculation, tumor volume was measured using the Antitumor test system II (Human Life). The 25 mice with tumors ranging from 90 to 130 mm3 were selected and randomized using the Antitumor test system II. From the day of randomization, vehicle (0.5 wt/vol% MC400) or KW-2449 (2.5, 5.0, 10, and 20 mg/kg) was orally administered to mice twice a day for 14 days. Tumor volume was measured twice a week during the treatment.
In vivo antileukemia effects on syngeneic transplantation
C3H/Hej mice were purchased from Charles River Japan. Fifteen C3H/Hej mice were intravenously inoculated with 2 × 106 of FLT3/ITD-GFP-32D cells and then randomly divided into 3 groups of 5 mice each. On the seventh day after inoculation, peripheral blood (PB) was collected from the mice. From the 10th day after inoculation, mice were treated with KW-2449 at 40 mg/kg (orally) twice a day, cytosine arabinoside (AraC) at 150 mg/kg (intravenously) daily, or vehicle for 4 days. Six hours after the last administration, PB was collected. Total RNA was extracted from each PB sample using a QIAamp RNA Blood Mini Kit (QIAGEN). cDNA was synthesized from each RNA sample using a random primer and Moloney murine leukemia virus reverse transcriptase (Super-Script II; Invitrogen) according to the manufacturer's recommendations. The expression level of the human FLT3 transcript was quantitated using a real-time fluorescence detection method on an ABI Prism 7000 sequence detection system (Applied Biosystems) as previously reported.10 After the collection of PB, spleens and bone marrow (BM) cells from femora were collected, and the total cell number from each femur was counted using a cell counter. To discriminate the FLT3/ITD-GFP-32D leukemia cells and normal BM cells, all collected cells were subjected to flow cytometry analysis after phycoerythrin-conjugated antihuman FLT3 monoclonal antibody (SF1.340; Immunotech) staining. In this flow cytometry analysis, GFP-positive cells were defined as residual leukemia in the femur. The weight of each collected spleen was measured.
Primary patient samples
BM samples from patients with AML or CML in blast crisis were subjected to Ficoll-Hypaque (Pharmacia LKB) density gradient centrifugation. All samples were morphologically confirmed to contain more than 90% leukemia cells after centrifugation on May-Grünwald Giemsa-stained cytospin slides, and then cryopreserved in liquid nitrogen before use. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki to use their samples for the present study as well as banking and molecular analysis, and approval was obtained from the ethics committees of Nagoya University and Ogaki Municipal Hospital for this study. Mutations of the FLT3 gene were examined as previously reported.8 Primary AML cells were incubated with RPMI1640 medium containing 10% fetal calf serum and 0.1 μM KW-2449 for 6 hours, and cell pellets were suspended with lysis buffer. Whole cell lysates were subjected to immunoblotting with antiphospho-FLT3 (Tyr591) (Cell Signaling Technology) and antiphospho-STAT5 antibodies. The membranes were incubated with the stripping buffer and then reprobed with anti-FLT3 (C20; Santa Cruz Biotechnology) and anti-STAT5 antibodies (Santa Cruz Biotechnology).
Colony formation analysis
Human AML cells (105 cells) were plated in MethoCult methylcellulose semisolid medium containing human stem cell factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-3 (H4534; Stem Cell Technologies) with or without KW-2449 (0.1 μM) and then incubated at 37°C for 14 days. Colonies with more than 20 cells were scored using an inverted microscope.
Human cord blood (CB) was collected after full-term deliveries with informed consent obtained in accordance with the Declaration of Helsinki and approved by the Review Board of Tokai Cord Blood Bank. Mononuclear cells were collected by the Ficoll-Hypaque (Pharmacia LKB) density gradient centrifugation. CB mononuclear cells (5 × 104 cells) were plated in complete MethoCult methylcellulose medium (H4435; Stem Cell Technologies) with an increasing concentration of KW-2449. After 14 days in culture, erythroid burst-forming units (BFU-E), colony-forming unit–granulocyte macrophage (CFU-GM), and colony-forming unit–granulocyte, erythrocyte, monocyte/macrophage, megakaryocyte (CFU-GEMM) colonies were counted.
Inhibitory effects on BCR/ABL-positive leukemia cells
K562, TCC-Y, and TCC/Ysr cells were incubated with an increasing concentration of KW-2449 or imatinib for 72 hours, and cell pellets were suspended with lysis buffer. Whole cell lysates were subjected to immunoblotting with antiphospho-ABL (Tyr245; Cell Signaling Technology), antiphospho-STAT5, and anti-poly(ADP-ribose) polymerase (PARP; Cell Signaling Technology) antibodies.
Human CML in blast crisis (CML-BC) cells with T315I-mutation were intravenously inoculated into nonobese diabetic (NOD)/SCID mice (CLEA Japan). On the 28th day after the inoculation, engraftment of leukemia cells in each mouse was confirmed by the detection of human CD45-positive cells in PB. On the next day, PB was collected from the leukemia cell-engrafted mice, and then the mice were treated with KW-2449 at 20 mg/kg twice a day, IM at 150 mg/kg daily, or vehicle for 5 days. Twelve hours after the last administration, PB was collected from each mouse. Total RNA was extracted from each PB sample, and cDNA was synthesized from each RNA sample using a random primer and Moloney murine leukemia virus reverse transcriptase as described in “In vivo antileukemia effects on syngeneic transplantation.” The expression level of the BCR/ABL transcript was quantitated using a real-time fluorescence detection method as previously reported.29 The GAPDH served as a control for cDNA quality. Relative gene expression levels were calculated using standard curves and adjusted based on the expression level of the GAPDH gene.
After the collection of PB, femora were subjected to pathologic examination. Residual leukemia cells were evaluated by the immunohistochemical staining with antihuman CD45 antibody (Dako North America) as previously reported.30 The animal experiments were approved by the institutional ethics committee for Laboratory Animal Research, Nagoya University School of Medicine and performed according to the guidelines of the institute.
Statistical analysis
Differences in continuous variables were analyzed with the Mann-Whitney U test for distribution among 2 groups or the Bonferroni test for distribution among more than 3 groups. Differences in therapeutic effects were analyzed with the repeated-measures analysis of variance method. These statistical analyses were performed with the StatView-J 5.0 software (Abacus Concepts).
Results
Development of KW-2449 and its kinase inhibition profile
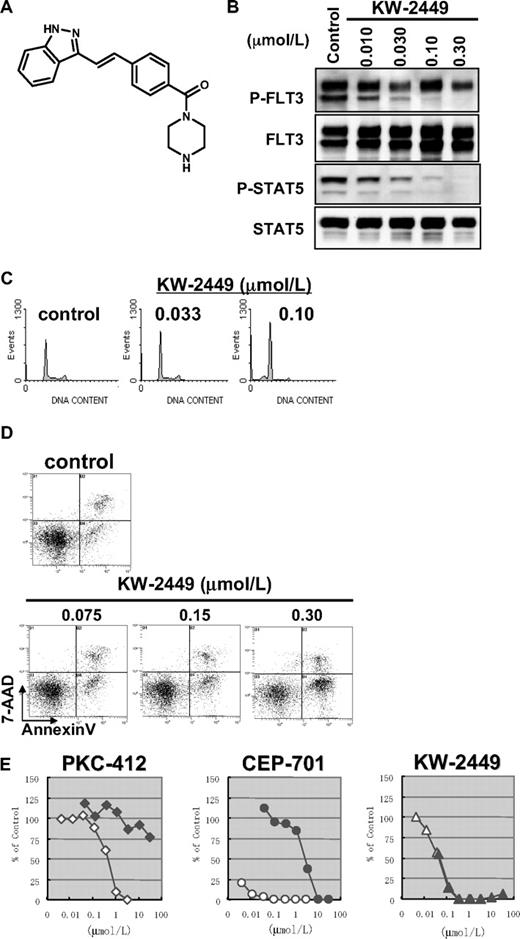
Our aim was to generate an orally available and highly potent FLT3 inhibitor with low toxicity profile for leukemia patients. For this goal, we screened the chemical libraries of Kyowa Hakko Kirin (previously Kyowa Hakko Kogyo) using several leukemia cells, which have several activated mutations in FLT3 or BCR-ABL translocation. As a result, we identified several chemo-types with different kinase inhibition profiles, intensively studied the structures of the identified chemo-types to improve the potency and selectivity, and then finally generated KW-2449 (Figure 1A).
Effects of KW-2449 on human leukemia cells with FLT3 mutation. (A) Chemical structure of KW-2449. (B) MOLM-13 cells, which express FLT3/ITD, were treated with KW-2449 at the indicated concentrations for 24 hours. For analysis of FLT3 and its phosphorylated form (P-FLT3), the blots of immunoprecipitated FLT3 SDS-PAGE samples were analyzed by antiphospho-tyrosine (P-Tyr) antibody and anti-FLT3 antibody. For detection of STAT5 and its phosphorylated form (P-STAT5), total cell lysate SDS-PAGE samples were analyzed by anti-P-STAT5 antibody and anti-STAT5 antibody as primary antibodies. Phosphorylation levels of FLT3 and STAT5 were decreased by KW-2449 in a dose-dependent manner. (C) MOLM-13 cells were treated with various concentrations of KW-2449 for 48 hours, and cell-cycle distribution was analyzed as described in “Growth inhibition profile and cell-cycle analysis.” (D) MOLM-13 cells were treated with various concentrations of KW-2449 for 48 hours, and apoptosis induction was analyzed by 7-amino-actinomycin D/annexin V staining. (E) Inhibitory effects of hAGP on growth inhibitory activity against MOLM-13 cells were compared between KW-2449 and potent FLT3 inhibitors, PKC-412 and CEP-701, as described in “Effects of hAGP on growth inhibitory activity by FLT3 inhibitors.” Growth inhibitory activity of KW-2449 was not affected by the presence of hAGP.
Effects of KW-2449 on human leukemia cells with FLT3 mutation. (A) Chemical structure of KW-2449. (B) MOLM-13 cells, which express FLT3/ITD, were treated with KW-2449 at the indicated concentrations for 24 hours. For analysis of FLT3 and its phosphorylated form (P-FLT3), the blots of immunoprecipitated FLT3 SDS-PAGE samples were analyzed by antiphospho-tyrosine (P-Tyr) antibody and anti-FLT3 antibody. For detection of STAT5 and its phosphorylated form (P-STAT5), total cell lysate SDS-PAGE samples were analyzed by anti-P-STAT5 antibody and anti-STAT5 antibody as primary antibodies. Phosphorylation levels of FLT3 and STAT5 were decreased by KW-2449 in a dose-dependent manner. (C) MOLM-13 cells were treated with various concentrations of KW-2449 for 48 hours, and cell-cycle distribution was analyzed as described in “Growth inhibition profile and cell-cycle analysis.” (D) MOLM-13 cells were treated with various concentrations of KW-2449 for 48 hours, and apoptosis induction was analyzed by 7-amino-actinomycin D/annexin V staining. (E) Inhibitory effects of hAGP on growth inhibitory activity against MOLM-13 cells were compared between KW-2449 and potent FLT3 inhibitors, PKC-412 and CEP-701, as described in “Effects of hAGP on growth inhibitory activity by FLT3 inhibitors.” Growth inhibitory activity of KW-2449 was not affected by the presence of hAGP.
KW-2449 inhibited FLT3 and ABL kinases with half-maximal inhibitory concentration (IC50) values of 0.0066 and 0.014 μM, respectively. In addition, it potently inhibited ABL-T315I, which is associated with IM resistance, with an IC50 value of 0.004 μM. On the other hand, KW-2449 had little effect on PDGFRβ, IGF-1R, EGFR, and various serine/threonine kinases even at a concentration of 1 μM. Among various serine/threonine kinases examined, KW-2449 inhibited Aurora A kinase with IC50 of 0.048 μM (Table 1) and Aurora B kinase with the equivalent potency (data not shown).
Kinase inhibitory profile of KW-2449
| Kinase . | KW-2449 . |
|---|---|
| Tyrosine kinase | |
| FLT3 | 0.0066 |
| FLT3/D835Y | 0.001 |
| KIT | 0.30 |
| PDFGRα | 1.7 |
| ABL | 0.014 |
| ABL-T315I | 0.004 |
| SRC | 0.40 |
| JAK2 | 0.15 |
| FGFR1 | 0.036 |
| Serine/threonine kinase | |
| Aurora A | 0.048 |
| Kinase . | KW-2449 . |
|---|---|
| Tyrosine kinase | |
| FLT3 | 0.0066 |
| FLT3/D835Y | 0.001 |
| KIT | 0.30 |
| PDFGRα | 1.7 |
| ABL | 0.014 |
| ABL-T315I | 0.004 |
| SRC | 0.40 |
| JAK2 | 0.15 |
| FGFR1 | 0.036 |
| Serine/threonine kinase | |
| Aurora A | 0.048 |
In vitro kinase inhibition IC50 (μmol/L). IC50 values of KW-2449 were determined by in vitro kinase assays as described in “Kinase inhibition profile.”
In vitro effects of KW-2449 on FLT3 mutated leukemia
In vitro kinase inhibition profile of KW-2449 indicated its extreme potency against FLT3 kinase. We first examined the effects of KW-2449 on several human leukemia cell lines with activated FLT3 and mutant FLT3-transfected cells. Because constitutive activation of FLT3 in leukemia cells is reportedly induced by mutation or coexpression of wild-type FLT3 (wt-FLT3) and FLT3 ligand (FL), we evaluated the growth inhibitory effect on mutant FLT3 (FLT3/ITD or FLT3/D835Y)–expressing and wt-FLT3– and FL-coexpressing (wt-FLT3/FL) murine myeloid-progenitor 32D cells. In addition, we evaluated the efficacy against FLT3/ITD-harboring human AML cell lines, MOLM-13 and MV4;11. Previously, we confirmed that FLT3 kinase is constitutively activated in these cell lines, and FI-700, a FLT3 selective inhibitor, can suppress the growth of mutated FLT3 transfected 32D cells as well as MOLM-13 and MV4;11 cells.31 As expected, KW-2449 showed growth inhibitory activities against FLT3/ITD-, FLT3/D835Y-, and wt-FLT3/FL-expressing 32D cells, MOLM-13 and MV4;11 with half-maximal growth inhibitory concentration (GI50) values of 0.024, 0.046, 0.014, 0.024, and 0.011 μM, respectively (Table 2). These results indicate that KW-2449 has the potent growth inhibitory activities against not only FLT3/ITD-expressing leukemia cells but also FLT3/KDM-activated and wild-type FLT3-overexpressing leukemia cells.
Growth inhibitory profile of KW-2449
| Cell lines . | KW-2449 . | Imatinib . |
|---|---|---|
| 32D transfectant | ||
| Mock (with IL-3) | > 10 | — |
| FLT3/ITD | 0.024 | — |
| FLT3/D835Y | 0.046 | — |
| Wt-FLT3/FL | 0.014 | — |
| Human leukemia | ||
| MOLM-13 | 0.024 | > 10 |
| MV4;11 | 0.011 | — |
| RS4;11 | 0.23 | 20 |
| HL-60 | 0.65 | > 10 |
| BCR/ABL+ leukemia | ||
| K562 | 0.27 | 0.24 |
| TCC-Y | 0.49 | 0.18 |
| TCC-Y/sr | 0.42 | 24 |
| Cell lines . | KW-2449 . | Imatinib . |
|---|---|---|
| 32D transfectant | ||
| Mock (with IL-3) | > 10 | — |
| FLT3/ITD | 0.024 | — |
| FLT3/D835Y | 0.046 | — |
| Wt-FLT3/FL | 0.014 | — |
| Human leukemia | ||
| MOLM-13 | 0.024 | > 10 |
| MV4;11 | 0.011 | — |
| RS4;11 | 0.23 | 20 |
| HL-60 | 0.65 | > 10 |
| BCR/ABL+ leukemia | ||
| K562 | 0.27 | 0.24 |
| TCC-Y | 0.49 | 0.18 |
| TCC-Y/sr | 0.42 | 24 |
Growth inhibition GI50 (μmol/L). GI50 values of KW-2449 and imatinib were determined by in vitro XTT assays. MOLM-13 and MV4;11 cells had FLT3/ITD. K562 and TCC-Y cells had wt-BCR/ABL, and TCC-Y/sr had the T315I mutation in the BCR/ABL gene.
— indicates not applicable.
It has been reported that PKC-412 and CEP-701, whose chemical structure contains indolocarbazole, tightly bind to hAGP. Although these compounds have been in clinical investigation as FLT3 inhibitors, the significant reduction of their inhibitory activity caused by tight hAGP binding is in part associated with the limited clinical efficacy despite their long exposure in vivo, as well as the potency in vitro.32,33 In these circumstances, we selected the compounds whose cellular efficacy was not attenuated by the presence of hAGP. Indeed, an addition of hAGP to culture media reduced the growth inhibitory effect of PKC-412 and CEP-701 more than 100- to 1000-fold, whereas the growth inhibitory activity of KW-2449 was not attenuated by hAGP (Figure 1E).
In accordance with growth inhibitory effect, KW-2449 suppressed the phosphorylations of FLT3 (P-FLT3) and its downstream molecule phospho-STAT5 (P-STAT5) in MOLM-13 cells in a dose-dependent manner (Figure 1B). Furthermore, KW-2449 increased the percentage of cells in the G1 phase of the cell cycle and reciprocally reduced the percentage of cells in the S phase, resulting in the increase of apoptotic cell population (Figure 1C-D). These results indicated that the dephosphorylation of constitutively active FLT3 kinase by KW-2449 induced the G1 arrest to leukemia cells with FLT3 activation, resulting in apoptosis. Apparent increase of sub-G1 apoptotic cells was also observed after KW-2449 exposure over 0.10 μM, at which concentration complete down-regulation of P-FLT3 was observed.
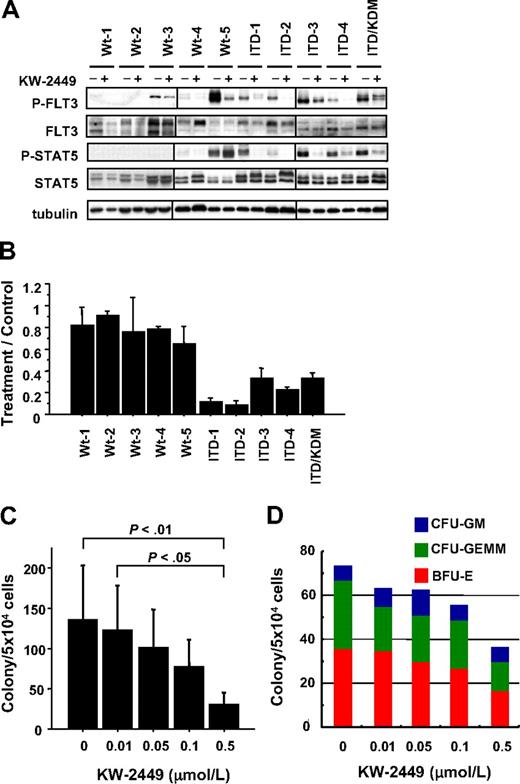
To confirm these effects on primary leukemia, we further analyzed the activities of KW-2449 using 10 human primary AML cells that consisted of 5 AML with wt-FLT3: 4 with FLT3/ITD and 1 with both FLT3/ITD and FLT3/KDM. In all AML cases with FLT3 mutation, KW-2449 (0.1 μM) reduced the phosphorylation levels both of FLT3 and STAT5 (Figure 2A). In accordance with the dephosphorylation level, the colony formations of AML cases with FLT3 mutation were inhibited by KW-2449 (Figure 2B). In contrast, the inhibitory effect of KW-2449 on the colony formations of all AML cases with wt-FLT3 was minimal at 0.1 μM (Figure 2B). In 2 cases with wt-FLT3 (Wt-3 and Wt-5), constitutive phosphorylations of FLT3 were observed, although KW-2449 did not inhibit their colony formations. In the Wt-3 case, the weak inhibitory effect on P-FLT3 might reflect the minimum effect on the colony formation. However, in the Wt-5 case, KW-2449 significantly reduced the level of P-FLT3, whereas the colony formation was not inhibited. In this case, constitutive phosphorylation of STAT5 was also observed, although KW-2449 did not reduce its phosphorylation level, indicating that the STAT5 was phosphorylated by another kinase signal. These results therefore suggested that KW-2449 can dephosphorylate constitutively active wt-FLT3 kinase but not inhibit the proliferation of leukemia cells if they were not mainly addicted to FLT3 the kinase.
Inhibitory effects of KW-2449 on primary AML and colony-forming cells. (A) Ten AML samples consisting of 5 with wild-type FLT3 (Wt-1 to Wt-5), 3 with FLT3/ITD (ITD-1 to ITD-4), and one with both FLT3/ITD and FLT3/KDM (ITD/KDM), were analyzed. Primary AML cells were incubated with or without KW-2449 at 0.1 μM for 6 hours, and then phosphorylation status of FLT3 and STAT5 was analyzed. KW-2449 reduced phosphorylation levels of FLT3 and STAT5 in all AML samples with FLT3 mutations. In the Wt-5 sample, KW-2449 reduced the phosphorylation level of FLT3 but not of STAT5. Vertical lines have been inserted to indicate a repositioned gel lane. (B) AML cells were suspended in methylcellulose semisolid medium containing human stem cell factor, GM-CSF, and interleukin-3 with or without 0.1 μM KW-2449. Colonies were counted after 14 days. Mean treatment/control ratio ± SD from 3 experiments in each sample are shown. In accordance with the down-regulation levels of FLT3 and STAT5 phosphorylations, KW-2449 inhibited the colony formations in all AML samples with FLT3 mutations. In the Wt-5 sample, weak inhibition of the colony formation seems to reflect the sustained STAT5 activation induced by another activation mechanism. (C) Mononuclear cells from human CB were plated in the complete methylcellulose semisolid medium with an increasing concentration of KW-2449. After 14 days of culture, BFU-E, CFU-GM, and CFU-GEMM colonies were counted. Mean total colony numbers ± SD at the indicated concentrations of KW-2449 are shown (n = 5). Although KW-2449 inhibited a total number of colonies in a dose-dependent manner, the Bonferroni test revealed that the statistical significances were found between control and at the 0.5 μM (P < .01), and at the .01 μM and at the 0.5 μM (P < .05). (D) The representative result of the distribution of BFU-E, CFU-GM, and CFU-GEMM colonies from 1 CB sample is shown. Although KW-2449 inhibited the colony formation of CB mononuclear cells in a dose-dependent manner, the distribution of BFU-E, CFU-GM, and CFU-GEMM colonies was not affected by the KW-2449 treatment.
Inhibitory effects of KW-2449 on primary AML and colony-forming cells. (A) Ten AML samples consisting of 5 with wild-type FLT3 (Wt-1 to Wt-5), 3 with FLT3/ITD (ITD-1 to ITD-4), and one with both FLT3/ITD and FLT3/KDM (ITD/KDM), were analyzed. Primary AML cells were incubated with or without KW-2449 at 0.1 μM for 6 hours, and then phosphorylation status of FLT3 and STAT5 was analyzed. KW-2449 reduced phosphorylation levels of FLT3 and STAT5 in all AML samples with FLT3 mutations. In the Wt-5 sample, KW-2449 reduced the phosphorylation level of FLT3 but not of STAT5. Vertical lines have been inserted to indicate a repositioned gel lane. (B) AML cells were suspended in methylcellulose semisolid medium containing human stem cell factor, GM-CSF, and interleukin-3 with or without 0.1 μM KW-2449. Colonies were counted after 14 days. Mean treatment/control ratio ± SD from 3 experiments in each sample are shown. In accordance with the down-regulation levels of FLT3 and STAT5 phosphorylations, KW-2449 inhibited the colony formations in all AML samples with FLT3 mutations. In the Wt-5 sample, weak inhibition of the colony formation seems to reflect the sustained STAT5 activation induced by another activation mechanism. (C) Mononuclear cells from human CB were plated in the complete methylcellulose semisolid medium with an increasing concentration of KW-2449. After 14 days of culture, BFU-E, CFU-GM, and CFU-GEMM colonies were counted. Mean total colony numbers ± SD at the indicated concentrations of KW-2449 are shown (n = 5). Although KW-2449 inhibited a total number of colonies in a dose-dependent manner, the Bonferroni test revealed that the statistical significances were found between control and at the 0.5 μM (P < .01), and at the .01 μM and at the 0.5 μM (P < .05). (D) The representative result of the distribution of BFU-E, CFU-GM, and CFU-GEMM colonies from 1 CB sample is shown. Although KW-2449 inhibited the colony formation of CB mononuclear cells in a dose-dependent manner, the distribution of BFU-E, CFU-GM, and CFU-GEMM colonies was not affected by the KW-2449 treatment.
The inhibitory effect of KW-2449 on normal hematopoiesis was also evaluated using human hematopoietic progenitors. Mononuclear cells from 5 independent CB were plated in complete methylcellulose semisolid medium with an increasing concentration of KW-2449. Although KW-2449 inhibited the colony formation of CB mononuclear cells in a dose-dependent manner, the distribution of BFU-E, CFU-GM, and CFU-GEMM colonies was not affected by the KW-2449 treatment (Figure 2C-D). The Bonferroni test revealed that statistically significant differences in the total number of colonies were found between control and at the 0.5 μM (P < .01) concentration, and at the 0.01 μM and at the 0.5 μM (P < .05) concentration. The reduction of a total colony number was at most 59.6% plus or minus 20.2% of the control, at 0.1 μM of KW-2449. These results indicated that the suppressive effect of KW-2449 on the normal hematopiesis was modest, whereas that on leukemia with FLT3 mutations was significant (Figure 2B-C).
In vivo effects of KW-2449 on leukemia cells with FLT3 mutation
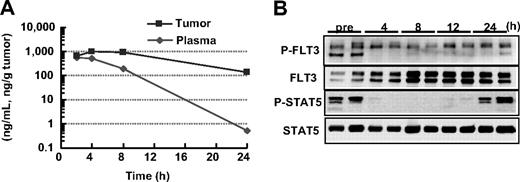
In vivo antileukemia activities of KW-2449 were evaluated using MOLM-13, FLT3-ITD AML, xenograft model. First, the concentrations of KW-2449 in both plasma and tumor after oral administration were sequentially examined in SCID mice bearing the subcutaneous MOLM-13 tumor. The tumor/plasma concentration ratio of KW-2449 tended to increase along with the time after administration and reached approximately 400, 24 hours after dosing (Figure 3A). The levels of P-FLT3 and P-STAT5 in the tumor were completely reduced from 4 to 12 hours after the administration of KW-2449 (Figure 3B). Although dephosphorylations of FLT3 and STAT5 were observed until 12 hours after administration, these returned to almost the basal level at 24 hours. These results suggested that the oral administration of KW-2449 at a twice daily schedule could be adequate for continuous inhibition of activated FLT3 in the mouse model.
Pharmacokinetic and pharmacodynamic effects of KW-2449 in FLT3-activated leukemia. (A) MOLM-13 cells (107 cells/mouse) were subcutaneously inoculated into 2 SCID mice. Ten days after inoculation, KW-2449 at 20 mg/kg was orally administered twice every 12 hours. The concentrations of KW-2449 in plasma and tumor were sequentially analyzed by LC/MS/MS after the final administration of KW-2449. (B) The transition of FLT3 and STAT5 phosphorylation levels in tumor were analyzed by Western blotting. For analysis of FLT3 and its phosphorylated form (P-FLT3), the blots of immunoprecipitated FLT3 SDS-PAGE samples were analyzed by antiphospho-tyrosine (P-Tyr) antibody and anti-FLT3 antibody. For detection of STAT5 and its phosphorylated form (P-STAT5), total tumor lysate SDS-PAGE samples were analyzed by anti–P-STAT5 antibody and anti-STAT5 antibody as primary antibodies. Dephosphorylations of FLT3 and STAT5 in MOLM-13 were observed until 12 hours after the last administration. Results from 2 mice at each point are shown.
Pharmacokinetic and pharmacodynamic effects of KW-2449 in FLT3-activated leukemia. (A) MOLM-13 cells (107 cells/mouse) were subcutaneously inoculated into 2 SCID mice. Ten days after inoculation, KW-2449 at 20 mg/kg was orally administered twice every 12 hours. The concentrations of KW-2449 in plasma and tumor were sequentially analyzed by LC/MS/MS after the final administration of KW-2449. (B) The transition of FLT3 and STAT5 phosphorylation levels in tumor were analyzed by Western blotting. For analysis of FLT3 and its phosphorylated form (P-FLT3), the blots of immunoprecipitated FLT3 SDS-PAGE samples were analyzed by antiphospho-tyrosine (P-Tyr) antibody and anti-FLT3 antibody. For detection of STAT5 and its phosphorylated form (P-STAT5), total tumor lysate SDS-PAGE samples were analyzed by anti–P-STAT5 antibody and anti-STAT5 antibody as primary antibodies. Dephosphorylations of FLT3 and STAT5 in MOLM-13 were observed until 12 hours after the last administration. Results from 2 mice at each point are shown.
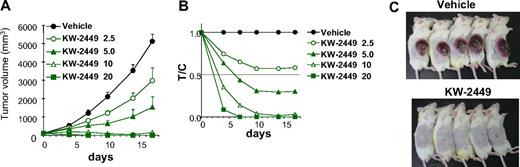
In the MOLM-13 tumor xenograft model, oral administration of KW-2449 for 14 days showed a potent and significant antitumor effect in a dose-dependent manner (Figure 4A). KW-2449 treatment at 2.5 and 5.0 mg/kg twice a day showed growth inhibition of tumors with the ratio of tumor volume in the treated to control mice minimum values (T/Cmin) of 0.57 and 0.29, respectively (Figure 4B). Furthermore, KW-2449 treatment at 10 mg/kg twice a day showed tumor regression with T/Cmin of 0.010 and treatment at 20 mg/kg twice a day completely eradicated tumors in all mice (Figure 4C).
In vivo efficacy of xenotransplanted tumors with FLT3/ITD. (A-C) MOLM-13 cells (107 cells/mouse) were subcutaneously inoculated into SCID mice. Five days after inoculation, tumor volume was measured. The 25 mice with tumors ranging from 90 to 130 mm3 were selected 5 days after inoculation and divided into 5 groups. Mice (n = 5 in each group) were orally administered with vehicle or KW-2449 (2.5, 5.0, 10, and 20 mg/kg) twice a day for 14 days. (A) Tumor volume was measured twice a week during the treatment. Mean tumor volume ± SD is shown. KW-2449 showed potent and significant antitumor effect in a dose-dependent manner. (B) Relative ratio of tumor volume (V) to initial tumor volume (V0) was represented (V/V0). Relative V/V0 ratio of a drug-treated group compared with a control group was represented as T/C. (C) KW-2449 treatment at 20 mg/kg twice a day showed complete regression and disappearance of tumors in all mice.
In vivo efficacy of xenotransplanted tumors with FLT3/ITD. (A-C) MOLM-13 cells (107 cells/mouse) were subcutaneously inoculated into SCID mice. Five days after inoculation, tumor volume was measured. The 25 mice with tumors ranging from 90 to 130 mm3 were selected 5 days after inoculation and divided into 5 groups. Mice (n = 5 in each group) were orally administered with vehicle or KW-2449 (2.5, 5.0, 10, and 20 mg/kg) twice a day for 14 days. (A) Tumor volume was measured twice a week during the treatment. Mean tumor volume ± SD is shown. KW-2449 showed potent and significant antitumor effect in a dose-dependent manner. (B) Relative ratio of tumor volume (V) to initial tumor volume (V0) was represented (V/V0). Relative V/V0 ratio of a drug-treated group compared with a control group was represented as T/C. (C) KW-2449 treatment at 20 mg/kg twice a day showed complete regression and disappearance of tumors in all mice.
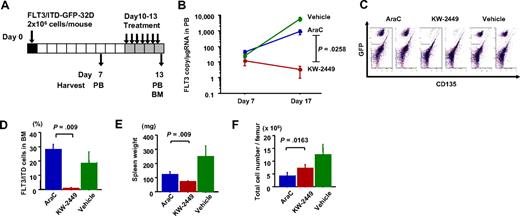
We next compared the effects of KW-2449 on mutant FLT3-expressing cells with a conventional antileukemic agent, AraC, using the syngeneic transplantation mouse model. Human FLT3/ITD-ires-GFP-expressing 32D (FLT3/ITD-GFP-32D) cells were intravenously inoculated into syngeneic C3H/Hej mice, and then KW-2449, AraC, or vehicle was administered to the mice 11 days after inoculation for 4 days (Figure 5A). At the seventh day after inoculation, mean FLT3 transcript levels in PB were 24.4 plus or minus 6.7, 11.8 plus or minus 5.7, and 42.0 plus or minus 21.7 copies/μg RNA in vehicle-, KW-2449- and AraC-treated mice, respectively. In all vehicle-treated mice, FLT3 transcript level increased, and the mean FLT3 transcript level in PB on day 13 was 5869.6 plus or minus 1640.1 copies/μg RNA. In contrast, KW-2449 treatment repressed the expansion of FLT3/ITD-GFP-32D cells as the decrease of FLT3 transcript levels was observed in all mice, and the mean FLT3 transcript level in PB was 3.25 plus or minus 2.29 copies/μg RNA on day 13. The increase in FLT3 transcripts level in all AraC-treated mice was lower than that in vehicle-treated mice, although the effect of AraC treatment was limited as the mean FLT3 transcript level in PB was 882.7 plus or minus 305.5 copies/μg RNA on day 13. These results demonstrated that the repressive effects by KW-2449 on the expansion of FLT3/ITD-GFP-32D cells were significantly stronger than those by AraC (P = .026 by the repeated-measures analysis of variance; Figure 5B). Flow cytometry analysis revealed that KW-2449 significantly eradicated FLT3/ITD-GFP-32D cells from BM (the mean percentages of FLT3/ITD-GFP-32D cells in BM after treatment were 30.0% ± 3.6% and 0.75% ± 0.75% in AraC- and KW-2449-treated mice, respectively; P = .009 by the Mann-Whitney U test; Figure 5C-D). Furthermore, the mean spleen weight of KW-2449-treated mice was significantly lighter than that of AraC-treated mice (71.4 ± 6.2 and 122.2 ± 20.4 mg, respectively; P = .009 by the Mann-Whitney U test; Figure 5E). Notably, the total number of nuclear cells in the BM of AraC-treated mice was significantly decreased compared with that of KW-2449-treated mice ([4.2 ± 1.3] × 106 and [7.3 ± 1.3] × 106 cells/femur, respectively; P = .016 by the Mann-Whitney U test; Figure 5F). In this model, we confirmed that KW-2449 could potently and selectively eradicate mutant FLT3-expressing leukemia cells both in PB and BM in contrast to the nonselective BM suppression of conventional cytotoxic agents such as AraC.
Inhibition effects on FLT3/ITD-GFP-32D cells in C3H/Hej-mice syngeneic transplantation model. (A) C3H/Hej mice were inoculated with 2 × 106 of FLT3/ITD-GFP-32D cells on day 0. From the 10th day after inoculation, mice were administrated with KW-2449 at 40 mg/kg (orally) twice a day, AraC at 150 mg/kg (intravenously) daily or vehicle for 4 days (n = 5 in each group). PB was collected from each mouse on day 7 and day 13. BM was collected on day 13. (B) Human FLT3 transcripts in PB were quantitated by a real-time fluorescence detection method. Mean transcript level ± SEM is shown. KW-2449 treatment significantly repressed the expansion of FLT3/ITD-GFP-32D cells compared with AraC treatment (P = .026 by the repeated-measures analysis of variance method). (C) The percentage of residual BM FLT3/ITD-GFP-32D cells in femur was compared among vehicle-, KW-2449-, and AraC-treated mice using flow cytometry. Representative results of flow cytometry are shown. (D) Mean percentages of residual FLT3/ITD-GFP-32D cells plus or minus SD in BM are shown. KW-2449 significantly eradicated FLT3/ITD-GFP-32D cells from BM compared with AraC treatment (P = .009 by the Mann-Whitney U test). (E) Mean spleen weight of each treated mouse ± SD is shown. The mean spleen weight of KW-2449-treated mice was significantly lighter than that of AraC-treated mice (P = .009 by the Mann-Whitney U test). (F) Mean total cell numbers in femur after the treatment ± SD are shown. The total number of nuclear cells in the BM of AraC-treated mice was significantly decreased compared with that of KW-2449–treated mice (P = .016 by the Mann-Whitney U test).
Inhibition effects on FLT3/ITD-GFP-32D cells in C3H/Hej-mice syngeneic transplantation model. (A) C3H/Hej mice were inoculated with 2 × 106 of FLT3/ITD-GFP-32D cells on day 0. From the 10th day after inoculation, mice were administrated with KW-2449 at 40 mg/kg (orally) twice a day, AraC at 150 mg/kg (intravenously) daily or vehicle for 4 days (n = 5 in each group). PB was collected from each mouse on day 7 and day 13. BM was collected on day 13. (B) Human FLT3 transcripts in PB were quantitated by a real-time fluorescence detection method. Mean transcript level ± SEM is shown. KW-2449 treatment significantly repressed the expansion of FLT3/ITD-GFP-32D cells compared with AraC treatment (P = .026 by the repeated-measures analysis of variance method). (C) The percentage of residual BM FLT3/ITD-GFP-32D cells in femur was compared among vehicle-, KW-2449-, and AraC-treated mice using flow cytometry. Representative results of flow cytometry are shown. (D) Mean percentages of residual FLT3/ITD-GFP-32D cells plus or minus SD in BM are shown. KW-2449 significantly eradicated FLT3/ITD-GFP-32D cells from BM compared with AraC treatment (P = .009 by the Mann-Whitney U test). (E) Mean spleen weight of each treated mouse ± SD is shown. The mean spleen weight of KW-2449-treated mice was significantly lighter than that of AraC-treated mice (P = .009 by the Mann-Whitney U test). (F) Mean total cell numbers in femur after the treatment ± SD are shown. The total number of nuclear cells in the BM of AraC-treated mice was significantly decreased compared with that of KW-2449–treated mice (P = .016 by the Mann-Whitney U test).
In vitro effects of KW-2449 on FLT3 wild-type leukemia
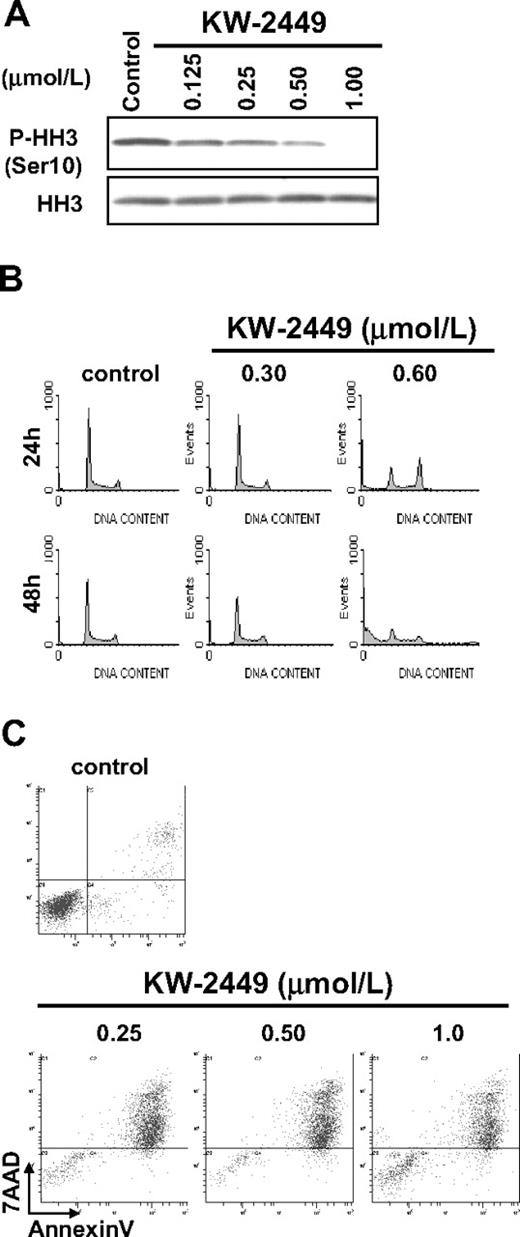
On the other hand, KW-2449 inhibited the growth of human ALL cell line RS4;11, which expresses unphosphorylated wt-FLT3, with the GI50 value of 0.23 μM (Table 2). Because KW-2449 shows potent Aurora A and Aurora B kinase inhibition, we evaluated whether the growth inhibitory effect on RS4;11 was induced by Aurora kinase inhibition. When the cell cycle was arrested in the M-phase by nocodazole, phosphorylated histone-H3 (P-HH3) was clearly observed in RS4;11, but it was decreased by the treatment with KW-2449 in a dose-dependent manner (Figure 6A). Cell cycle distribution analysis indicated that KW-2449 (0.60 μM) induced G2/M arrest and apparent increase of sub-G1 apoptotic cells after 24 hours and 48 hours of exposure, respectively (Figure 6B). Even at 0.30 μM, KW-2449 slightly decreased the population of S-phase cells from 49.0% to 40.6% after 72 hours (histogram data not shown). The increase of annexin V–positive (early apoptotic) cells was also observed at the GI50 value against RS4;11 cells (Figure 6C). These results suggested that KW-2449 has a growth inhibitory potency against leukemia cells even without activated FLT3 through the inhibition of Aurora kinase, although its potency was 5- to10-fold lower than that against those with activated FLT3 kinase.
Effects of KW-2449 on human leukemia cells without FLT3 mutation. (A) RS4;11 cells, which express wild-type FLT3/ITD, were treated with KW-2449 at the indicated concentrations for 30 minutes. Total and phosphorylation levels of HH3 were analyzed by Western blotting. (B) RS4;11 cells were treated with various concentrations of KW-2449 for 48 hours, and cell-cycle distribution was analyzed. (C) RS4;11 cells were treated with various concentration of KW-2449 for 48 hours, and apoptosis induction was analyzed.
Effects of KW-2449 on human leukemia cells without FLT3 mutation. (A) RS4;11 cells, which express wild-type FLT3/ITD, were treated with KW-2449 at the indicated concentrations for 30 minutes. Total and phosphorylation levels of HH3 were analyzed by Western blotting. (B) RS4;11 cells were treated with various concentrations of KW-2449 for 48 hours, and cell-cycle distribution was analyzed. (C) RS4;11 cells were treated with various concentration of KW-2449 for 48 hours, and apoptosis induction was analyzed.
Effects of KW-2449 on wt and T315I-mutated BCR/ABL-expressing leukemia cells
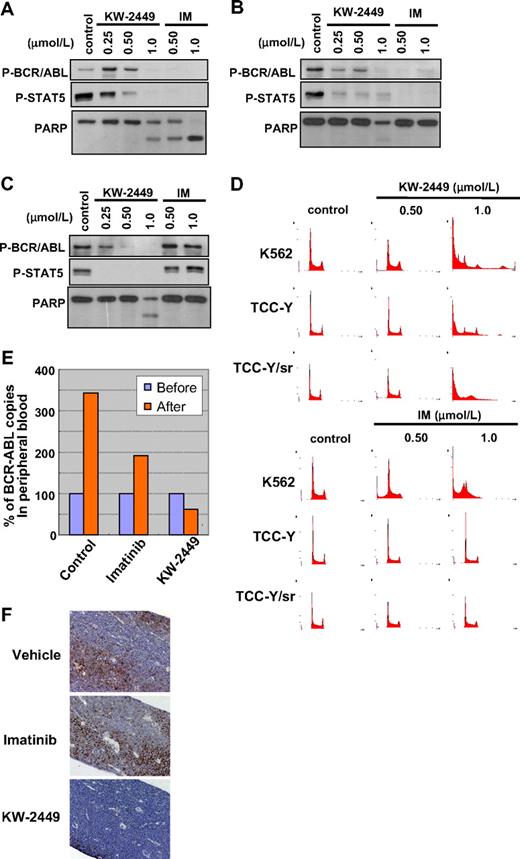
IM resistance is a critical issue to be resolved in the treatment of patients with BCR/ABL-positive leukemia. Because KW-2449 showed potency against both ABL and ABL-T315I kinases in the in vitro kinase assays, we evaluated its growth inhibitory effects on wt (K562 and TCC-Y) and T315I-mutated (TCC-Y/sr) BCR/ABL-expressing human leukemia cell lines. IM inhibited the growth of K562 and TCC-Y cells with GI50 values of 0.24 and 0.18 μM, respectively, whereas its GI50 value against TCC-Y/sr was 24 μM, which was approximately 100-fold higher than against K562 and TCC-Y cells. However, KW-2449 equally inhibited the growth of wt and T315I-mutated BCR/ABL-expressing leukemia cells: GI50 values were 0.27, 0.49, and 0.42 μM in K562, TCC-Y, and TCC-Y/sr cells, respectively (Table 2). In K562 cells, IM decreased the phosphorylation levels of BCR/ABL and STAT5 (Figure 7A), increased the number of the G1 phase-arrested cells at 0.5 μM, and induced apoptosis, which was also shown by an increase of cleaved PARP (Figure 7A,D). On the other hand, KW-2449 induced G2/M phase-arrested cells at 0.50 μM and increased the sub-G1 and polyploidy cells at 1.0 μM (Figure 7D). These inductions of G2/M arrest and polyploidy in K562 cells are presumed to be caused both by the Aurora A and Aurora B inhibitory profile of KW-2449. In TCC-Y cells, the IM treatment at 0.5 and 1.0 μM decreased the phosphorylation levels of BCR/ABL and STAT5, whereas it did not increase the apoptotic or the G1 phase-arrested cells. In contrast, KW-2449 decreased the phosphorylation levels of BCR/ABL and STAT5 from 0.25 μM and induced the G2/M-arrested cells at 0.25 μM (data not shown), as well as apoptosis at 1.0 μM (Figure 7B,D). In TCC-Y/sr cells, IM did not affect the phosphorylation levels of BCR/ABL and STAT5 as well as apoptosis and the cell cycle, whereas KW-2449 decreased both phosphorylation levels from 0.25 and 0.5 μM, respectively. Furthermore, KW-2449 apparently induced apoptosis at 1.0 μM, which was shown by PARP cleavage and the sub-G1 population (Figure 7C-D).
Inhibitory effects of KW-2449 on BCR/ABL-positive leukemia cells. We compared inhibitory effects on wt (K562 and TCC-Y) and T315I-mutated (TCC-Y/sr) BCR/ABL-expressing human leukemia cells between KW-2449 and imatinib (IM). (A) In K562 cells, KW-2449 and IM equally decreased the phosphorylation levels of BCR/ABL and STAT5 and increased cleaved PARP. (B) In TCC-Y cells, IM decreased the phosphorylation levels of BCR/ABL and STAT5, but did not increase cleaved PARP. In contrast, KW-2449 decreased the phosphorylation levels of BCR/ABL and STAT5 and increased cleaved PARP. (C) In TCC-Y/sr cells, IM did not affect the phosphorylation levels of BCR/ABL and STAT5, whereas KW-2449 decreased both phosphorylation levels and increased cleaved PARP. (D) DNA contents were also compared between KW-2449 and IM treatments. IM increased the number of the G1-arrested cells only in K562 cells. However, KW-2449 induced the G2/M-arrested cells in K562, TCC-Y, and TCC-Y/sr cells. (E) We compared the antileukemic efficacy in NOD/SCID mice xenotransplanted with human CML in blast crisis cells harboring the T315I mutation after IM treatment. The treatment effects on the leukemia cells in PB are shown by the after/before BCR/ABL transcript ratio. After the treatment, the BCR/ABL transcript levels in PB increased to 3.391 plus or minus 1.071 and 1.927 plus or minus 0.332 times as much as those before the treatment in the vehicle- and IM-treated mice, respectively. In contrast, KW-2449 significantly decreased BCR/ABL transcript levels as to 0.553 ± 0.288 times as much as those before the treatment compared with the vehicle- and IM-treated mice (P = .001 and P = .003 by the unpaired t test, respectively). (F) Residual leukemia cells in femora were evaluated by the immunohistochemical staining with human CD45. KW-2449 more potently eradicated leukemia cells in BM than IM.
Inhibitory effects of KW-2449 on BCR/ABL-positive leukemia cells. We compared inhibitory effects on wt (K562 and TCC-Y) and T315I-mutated (TCC-Y/sr) BCR/ABL-expressing human leukemia cells between KW-2449 and imatinib (IM). (A) In K562 cells, KW-2449 and IM equally decreased the phosphorylation levels of BCR/ABL and STAT5 and increased cleaved PARP. (B) In TCC-Y cells, IM decreased the phosphorylation levels of BCR/ABL and STAT5, but did not increase cleaved PARP. In contrast, KW-2449 decreased the phosphorylation levels of BCR/ABL and STAT5 and increased cleaved PARP. (C) In TCC-Y/sr cells, IM did not affect the phosphorylation levels of BCR/ABL and STAT5, whereas KW-2449 decreased both phosphorylation levels and increased cleaved PARP. (D) DNA contents were also compared between KW-2449 and IM treatments. IM increased the number of the G1-arrested cells only in K562 cells. However, KW-2449 induced the G2/M-arrested cells in K562, TCC-Y, and TCC-Y/sr cells. (E) We compared the antileukemic efficacy in NOD/SCID mice xenotransplanted with human CML in blast crisis cells harboring the T315I mutation after IM treatment. The treatment effects on the leukemia cells in PB are shown by the after/before BCR/ABL transcript ratio. After the treatment, the BCR/ABL transcript levels in PB increased to 3.391 plus or minus 1.071 and 1.927 plus or minus 0.332 times as much as those before the treatment in the vehicle- and IM-treated mice, respectively. In contrast, KW-2449 significantly decreased BCR/ABL transcript levels as to 0.553 ± 0.288 times as much as those before the treatment compared with the vehicle- and IM-treated mice (P = .001 and P = .003 by the unpaired t test, respectively). (F) Residual leukemia cells in femora were evaluated by the immunohistochemical staining with human CD45. KW-2449 more potently eradicated leukemia cells in BM than IM.
We next compared the effects of KW-2449 on human CML-BC cells harboring T315I mutation with IM, using the xenotransplantation mouse model. After confirming the engraftment of human CML-BC cells, NOD/SCID mice were administered with KW-2449, IM, or vehicle for 5 days. After the treatment, the BCR/ABL transcript levels in PB increased to 3.391 plus or minus 1.071 and 1.927 plus or minus 0.332 times as much as those before the treatment in the vehicle- and IM-treated mice, respectively. In contrast, KW-2449 significantly decreased BCR/ABL transcript levels as to 0.553 plus or minus 0.288 times as much as those before treatment compared with the vehicle- and IM-treated mice (P = .001 and P = .003 by the unpaired t test, respectively; Figure 7E). Furthermore, the immunohistochemical analysis showed that KW-2449 dramatically eradicated leukemia cells in BM (Figure 7F). In addition, KW-2449 treatment significantly prolonged the survival time of TCC-Y/sr-inoculated SCID mice (data not shown). These results collectively suggested that KW-2449 potently suppresses the growth both of wt- and T315I-mutated BCR/ABL-expressing leukemia cells by dual-inhibitory activities against BCR/ABL and Aurora kinases.
Discussion
Here we describe how KW-2449 potently and selectively inhibits the growth of leukemia cells harboring constitutively activated FLT3 kinase both in vitro and in vivo. As described previously, KW-2449 was selected from chemical libraries of Kyowa Hakko Kirin as a highly potent compound whose GI50 values against MOLM-13 and FLT3-D835Y-expressing 32D cells were less than 0.10 μM. In parallel, we evaluated the growth inhibitory activities against BCR/ABL-positive K562 cells and several hematologic malignant cell lines. As shown in this study, KW-2449 inhibited FLT3, ABL, and ABL-T315I kinases. In addition to these tyrosine kinases, which are involved in oncogenic addiction of several leukemia cells, KW-2449 has inhibitory effect on Aurora kinase, which is a key regulatory kinase in mitosis. Because KW-2449 potently inhibited the proliferation of various hematologic malignant cells, including wt-BCR/ABL– and T315I-mutated BCR/ABL-expressing cells, with GI50 values ranging from 0.014 to 0.65 μM, we evaluated which kinase was targeted in each malignant cell. To address this issue, we analyzed the phosphorylation status of possibly targeted kinases and the cell cycle distribution after KW-2449 treatment. In mutant FLT3-expressing leukemia cells, the reduction of P-FLT3 level was observed from less than 0.030 μM of KW-2449, which was consistent with its growth inhibitory and the G1-arrest effects. In leukemia cells without FLT3 activation such as RS4;11, the sensitivity of KW-2449 was 5- to 10-fold lower than that in mutant FLT3-expressing leukemia cells. In these cells, KW-2449 induced the G2/M arrest or polyploidy and apoptosis at approximately GI50 value (0.25 μM) via Aurora kinase inhibition, which was detected by the reduction of P-HH3. It has been reported that P-HH3 is the target molecule of Aurora B kinase, and the decrease of P-HH3 level was observed from 0.125 μM (Figure 6A), whereas G2/M arrest, an indicator of Aurora kinase A inhibition, was clear at 0.60 μM of KW-2449. It suggested that both Aurora B and Aurora A kinase inhibition by KW-2449 contributes antileukemia effects in FLT3 wild-type. These results collectively suggested that KW-2449 potently suppressed the growth of leukemia cells immortalized by FLT3 activation via FLT3 inhibition alone at a lower concentration, whereas the growth suppression of FLT3-inactivated leukemia cells was induced by Aurora inhibition at a higher concentration. It has been reported that several kinase inhibitors, such as MK-0457 (VX-680), simultaneously suppress both FLT3 and Aurora kinases.34 When we examined the effects of MK-0457 on mutant FLT3-expressing leukemia cells, it induced the G2/M arrest at the GI50 values, indicating that its primary cellular target was Aurora kinase, but not FLT3 even in the constitutively FLT3-activated cells (data not shown). However, KW-2449 first inhibits FLT3 kinase with approximately 10-fold higher potency than Aurora kinase. Therefore, this characteristic mode of inhibitory action may be advantageous over the adverse events associated with the Aurora kinase inhibition.
The clinical efficacy of both PKC-412 and CEP-701 given as monotherapy was reportedly unimpressive despite their high potency in the in vitro studies and extensive exposure in humans. This was partly explained by their structural problem (both compounds are well known as tight binders to hAGP in human plasma) because they contain indolocarbazole motif and result in the significant reduction of their biologic activities in human bodies. In contrast, the growth inhibitory activity of KW-2449 was not attenuated by hAGP, indicating the advantage to keep the biologically active concentration in human plasma.
It is well known that IM that targets the adenosine triphosphate-binding site of the kinase domain of BCR/ABL, inducing remissions in patients with chronic-phase CML. However, whereas responses in the chronic phase were durable, remissions observed in blast crisis patients were typically short-lived, with relapse occurring within 6 months despite continued therapy. Thus, IM resistance is becoming an increasingly recognized problem for the treatment of patients with BCR/ABL-positive leukemia. Several IM-resistant mechanisms, such as acquired mutation in the BCR/ABL gene, overexpression of BCR/ABL, hAGP binding, and the emerging other kinase activations, have been reported.35 To overcome the resistance, ABL kinase inhibitors in the second generation have been investigated, and some of them showed significant clinical response to IM-resistant or refractory patients, although the resistance of T315I-mutated BCR/ABL kinase remains to be resolved.36 We therefore evaluated the efficacy of KW-2449 for leukemia cells with T315I-mutated BCR/ABL both in vitro and in vivo. KW-2449 at 0.25 μM showed the decrease of P-ABL and P-STAT5 in K562, TCC-Y, and TCC-Y/sr cells, whereas IM had little effect on these signaling molecules in T315I-mutated leukemia (Figure 7A). In addition to inhibitory activity of KW-2449 to T315I-mutated BCR/ABL, Aurora kinase inhibition, which was indicated by cell-cycle distribution in TCC-Y/sr, could contribute to the release of IM resistance. On the other hand, whereas IM showed G1 arrest and ABL inhibition in wt-BCR/ABL cells, it had limited activity both in cell cycle and cell signaling in T315I-mutated BCR/ABL cells. These data indicated that the inhibition of wt- and T315I-mutated BCR/ABL kinase by KW-2449 at lower concentrations showed limited effects on cell viability, whereas the additional inhibitory effects on Aurora kinase by KW-2449 at higher concentrations modulated the survival and proliferation of the IM-resistant leukemia cells. These multifunctional action mechanisms, as well as the order of potency against various kinases, which were involved in oncogenic addiction and drug-resistance, would contribute to overcome the IM resistance.
It has been reported that potent and selective Aurora kinase inhibitors show remarkable growth inhibition of a variety of cancer cells in vitro, although several severe adverse events such as hematopoietic toxicity have been observed in the early-phase clinical trials. However, our results suggest that the additive and/or simultaneous inhibition of Aurora kinase at a lower potency than mainly targeted kinases might contribute to increase the growth inhibitory effects on cancer cells without severe adverse effects.
In conclusion, targeted inhibition of FLT3 kinase with KW-2449 induced the potent growth inhibition of leukemia cells transformed by the constitutive activation of FLT3 kinase. KW-2449 also showed growth inhibitory effects against FLT3 leukemia by its Aurora kinase inhibition. In addition, simultaneous inhibition of T315I-mutated BCR/ABL and Aurora kinases by KW-2449 also induced the growth inhibition of IM-resistant leukemia cells. Currently, KW-2449 is being investigated in a phase 1/2 study in patients with relapsed or refractory AML (NCT00779480). The present results, nevertheless, provide an important insight into clinical investigations for the treatment of patients with BCR/ABL-positive leukemia acquiring the IM resistance, including the T315I-mutation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank H. Kosugi for collecting clinical samples and S. Yamaji, E. Koshimura, M. Asano, and K. Higuchi for technical assistance.
This work was supported by the National Institute of Biomedical Innovation, Ministry of Health, Labor and Welfare, Ministry of Education, Culture, Sports, Science and Technology on the Scientific Research and the 21st Century COE Program Integrated Molecular Medicine for Neuronal and Neoplastic Disorders, Japan.
Authorship
Contribution: Y. Shiotsu designed experiments; screened chemical libraries; performed cell-based assay, Western blot, and animal studies; analyzed data; generated figures; and wrote the manuscript; H.K. designed experiments; performed Western blot, colony assay, animal studies, and quantitative real-time RT-PCR; analyzed data; generated figures; and wrote the manuscript; Y.I. collected clinical samples and performed Western blot and FCM; R.T. performed animal studies, quantitative real-time RT-PCR, and pathologic analysis; M.S. performed FCM analysis and Western blot; H.U. screened chemical libraries; K.I. performed Western blot and animal studies; Y. Mori performed colony assay; K.O. collected clinical samples and performed animal studies and quantitative real-time RT-PCR; Y. Minami performed cell cycle analysis; A.A. performed animal studies; H.M. analyzed the LC/MS/MS; T.A. and S.A. provided input into experiment design; Y.K. provided input into chemical synthesis; Y. Sato established imatinib-resistant cell lines; and T.N. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: Y. Shiotsu, M.S., H.U., K.I., H.M., T.A., Y.K., and S.A. are employed by Kyowa Hakko Kirin Co Ltd. H.K. has a consultancy with Kyowa Hakko Kirin Co Ltd.
Correspondence: Yukimasa Shiotsu, Fuji Research Park, Kyowa Hakko Kirin, 1188 Shimotogari, Nagaizumi-cho, Sunto-gun, Shizuoka 411-8731, Japan; e-mail: yukimasa.shiotsu@kyowa-kirin.co.jp.
References
Author notes
*Y. Shiotsu and H.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal