Abstract
CC-chemokine receptor 7 (CCR7) is expressed on the surface of naive T cells, and plays a critical role in their movement into secondary lymphoid tissue. Here, we show that murine T cells lacking CCR7 (CCR7−/−) generate attenuated graft-versus-host disease (GVHD) responses compared with wild-type (WT) cells, with the difference varying inversely with the degree of major histocompatibility complex (MHC) disparity between the donor and recipient. CCR7−/− T cells exhibited an impaired ability to traffic to recipient lymph nodes, with an increased capacity to home to the spleen. CCR7−/− T cells, however, demonstrated a reduced ability to undergo in vivo expansion in the spleen due to impaired interactions with splenic antigen-presenting cells. On a cellular level, CCR7−/− T cells were functionally competent, demonstrating a normal in vitro proliferative capacity and a preserved ability to produce inflammatory cytokines. Importantly, CCR7−/− T cells were capable of generating robust graft-versus-leukemia (GVL) responses in vivo, as well as complete donor T-cell reconstitution. CCR7−/− regulatory T cells were able to protect against lethal GVHD when administered before WT conventional T cells. Our data suggest that CCR7 inhibition in the early posttransplantation period may represent a feasible new therapeutic approach for acute GVHD attenuation without compromising GVL responses.
Introduction
Graft-versus-host disease (GVHD) is the greatest complication limiting the clinical utility of allogeneic hematopoietic stem cell transplantation (HSCT). Mechanistically, GVHD involves the early trafficking of donor naive T cells (Tn cells) to recipient secondary lymphoid tissue (SLT) where they undergo activation and expansion, and their subsequent migration to peripheral target organs where they elicit injury.1-3 Some uncertainty exists, however, as to the relative contributions of the various recipient SLTs to GVHD pathogenesis. Several studies have shown that donor Tn cells are imprinted with a particular adhesion molecule profile within a specific lymphatic site that serves to direct the cell to a corresponding area of peripheral inflammation. For example, recipient Peyer patches (PPs) and mesenteric lymph nodes (MLNs) may be important for the induction of gastrointestinal GVHD.4,5 Other studies, however, have suggested a more significant redundancy among the various recipient lymphoid tissues, without a direct link between a given lymphatic organ and a specific GVHD manifestation. Notably, irradiated B6 lymphotoxin-α receptor–deficient mice, which lack PPs and MLNs but possess an intact spleen, appear to develop intestinal acute GVHD that is similar to that of wild-type (WT) B6 recipients when given transplants across a complete major histocompatibility complex (MHC) mismatch.6
Regardless, the movement of donor T cells into recipient lymphoid organs is critical for maximal GVHD induction, as animals lacking all SLTs have consistently been shown to generate absent or attenuated inflammatory responses.6,7 The trafficking of donor T cells into SLT is in turn dependent on the exact array of adhesion molecules expressed by the cell. In general, naive and central memory T cells express a receptor profile that allows for their efficient migration into SLT, where they sample antigen on resident antigen-presenting cells (APCs). Upon activation, T cells down-regulate these homeostatic trafficking receptors and express other adhesion molecules that direct them to peripheral sites of inflammation.3
CC-chemokine receptor 7 (CCR7) is a G protein–coupled receptor expressed on naive T cells, B cells, and activated dendritic cells (DCs), and plays an important role in their trafficking into SLT.8 In the case of T cells, the binding of CCR7 to either CCL19 or CCL21 allows for lymphocyte firm arrest on lymph node (LN) high endothelial venules by stabilizing the binding of T-cell LFA-1 to vascular ICAM-1.9,10 In addition, CCR7 functions to direct T cells to appropriate T cell–rich zones within the LN paracortex after egress from the circulation, and is critical for the movement of T cells to their proper anatomical location within the spleen. Not surprisingly, mice knocked out at the CCR7 locus demonstrate numerous lymphoid abnormalities, including greatly reduced T cell numbers within peripheral LNs and PPs and a near-total absence of T cells within the splenic white pulp.8 Given its multiplicity of effects on the organization of SLTs, we hypothesized that the absence of CCR7 on donor T cells might overcome any lymphatic redundancy present in transplant recipients, and allow for a reduction in GVHD severity.
Here, we show that CCR7−/− T cells generate greatly attenuated GVHD responses due to both impaired migration of donor Tn cells into recipient LNs and PPs, and abnormal Tn-cell proliferation in the spleen. Importantly, however, CCR7−/− Tn cells retain the ability to mount aggressive graft-versus-leukemia (GVL) effects and to generate complete donor T-cell reconstitution. In addition, CCR7−/− regulatory T cells (Treg cells) remain capable of protecting against lethal GVHD when administered before conventional WT Tn cells. Taken together, these data suggest that CCR7 inhibition may represent a viable treatment option for the prevention of acute GVHD in the clinical setting.
Methods
Mice
C57BL/6, BALB/c. BALB.B, B6.PL-Thy1a/CyJ, and B6xDBA/2 F1 mice were purchased from The Jackson Laboratory. Enhanced green fluorescent protein (eGFP)–expressing C57BL/6 mice were generated as described.2 CCR7−/− mice backcrossed 4 times onto a C57BL/6 background (B6.129P2-CCR7tm1Dgen) were obtained from The Jackson Laboratory and further backcrossed 7 to 8 generations. eGFP-expressing CCR7−/− mice were generated by crossing CCR7−/− C57BL/6 mice with eGFP-expressing C57BL/6 mice. All experiments were performed in accordance with protocols approved by The University of North Carolina Institutional Animal Care and Use Committee.
Transplantation systems
Donor T cell–depleted (TCD) bone marrow (BM) cells were prepared as previously described.11 Whole CD25-depleted splenic effector T cells were prepared using a total T-cell isolation column kit (Cedarlane Laboratories) followed by negative selection of CD25- and B220-expressing cells using antibodies coupled to ferromagnetic beads and magnetic separation of these cells using a magnetic-activated cell sorter (MACS) column (Miltenyi Biotec).12 Tn cell purity was measured by flow cytometry before transplantation. For whole splenic Tn cell transplants, the donor CD4+ to CD8+ ratio approximated 1:1 following our standard purification procedure. Donor Treg cells were prepared as previously described using a column purification method.13
P815 tumor model
P815 cells (ATCC; TIB-64) are murine mastocytoma cells derived from DBA/2 (H-2d) mice.14 P815 cells were cultured in RPMI-1640 media (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 100 U/mL of penicillin and streptomycin for 7 to 10 days before all transplantations. Initial tumor dose experiments were conducted to determine an optimal P815 inoculum that would consistently result in 100% recipient mortality when irradiated B6D2 mice were given transplants of TCD B6 BM cells without Tn cells (25 000 tumor cells per recipient).
Organ cytokine analysis
Recipient animals were anesthetized and perfused with phosphate-buffered saline (PBS). Whole organs (or in the case of the liver, the hepatic middle lobe immediately adjacent to the gallbladder) were removed. Cytokine levels were measured in the homogenates using enzyme-linked immunosorbent assay (ELISA) kits directed against IFN-γ and TNF-α (Biolegend).
Flow cytometry and immunofluorescence microscopy
The following antibodies were purchased from eBioscience: anti-CD4 (RM 4.5), anti-CD8 (53-6-7), anti-B220 (RA3-6B2), anti-CD25 (PC61), anti-CD62L (Mel-14), anti-CD44 (IM7), anti-Kb (AF6-88.5.5.3), anti–IFN-γ (XMG1.2), anti–IL-13 (eBio13A), anti–IL-10 (JES5-16E3), anti–TNF-α (MP6-XT22), anti-CD107a (eBioABL-93), anti-CD107b (eBio1D4B), anti-Thy1.1 (HIS51), and anti-CD11c (N418). Antibody to mouse Kd (SF1-1.1) was purchased from Biolegend. Cy3 goat anti–Armenian hamster was purchased from Jackson ImmunoResearch Laboratories. Flow cytometric acquisition was performed on a Cyan flow cytometer using Summit software (Dako).
Intracellular cytokine staining
Cells from recipient spleens and MLNs were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA), 500 ng/mL ionomycin, and 3 μg/mL brefeldin A (Sigma-Alrich) for 4 hours, with or without anti-CD107a and anti-CD107b antibody. Cells were then washed and stained for Kd, CD4, or CD8, and intracellular cytokine staining was subsequently performed using a Cytofix/Cytoperm kit (BD Biosciences).
Mixed lymphocyte reactions
A total of 2 × 105 highly pure CD4+CD25− or CD8+CD25− were obtained from donor spleens using fluorescence-activated cell sorting and cocultured with an equal number of irradiated, TCD B6D2 stimulator cells for 96 hours. During the last 16 to 20 hours of incubation, 0.037 MBq (1 μCi) of [3H]thymidine (Amersham Pharmacia Biotech) was added per well. Cells were harvested onto filters, and [3H]thymidine incorporation was measured by scintillation counting.
Stereomicroscopy
Animals were anesthetized, and the organs were imaged with a Zeiss Stereo Lumar V12 microscope with eGFP bandpass filter (Carl Zeiss) at room temperature (RT). eGFP intensities were determined with AxioVision (Carl Zeiss) software. For all time points and organs, WT and CCR7−/− Tn cell recipients were assessed using identical camera exposure times (exp) and similar magnifications (mag). Specific values were as follows: Spleen day +15, exp = 824 milliseconds, mag = 15 × to 18 ×; MLN day +15, exp = 263 milliseconds, mag = 13 × to 15 ×; PP day +15, exp = 318 milliseconds, mag = 22 × to 27 ×; lung day +15, exp = 1.3 seconds, mag = 17 ×; liver day +15, exp = 3.1 seconds, mag = 34 × to 40 ×; colon day +15, exp = 2.1 seconds, mag = 12 ×; spleen day +3, exp = 3.5 seconds, mag = 18 × to 32 ×; MLN day +3, exp = 2.5 seconds, mag = 15 ×; and PP day +3, exp = 976 milliseconds, mag = 32 × to 33 ×.
Organ eGFP quantification
Recipient organs were homogenized, and absolute eGFP levels were measured using an ELISA kit directed against GFP/eGFP (Cell Biolabs). To ensure that there were no intrinsic differences in eGFP expression between B6 eGFP+ and CCR7−/− eGFP+ cells, 1 × 107 splenocytes were isolated from 2 of each mouse strain, the cells were pelleted by centrifugation and homogenized, and absolute eGFP levels within the homogenates were measured. The eGFP levels from equal numbers of cells from both strains were found to be nearly identical (data not shown).
Fluorescence microscopy
Recipient spleens were immersed in 4% formalin, 10% sucrose PBS solution for 2 hours at 4°C before being flash-frozen over liquid nitrogen in optimal cutting temperature solution.15 Subsequently 8-μm sections were cut on a cryostat at −20°C and allowed to dry overnight at 25°C. On the day of immunostaining, slides were fixed in cold acetone for 10 minutes, then washed for 5 minutes in PBS. Slides were blocked using 10% goat serum, 2% bovine serum albumin, and 5% FC-block solution, and stained with an Armenian hamster anti–mouse CD11c/Cy3-conjugated goat anti–hamster antibody combination. Slides were visualized on an Olympus BX61 fluorescence microscope using Improvision Velocity Software and a Hammatsu ORCA RC camera at RT. Images were obtained at 4× and 10× magnifications with the following exposures: 4×, 1.5 to 1.8 seconds for Cy3, 700 to 760 milliseconds for eGFP; 10×, 700 to 800 milliseconds for Cy3, 500 to 600 milliseconds for eGFP.
Statistical analysis
Survival curves were constructed using the method of Kaplan and Meier. Overall survival was compared using Fisher exact test. Where indicated, median survival times were compared using the log-rank test. To compare mean scores that fell within a predetermined range (ie, GVHD scores ranging from 0-10, and pathology scores ranging from 0-4), the nonparametric Mann-Whitney test was used. All other continuous variables, including cytokine levels, eGFP levels, and cell ratios, were compared using the 2-tailed Student t test in accordance with previously published reports.16-19 P values less than .05 were considered significant. Unless otherwise indicated, error bars represent SEM.
Results
In the absence of CCR7, T cells generate attenuated GVHD responses
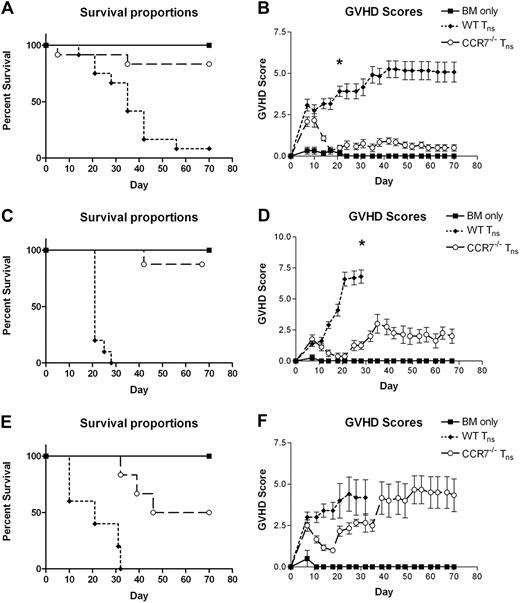
Initially, we set out to determine whether the absence of CCR7 on donor Tn cells would affect on their ability to induce lethal GVHD. Using a well-described C57BL/6 (H-2b, termed B6) into B6xDBA/2 F1 (H-2bxd; termed B6D2) haplotype-matched murine transplantation model, we found that WT and CCR7−/− Tn cells generated nearly identical GVHD responses during the first 7 to 10 days after transplantation. Thereafter, those mice receiving CCR7−/− Tn cells demonstrated an attenuation of their GVHD, with 83% surviving until the completion of the experiment (Figure 1A-B). In contrast, those animals receiving WT Tn cells exhibited relentlessly progressive disease with a 92% mortality rate by day +70 (P < .001).
Survival and GVHD scores of animals receiving TCD BM alone or BM plus T cells from WT B6 or CCR7−/− B6 donors. (A-B) B6D2 recipients were lethally irradiated to 9.5 Gy (950 rads) on day −1, and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted whole (CD4+ and CD8+) splenic Tn cells from either WT B6 or CCR7−/− B6 donors on day 0 by tail vein injection. Recipient animals were followed for survival and scored for GVHD twice weekly using a validated clinical scoring system.20 Animals were assigned a score from 0 to 2 for each of 5 GVHD parameters: weight loss, activity, fur-ruffling, kyphosis, and skin lesions. Scores ranged from 0 (minimum) to 10 (maximum). Error bars depict SEM. Data are combined from 3 separate transplantation experiments. n = 12 animals per group. (A) P < .001 for comparison of overall survival proportions at the end of the study observation period between BM/WT Tn and BM/CCR7−/− Tn groups by Fisher exact test. (B) *P < .001 for comparison of GVHD scores between BM/WT Tn and BM/CCR7−/− Tn groups by the Mann-Whitney test. (C-D) BALB.B recipients were lethally irradiated to 9.5 Gy (950 rads) on day −1, and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 5 × 106 CD25-depleted whole Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. Data are combined from 2 separate transplantation experiments. n = 6 BM, 10 BM/WT Tn, 8 BM/CCR7−/− Tn. (C) P < .001 for overall survival comparison between BM/WT Tn and BM/CCR7−/− Tn groups. (D) *P < .001 for comparison of GVHD scores. (E-F) BALB/c recipients were lethally irradiated to 8 Gy (800 rads) in a single fraction on day −1, and then administered 5 × 106 TCD BM cells from WT B6 donors with or without 6 × 105 CD25-depleted whole Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. n = 2 BM, 5 BM/WT Tn, 6 BM/CCR7−/− Tn. (E) P = .182 for overall survival comparison using the Fisher exact test, and P = .002 for median survival comparison using the log-rank test between BM/WT Tn and BM/CCR7−/− Tn groups.
Survival and GVHD scores of animals receiving TCD BM alone or BM plus T cells from WT B6 or CCR7−/− B6 donors. (A-B) B6D2 recipients were lethally irradiated to 9.5 Gy (950 rads) on day −1, and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted whole (CD4+ and CD8+) splenic Tn cells from either WT B6 or CCR7−/− B6 donors on day 0 by tail vein injection. Recipient animals were followed for survival and scored for GVHD twice weekly using a validated clinical scoring system.20 Animals were assigned a score from 0 to 2 for each of 5 GVHD parameters: weight loss, activity, fur-ruffling, kyphosis, and skin lesions. Scores ranged from 0 (minimum) to 10 (maximum). Error bars depict SEM. Data are combined from 3 separate transplantation experiments. n = 12 animals per group. (A) P < .001 for comparison of overall survival proportions at the end of the study observation period between BM/WT Tn and BM/CCR7−/− Tn groups by Fisher exact test. (B) *P < .001 for comparison of GVHD scores between BM/WT Tn and BM/CCR7−/− Tn groups by the Mann-Whitney test. (C-D) BALB.B recipients were lethally irradiated to 9.5 Gy (950 rads) on day −1, and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 5 × 106 CD25-depleted whole Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. Data are combined from 2 separate transplantation experiments. n = 6 BM, 10 BM/WT Tn, 8 BM/CCR7−/− Tn. (C) P < .001 for overall survival comparison between BM/WT Tn and BM/CCR7−/− Tn groups. (D) *P < .001 for comparison of GVHD scores. (E-F) BALB/c recipients were lethally irradiated to 8 Gy (800 rads) in a single fraction on day −1, and then administered 5 × 106 TCD BM cells from WT B6 donors with or without 6 × 105 CD25-depleted whole Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. n = 2 BM, 5 BM/WT Tn, 6 BM/CCR7−/− Tn. (E) P = .182 for overall survival comparison using the Fisher exact test, and P = .002 for median survival comparison using the log-rank test between BM/WT Tn and BM/CCR7−/− Tn groups.
To confirm and extend these findings, a minor MHC-mismatched B6 into BALB.B (H-2b) system was used, which more closely resembles the degree of histocompatibility matching typically found in the clinical HSCT setting (Figure 1C-D). Once again, we found a striking difference in outcomes, with recipients of CCR7−/− Tn cells demonstrating a profound survival advantage and much lower GVHD scores (P < .01, Figure 1C; P < .001, Figure 1D).
We then examined how the absence of CCR7 would affect the ability of Tn cells to mediate lethal GVHD in a completely mismatched B6 into BALB/c (H-2d) model (Figure 1E-F). In this setting, we found the influence of CCR7 on GVHD induction to be somewhat less striking. Although median survival was clearly improved in recipients of CCR7−/− Tn cells compared with those getting WT cells (P = .002; Figure 1E), we did not find a statistical difference in overall survival (P = .182). Further, whereas the onset of severe GVHD was substantially delayed in CCR7−/− Tn cell recipients, these mice eventually went on to develop skin inflammation and weight loss. In total, these findings suggest that the absence of CCR7 on donor T cells affects on their ability to induce GVHD, with the extent of this effect varying with the degree of MHC mismatch between the donor and recipient strains.
CCR7−/− T cells retain a significant GVL effect
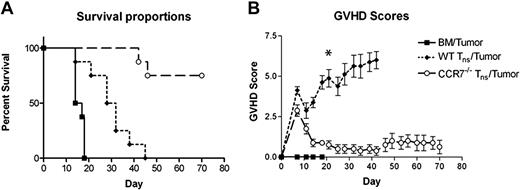
We next set out to determine whether the absence of CCR7 would affect on the ability of donor T cells to mount antitumor responses in vivo. For these experiments, we returned to our haplotype-matched B6 into B6D2 HSCT model, but challenged recipient animals at the time of transplantation with 25 000 P815 murine mastocytoma cells (H-2d), a tumor line with a propensity to metastasize to the spleen, liver, and spinal cord. As seen in Figure 2A, those mice receiving TCD BM plus tumor cells without Tn cells all died by transplantation day +20. At the time of death, these animals demonstrated massive splenomegaly with diffuse tumor infiltration of the liver. In contrast, animals receiving WT Tn cells at the time of P815 challenge all died of GVHD, with no tumor identified at the time of death and all animals exhibiting high GVHD scores. Importantly, 75% of the mice administered P815 cells and CCR7−/− Tn cells survived long term with no sign of malignancy and only minimal evidence of GVHD clinically (P = .001).
Survival and GVHD scores of B6D2 mice receiving TCD BM cells with or without Tn cells from WT B6 or CCR7−/− B6 donors with P815 murine mastocytoma cells. B6D2 recipients were lethally irradiated and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. In addition, all animals simultaneously received 25 000 P815 murine mastocytoma cells in the same inoculum. Data are combined from 2 separate transplantation experiments. n = 8 animals per group. (A) P = .001 for overall survival comparison between WT Tn cell/tumor and CCR7−/− Tn cell/tumor groups. (B) *P < .001 for comparison of GVHD scores.
Survival and GVHD scores of B6D2 mice receiving TCD BM cells with or without Tn cells from WT B6 or CCR7−/− B6 donors with P815 murine mastocytoma cells. B6D2 recipients were lethally irradiated and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. In addition, all animals simultaneously received 25 000 P815 murine mastocytoma cells in the same inoculum. Data are combined from 2 separate transplantation experiments. n = 8 animals per group. (A) P = .001 for overall survival comparison between WT Tn cell/tumor and CCR7−/− Tn cell/tumor groups. (B) *P < .001 for comparison of GVHD scores.
Recipients of CCR7−/− Tn cells demonstrate less hepatic/gastrointestinal inflammation and less colonic inflammatory cytokine production
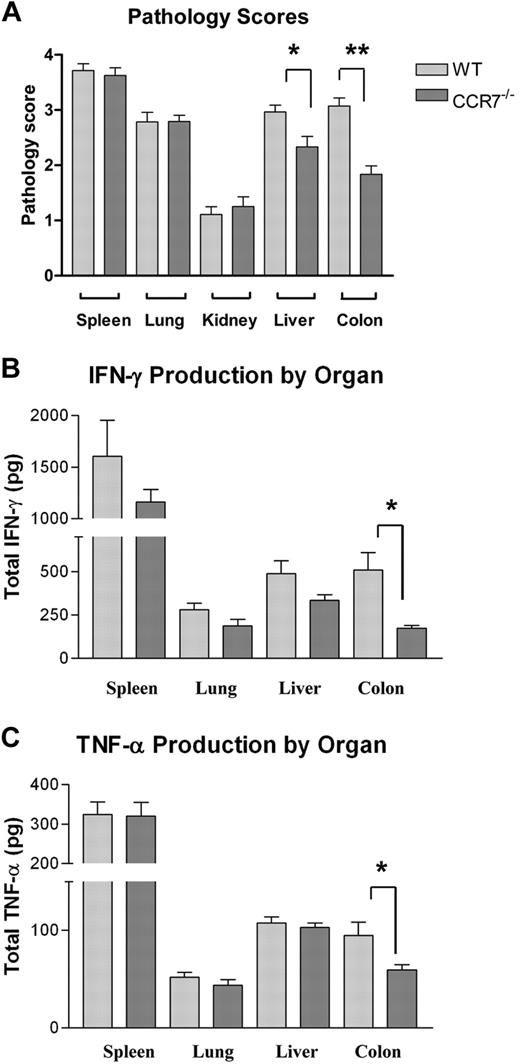
To evaluate the mechanism by which the absence of CCR7 on donor Tn cells attenuated GVHD severity, lethally irradiated B6D2 mice received transplants of TCD B6 BM with or without WT or CCR7−/− B6 Tn cells, and their organs were harvested between transplantation days 18 and 21 for histopathology (Figure 3A). A separate cohort of recipients underwent transplantation similarly, but their organs were homogenized for cytokine analysis on day +15 (Figure 3B-C). B6D2 recipients that underwent transplantation with CCR7−/− T cells had a significant reduction in pathology scores within the liver (P = .017) and to an even greater extent in the colon (P < .001) compared with animals given WT cells. In addition, less interferon-γ and TNF-α were observed in the colons of CCR7−/− Tn recipients (P = .017 and P = .052, respectively).
Organ pathology scores and inflammatory cytokine levels in B6D2 mice receiving TCD BM cells plus Tn cells from WT B6 or CCR7−/− B6 donors. B6D2 recipients were lethally irradiated and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. Error bars indicate SEM. (A) Recipient animals were killed between days 18 and 21, and their organs were extracted for pathologic analysis. Organs were assigned a histologic score between 0 and 4 by a pathologist blinded to treatment group using a system described previously. n = 14 WT Tn cell recipients and 12 CCR7−/− Tn cell recipients. *P = .017, **P < .001 by Mann-Whitney test. (B-C) Recipient animals were killed on transplantation day +15, and their organs were homogenized in a PBS/protease-inhibitor solution. TNF-α and IFN-γ levels were then quantified by ELISA. (B) *P = .017 by Student t test. (C) *P = .052.
Organ pathology scores and inflammatory cytokine levels in B6D2 mice receiving TCD BM cells plus Tn cells from WT B6 or CCR7−/− B6 donors. B6D2 recipients were lethally irradiated and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. Error bars indicate SEM. (A) Recipient animals were killed between days 18 and 21, and their organs were extracted for pathologic analysis. Organs were assigned a histologic score between 0 and 4 by a pathologist blinded to treatment group using a system described previously. n = 14 WT Tn cell recipients and 12 CCR7−/− Tn cell recipients. *P = .017, **P < .001 by Mann-Whitney test. (B-C) Recipient animals were killed on transplantation day +15, and their organs were homogenized in a PBS/protease-inhibitor solution. TNF-α and IFN-γ levels were then quantified by ELISA. (B) *P = .017 by Student t test. (C) *P = .052.
CCR7−/− T cells possess an intact ability to undergo activation and cytokine production
Previous reports have suggested that CCR7−/− T cells possess an intrinsic inability to undergo normal cellular activation and cell cycling.21 To determine whether such functional abnormalities would provide an explanation for our findings, we examined the surface expression of various cellular activation markers on both CD4+ and CD8+ donor T cells within B6D2 spleens and MLNs 7 and 14 days after transplantation, and evaluated the cytokine expression profile of these cells after ex vivo stimulation with PMA and ionomycin (representative data of donor cells extracted from recipient spleens on transplantation day +14 are depicted in supplemental Figure 1A-D, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We also performed in vitro mixed lymphocyte reactions (MLRs) with freshly purified WT and CCR7−/− CD4+ CD25− and CD8+ CD25− lymphocytes, plus irradiated, TCD B6D2 splenocytes as stimulator cells (supplemental Figure 1E). WT and CCR7−/− T cells demonstrated a similar ability to undergo activation and proliferation in vitro and a similar phenotype characterized by high levels of surface CD44 and low expression of L-selectin after transplantation. Both CD4+ cell populations showed a T helper cell 1 (Th1)–polarized intracellular cytokine expression profile characterized by abundant IFN-γ and TNF-α and little IL-13 or IL-10, and CD8+ T cells demonstrated nearly identical levels of surface CD107, a surrogate for cytotoxic T lymphocyte (CTL) lytic activity22 (supplemental Figure 1A-D; data not shown). Further, CCR7−/− Tn cells were found to produce complete donor reconstitution of the CD4+ and CD8+ compartments by transplantation day +40, although the rate of reconstitution was delayed compared with WT cells (supplemental Figure 1F). Taken together, these findings suggested that in the absence of CCR7, T cells are functional, with an immunophenotype and in vitro cytokine production profile that are similar to those of WT cells.
CCR7−/− T cells demonstrate an impaired ability to accumulate within recipient tissues after HSCT
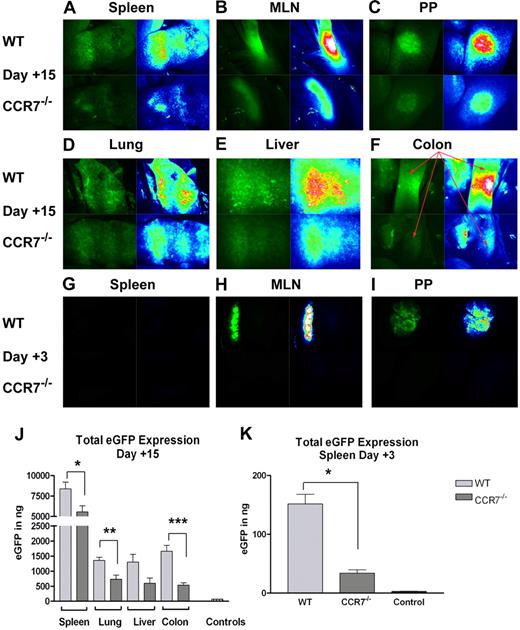
We next focused on any differences in T-cell in vivo trafficking that might exist in the absence of CCR7. For these studies, we made use of B6 and CCR7−/− mice that were transgenic for eGFP and imaged specific organs by fluorescence stereomicroscopy as previously described.2 Upon examining B6D2 recipient mice on transplantation day +15, a time point just before those when our histopathology studies were performed (Figure 3A), we observed a higher fluorescence intensity in the spleens, MLNs, PPs, livers, lungs, and especially colons of those animals receiving WT cells versus those administered CCR7−/− Tn cells (Figure 4A-F). In addition, we quantified the levels of eGFP expressed within several of these organs using an anti-eGFP ELISA (Figure 4J). In accordance with our microscopy findings, we detected significantly less eGFP expression within the spleens, lungs, and colons of those mice receiving CCR7−/− cells, with a strong trend toward less eGFP observed in the liver (P = .044, P = .011, P = .002, and P = .06, respectively).
Accumulation of WT versus CCR7−/− Tn cells within B6D2 recipients. B6D2 recipients were lethally irradiated and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted eGFP+ Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. (A-F) Recipient mice were anesthetized on transplantation day +15, and donor T-cell trafficking was studied using stereofluorescence microscopy. Tissues from 1 of 3 representative WT Tn cell recipients are depicted in the top panels, and tissues from 1 of 3 representative CCR7−/− Tn cell recipients are depicted in the bottom panels. Images on the left of each panel depict actual eGFP fluorescence, while those on the right indicate the intensity of the eGFP signal (white > red > yellow > green > blue > black). (G-I) Recipient mice were anesthetized on transplantation day +3, and donor T-cell trafficking to SLTs was evaluated using stereofluorescence microscopy. Images are taken from 1 of 3 representative WT Tn cell recipients and from 1 of 3 CCR7−/− Tn recipients. (J) Recipient animals were euthanized on transplantation day +15, and their organs were homogenized in a PBS/protease-inhibitor solution. After appropriate dilutions, eGFP levels were measured in each site using an anti-GFP ELISA kit. n = 4 per group. *P = .044; **P = .011; ***P = .002. Organs were pooled from a B6D2 mouse that received a transplant of TCD BM cells plus non–eGFP-expressing B6 Tn cells for control purposes. (K) Recipient animals were killed on transplantation day 3, and their spleens were homogenized for analysis by anti-GFP ELISA. n = 3 per group. *P = .002. Error bars for panels J and K indicate SEM.
Accumulation of WT versus CCR7−/− Tn cells within B6D2 recipients. B6D2 recipients were lethally irradiated and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 4 × 106 CD25-depleted eGFP+ Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. (A-F) Recipient mice were anesthetized on transplantation day +15, and donor T-cell trafficking was studied using stereofluorescence microscopy. Tissues from 1 of 3 representative WT Tn cell recipients are depicted in the top panels, and tissues from 1 of 3 representative CCR7−/− Tn cell recipients are depicted in the bottom panels. Images on the left of each panel depict actual eGFP fluorescence, while those on the right indicate the intensity of the eGFP signal (white > red > yellow > green > blue > black). (G-I) Recipient mice were anesthetized on transplantation day +3, and donor T-cell trafficking to SLTs was evaluated using stereofluorescence microscopy. Images are taken from 1 of 3 representative WT Tn cell recipients and from 1 of 3 CCR7−/− Tn recipients. (J) Recipient animals were euthanized on transplantation day +15, and their organs were homogenized in a PBS/protease-inhibitor solution. After appropriate dilutions, eGFP levels were measured in each site using an anti-GFP ELISA kit. n = 4 per group. *P = .044; **P = .011; ***P = .002. Organs were pooled from a B6D2 mouse that received a transplant of TCD BM cells plus non–eGFP-expressing B6 Tn cells for control purposes. (K) Recipient animals were killed on transplantation day 3, and their spleens were homogenized for analysis by anti-GFP ELISA. n = 3 per group. *P = .002. Error bars for panels J and K indicate SEM.
Given that higher numbers of WT cells were present in recipient MLNs, PPs, and spleens 2 weeks after transplantation, we wondered if a similar difference in accumulation in SLT could be detected at a time point closer to transplantation when donor T cells would have had less time to undergo in vivo expansion. When we examined B6D2 recipients on day +3, we again observed higher numbers of WT cells within the MLN and PPs (Figure 4H-I). Using anti-eGFP ELISA, we also detected more eGFP in the spleens of those mice receiving WT cells (P = .002; Figure 4K). Thus, we confirmed the important role of CCR7 in the accumulation of Tn cells within LNs, and surprisingly found that CCR7 was important for the early collection of donor Tn cells in the spleen.
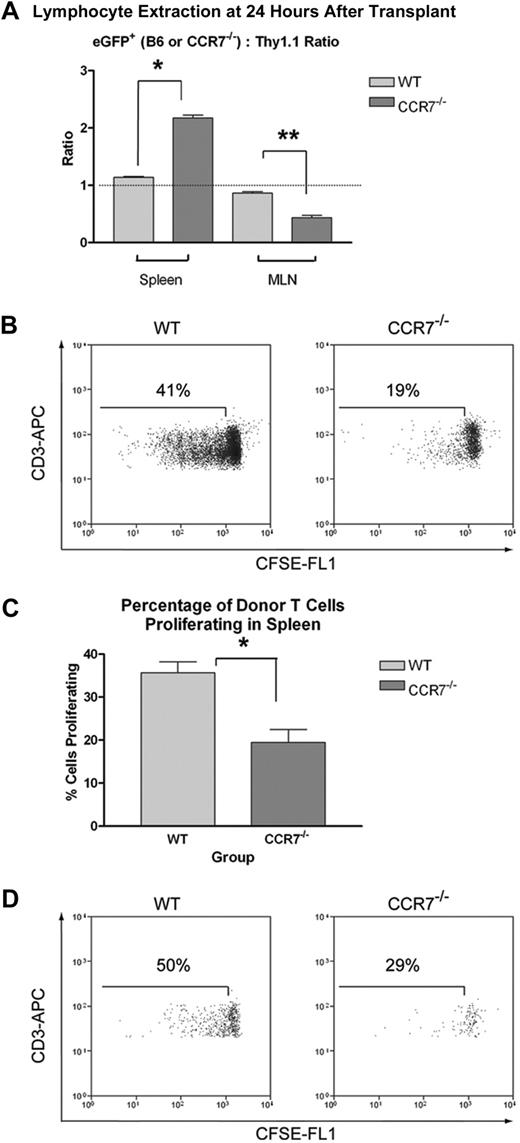
CCR7−/− Tn cells show increased trafficking to the spleen and reduced homing to LNs, with less in vivo expansion in both sites
Although the greater number of WT cells within recipient MLNs and spleens as early as transplantation day +3 suggested a homing advantage to both of these sites, we could not rule out the possibility that differential in vivo expansion was also contributing to the difference. To definitively examine the issue of T-cell homing, we made use of a competitive lymphocyte trafficking approach.16 With this method, Thy1.2+ eGFP+ WT or eGFP+ CCR7−/− Tn cells are coinjected with an equal number of Thy1.1+ Tn cells, and the ratio of eGFP+ to Thy1.1+ cells within recipient tissues are determined by flow cytometry within 24 hours of adoptive transfer, before any substantial T-cell expansion would be expected. As a result, absolute numbers of CCR7−/− and WT eGFP+ cells need not be directly compared, thereby avoiding confounding issues such as variable recipient animal size, circulatory system anatomy, and differences in the number and size of retrieved LNs.
As anticipated, eGFP+ WT B6 and Thy1.1+ Tn cells were found to home equally well to both lymphoid sites, with the ratio of these 2 populations approaching 1:1 in the spleen and MLNs. In contrast, the eGFP+ CCR7−/−/Thy1.1+ ratio was found to be reduced in the MLN, but augmented in the spleen, demonstrating an impaired ability for CCR7−/− cells to traffic into LNs, with an increase in their splenic homing (Figure 5A). This finding would be in keeping with previous reports showing higher overall numbers of T cells within the spleens of CCR7−/− mice, with reduced numbers in the peripheral LNs.8 It also strongly suggested that our finding decreased numbers of CCR7−/− T cells in the spleen at later time points was not due to impaired trafficking.
Trafficking of donor T cells to recipient SLTs 24 hours after transplantation, and their subsequent in vivo expansion. (A) B6D2 recipients were lethally irradiated and then administered 4 × 106 non–eGFP-expressing B6.PL-Thy1a/CyJ Thy1.1+ Tn plus either 4 × 106 eGFP+ WT B6 Tn or 4 × 106 eGFP+ CCR7−/− B6 Tn cells. Recipient animals were killed after 24 hours, and lymphocytes were extracted from the spleen and MLNs. These cells were then stained for flow cytometry using PerCp-Cy5.5–conjugated anti-Thy1.1 and APC-conjugated anti-CD3 antibodies. The ratios of CD3+eGFP+/CD3+Thy1.1+ events were then determined for all of the animals in each treatment group. For spleens, n = 6 per group. For MLNs, n = 3 LN pairs per group. *P < .001. **P = .005. Error bars indicate SEM. (B-C) B6D2 recipients were lethally irradiated and then administered 3 × 106 unlabeled TCD BM cells from WT B6 donors plus 3 × 106 CFSE-labeled Tn cells from either WT or CCR7−/− B6 donors. Recipient animals were killed on transplantation day +3, and lymphocytes were extracted from the spleen for flow cytometry. (B) Histograms of cells isolated from the spleen from 1 of 3 representative WT Tn cell recipients and from 1 of 3 representative CCR7−/− Tn cell recipients are depicted, using a CD3+ Kd-negative (donor cell) live lymphocyte gate. (C) The mean percentage of proliferating donor T cells within the spleens of WT (left) and CCR7−/− Tn cell (right) recipients are also shown. Error bars indicate SEM. *P = .014. (D) Recipient animals were killed on transplantation day +3, and the MLNs from each of 3 animals per treatment group were pooled for flow cytometry. MLN plots are depicted using a CD3+ Kd-negative (donor cell) live lymphocyte gate.
Trafficking of donor T cells to recipient SLTs 24 hours after transplantation, and their subsequent in vivo expansion. (A) B6D2 recipients were lethally irradiated and then administered 4 × 106 non–eGFP-expressing B6.PL-Thy1a/CyJ Thy1.1+ Tn plus either 4 × 106 eGFP+ WT B6 Tn or 4 × 106 eGFP+ CCR7−/− B6 Tn cells. Recipient animals were killed after 24 hours, and lymphocytes were extracted from the spleen and MLNs. These cells were then stained for flow cytometry using PerCp-Cy5.5–conjugated anti-Thy1.1 and APC-conjugated anti-CD3 antibodies. The ratios of CD3+eGFP+/CD3+Thy1.1+ events were then determined for all of the animals in each treatment group. For spleens, n = 6 per group. For MLNs, n = 3 LN pairs per group. *P < .001. **P = .005. Error bars indicate SEM. (B-C) B6D2 recipients were lethally irradiated and then administered 3 × 106 unlabeled TCD BM cells from WT B6 donors plus 3 × 106 CFSE-labeled Tn cells from either WT or CCR7−/− B6 donors. Recipient animals were killed on transplantation day +3, and lymphocytes were extracted from the spleen for flow cytometry. (B) Histograms of cells isolated from the spleen from 1 of 3 representative WT Tn cell recipients and from 1 of 3 representative CCR7−/− Tn cell recipients are depicted, using a CD3+ Kd-negative (donor cell) live lymphocyte gate. (C) The mean percentage of proliferating donor T cells within the spleens of WT (left) and CCR7−/− Tn cell (right) recipients are also shown. Error bars indicate SEM. *P = .014. (D) Recipient animals were killed on transplantation day +3, and the MLNs from each of 3 animals per treatment group were pooled for flow cytometry. MLN plots are depicted using a CD3+ Kd-negative (donor cell) live lymphocyte gate.
To elucidate a mechanism for the lower numbers of CCR7−/− T cells in the spleen on days +3 and +15, we evaluated the proliferation of CCR7−/− and WT T cells within SLTs early after transplantation. For this work, we labeled both B6 WT and CCR7−/− Tn cells with carboxyfluorescein succinimidyl ester (CFSE) immediately before transplantation, and assessed the extent of CFSE dilution within B6D2 recipient spleens and MLNs on day +3. As shown (Figure 5B-D), WT cells had lower levels of residual CFSE within both sites compared with CCR7−/− T cells, demonstrating that donor T-cell proliferation is impaired in the spleen and MLNs in the absence of CCR7.
CCR7−/− donor T cells interact less efficiently with splenic DCs than WT cells
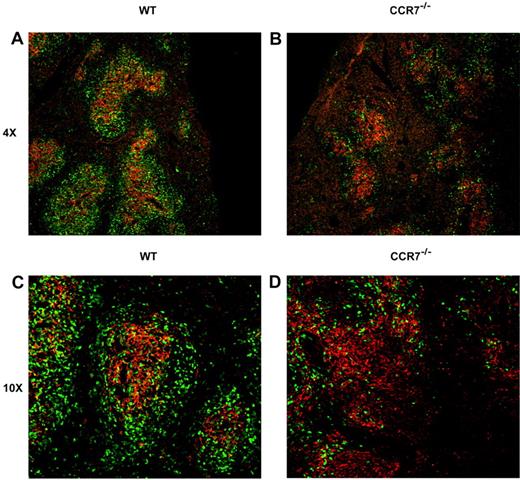
Next, we set out to identify a potential mechanism for the impaired donor T-cell proliferation observed in the absence of CCR7 within the recipient spleen, despite a normal capacity for cellular division in vitro (supplemental Figure 1E). Based on previous reports showing an important role for CCR7 in the normal organization of SLTs,8 we hypothesized that CCR7−/− donor T cells might home to recipient spleens after HSCT, but localize poorly to DC-rich zones within the white pulp. To address this possibility, B6D2 recipients received transplants of Tn cells from eGFP+ WT or eGFP+ CCR7−/− donors, and their spleens were extracted for immunofluorescence microscopy on transplantation days +4 and +7. DCs were stained with an anti-CD11c antibody, and donor T cells were identified by their eGFP signal. As seen in Figure 6A and 6C, by transplantation day +4, WT donor T cells had efficiently localized into the splenic white pulp, forming discrete, circular T-cell aggregates overlapping with resident DCs. In contrast, CCR7−/− donor T cells were found to colocalize much less frequently with splenic DCs, demonstrating a more diffuse infiltration pattern (Figure 6B,D). These early differences became somewhat less striking 7 days after transplantation (supplemental Figure 2), demonstrating that although CCR7−/− T-cell activation is both diminished and delayed compared with WT cells, it is not entirely impaired.
Donor T cell/DC interactions within recipient spleens on transplantation day +4. B6D2 recipients were lethally irradiated and administered 3 × 106 TCD BM cells from WT B6 donors with 4 × 106 CD25-depleted eGFP+ Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. Recipients were killed on transplantation day +4, and their spleens removed for immunofluorescence microscopy. Images are taken from 1 of 3 representative WT Tn cell recipients and from 1 of 3 CCR7−/− Tn cell recipients. Donor cells are identified by their eGFP positivity (green). An anti-CD11c primary/Cy3-conjugated secondary antibody combination was used to visualize CD11c-expressing cells, which are predominantly DCs (red). (A,C) Recipients of WT Tn cells. (B,D) Recipients of CCR7−/− cells. Original magnifications, ×4 (A-B) and ×10 (C-D).
Donor T cell/DC interactions within recipient spleens on transplantation day +4. B6D2 recipients were lethally irradiated and administered 3 × 106 TCD BM cells from WT B6 donors with 4 × 106 CD25-depleted eGFP+ Tn cells from either WT B6 or CCR7−/− B6 donors on day 0. Recipients were killed on transplantation day +4, and their spleens removed for immunofluorescence microscopy. Images are taken from 1 of 3 representative WT Tn cell recipients and from 1 of 3 CCR7−/− Tn cell recipients. Donor cells are identified by their eGFP positivity (green). An anti-CD11c primary/Cy3-conjugated secondary antibody combination was used to visualize CD11c-expressing cells, which are predominantly DCs (red). (A,C) Recipients of WT Tn cells. (B,D) Recipients of CCR7−/− cells. Original magnifications, ×4 (A-B) and ×10 (C-D).
Treg cells can protect against lethal GVHD in irradiated HSC transplant recipients in the absence of CCR7
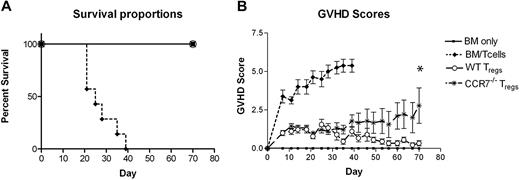
Up to this point, our experiments had focused on conventional T cells and the impact of CCR7 on the ability of donor Tn cells to induce GVHD and GVL responses. While these data suggested that blocking CCR7 function in the immediate pre- and posttransplantation periods might prove to be a feasible approach for improving transplantation outcomes, the effect that such an intervention would have on the ability of Foxp3-expressing Treg cells to suppress GVHD responses remained uncertain. To address the contribution of CCR7 to the in vivo function of Treg cells in the HSCT setting, we administered WT B6 Tn cells with WT B6 or CCR7−/− Treg cells to lethally irradiated B6D2 mice. As expected, WT B6 Treg cells afforded 100% survival of B6D2 recipients (Figure 7A). Importantly, however, recipients given CCR7−/− donor Treg cells also demonstrated 100% survival, indicating that naive Treg cells lacking CCR7 remain capable of substantial in vivo immune suppression in lethally irradiated hosts.
Survival and GVHD scores of B6D2 mice receiving WT or CCR7−/− Treg cells before WT Tn cell dosing. (A-B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 1 × 106 column-purified CD4+CD25+ Treg cells from WT B6 or CCR7−/− B6 mice on day 0. A total of 4 × 106 CD25-depleted Tn cells from WT B6 donors were then administered on day +2.23 n = 4 BM, 7 BM/Tn, 9 BM/Tn/WT Treg, 9BM/Tn/CCR7−/− Treg. (B) *P = .094 for comparison of GVHD scores between WT Treg and CCR7−/− Treg groups on day +70. Error bars indicate SEM.
Survival and GVHD scores of B6D2 mice receiving WT or CCR7−/− Treg cells before WT Tn cell dosing. (A-B) B6D2 recipients were lethally irradiated on day −1 and then administered 3 × 106 TCD BM cells from WT B6 donors with or without 1 × 106 column-purified CD4+CD25+ Treg cells from WT B6 or CCR7−/− B6 mice on day 0. A total of 4 × 106 CD25-depleted Tn cells from WT B6 donors were then administered on day +2.23 n = 4 BM, 7 BM/Tn, 9 BM/Tn/WT Treg, 9BM/Tn/CCR7−/− Treg. (B) *P = .094 for comparison of GVHD scores between WT Treg and CCR7−/− Treg groups on day +70. Error bars indicate SEM.
Discussion
For the past 20 years, investigators have sought methods to separate the deleterious effects of GVHD from beneficial GVL responses. Here, we demonstrate that the absence of CCR7 on Tn cells leads to impaired T-cell migration into recipient MLNs and PPs and infrequent T-cell/DC interactions within the spleen, which result in impaired donor T-cell expansion. These effects appear to be sufficient for a general attenuation of GVHD severity while still allowing for the elimination of tumor cells.
Several of our results were somewhat unexpected. Previous work had suggested that donor T-cell homing to recipient LNs and/or PPs is not required for lethal GVHD in the presence of an intact spleen, with the latter lymphoid site being sufficient for aggressive inflammatory responses in most if not all peripheral GVHD target organs.6 Because CCR7 had previously been shown to be critical for Tn cell homing to LNs and PPs but not for overall splenic trafficking, we hypothesized that CCR7−/− Tn cells might generate GVHD effects that were identical to those of WT cells. Our GVHD survival data showing improved outcomes with CCR7−/− Tn cells were initially surprising, particularly after we confirmed that their splenic homing remained intact after transplantation. Our subsequent studies, however, appear to have reconciled this paradox by demonstrating that early T-cell proliferation within SLTs is also critical for maximal GVHD, and that approaches to diminish this expansion can substantially alter the incidence and severity of acute GVHD independent of effects on T-cell migration.
Also unexpected was the finding that T cells lacking CCR7 were still capable of substantial antitumor activity in vivo. This was most likely because (1) CCR7−/− Tn cells remained capable of migrating to the liver and spleen, important sites of P815 tumor growth; and that (2) higher T-cell numbers are required for lethal GVHD compared with GVL effects.24 Although CCR7−/− T-cell numbers were reduced relative to WT cells within all SLTs and GVHD target organs 2 weeks after transplantation, CCR7 Tn cells still demonstrated a residual ability to accumulate in those sites and to generate inflammation (Figures 3–4). It is likely that these immune responses were sufficient to eliminate small numbers of tumor cells, but inadequate to produce enough end-organ damage to consistently result in GVHD mortality.
Interestingly, separate anatomic sites appeared to be differentially affected by the absence of CCR7 on Tn cells. Significant differences in pathology scores were noted within the colons and livers of those animals given CCR7−/− Tn cells versus WT cells, whereas similar degrees of histologic injury were observed in recipient spleens and lungs (Figure 3A). Somewhat to our surprise, these variable pathologic differences were observed even though CCR7−/− Tn cell accumulation was generally less than that of WT cells within every SLT and GVHD target organ examined (Figure 4). Although the mechanism underlying this disparity is not entirely clear, several different hypotheses may explain this finding. First, GVHD pathology may be dependent on a threshold number of donor T cells within a specific organ. In such a scenario, any effector T cells accumulating beyond that threshold would be expected to have little effect on the ultimate pathologic score observed in that location, even though the kinetics of the GVHD inflammatory response might be accelerated. Alternatively, it is possible that GVHD pathology correlates better with inflammatory cytokine production than donor T-cell accumulation in certain organs. In our experiments, the greatest differences in both donor T-cell accumulation and IFN-γ/TNF-α production were observed in the colon. We believe that the improved survival outcomes seen in those animals receiving CCR7−/− Tn cells were primarily due to the reduced histopathology observed in that site, since gastrointestinal injury is an important cause of recipient mortality in acute GVHD.
Finally, we were surprised by the finding that CCR7−/− Treg cells were able to prevent lethal GVHD in murine HSC transplant recipients. Previous reports had demonstrated that although CCR7 is not required for the thymic development of Treg cells or for their ability to suppress T-cell expansion in vitro, CCR7−/− Treg cells are impaired in their capacity to suppress immune responses in nonirradiated animals secondary to an inability to efficiently enter LNs and to traffic to appropriate T-cell zones within SLTs.25 Our data would suggest that in the lymphopenic and inflammatory environment that follows ablative conditioning, Treg cells may be capable of trafficking into lymphoid sites using adhesion/chemokine receptors other than CCR7. Alternatively, donor Treg cells might directly suppress developing alloimmune responses within peripheral GVHD target organs26 and rely less on early trafficking into SLTs. Future experiments will focus in greater depth on the influence of CCR7 on the regulatory T-cell arm in the transplantation setting, with an emphasis on where these cells function to suppress GVHD.
While we were pursuing these studies, Anderson et al published work specifically designed to evaluate whether trafficking receptor differences between Tn cells and memory T cells were responsible for the disparate GVHD outcomes produced by the 2 cell types.7 For a portion of these experiments, the investigators used CCR7−/−BALB/c mice as donors and B6 mice knocked-out at the glucosyltransferase 2-3 loci (which lack peripheral LN addressin, the ligand for L-selectin) as recipients (GST 2-3−/−). There, they found CD4+ CCR7−/− Tn cells to be capable of mediating lethal GVHD in GST 2-3−/− animals. Although our own experiments with haplotype and minor MHC-mismatched transplantation systems clearly yielded different outcomes, our findings using a completely MHC-mismatched model (B6 into BALB/c) would be consistent with their data, as CCR7−/− Tn cells were able to elicit substantial GVHD in that setting (Figure 1E-F). The unfavorable outcomes observed in these 2 model systems were likely the result of the extreme histocompatibility differences between the donor and recipient strains, which appear to have allowed for enough T-cell expansion to overcome the beneficial effects of the absence of CCR7. Our results are somewhat divergent, however, in that we found CCR7−/− Tn cells to generate GVHD responses that differed qualitatively from those of WT cells, even in the setting of a complete MHC mismatch. Namely, the onset of severe GVHD was delayed in those animals receiving CCR7−/− cells, and their median survival times were significantly prolonged. The reason for these disparities is not entirely clear, and is most likely related to differences between the recipient mice in the 2 models used.
In summary, we have demonstrated superior HSCT outcomes with CCR7−/− donor T cells in clinically relevant, minor and haplotype MHC-mismatched murine model systems. Importantly, the absence of CCR7 appears to have little impact on the ability of Tn cells to mount robust GVL responses, or on the in vivo suppressive ability of donor Treg cells in irradiated hosts. While long-term inhibition of CCR7 would most likely have deleterious consequences due to the critical role of CCR7 in the homeostatic migration of T- and B-lymphocytes, short-term targeting of this chemokine receptor for approximately 4 to 6 weeks after stem cell transplantation could significantly affect the occurrence of acute GVHD with minimal long-term effects.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the following National Institutes of Health grants: R01 CA102052 and R01 AI064363 to J.S.S.; R01 HL56067, R01 AI 34495, R01 CA72669, and P01 CA142106 to B.R.B., F32 AI072827-01A2 to J.M.C., and T32 HL007149 to J.M.C. and M.J.C.
National Institutes of Health
Authorship
Contribution: J.M.C. performed experiments and wrote the manuscript; M.J.C. performed experiments; A.P.-M. analyzed the histopathology specimens; M.L.W. performed experiments and derived the CCR7−/−eGFP+ mouse line; J.E.B. performed experiments; B.R.B. reviewed and edited the manuscript; and J.S.S. conceived and supervised the project and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James M. Coghill, CB No. 7305, 3rd Fl POB, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: jcoghill@unch.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal