Abstract
The role of allogeneic stem cell transplantation in chronic myeloid leukemia is being reevaluated. Whereas drug treatment has been shown to be superior in first-line treatment, data on allogeneic hematopoietic stem cell transplantation (allo SCT) as second-line therapy after imatinib failure are scarce. Using an interim safety analysis of the randomized German CML Study IV designed to optimize imatinib therapy by combination, dose escalation, and transplantation, we here report on 84 patients who underwent consecutive transplantation according to predefined criteria (low European Group for Blood and Marrow Transplantation [EBMT] score, imatinib failure, and advanced disease). Three-year survival after transplantation of 56 patients in chronic phase was 91% (median follow-up: 30 months). Transplantation-related mortality was 8%. In a matched pair comparison of patients who received a transplant and those who did not, survival was not different. Three-year survival after transplantation of 28 patients in advanced phase was 59%. Eighty-eight percent of patients who received a transplant achieved complete molecular remissions. We conclude that allo SCT could become the preferred second-line option after imatinib failure for suitable patients with a donor. The study is registered at the National Institutes of Health, http://clinicaltrials.gov: NCT00055874.
Introduction
Imatinib as a selective BCR-ABL tyrosine kinase inhibitor (TKI) has replaced allogeneic hematopoietic stem cell transplantations (allo SCTs) as first-line therapy for patients with chronic myeloid leukemia (CML).1 As a consequence, the number of allo SCTs has decreased substantially.2,3 Most recent studies have concentrated on a potential negative effect of imatinib on the outcome after allo SCT.4,5 Although observation time and overall survival data of such studies are limited, they indicate that imatinib therapy is not harmful for transplantation.2,4-9 Still, allo SCT remains an important treatment option. According to the International Randomized Study of Interferon Versus STI571 (IRIS), 40% of the patients are no longer on study imatinib at 7 years,10 due to unsatisfactory response, imatinib failure, or other reasons.11 The patients are in need of second-line treatments. The best approach in these situations has not yet been defined in prospective controlled studies. Allo SCT is one option. Fear of excess mortality with allo SCT and ease of drug administration frequently preclude transplantation. In a randomized study, the German CML Study Group has shown that interferon α (IFN)–based drug treatment is superior to allo SCT as first-line therapy.12 Data on allo SCT in the imatinib era are scarce; controlled data are warranted. There are hints that pretransplantation imatinib might improve outcome4 in line with earlier observations that reduction of leukemia load by IFN before transplantation is associated with better transplantation outcome.13 We made use of an interim safety analysis of the randomized German CML Study IV, designed to optimize imatinib-based therapy,14 to determine the role of allo SCT in the imatinib era within the context of a prospective controlled multicenter trial and according to predefined criteria. We show that survival rates in chronic phase (CP) CML 3 years after transplantation are not different from those achieved with drug treatment alone, but with high rates of molecular response. In advanced phase disease, transplantation outcome is better than hitherto reported.
Methods
Study design
This report is based on a planned interim safety analysis of the German CML Study IV, a 5-arm randomized multicenter trial, designed to compare imatinib 400 mg versus imatinib in combination with IFN versus imatinib in combination with cytarabine (araC) versus imatinib after IFN failure versus imatinib 800 mg and to determine the role of transplantation in the imatinib era. The criteria for transplantation are, as first-line therapy, high disease risk (Euro score)15 and/or low transplantation risk (European Group for Blood and Marrow Transplantation [EBMT] score 0-1)16 and, as second-line therapy, imatinib failure and advanced disease (accelerated phase or blast crisis). Primary goals of CML Study IV are comparative determination of survival, time to progression, and rates of hematologic, cytogenetic, and molecular remissions between treatment arms and comparative evaluation of imatinib-based treatment strategies with and without allogeneic transplantation. Secondary goals include determinations of remission duration, toxicities and course of terminal phase, development of a prognostic score, evaluation of new therapies for imatinib resistance, and long-term observation of patients who received a transplant and of patients in complete cytogenetic remission.
The protocol followed the Declaration of Helsinki and was approved by the ethics committee of the Medizinische Fakultät Mannheim of the University of Heidelberg and by local ethics committees of participating centers. Written informed consent was obtained from all patients before entering the study. The study is registered at the National Institutes of Health, http://clinicaltrials.gov: NCT00055874.
Patient population
By November 2008, 1242 patients were randomized and 84 received a transplant, as outlined in Figure 1. (1) Nineteen patients (23%) underwent elective transplantation in first CP with low EBMT score and/or high Euro risk score; 14 of these fulfilled the transplantation criteria of the protocol, 2 had borderline criteria, and 3 underwent transplantation on patients' wish. (2) Thirty-seven patients (44%) underwent transplantation after imatinib failure in first CP. (3) Twenty-eight patients (33%) underwent transplantation in advanced disease, including 25 patients in blast crisis and 3 patients in accelerated phase; 11 of these patients achieved a second and 1, a third CP before transplantation. Patients were derived equally from all 5 randomization/treatment groups (Figure 1). Imatinib failure comprised imatinib intolerance and resistance according to European LeukemiaNet (ELN) definition.17
Overview of randomized and evaluable patients. By November 30, 2008, 1242 patients were randomized. Eleven of 312 patients randomized to imatinib 400 mg, 11 of 304 randomized to imatinib 800 mg, 20 of 338 randomized to imatinib in combination with IFN, 22 of 158 randomized to imatinib in combination with araC, and 20 of 131 randomized to imatinib after IFN failure underwent transplantation. The 84 patients who underwent transplantation were analyzed in 3 groups according to the reason of allo SCT: group I (early transplantation in low-risk patients, EBMT score 0-1), group II (imatinib failure in first CP), and group III (advanced disease).
Overview of randomized and evaluable patients. By November 30, 2008, 1242 patients were randomized. Eleven of 312 patients randomized to imatinib 400 mg, 11 of 304 randomized to imatinib 800 mg, 20 of 338 randomized to imatinib in combination with IFN, 22 of 158 randomized to imatinib in combination with araC, and 20 of 131 randomized to imatinib after IFN failure underwent transplantation. The 84 patients who underwent transplantation were analyzed in 3 groups according to the reason of allo SCT: group I (early transplantation in low-risk patients, EBMT score 0-1), group II (imatinib failure in first CP), and group III (advanced disease).
Statistical analysis
Probabilities of overall survival were calculated by the Kaplan-Meier method and compared by the log-rank statistics. Cumulative incidences of transplantation-related mortality (TRM) were calculated under consideration of competing risks, as suggested by Gooley et al.18 All analyses were performed with the SAS software Version 9.1.3 (SAS Institute). Survival was censored at the date of last follow-up.
Matched pair analysis
Fifty-six patients underwent transplantation in first CP (groups I and II). To each of 53 patients who received a transplant, 2 imatinib-treated patients could be matched with regard to age, sex, risk profile, disease phase, and interval to transplantation (Table 1). Because no relevant differences with regard to progression or survival are known, there was no differentiation between imatinib dosages or treatment with or without an additional drug. In case more than 2 imatinib-treated patients were qualified, a random drawing among all candidates was performed. All 106 imatinib-treated patients had been in first CP at the time when their corresponding partner underwent transplantation. The time between diagnosis and transplantation was appropriately matched. A patient who underwent transplantation in first chronic phase on day X has a guaranteed survival time from diagnosis to day X. To ensure a fair comparison, the 2 matched imatinib-treated partners had to have survived and been in first chronic phase on day X, too.
Patient characteristics of 106 matched imatinib-treated patients and 53 patients who underwent transplantation in first CP (matched pair analysis)
| . | Transplantation . | Imatinib . |
|---|---|---|
| No. | 53 | 106 |
| Sex, % male | 56.6 | 56.6 |
| Median age, y | 37 | 36.5 |
| EURO risk score, % | ||
| Low | 41.5 | 41.5 |
| Intermediate | 35.8 | 35.8 |
| High | 22.6 | 22.6 |
| . | Transplantation . | Imatinib . |
|---|---|---|
| No. | 53 | 106 |
| Sex, % male | 56.6 | 56.6 |
| Median age, y | 37 | 36.5 |
| EURO risk score, % | ||
| Low | 41.5 | 41.5 |
| Intermediate | 35.8 | 35.8 |
| High | 22.6 | 22.6 |
Matching criteria were age, sex, risk profile, disease phase, and interval to transplantation.
CP indicates first chronic phase.
Cytogenetic and molecular analysis
Cytogenetic analyses were performed by chromosome banding analysis of at least 20 marrow cell metaphases, after short-term culture (24 or 48 hours or both) with standard G or Q banding techniques. Molecular diagnostics for residual BCR-ABL mRNA transcripts followed the procedures recommended by Hughes et al19 and Cross et al.20
Results
Eighty-four CML patients who underwent transplantation consecutively according to predefined criteria within the randomized treatment optimization study IV of the German CML Study Group are analyzed for outcome. Patients' characteristics and transplantation and outcome details are summarized in Table 2. The median age of all 84 patients who underwent transplantation was 37 years (range, 16-62 years); 65% were male. Transplantations were performed in 2003 to 2008, with 80% of transplantations completed before 2007.
Patient and transplantation characteristics of 84 patients who underwent transplantation between 2003 and 2008
| . | Allo SCT in 1st CP . | Total allo SCT in 1st CP . | Allo SCT in advanced phases . | |
|---|---|---|---|---|
| Early allo SCT . | Imatinib failure in 1st CP . | |||
| No. | 19 | 37 | 56 | 28 |
| Euro score15 (%) | ||||
| High | 6 (32) | 10 (27) | 15 (27) | 9 (32) |
| Intermediate | 3 (15) | 13 (35) | 17 (30) | 8 (29) |
| Low | 10 (53) | 14 (38) | 24 (43) | 11 (39) |
| Sokal score21 (%) | ||||
| High | 6 (32) | 17 (46) | 23 (41) | 11 (39) |
| Intermediate | 2 (10) | 7 (19) | 9 (16) | 7 (25) |
| Low | 11 (58) | 13 (35) | 24 (43) | 10 (36) |
| Sex (% male) | 12 (63) | 21 (57) | 33 (59) | 22 (79) |
| Age at diagnosis, y, median (range) | 35 (16-56) | 38 (21-56) | 37 (16-56) | 38 (18-62) |
| Time to transplantation mo, median (range) | 9.0 (4.8-23.6) | 17.6 (5.0-53.7) | 14.2 (4.8-53.7) | 12.8 (3.5-55.1) |
| EBMT score16 (%) | ||||
| 0-1 | 8 (42) | 4 (11) | 12 (21) | 0 |
| 2 | 3 (16) | 4 (11) | 7 (13) | 1 (4) |
| 3-4 | 8 (42) | 27 (73) | 35 (62) | 9 (32) |
| 5 or higher | 0 | 2 (5) | 2 (4) | 18 (64) |
| Best response before SCT (%) | ||||
| CHR | 18 (95)* | 30 (81) | 48 (86) | 15 (54) |
| Any CyR less than 95% Ph+ metaphases | 11/15 (73) | 27/35 (77) | 38/50 (76) | 10/24 (42) |
| Minor CyR 35%-65% Ph+ metaphases | 3/15 (20) | 2/35 (6) | 5/50 (10) | 2/24 (8) |
| MCyR 1%-34% Ph+ metaphases | 3/15 (20) | 8/35 (23) | 11/50 (22) | 3/24 (13) |
| CCyR 0% Ph+ metaphases | 5/15 (33) | 11/35 (31) | 16/50 (32) | 5/24 (21) |
| MMR | 2/16 (13) | 3/32 (9) | 5/48 (10) | 2/19 (10) |
| Molecular response after SCT (%) | ||||
| CMR | 13/16 (81) | 25/28 (89) | 38/44 (86) | 14/15 (93) |
| Transplant source (%) | ||||
| Sibling | 10 (53) | 11 (30) | 21 (37) | 9 (32) |
| Unrelated | 9 (47) | 26 (70) | 35 (63) | 19 (68) |
| PB | 13 (68) | 28 (76) | 41 (73) | 23 (82) |
| BM | 6 (32) | 9 (24) | 15 (27) | 5 (18) |
| Conditioning therapy (%)† | ||||
| Standard | 15 (79) | 24 (65) | 39 (70) | 18 (64) |
| Reduced | 3 (16) | 5 (13) | 8 (14) | 3 (11) |
| Other/na | 1 (5) | 6/2 (22) | 7/2 (16) | 6/1 (25) |
| GvHD, at any time point (%) | ||||
| All | 11 (58) | 25 (68) | 36 (54) | 20 (71) |
| Grade 3-4 | 4 (20) | 7 (19) | 11 (20) | 10 (35) |
| Chronic | 7 (35) | 13 (36) | 20 (36) | 6 (21) |
| Mortality | ||||
| All | 2 | 2 | 4 | 10 |
| TRM | 2 | 2 | 4 | 5 |
| CML | 0 | 0 | 0 | 4 |
| Unclass | 0 | 0 | 0 | 1 |
| Survival at 3 years after allo SCT, % | 88.2 | 94.1 | 91.4 | 58.8 |
| . | Allo SCT in 1st CP . | Total allo SCT in 1st CP . | Allo SCT in advanced phases . | |
|---|---|---|---|---|
| Early allo SCT . | Imatinib failure in 1st CP . | |||
| No. | 19 | 37 | 56 | 28 |
| Euro score15 (%) | ||||
| High | 6 (32) | 10 (27) | 15 (27) | 9 (32) |
| Intermediate | 3 (15) | 13 (35) | 17 (30) | 8 (29) |
| Low | 10 (53) | 14 (38) | 24 (43) | 11 (39) |
| Sokal score21 (%) | ||||
| High | 6 (32) | 17 (46) | 23 (41) | 11 (39) |
| Intermediate | 2 (10) | 7 (19) | 9 (16) | 7 (25) |
| Low | 11 (58) | 13 (35) | 24 (43) | 10 (36) |
| Sex (% male) | 12 (63) | 21 (57) | 33 (59) | 22 (79) |
| Age at diagnosis, y, median (range) | 35 (16-56) | 38 (21-56) | 37 (16-56) | 38 (18-62) |
| Time to transplantation mo, median (range) | 9.0 (4.8-23.6) | 17.6 (5.0-53.7) | 14.2 (4.8-53.7) | 12.8 (3.5-55.1) |
| EBMT score16 (%) | ||||
| 0-1 | 8 (42) | 4 (11) | 12 (21) | 0 |
| 2 | 3 (16) | 4 (11) | 7 (13) | 1 (4) |
| 3-4 | 8 (42) | 27 (73) | 35 (62) | 9 (32) |
| 5 or higher | 0 | 2 (5) | 2 (4) | 18 (64) |
| Best response before SCT (%) | ||||
| CHR | 18 (95)* | 30 (81) | 48 (86) | 15 (54) |
| Any CyR less than 95% Ph+ metaphases | 11/15 (73) | 27/35 (77) | 38/50 (76) | 10/24 (42) |
| Minor CyR 35%-65% Ph+ metaphases | 3/15 (20) | 2/35 (6) | 5/50 (10) | 2/24 (8) |
| MCyR 1%-34% Ph+ metaphases | 3/15 (20) | 8/35 (23) | 11/50 (22) | 3/24 (13) |
| CCyR 0% Ph+ metaphases | 5/15 (33) | 11/35 (31) | 16/50 (32) | 5/24 (21) |
| MMR | 2/16 (13) | 3/32 (9) | 5/48 (10) | 2/19 (10) |
| Molecular response after SCT (%) | ||||
| CMR | 13/16 (81) | 25/28 (89) | 38/44 (86) | 14/15 (93) |
| Transplant source (%) | ||||
| Sibling | 10 (53) | 11 (30) | 21 (37) | 9 (32) |
| Unrelated | 9 (47) | 26 (70) | 35 (63) | 19 (68) |
| PB | 13 (68) | 28 (76) | 41 (73) | 23 (82) |
| BM | 6 (32) | 9 (24) | 15 (27) | 5 (18) |
| Conditioning therapy (%)† | ||||
| Standard | 15 (79) | 24 (65) | 39 (70) | 18 (64) |
| Reduced | 3 (16) | 5 (13) | 8 (14) | 3 (11) |
| Other/na | 1 (5) | 6/2 (22) | 7/2 (16) | 6/1 (25) |
| GvHD, at any time point (%) | ||||
| All | 11 (58) | 25 (68) | 36 (54) | 20 (71) |
| Grade 3-4 | 4 (20) | 7 (19) | 11 (20) | 10 (35) |
| Chronic | 7 (35) | 13 (36) | 20 (36) | 6 (21) |
| Mortality | ||||
| All | 2 | 2 | 4 | 10 |
| TRM | 2 | 2 | 4 | 5 |
| CML | 0 | 0 | 0 | 4 |
| Unclass | 0 | 0 | 0 | 1 |
| Survival at 3 years after allo SCT, % | 88.2 | 94.1 | 91.4 | 58.8 |
allo SCT indicates allogeneic stem cell transplantation; 1st CP, first chronic phase; EBMT, European Group for Blood and Marrow Transplantation; CHR, complete hematologic remission; CyR, cytogenetic response; Ph+, Philadelphia chromosome positive; MCyR, major cytogenetic remission; CCyR, complete cytogenetic remission; MMR, major molecular remission; CMR, complete molecular remission; PB, peripheral blood; BM, bone marrow; GVHD, graft-versus-host disease; na, not available; TRM, transplantation-related mortality; and unclass, unclassifiable; and CML, chronic myeloid leukemia.
Spleen size not evaluable in 4 cases.
Cyclosporin A ± busulfan ± antithymocyte globulin ± TBI and others.
Criteria for transplantation were early disease in young patients (EBMT score 0-1; group I), imatinib failure (group II), and advanced disease (group III); see “Patient population.” All patients received imatinib before transplantation except 3 patients in group I who received IFN only. All randomized therapies were evenly represented. Eleven patients (5 in group II, 6 in group III) were treated with dasatinib, nilotinib, and/or bosutinib second or third line before transplantation; 22 (group III), with chemotherapy (mitoxantrone [n = 4], methotrexate [n = 2], etoposide [n = 2], acute lymphoblastic leukemia induction [daunorubicin, vincristine and PEG-asparaginase; n = 4], hydroxyurea [n = 5], AIDA [all-trans retinoic acid and idarubicin; n = 1], fludarabine [n = 1], and radiation therapy of extramedullary manifestations [n = 1]; chemotherapy type not specified [n = 2]).
Donors were unrelated in 54 cases (64%) and related in 30 cases (36%). Most patients received busulfan/cyclophosphamide or cyclophosphamide/total body irradiation (TBI)–based standard conditioning (n = 57, 68%); 12% (n = 10), fludarabine/busulfan or TBI-based reduced-intensity conditioning; 15% (n = 13), conditioning with other regimens including cyclophosphamide and/or TBI; and 5% (n = 4), conditioning with unknown regimens (supplemental Table, available on the Blood website; see the Supplemental Materials link at the top of the online article). Graft-versus-host disease (GVHD) prophylaxis was primarily with cyclosporine A and short methotrexate with or without antithymocyte globulin for patients with standard conditioning, and was cyclosporine and mycophenolate mofetil for patients with reduced-intensity conditioning. Sixty-four transplants (76%) were derived from peripheral blood. Supportive care was provided according to institutional recommendations; most centers followed the EBMT guidelines (http://www.ebmt.org).
The cytogenetic status was analyzed before transplantation to define tumor load. Close to 70% of CP patients had achieved a cytogenetic response (CyR); 10%, a major molecular remission (MMR). In advanced phase patients 42% had achieved a CyR and 10%, an MMR.
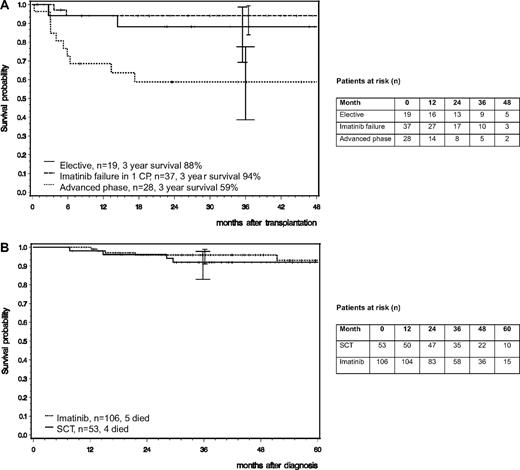
Three-year survival probability after transplantation of the 56 patients in chronic phase was 91% (88.2% in group I [CI: 69.3%-98.7%] and 94.1% in group II [CI: 83.8%-99.4%]; Figure 2A). Median follow-up after transplantation was 30 months (group I: 37 months [range 1-60 months], group II: 26 months [range, 1-50 months]). Four patients died: 2 in group I and 2 in group II due to infections (n = 2) and GVHD (n = 2), resulting in a cumulative TRM of 8%. It appears that all deaths in CP patients were transplantation related.
Survival probability. (A) After allo-SCT. Patients with elective transplantation in first CP (n = 20; group I) and patients who underwent transplantation after imatinib failure in first CP (n = 36; group II) had a 3-year survival probability of 88% and 94% (CI: 69.3-98.7 and 83.9-99.4), respectively; patients who underwent transplantation in advanced disease (n = 28; group III) had a 3-year survival probability of 59% (CI: 38.6-77.5). Tick marks indicate last observation of living patients. (B) Matched pair analysis. For each of 53 patients who underwent transplantation, 2 matched imatinib-treated patients (n = 106 in total) were found who were in first CP at the time of transplantation of the matched partner. Observation intervals of patients who did not undergo transplantation were appropriately matched with those who did for the time between diagnosis and transplantation. A patient who underwent transplantation in first chronic phase on day X has a guaranteed survival time from diagnosis to day X. To ensure a fair comparison, the 2 matched imatinib-treated partners had to have survived and been in first chronic phase on day X, too. Survival probabilities showed no difference. Tick marks indicate last observation of living patients.
Survival probability. (A) After allo-SCT. Patients with elective transplantation in first CP (n = 20; group I) and patients who underwent transplantation after imatinib failure in first CP (n = 36; group II) had a 3-year survival probability of 88% and 94% (CI: 69.3-98.7 and 83.9-99.4), respectively; patients who underwent transplantation in advanced disease (n = 28; group III) had a 3-year survival probability of 59% (CI: 38.6-77.5). Tick marks indicate last observation of living patients. (B) Matched pair analysis. For each of 53 patients who underwent transplantation, 2 matched imatinib-treated patients (n = 106 in total) were found who were in first CP at the time of transplantation of the matched partner. Observation intervals of patients who did not undergo transplantation were appropriately matched with those who did for the time between diagnosis and transplantation. A patient who underwent transplantation in first chronic phase on day X has a guaranteed survival time from diagnosis to day X. To ensure a fair comparison, the 2 matched imatinib-treated partners had to have survived and been in first chronic phase on day X, too. Survival probabilities showed no difference. Tick marks indicate last observation of living patients.
To compare survival of patients who received a transplant with that of those who did not, a matched pair analysis was performed. At 3 years, survival after diagnosis of 53 patients who underwent transplantation was not different from that of 106 matched patients who did not (91.9% [CI: 82.9%-97.8%] vs 95.9% [CI: 91.1%-98.9%]; Figure 2B). Of the 106 matched patients, 4 received a second-line TKI before matching, 4 progressed and received chemotherapy, 2 died on imatinib, and 96 are still in continued CP on imatinib.
Three-year survival probability after transplantation of the 28 patients in advanced phase was 59% (CI: 38.6%-77.5%; Figure 2A). Median follow-up after transplantation was 24 months (range, 0-50 months). Ten patients died: 4 deaths were disease related, 5 were treatment related, and 1 was unclassifiable. There was no correlation of TRM with prior chemotherapy.
GVHD (all phases) was reported in 56 patients (67%), 20 (23%) of these with grade 3 or 4. Chronic GVHD was reported in 26 patients (31%; 46% of patients with GVHD).
Residual disease after transplantation by quantitative and/or qualitative reverse-transcription polymerase chain reaction was determined in 59 of 70 living patients (median follow-up, 28 months; range, 1-60 months). Complete molecular remission was achieved in 52 patients (88%) at the last molecular follow-up, and in 26 of 33 patients (79%) 2 years after transplantation.
Discussion
The important finding of this report is the low TRM and the very good long-term survival of patients who underwent transplantation. Data were derived prospectively from a clinical study in a multicenter setting. Indications for transplantation were according to predefined criteria. Because patients underwent transplantation at various institutions, a single-center effect can be excluded. The reasons for this surprisingly favorable outcome are manifold. They are in line with a recent report of a multicenter study, using treosulfan-based regimens, that reported a TRM of less than 10% at 3 years for patients with EBMT risk score 0 to 2.22 The findings fit with recent reports of reduced TRM, in general, achieved by progress in transplantation procedures, including better human leukocyte antigen typing, more careful patient selection, and improved supportive care.
There is an additional element. Reduction of leukemia load before transplantation by imatinib might have contributed to this outcome, resulting in one of the lowest reported TRM for CP CML patients (8%). This compares favorably with our earlier randomized study of allo SCT after IFN-based drug treatment (CML Study III) in which a TRM of 26% was observed.12 In our current cohort, 36 of 52 patients who underwent transplantation in first CP showed at least some cytogenetic response before transplantation. In support of this interpretation are the significantly better survival probability after allo SCT for patients with reduced tumor load under IFN (5-year survival: 63.6% vs 49.2%),13 a positive impact of prior imatinib on the outcome of allo SCT in CP CML,4 and the observation in adult Ph-positive acute lymphoblastic leukemia that the extent of minimal residual disease under imatinib determines outcome after allo SCT.22,23
The results of an overall survival higher than 90% at 2 years are also remarkable in view of the fact that the majority of CP patients underwent transplantation with an EBMT score equal to or higher than 3. Historically, an EBMT score of greater than or equal to 3 was associated with a 50% or less 2-year survival.16 This score, by definition, is influenced by transplantation more than 12 months after diagnosis and by transplants from unrelated donors. It is also noteworthy that more patients received transplants from peripheral blood than from bone marrow.
Earlier studies showed lower survival rates after allo SCT. EBMT data of the years 2000 to 2003 report 2-year survival rates of 74% for sibling and 63% for unrelated allo SCT in first CP.2 Center for International Blood and Marrow Transplant Research (CIBMTR) data of the years 1999 to 2004 report survival rates of 79% and 72% at 1 and 2 years, respectively.4 Our earlier CML Study III reported 2- and 5-year survival rates of 76% and 62%, respectively.12 The results reported here indicate that by consideration of other known risk factors (eg, comorbidity score, cytomegalovirus serostatus, polymorphisms of cytokine genes, and BMI-1), mortality risk could be further reduced.24,25
The transplantation outcome in advanced phase patients basically is in line with earlier observations that the best long-term survival results in blast crisis are achieved by allo SCT and that most long-term survivors in blast crisis received an allo SCT, mostly in second CP.26,27
Survival of CP patients in the transplantation cohort is not worse than that of matched patients who did not undergo transplantation of the same study. Considering that the cohort that underwent transplantation probably includes more patients with imatinib failure than the imatinib-treated cohort, transplantation results may be an underestimation. The value of allo SCT is further supported by the quality of molecular remissions, which is better than that observed with imatinib (CMR rates of 30%-35% at 5 years in all imatinib-treated patients are reported for CML Study IV17 ). The complete molecular remission rate of 88% indicates the curative potential of allo SCT.
We conclude that reduction of tumor load by initial imatinib therapy and improvements in transplantation procedures translate into improved outcome of patients after hematopoietic stem cell transplantation. As a limitation to our results, it has to be stated that patients were not randomized to receive a hematopoietic stem cell transplant. Due to the elective option for transplantation, results have to be confirmed by additional data. However, in view of the curative potential of transplantation and survival results that were equally good as with imatinib treatment, allo SCT could become the preferred second-line option after failure of first-line TKI therapy for suitable patients with a donor.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The contribution of Angelika Adler, Sabine Dean, Matthias Dumke, Christine Folz, Michaela Hausmann, Elke Matzat, Regina Pleil-Lösch, Inge Stalljann, and all CML centers (see the supplemental Appendix in the online article) is gratefully acknowledged.
This study was supported by the Deutsche Krebshilfe (no. 106642), Novartis, Deutsches Kompetenznetz für Akute and Chronische Leukämien (BMBF 01GI0270), Deutsche José-Carreras Leukämiestiftung (DJCLS H06/04v, H03/01), the European LeukemiaNet (LSHC-CT-2004-503216), Roche, and Essex Pharma.
Authorship
Contribution: S. Saussele and R.H. designed the research, interpreted the data, and wrote the paper; M.L., A.G., A.H., M.P., and J.H. designed the research, interpreted the data, and contributed to writing the paper; C.H., B.S., M.C.M., and S. Schnittger performed research and conducted molecular and cytogenetic analyses; A.L. and N.P. performed research and interpreted data; D.W.B., D.B., R.S., H.-J.K., A.D.H., C.F., E.H., G.S., A.R.Z., R.A., L.K., and R.D. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of German CML Study Group participants, see the supplemental Appendix in the online article.
Correspondence: Rüdiger Hehlmann, Medizinische Fakultät Mannheim, Universität Heidelberg, Pettenkoferstr 22, D-68169 Mannheim, Germany; e-mail: r.hehlmann@urz.uni-heidelberg.de.