Abstract
T-cell immunotherapy that takes advantage of Epstein-Barr virus (EBV)–stimulated immunity has the potential to fill an important niche in targeted therapy for EBV-related cancers. To address questions of long-term efficacy, safety, and practicality, we studied 114 patients who had received infusions of EBV-specific cytotoxic T lymphocytes (CTLs) at 3 different centers to prevent or treat EBV+ lymphoproliferative disease (LPD) arising after hematopoietic stem cell transplantation. Toxicity was minimal, consisting mainly of localized swelling at sites of responsive disease. None of the 101 patients who received CTL prophylaxis developed EBV+ LPD, whereas 11 of 13 patients treated with CTLs for biopsy-proven or probable LPD achieved sustained complete remissions. The gene-marking component of this study enabled us to demonstrate the persistence of functional CTLs for up to 9 years. A preliminary analysis indicated that a patient-specific CTL line can be manufactured, tested, and infused for $6095, a cost that compares favorably with other modalities used in the treatment of LPD. We conclude that the CTL lines described here provide safe and effective prophylaxis or treatment for lymphoproliferative disease in transplantation recipients, and the manufacturing methodology is robust and can be transferred readily from one institution to another without loss of reproducibility. The current trial was registered at www.clinicaltrials.gov as #NCT00058812.
Introduction
Targeted immunotherapy has the potential to eradicate tumor cells in the absence of collateral damage to normal tissues. Whereas adoptive therapy with monoclonal antibodies has begun to fulfill this potential and indeed has become an established treatment for many malignancies,1 monoclonal antibodies have several limitations related to their biodistribution and modest tumoricidal activity. These restrictions may be overcome by adoptive immunotherapy with cytotoxic T lymphocytes (CTLs), which can actively migrate through microvascular walls to reach sequestered concentrations of tumor cells, self-amplify on encountering tumor cell target antigens, and kill tumors by a range of cytotoxic effector mechanisms.2,3 Epstein-Barr virus–positive lymphoproliferative disease (EBV-LPD) arises in the immunocompromised host after hematopoietic stem cell or solid organ transplantation and provides an excellent model for evaluating the clinical potential of targeted T-cell therapies.4-6 The EBV-associated lymphomas that develop after transplantation are highly immunogenic tumors that express immunodominant viral antigens, such as the EBNA3 proteins, and therefore should be susceptible to control with adoptively transferred EBV-specific CTLs.7 Consequently, we devised a strategy to prevent this complication by infusing polyclonal EBV-CTL lines to reconstitute immunity to EBV in patients who were undergoing hematopoietic stem cell transplantation (HSCT) and were at high risk of developing EBV-LPD and to actively treat patients who had already developed clinically apparent lymphoma.8
Our initial studies of 39 patients who received polyclonal EBV-CTLs generated from the transplantation donor showed that the adoptively transferred cells could expand by at least 2 to 3 logs, reconstitute immunity to EBV, prevent EBV-LPD by destroying EBV-infected target cells, and eradicate established EBV-associated lymphoma.8-10 The infused cells were genetically marked with a retroviral vector encoding the neomycin resistance gene (neo), allowing us to show that the CTLs could persist for up to 3 years and traffic to sites of disease.9,10 Although these preliminary findings were encouraging, they did not reveal the true rate of response or demonstrate their durability. Moreover, as a single-center study, it did not reveal whether adoptive T-cell therapy would be sufficiently robust to become a more generally applicable intervention. To address these questions of long-term efficacy, safety, and practicality, we studied 114 patients (including the first 39) who had received EBV-specific T-cell therapy in 3 different centers with a median follow-up of 10 years (range, 3-15 years). We now report an effectiveness of 100% for EBV-specific CTLs used as prophylaxis against EBV-LPD and of more than 80% when used as therapy. We conclude that this is a robust, safe, and effective strategy for preventing or treatingEBV-associated lymphoproliferative disorders in patients undergoing HSCT.
Methods
Patient details
Between 1993 and 2005, 114 patients were enrolled on studies at 3 institutions to adoptively transfer EBV-CTLs for the prophylaxis or therapy of EBV-associated LPD after HSCT. The initial study at St Jude Children's Research Hospital (SJCRH) targeted patients who were at high risk of developing EBV-LPD after undergoing CD6+ and CD8+ T cell–depleted HSCT from an unrelated or mismatched family member, or had already developed posttransplantation lymphoma.11 This study was transferred to Baylor College of Medicine, Texas Children's Hospital, and the Methodist Hospital, Houston after authors H.E.H., C.M.R., and M.K.B. relocated, whereas the approach was continued at SJCRH under a new IND (Investigational New Drug; J.L.H. and K.S.S.). After both institutions discontinued the T-cell depletion methodology and rituximab became available, the Baylor study evolved to focus on prophylaxis for high-risk patients undergoing unrelated or mismatched family member HSCT for immunodeficiency or with a past history of EBV lymphoma or rituximab-resistant EBV-LPD. A third study, opened at the Hospital for Sick Children, London, United Kingdom, in 2002 (P.J.A.), targeted these same patient populations. The studies in the United States were approved by the Food and Drug Administration and local institutional review boards. The initial SJCRH study used gene marking (in the first 26 patients) and therefore was also approved by the Recombinant DNA Advisory Committee of the National Institutes of Health and the Institutional Biosafety Committee. The study at the Hospital for Sick Children was approved by the hospital ethics committee and the Medicines and Healthcare Regulatory Authority, and all participants or their guardians gave informed consent on enrollment in accordance with the Declaration of Helsinki. This long-term follow-up analysis, combining data from the 3 sites, was also approved by the institutional review boards at Baylor and SJCRH.
Of the 114 subjects enrolled on these protocols, the first 39 were subjects of a 3-year follow-up study.10 Altogether, 101 patients were treated prophylactically, 90 because they had received transplantations from matched unrelated donors or mismatched family members on CD6+,CD8+ T-cell depletion protocols from 1993 to 2000 and 11 from 2000 to 2005 because of a diagnosis of immune deficiency, such as X-linked lymphoproliferative disease (XLP) or a history of EBV lymphoma that conferred a high risk of developing EBV-LPD after transplantation. We treated 13 patients therapeutically, for EBV-LPD diagnosed from biopsy results or from a rising EBV-DNA level associated with symptoms, signs, and radiologic evidence consistent with the diagnosis of lymphoma (fever, adenopathy, imaging findings, or an increasing lactate dehydrogenase [LDH] level). Many patients were off immunosuppression at the time they received CTLs, and in the remainder we attempted to reduce immunosuppression before infusion. Six patients received 4 doses of 107 CTLs/m2, whereas 6 others received 2 doses of 107 CTLs/m2 and 2 doses of 5 × 107 CTLs/m2 on the initial dose escalation study. Because both of these dose levels appeared safe and were associated with a reduced viral load and complete remission of EBV-LPD, the dose was first deescalated to 2 doses of 2 × 107 cells/m2 and then to 1 dose of 2 × 107 cells/m2. Twenty-six patients (23 in the prophylaxis group and 3 in the treatment group) received genetically modified CTLs to allow long-term monitoring of T-cell fate (Table 1). The clinical characteristics of the 114 patients are described in Table 2.
Patients who received genetically marked CTLs
| UPN . | Diagnosis . | Transplantation . | Status . | Follow-up . | Persistence, months . |
|---|---|---|---|---|---|
| 227 | CML (CP2) | 4/6 MMFM | Died (sepsis) | 187 days | 6 |
| 221 | ALL (CR3) | 6/6 MUD | Alive | 15 years | 105 |
| 219 | ALL (CR2) | 6/6 MUD | Alive | 15 years | 85 |
| 239 | AML in relapse | 4/6 MMFM | Alive | 15 years | 44 |
| 240 | ALL in relapse | 6/6 MUD | Died (relapse) | 99 days | 1 |
| 230 | CML (CP1) | 6/6 MUD | Alive | 14 years | 12 |
| 238 | Secondary AML | 6/6 MUD | Died (relapse) | 873 days | 6 |
| 253 | Secondary AML | 6/6 MUD | Alive | 14 years | Never detected |
| 259 | Secondary AML | 5/6 MUD | Died (relapse) | 154 days | 1 |
| 269 | CML (CP1) | 5/6 MMFM | Died (sepsis) | 239 days | 3 |
| 277 | AML in relapse | 6/6 MUD | Alive | 14 years | 57 |
| 282 | CML (AP) | 5/6 MUD | Alive | 14 years | 55 |
| 293 | CML (CP1) | 6/6 MUD | Alive | 13 years | 70 |
| 296 | MDS | 6/6 MUD | Alive | 13 years | 37 |
| 303 | Secondary AML | 6/6 MUD | Died (relapse) | 196 days | 4 |
| 308 | Secondary AML | 6/6 MUD | Alive | 13 years | 80 |
| 314 | ALL (CR2) | 4/6 MMFM | Alive | 13 years | 78 |
| 318 | MDS | 6/6 MUD | Alive | 13 years | 45 |
| 337 | ALL (CR1) | 5/6 MUD | Died (motor vehicle accident) | 10 years | 82 |
| 317 | AML in relapse | 5/6 MUD | Died (relapse) | 283 days | 1 |
| 345 | MDS | 6/6 MUD | Alive | 13 years | 8 |
| 324 | MDS | 6/6 MUD | Alive | 13 years | 55 |
| 347 | CML (CP1) | 5/6 MUD | Alive | 13 years | 72 |
| 327 | ALL (CR3) | 6/6 MUD | Alive | 13 years | 53 |
| 332 | ALL (CR1) | 5/6 MUD | Died (relapse) | 714 days | 5 |
| 365 | CML (CP1) | 5/6 MUD | Alive | 13 years | 46 |
| UPN . | Diagnosis . | Transplantation . | Status . | Follow-up . | Persistence, months . |
|---|---|---|---|---|---|
| 227 | CML (CP2) | 4/6 MMFM | Died (sepsis) | 187 days | 6 |
| 221 | ALL (CR3) | 6/6 MUD | Alive | 15 years | 105 |
| 219 | ALL (CR2) | 6/6 MUD | Alive | 15 years | 85 |
| 239 | AML in relapse | 4/6 MMFM | Alive | 15 years | 44 |
| 240 | ALL in relapse | 6/6 MUD | Died (relapse) | 99 days | 1 |
| 230 | CML (CP1) | 6/6 MUD | Alive | 14 years | 12 |
| 238 | Secondary AML | 6/6 MUD | Died (relapse) | 873 days | 6 |
| 253 | Secondary AML | 6/6 MUD | Alive | 14 years | Never detected |
| 259 | Secondary AML | 5/6 MUD | Died (relapse) | 154 days | 1 |
| 269 | CML (CP1) | 5/6 MMFM | Died (sepsis) | 239 days | 3 |
| 277 | AML in relapse | 6/6 MUD | Alive | 14 years | 57 |
| 282 | CML (AP) | 5/6 MUD | Alive | 14 years | 55 |
| 293 | CML (CP1) | 6/6 MUD | Alive | 13 years | 70 |
| 296 | MDS | 6/6 MUD | Alive | 13 years | 37 |
| 303 | Secondary AML | 6/6 MUD | Died (relapse) | 196 days | 4 |
| 308 | Secondary AML | 6/6 MUD | Alive | 13 years | 80 |
| 314 | ALL (CR2) | 4/6 MMFM | Alive | 13 years | 78 |
| 318 | MDS | 6/6 MUD | Alive | 13 years | 45 |
| 337 | ALL (CR1) | 5/6 MUD | Died (motor vehicle accident) | 10 years | 82 |
| 317 | AML in relapse | 5/6 MUD | Died (relapse) | 283 days | 1 |
| 345 | MDS | 6/6 MUD | Alive | 13 years | 8 |
| 324 | MDS | 6/6 MUD | Alive | 13 years | 55 |
| 347 | CML (CP1) | 5/6 MUD | Alive | 13 years | 72 |
| 327 | ALL (CR3) | 6/6 MUD | Alive | 13 years | 53 |
| 332 | ALL (CR1) | 5/6 MUD | Died (relapse) | 714 days | 5 |
| 365 | CML (CP1) | 5/6 MUD | Alive | 13 years | 46 |
CTL indicates cytotoxic T lymphocyte; UPN, unique patient number; CML, chronic myeloid leukemia; ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; MDS, myelodysplasia; MMFM, mismatched family donor; MUD, matched unrelated donor; CR, complete remission; CP, chronic phase; and AP, accelerated phase.
Clinical characteristics of 114 patients who received EBV-specific CTLs after transplantation
| Characteristic . | CTL recipients . |
|---|---|
| Age at transplantation, y [mean (range)] | 8.4 (0.5-38) |
| Male:female | 69:45 |
| Diagnosis | |
| Acute lymphoblastic leukemia | 29 |
| Acute myeloid leukemia | 33 |
| Myelodysplasia | 11 |
| Acute undifferentiated leukemia | 1 |
| Chronic myeloid leukemia | 12 |
| Hodgkin disease | 1 |
| Non-Hodgkin lymphoma | 3 |
| Aplastic anemia | 1 |
| Paroxysmal nocturnal hemoglobinuria | 2 |
| Histiocytic disorders | 3 |
| Hurler syndrome | 2 |
| X-linked lymphoproliferative disease | 6 |
| Wiskott-Aldrich syndrome | 5 |
| Osteogenesis imperfecta | 3 |
| Common immunodeficiency | 2 |
| Type of transplantation | |
| Unrelated donor | |
| 10/10 match | 3 |
| 9/10 match | 4 |
| 6/6 match | 63 |
| 5/6 match | 30 |
| Family member | |
| Syngeneic | 1 |
| 5/6 match | 6 |
| 4/6 match | 4 |
| 3/6 match | 3 |
| HSC product | |
| Marrow | 108 |
| PBSC | 6 |
| Characteristic . | CTL recipients . |
|---|---|
| Age at transplantation, y [mean (range)] | 8.4 (0.5-38) |
| Male:female | 69:45 |
| Diagnosis | |
| Acute lymphoblastic leukemia | 29 |
| Acute myeloid leukemia | 33 |
| Myelodysplasia | 11 |
| Acute undifferentiated leukemia | 1 |
| Chronic myeloid leukemia | 12 |
| Hodgkin disease | 1 |
| Non-Hodgkin lymphoma | 3 |
| Aplastic anemia | 1 |
| Paroxysmal nocturnal hemoglobinuria | 2 |
| Histiocytic disorders | 3 |
| Hurler syndrome | 2 |
| X-linked lymphoproliferative disease | 6 |
| Wiskott-Aldrich syndrome | 5 |
| Osteogenesis imperfecta | 3 |
| Common immunodeficiency | 2 |
| Type of transplantation | |
| Unrelated donor | |
| 10/10 match | 3 |
| 9/10 match | 4 |
| 6/6 match | 63 |
| 5/6 match | 30 |
| Family member | |
| Syngeneic | 1 |
| 5/6 match | 6 |
| 4/6 match | 4 |
| 3/6 match | 3 |
| HSC product | |
| Marrow | 108 |
| PBSC | 6 |
EBV indicates Epstein-Barr virus; CTL, cytotoxic T lymphocyte; HSC, hematopoietic stem cell; and PBSC, peripheral blood stem cell.
CTL production and characterization
B-Lymphoblastoid cell lines (LCLs) and EBV-specific CTLs were generated and characterized according to current Good Manufacturing Practice guidelines as previously described.10,12,13 There were no changes in manufacturing over the course of the study. In the first 26 recipients, the cells were transduced with the neo-containing G1Na retroviral vector (Genetic Therapy Inc), which confers resistance to neomycin and its G418 analog.8
Monitoring EBV DNA
Immunophenotyping
Immunophenotyping was performed as previously described.15
Chromium release assay
EliSpot assays
We analyzed γ-interferon production in response to stimulation with antigen as previously described.13,17 EBV peptides (Genemed Synthesis) were used in enzyme-linked immunosorbent spot (EliSpot) assays to determine the frequency of epitope specific T cells in CTL lines or patients' peripheral blood mononuclear cells (PBMCs).16-19 We currently perform immune studies at 2, 4, and 6 weeks and 3 months after infusion.
Real-time PCR amplification for neo and RCR
All PCR amplification primers were synthesized by Integrated DNA Technologies. The forward and reverse primers and probes specific for the G1Na vector were MP429, MP430, and MP428, respectively, and the G1Na-probe was 5′VIC and 3′MGBHQ20 conjugated. The primers and probe specific for the CRP gene were MP282, MP283, and MP281, respectively. The CRP-probe was 3′FAM and 5′BHQ conjugated. A standard curve was generated with cloned K562 cells after transduction with the G1Na clinical vector. Retroviral integration in each colony was characterized by genomic Southern blot analysis. A clone with a single integrant (T3) was selected and used as a standard by dilution with nontransduced K562 cells to the following clinically relevant dilution range: 0.01%, 0.069%, 0.29%, 1.27%, 5.4%, 33%, and 100%. The reaction consisted of 500nM MP429, MP430, 100 nM MP428, MP282, and MP283, and 40nM MP281, and 250 ng of genomic DNA with TaqMan PCR mastermix (Applied Biosystems) diluted to 1 time with nuclease free water.
Peripheral blood genomic DNA was tested in duplicate by PCR or reverse-transcribed (RT)–PCR to detect the MoMLV gag pol env coding sequence. Replication competent retrovirus (RCR) testing was performed every 3 months for a year and then annually until 2001, since samples have been archived.
Integration site analysis
Integration site analysis used a sensitive and specific flanking-sequence exponential anchored-PCR amplification as previously described.21
Toxicity monitoring
All patients were monitored for toxicity before and after each CTL infusion. Graft-versus-host-disease (GVHD) was graded by standard criteria.22 Patients who received gene-marked cells were monitored annually for 15 years.
Statistical methods
We relied on the descriptive statistics (means, ranges, and SD) to analyze most of the datasets. Comparisons of continuous variables between groups were performed by the 2-sample t test or the nonparametric Wilcoxon rank-sum test. Overall survival was defined as the time from transplantation to death of any cause. Kaplan-Meier analysis was used to estimate the overall survival curve. The cumulative incidence of EBV-LPD after transplantation was analyzed and plotted by the competing risk method as described in Gray.23 All P values are 2-sided, with P less than .05 considered statistically significant. All statistical analyses were performed with R, STATA 9.0, and SAS 9.2 software packages.
Results
Characterization of T-cell lines
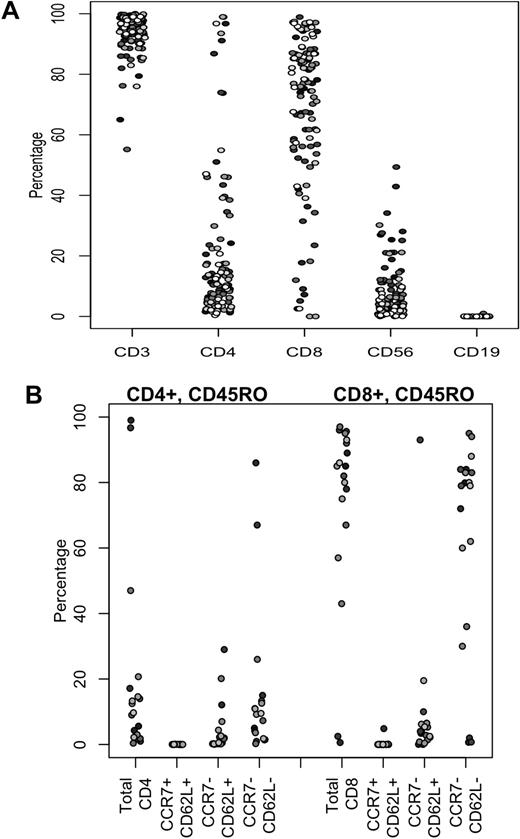
Before infusing the CTLs, we characterized their phenotype and ex vivo function. The majority of cells, 93.7% (± 6.8%; mean ± SD) expressed CD3, whereas 69.1% (± 25.8%) expressed CD8 and 19.6% (± 24.5%) expressed CD4 (Figure 1A). Because recent studies suggest that cells derived from the central memory compartment may be important for the long-term persistence of adoptively transferred T cells,24 we also assayed memory markers on the 20 most recently generated CTL lines. In normal EBV-seropositive persons, circulating EBV latent epitope-specific memory cells contain both central and effector memory subsets.25 Figure 1B shows that, in the CTL lines produced from such donors, the majority of both CD4+ and CD8+ cells express an effector memory phenotype (CCR7−,CD62L−), although a minority CD62L+ subset remains. In cytotoxicity assays, the lines showed consistently strong activity against autologous LCL (54.6% ± 12.6% killing at an effector/target ratio of 20:1), whereas the result for autologous phytohemagglutinin blasts (1.9% ± 2.6%), was well below the release criterion of less than 10%. The phenotype and ex vivo function of these EBV-specific CTLs did not vary appreciably by production site or manufacturing team (data not shown).
Immunophenotypes of EBV-specific CTLs. (A) Phenotypic composition of CTL lines. Percentages of CD3+, CD4+, and CD8+ T cells, natural killer cells (CD3−CD56+), and residual CD19+ B cells are shown. Each symbol represents a cell line infused into a single subject. (B) Expression of naive, central memory, and effector memory surface markers on CD4+ and CD8+ populations of the 20 most recently generated EBV-CTL lines.
Immunophenotypes of EBV-specific CTLs. (A) Phenotypic composition of CTL lines. Percentages of CD3+, CD4+, and CD8+ T cells, natural killer cells (CD3−CD56+), and residual CD19+ B cells are shown. Each symbol represents a cell line infused into a single subject. (B) Expression of naive, central memory, and effector memory surface markers on CD4+ and CD8+ populations of the 20 most recently generated EBV-CTL lines.
GVHD and other adverse effects
None of the patients had an immediate adverse reaction to the CTL infusions. Because the cells were derived from transplantation donors with up to a 3-allele HLA mismatch, we assessed the incidence and severity of postinfusion GVHD defining acute GVHD by consensus criteria and chronic GVHD by day of onset after transplantation using the Seattle criteria.22,26 Patients were excluded from study entry if their GVHD was grade 2 or greater, but 51 of 114 patients had had GVHD at some time preceding CTL infusion (grade 1 in 36, grade 2 in 13, grade 3 in 2). Eight of these 51 patients developed a recurrence of GVHD (grade 1 in 6 patients and grade 2 in 2 patients), all of whom responded to GVHD therapy (Table 3). None of the patients developed de novo GVHD after CTL infusions. Of 108 evaluable patients, 11 developed limited chronic GVHD and 2 extensive chronic GVHD. Ninety-five did not develop this complication.
Severity and frequency of GVHD
| GVHD . | No. of patients . |
|---|---|
| Acute GVHD (pre-CTL infusion) | |
| None | 63 |
| Grade 1 | 36 |
| Grade 2 | 13 |
| Grade 3 | 2 |
| Grade 4 | 0 |
| Total | 114 |
| Acute GVHD (post-CTL infusion) | |
| None | 106 |
| Grade 1 | 6 |
| Grade 2 | 2 |
| Grade 3 | 0 |
| Grade 4 | 0 |
| Total | 114 |
| Chronic GVHD | |
| None | 95 |
| Limited | 11 |
| Extensive | 2 |
| Total | 108 |
| GVHD . | No. of patients . |
|---|---|
| Acute GVHD (pre-CTL infusion) | |
| None | 63 |
| Grade 1 | 36 |
| Grade 2 | 13 |
| Grade 3 | 2 |
| Grade 4 | 0 |
| Total | 114 |
| Acute GVHD (post-CTL infusion) | |
| None | 106 |
| Grade 1 | 6 |
| Grade 2 | 2 |
| Grade 3 | 0 |
| Grade 4 | 0 |
| Total | 114 |
| Chronic GVHD | |
| None | 95 |
| Limited | 11 |
| Extensive | 2 |
| Total | 108 |
GVHD indicates graft-versus-host disease; and CTL, cytotoxic T lymphocyte.
The main adverse event in this study was localized swelling at sites of disease during a therapeutic response. This toxicity was severe in only 2 of 4 subjects. One was a patient with bulky pharyngeal disease, who had a vigorous inflammatory response with an immune infiltrate of genetically marked cells apparent on biopsy. This response produced progressive airway obstruction and mucosal sloughing, ultimately requiring mechanical ventilation.10 This patient subsequently made a complete recovery and remains well more than 10 years later. The other was a patient who developed enlarged adenoids after CTL infusion that caused partial respiratory obstruction in whom necrotic tissue was found on biopsy. One other patient developed a left lower lobe pulmonary infiltrate a week after receiving CTLs, and 1 had a transient increase in hepatic transaminases. None of these complications resulted in long-term sequelae, and all 4 patients were well 8 to 13 years after CTL infusion.
Antiviral activity in patients receiving CTLs as prophylaxis for EBV-LPD
Elevated EBV-DNA levels are highly predictive of the development of EBV-LPD in recipients of T cell–depleted transplantations, and the sensitivity remains high but with lower specificity in recipients of unmanipulated hemopoietic stem cell products.14 Prophylactic infusions decreased the EBV load in 11 of the 12 patients who had elevated levels of EBV-DNA but no other evidence of EBV-LPD before infusion. In the one exception, EBV-DNA remained elevated at more than 1500 copies/μg of PBMC-DNA for more than a year despite an additional dose of CTLs, but then normalized. This patient did not develop LPD.
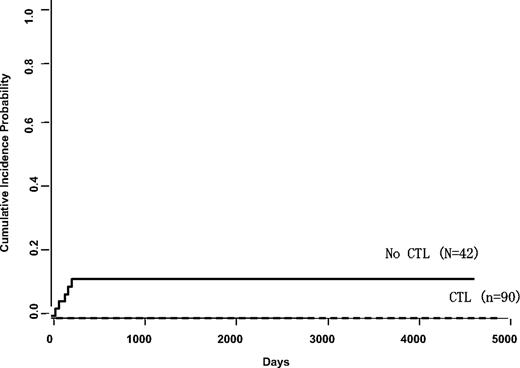
Altogether, 101 patients received EBV-specific CTLs as prophylactic therapy, 90 of whom had undergone T cell–depleted HSCT with a regimen associated with an 11% incidence of EBV posttransplantation lymphoproliferative disorder.10,11 The remaining 11 patients received CTLs because of diagnoses conferring a high risk of LPD development; 7 patients had a history of EBV-associated lymphoma before transplantation, requiring treatment with rituximab or chemotherapy. Neither group developed LPD over 15 years of follow-up, compared with 5 of 42 patients (11%) who were enrolled on the same transplantation protocol as 90 of the patients but did not receive CTLs (Figure 2).
Incidence of LPD in patients receiving CD6+CD8+ T cell–depleted marrow with or without EBV CTL prophylaxis. Kaplan-Meier analysis was based on 90 patients who received CTLs prophylactically compared with 42 who were enrolled on the same transplantation protocol but who did not receive CTLs. These patients were treated on Institutional Review Board-approved transplantation protocols open to patients with hematologic malignancies at St Jude Children's Research Hospital (1993-2000) or Baylor College of Medicine (1998-2000) where they received marrow from a matched unrelated donor or mismatched family member that had been depleted of T cells with antibodies to CD6 and CD8 after conditioning with cyclophosphamide, cytarabine, total body irradiation, and antithymocyte globulin.11 All recipients received cyclosporine A at a dosage adjusted to attain plasma concentrations of 250 to 350 ng/mL. The control patients were enrolled on the same study but had either declined enrollment on the CTL prophylaxis study or were not eligible. The difference in incidence rates is highly significant.
Incidence of LPD in patients receiving CD6+CD8+ T cell–depleted marrow with or without EBV CTL prophylaxis. Kaplan-Meier analysis was based on 90 patients who received CTLs prophylactically compared with 42 who were enrolled on the same transplantation protocol but who did not receive CTLs. These patients were treated on Institutional Review Board-approved transplantation protocols open to patients with hematologic malignancies at St Jude Children's Research Hospital (1993-2000) or Baylor College of Medicine (1998-2000) where they received marrow from a matched unrelated donor or mismatched family member that had been depleted of T cells with antibodies to CD6 and CD8 after conditioning with cyclophosphamide, cytarabine, total body irradiation, and antithymocyte globulin.11 All recipients received cyclosporine A at a dosage adjusted to attain plasma concentrations of 250 to 350 ng/mL. The control patients were enrolled on the same study but had either declined enrollment on the CTL prophylaxis study or were not eligible. The difference in incidence rates is highly significant.
Antitumor effects of CTL infusions
Thirteen patients received CTLs as therapy for proven or probable EBV-LPD. Probable EBV-LPD was defined according to published guidelines27 as an elevated EBV DNA level associated with clinical symptoms (adenopathy or fever or masses on imaging) but without biopsy confirmation, whereas proven LPD required biopsy confirmation. The clinical details of these cases are summarized in Table 4. Two of the 13 patients failed to respond and died of progressive EBV-associated lymphoma. One had extensive central nervous system disease and died 8 days after infusion of CTLs, whereas the other had a 244 base pair deletion in the immunodominant EBNA 3B gene that abolished the 2 epitopes recognized by her HLA11-restricted donor line.28 The remaining 11 patients achieved complete remissions that were sustained with no recurrences.
Clinical outcome in 13 patients who received EBV-CTLs as therapy for proven or probable EBV-LPD
| Patient no. . | Donor . | Diagnostic findings . | Response to CTLs . | Recurrence . | Complications . | Long-term outcome . |
|---|---|---|---|---|---|---|
| 238 | 6/6 URD | Fever, lymphadenopathy, and lung nodules; biopsy-proven EBV- LPD | CR | None | None | Died of relapsed secondary AML at 2 years |
| 345 | 6/6 URD | Fever, lymphadenopathy, abnormal liver function, and elevated EBV-DNA | CR | None | Transient increase in transaminases | Alive at 13 years |
| 324 | 6/6 URD | Fever, extensive bulky, and widespread adenopathy; biopsy-proven EBV-LPD | CR | None | Swelling and sloughing during response requiring ventilation | Alive at 13 years |
| 401 | 6/6 URD | Fever and enlarged adenoids; biopsy-proven EBV-LPD | CR | None | Swelling during response | Alive at 12 years |
| 426 | 5/6 URD | Fever, adenopathy, and extensive pulmonary nodules; biopsy-proven EBV-LPD | No response | Progressive disease | NA | Died of progressive EBV-LPD resistant to CTLs at 25 days |
| 2–1 | 6/6 URD | Increasing LDH and EBV-DNA levels suggested probable LPD for which CTL clones were administered (initial CTL line did not meet release criteria because of > 10% killing of PHA blasts); subsequent development of biopsy-proven LPD led to infusion of polyclonal line | CR | None | None | Died of respiratory failure secondary to another infection at 152 days; autopsy showed no evidence of EBV- LPD |
| 2–2 | 5/6 URD | Fever, adenopathy, spleen lesions, elevated EBV-DNA | CR | None | Exacerbation of preexisting GVHD | Died of Enterococcus faecalis sepsis at 250 days |
| 2–3 | 5/6 URD | Widespread biopsy-proven EBV-LPD with extensive CNS disease | No response | Progressive disease | None | Died of progressive EBV-LPD at 8 days |
| 2–4 | 6/6 URD | Biopsy proven EBV-LPD | CR | None | None | Alive at 10 years |
| CAGT-1 | 6/6 URD | Fevers and elevated EBV DNA | CR | None | None | Alive at 9 years |
| CAGT-2 | 6/6 URD | Fever, pulmonary infiltrates, and increased EBV-DNA level | CR | None | Small pleural effusion | Rituximab consolidation after steroid therapy; alive at 10 years |
| CAGT-677 | 6/6 URD | CNS lesions; biopsy-proven monoclonal EBV+ lymphoma | CR | None | None | Received hydroxyurea until CTLs available; alive at 7 years |
| CAGT-823 | 6/6 URD | Fever, pharyngitis, adenopathy, increased LDH, and elevated EBV-DNA level | CR | None | None | Alive at 5 years |
| Patient no. . | Donor . | Diagnostic findings . | Response to CTLs . | Recurrence . | Complications . | Long-term outcome . |
|---|---|---|---|---|---|---|
| 238 | 6/6 URD | Fever, lymphadenopathy, and lung nodules; biopsy-proven EBV- LPD | CR | None | None | Died of relapsed secondary AML at 2 years |
| 345 | 6/6 URD | Fever, lymphadenopathy, abnormal liver function, and elevated EBV-DNA | CR | None | Transient increase in transaminases | Alive at 13 years |
| 324 | 6/6 URD | Fever, extensive bulky, and widespread adenopathy; biopsy-proven EBV-LPD | CR | None | Swelling and sloughing during response requiring ventilation | Alive at 13 years |
| 401 | 6/6 URD | Fever and enlarged adenoids; biopsy-proven EBV-LPD | CR | None | Swelling during response | Alive at 12 years |
| 426 | 5/6 URD | Fever, adenopathy, and extensive pulmonary nodules; biopsy-proven EBV-LPD | No response | Progressive disease | NA | Died of progressive EBV-LPD resistant to CTLs at 25 days |
| 2–1 | 6/6 URD | Increasing LDH and EBV-DNA levels suggested probable LPD for which CTL clones were administered (initial CTL line did not meet release criteria because of > 10% killing of PHA blasts); subsequent development of biopsy-proven LPD led to infusion of polyclonal line | CR | None | None | Died of respiratory failure secondary to another infection at 152 days; autopsy showed no evidence of EBV- LPD |
| 2–2 | 5/6 URD | Fever, adenopathy, spleen lesions, elevated EBV-DNA | CR | None | Exacerbation of preexisting GVHD | Died of Enterococcus faecalis sepsis at 250 days |
| 2–3 | 5/6 URD | Widespread biopsy-proven EBV-LPD with extensive CNS disease | No response | Progressive disease | None | Died of progressive EBV-LPD at 8 days |
| 2–4 | 6/6 URD | Biopsy proven EBV-LPD | CR | None | None | Alive at 10 years |
| CAGT-1 | 6/6 URD | Fevers and elevated EBV DNA | CR | None | None | Alive at 9 years |
| CAGT-2 | 6/6 URD | Fever, pulmonary infiltrates, and increased EBV-DNA level | CR | None | Small pleural effusion | Rituximab consolidation after steroid therapy; alive at 10 years |
| CAGT-677 | 6/6 URD | CNS lesions; biopsy-proven monoclonal EBV+ lymphoma | CR | None | None | Received hydroxyurea until CTLs available; alive at 7 years |
| CAGT-823 | 6/6 URD | Fever, pharyngitis, adenopathy, increased LDH, and elevated EBV-DNA level | CR | None | None | Alive at 5 years |
EBV-CTL indicates Epstein-Barr virus–cytotoxic T lymphocyte; EBV-LPD, Epstein-Barr virus–lymphoproliferative disease; URD, unrelated donor; CR, complete remission; AML, acute myeloid leukemia; NA, not applicable; LDH, lactate dehydrogenase; and CAGT, Center for Cell and Gene Therapy.
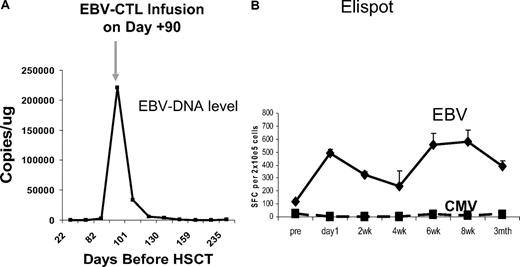
Figure 3 shows a representative immunovirologic response in a patient (CAGT-823) with XLP who developed probable EBV-LPD. After infusion of donor-derived CTLs, there was a rapid decrease in the EBV-DNA level associated with an increase in EBV-specific immune activity, as measured in an EliSpot assay, and resolution of fevers, adenopathy, and pharyngitis. This patient's line contained a substantial cell population detected with the RAK pentamer. Cells positive for this peptide were composed of 12.2% of all the patients' CD3+ cells at 2 weeks after infusion and were still detectable at a level of 2.7% of total CD3+ cells at 6 months after infusion. RAK is a lytic epitope derived from BZLF1 that was likely one of many specificities of the patient's line, detected because of its immunodominance and not necessarily because it was protective.
Representative clinical response. An 18-month-old boy with XLP presented at day 87 after matched unrelated donor transplantation with pharyngeal swelling, an elevated lactate dehydrogenase level, and a rapidly increasing EBV-DNA load. (A) After receiving 2 × 107 CTLs/m2 on day 90, he showed rapid clinical improvement and a decrease in EBV DNA level to baseline. (B) Coincident with this response, there was an increase in EBV-specific activity, as measured with a γ-interferon EliSpot assay, whereas CMV activity remained undetectable.
Representative clinical response. An 18-month-old boy with XLP presented at day 87 after matched unrelated donor transplantation with pharyngeal swelling, an elevated lactate dehydrogenase level, and a rapidly increasing EBV-DNA load. (A) After receiving 2 × 107 CTLs/m2 on day 90, he showed rapid clinical improvement and a decrease in EBV DNA level to baseline. (B) Coincident with this response, there was an increase in EBV-specific activity, as measured with a γ-interferon EliSpot assay, whereas CMV activity remained undetectable.
Long-term persistence and functionality of transferred cells
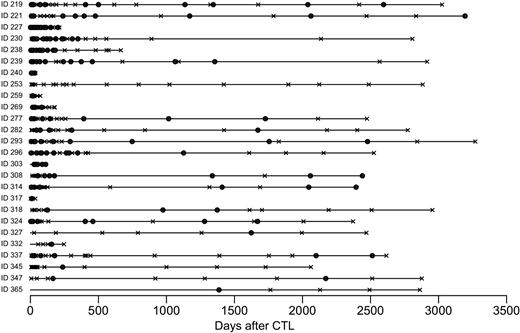
The first 26 patients treated from 1993 to 1996 received between 2 × 107 and 1.2 × 108 EBV-specific CTL per meter squared that were marked at a low level (mean transgenic cells, 2.6%; range, 0.1%-9.6%) with the retroviral vector GINa to allow tracking of the infused cells (Table 1). Patients were monitored 1 or 2 times weekly for 6 weeks, then every 3 months for a year, and then annually for 8 to 10 years, with samples then archived each year until 15 years. The retroviral integrant could be detected by real-time PCR analysis in the peripheral blood of 25 of these patients. Although levels were highest in the early postinfusion period, gene-marked cells could be detected in peripheral blood for as long as 105 months after CTL infusion (Figure 4).
Long-term detection of marked CTLs. Circles within bars represent detection of the retroviral integrant by real-time PCR analysis of either peripheral blood or a reactivated line; black X, assays where the marker gene was not detected. Each bar represents an individual patient. Patients were monitored every 1 to 2 weeks for 8 weeks and then 3 monthly for a year, and then annually.
Long-term detection of marked CTLs. Circles within bars represent detection of the retroviral integrant by real-time PCR analysis of either peripheral blood or a reactivated line; black X, assays where the marker gene was not detected. Each bar represents an individual patient. Patients were monitored every 1 to 2 weeks for 8 weeks and then 3 monthly for a year, and then annually.
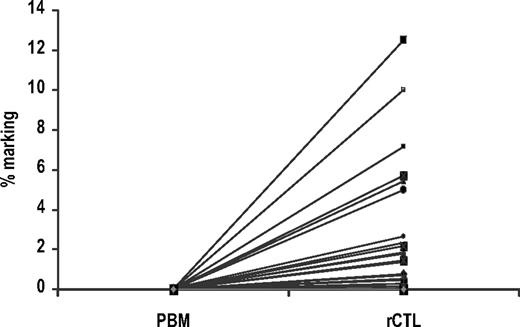
PBMCs were drawn at 1 week to 55 months after infusion and stimulated with irradiated autologous EBV-LCLs and interleukin-2 to mimic exposure to the relevant antigen in vivo. We observed a several hundred-fold increase in the frequency of gene-marked cells before and after stimulation with EBV-LCLs (prestimulation mean marking frequency of 0.0008%, compared with 1.49% after stimulation; Figure 5). In 20 peripheral blood samples initially negative for gene-marked cells, ex vivo stimulation with EBV-LCLs expanded the marked population to a level more than the 1/10 000 threshold for RT-PCR detection. This rapid increase in signal illustrates that infused gene-marked EBV-CTLs can expand in response to EBV antigens even after their initial culture, transduction, infusion, and persistence in vivo, at a rate similar to that of unmanipulated EBV-specific T cells. Both CD4+ and CD8+ gene-modified T lymphocytes were detected in this long-lived circulating memory pool after separation of the reactivated EBV-CTLs with magnetic beads (data not shown).
Long-term functionality. Change in the frequency of gene-marked cells in 46 peripheral blood samples from 18 patients before and after ex vivo stimulation with EBV-LCLs. The median marking efficiency before prestimulation was 0.0008% (SEM = 0.0007%) compared with 1.49% (SEM = 0.40%) after stimulation (P < .001 by the Wilcoxon signed-rank test). In 20 samples that were initially negative for gene-marked cells, ex vivo stimulation with EBV-LCLs expanded the marked population to a level above the 1/10 000 threshold for quantitative RT-PCR detection.
Long-term functionality. Change in the frequency of gene-marked cells in 46 peripheral blood samples from 18 patients before and after ex vivo stimulation with EBV-LCLs. The median marking efficiency before prestimulation was 0.0008% (SEM = 0.0007%) compared with 1.49% (SEM = 0.40%) after stimulation (P < .001 by the Wilcoxon signed-rank test). In 20 samples that were initially negative for gene-marked cells, ex vivo stimulation with EBV-LCLs expanded the marked population to a level above the 1/10 000 threshold for quantitative RT-PCR detection.
The expansion in marked cells on exposure to EBV antigens ex vivo is paralleled by events in vivo at times remote from infusion. In 4 patients, in whom the marker gene could be detected at 6 to 42 months after infusion, we were able to measure the EBV load (by RT-PCR) in peripheral blood samples and show that EBV reactivation was concomitant with (or shortly preceded) the rise in marker signal. These results indicate that increased antigenic stimulation in vivo can induce expansion of the infused marked EBV-CTLs. Most importantly, from a safety perspective, as the EBV-DNA levels fell in the peripheral blood of these patients, so too did their level of gene marking. Hence, infused CTLs remain dependent on antigenic stimulation for their expansion and survival, behaving as normal memory T cells rather than as autonomous mutants. Taken together, these findings suggest that adoptively transferred cells entered the memory pool and retained their physiologic behavior, despite ex vivo selection and transduction.
Integration site analysis
Although we expected to detect multiple distinct retroviral integration sites in the infused polyclonal T-cell lines, the T-cell preparations were expanded several-fold after retroviral transduction, raising the possibility that oligoclonal or even monoclonal populations might be selected if a T cell developed a growth advantage because of modulation of a critical regulatory gene(s) after retroviral integration. We therefore used flanking sequence exponential anchored (FLEA)–PCR21 to analyze integration sites before and after infusion. We subjected genomic DNA from 3 therapeutic products to FLEA-PCR and subcloned the PCR smears to generate a plasmid library. Twenty-four plasmid clones containing external sequences from each library were sequenced. As hoped for, distinct integrant-host junctions (IHJs) were detected, representing the progeny of each individual cell that was initially transduced.
We then studied the IHJ sequences that persisted in 6 patients at 2 or 3 time points after infusion to determine whether a single IHJ clone emerged as dominant over time. We also analyzed samples taken from T-cell cultures reactivated from blood by stimulation with EBV-LCLs ex vivo to increase the frequency of gene-marked cells. PCR smears were subcloned and sequenced. IHJs were validated by the presence of 32 base pairs of LTR, beginning with “TGAAAG.” We isolated a single IHJ from each sample, excluding 1 reactivated sample that contained 4 distinct IHJs (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, all IHJs analyzed at each distinct time point were unique, suggesting that a multiplicity of clones were present but that sampling limitations, PCR bias, or a variation in circulation kinetics affected detection. Most of the clones detected in the infused lines were never detected in vivo. Hence, there was no evidence for progressive clonal dominance in vitro or in vivo.
To discover whether any of the cells persisting in vivo contained IHJs that were potentially able to affect T-cell growth and survival, the human sequences preceding the retroviral LTR in peripheral blood and ex vivo-expanded CTLs were compared with the human genome. Genes potentially affected by retroviral integration include 2 transcriptional control genes (NM030915.1. and SHOX), 2 genes coding for RNA binding proteins (CUGBP2 and RBM8a), and a kinase (TINN13K; supplemental Table 2). None of these genes has any known associations with lymphoproliferative disorders or participates in the control of T-cell proliferation, nor do they match murine genes in the murine retroviral insertional mutagenesis database. This diverse group of genes argues against the persistence of transduced T cells being attributable to the modulation of a particular endogenous gene.
Long-term follow-up
With a median follow-up of 10.5 years, the estimated overall survival rates (with 95% confidence intervals) for all patients who received EBV-specific CTLs were 69% (60%-77%) at 5 years and 67% (57%–76%) at 10 years (Figure 6). Seventy-nine patients were alive at 3 to 15 years of follow-up. Relapse or persistence of the primary malignancy was the major cause of death (n = 24), and other outcomes are reported in Table 5.
Overall survival of the 114 patients receiving CTLs. The mean Kaplan-Meier estimate (with 95% confidence levels) at 3, 5, and 10 years was 70% (61%-78%), 69% (60%-77%), and 67% (57%-76%), respectively. Tic marks on the survival curves indicate patients still at risk; dashed lines outline the 95% confidence levels.
Overall survival of the 114 patients receiving CTLs. The mean Kaplan-Meier estimate (with 95% confidence levels) at 3, 5, and 10 years was 70% (61%-78%), 69% (60%-77%), and 67% (57%-76%), respectively. Tic marks on the survival curves indicate patients still at risk; dashed lines outline the 95% confidence levels.
Causes of death in 114 patients who received EBV-specific CTLs
| Primary cause of death . | No. of patients . |
|---|---|
| Alive | 79 |
| Recurrence or persistence of primary disease | 24 |
| Motor vehicle accident | 1 |
| Infection | 3 |
| EBV-LPD | 2 |
| Regimen-related toxicity | 5 |
| Total | 114 |
| Primary cause of death . | No. of patients . |
|---|---|
| Alive | 79 |
| Recurrence or persistence of primary disease | 24 |
| Motor vehicle accident | 1 |
| Infection | 3 |
| EBV-LPD | 2 |
| Regimen-related toxicity | 5 |
| Total | 114 |
EBV indicates Epstein-Barr virus; CTL, cytotoxic T lymphocyte; and EBV-LPD, Epstein-Barr virus–lymphoproliferative disease.
Seventeen of the 26 patients who received gene-marked cells were alive at 12 to 15 years after infusion, and none showed any evidence of harm from the gene-marking process (Table 1) Follow-up RCR testing was negative. Six of the 9 deaths resulted from relapse of the underlying malignancy, whereas 2 deaths were the result of opportunistic infections and the ninth to a motor vehicle accident. Yearly assessment of survivors has not revealed clinical evidence of retrovirally induced lymphoproliferation or secondary tumors.
Cost-effectiveness
In 2009, the cost for manufacturing, testing, and infusing of an EBV CTL line was $6095 (Table 6), excluding professional time. Although each line is a patient-specific product, this cost compares favorably with estimates for other modalities used in the treatment of EBV-LPD, such as CD20 monoclonal antibody therapy for which the charge is $9000 per dose.
Cost of CTL line
| Item . | Cost, $ . |
|---|---|
| LCL line reagents | 312 |
| EBV CTL line reagents | 560 |
| Testing | 2643 |
| Donor infectious disease testing | 322 |
| HLA typing | 700 |
| Bacterial | 78 |
| Fungal | 154 |
| Bacterial CFR | 39 |
| Fungal CFR | 39 |
| Mycoplasma (PCR) | 534 |
| Endotoxin | 300 |
| Cytotoxicity assay | 250 |
| Phenotyping | 227 |
| 2643 | |
| GMP charge (technician labor, facility fee) | 2280 |
| Infusion charge | 300 |
| Total | 6095 |
| Item . | Cost, $ . |
|---|---|
| LCL line reagents | 312 |
| EBV CTL line reagents | 560 |
| Testing | 2643 |
| Donor infectious disease testing | 322 |
| HLA typing | 700 |
| Bacterial | 78 |
| Fungal | 154 |
| Bacterial CFR | 39 |
| Fungal CFR | 39 |
| Mycoplasma (PCR) | 534 |
| Endotoxin | 300 |
| Cytotoxicity assay | 250 |
| Phenotyping | 227 |
| 2643 | |
| GMP charge (technician labor, facility fee) | 2280 |
| Infusion charge | 300 |
| Total | 6095 |
CTL indicates cytotoxic T lymphocyte; LCL, lymphoblastoid cell line; EBV-CTL, Epstein-Barr virus–cytotoxic T lymphocyte; CFR, Code of Federal Regulations; PCR, polymerase chain reaction; and GMP, Good Manufacturing Practices.
Discussion
Broader application of antigen-specific CTL infusions in patients with viral infection or malignancy will require intervention strategies that are both safe and effective, a transferable technology, and costs that are proportionate to the benefits. In this study, we used each of these criteria to evaluate EBV-specific CTLs as a means to prevent or treat LPD after HSCT in 114 patients at 3 institutions. We found the CTLs provided safe and effective prophylaxis in 101 patients, all of whom were at risk for EBV-LPD. None developed lymphoma, irrespective of risk factors that included T-cell depletion, primary immunodeficiency, or prior EBV+ lymphoma. The CTLs were also effective therapy in 11 of 13 patients with proven or probable LPD. Effective prophylaxis and therapeutic response were achieved with minimal toxicity, excluding inflammation at tumor sites, which required clinically significant intervention in 2 patients only. Finally, the infused cells were long lived and provided persistent activity against viral reactivation with no evidence of monoclonal outgrowth.
When used as therapy for EBV-LPD, unmanipulated donor T cells can cause significant morbidity resulting from alloreactivity.29,30 By contrast, infusion of CTLs proved to be safe with no appreciable alloreactivity seen in either the prophylaxis or treatment group and without the de novo development of acute GVHD after CTL infusions. Eight patients with a previous GVHD episode had a reactivation that was chiefly limited to grade 1. This reactivation rate is comparable with that seen in other patients on the same transplantation protocol who did not receive CTLs. The cumulative incidence of chronic GVHD was 14%, not significantly different from the incidence of 23.5% observed in patients treated on the selective T-cell depletion used with most of the CTL recipients.11 Indeed, the cumulative incidences of both recurrent acute GVHD and chronic GVHD are lower than those reported in the literature for recipients of T cell–depleted transplantations with similar degrees of T-cell depletion.31,32 Significant inflammation during therapeutic responses in 2 patients with bulky disease constituted the most severe toxicity.
Genotoxicity is a common concern in studies using retroviral vectors to transfer transgenes after the development of T-cell lymphoproliferation, as in the γ-chain SCID studies.33,34 In our study, the gene-marking component allowed us to show that transgenic cells could survive for up to 9 years. Moreover, the adoptively transferred cells continued to respond appropriately to antigenic stimulation, whereas analysis of retroviral IHJs showed no evidence of persistent clones that could be attributable to the site of retroviral integration. After CTL infusion, the majority of patients had only a single integration site detected in each cell sample; however, repeat samplings of the same patient often revealed different integrants. We have extensively validated the accuracy and repeatability of the FLEA-PCR technique by analyzing individual clones and their mixtures,21 resulting in the detection of the expected IHJ. Hence, the likeliest explanation for our detection of unique integrants at each sampling time is that a polyclonal population of transduced EBV-CTLs survives in vivo, so that individual samples of peripheral blood typically contain only a single integrant. This suggests a limitation of the sampling procedure or that a given memory T-cell clone circulates only intermittently.
The substantial expansion and long-term persistence and function of the CTLs used in this study may have several different explanations. First, we infused EBV-CTLs soon after stem cell transplantation, into a milieu where the preexisting lymphoid pool (including regulatory T cells) had been profoundly depleted, and in which lymphoid regeneration was proceeding apace. It was recently shown, moreover, that lymphocyte depletion, even by nonmyeloablative chemotherapy and in the absence of an allogeneic stem cell graft, favors the expansion and function of infused tumor-reactive T cells.35 However, infusion into a lymphodepleted environment per se is not sufficient to guarantee expansion and prolonged persistence of CTL: for example, CMV-specific CD8+ T-cell clones had a more limited capacity to survive and expand in vivo than did a polyclonal mixture of CD4+ and CD8+ T cells with the same specificity.36,37 Recently, tetramer-selected CD8+ CMV-specific T cells were reported to expand in stem cell recipients after infusion without an apparent requirement for CD4+ T cells.38 Our patients received a combination of CD4+ and CD8+ cells, and an interaction between helper and effector populations is probably beneficial for the expansion and survival of EBV-specific T cells in vivo. Prolonged persistence of gene-modified EBV-CTLs may also be attributable to chronic stimulation by EBV-infected B cells in EBV-seropositive persons. The importance of antigen was recently demonstrated by studies of adoptively transferred adenovirus-specific T cells, whose in vivo expansion in stem cell recipients could be detected only in patients with concurrent adenovirus infections.16 The presence on antigen-presenting cells of costimulatory molecules that favor T-cell growth and survival will also facilitate T-cell killing of EBV-infected B cells and may contribute to the longer persistence of CTLs compared with those specific for melanoma.39 In contrast to other studies,40 an immune response to the xenogeneic transgene was not generated in this effort, and neo-expressing T cells persisted long-term in most patients. The T cells also retained their ability to express the transgene after antigenic stimulation ex vivo. Although transgene expression is probably down-modulated in cells not exposed to antigens, the gene nonetheless retains the capacity to be reexpressed after antigenic stimulation.
The methodology for manufacture of CTLs proved to be robust, and procedures could be transferred to another institution and to a different group of investigators at the original institution with reproducible and consistent manufacturing results and clinical outcomes. It was also possible to manufacture cells in one center and ship them to another center for infusion. This exportability has been confirmed in studies from other centers that have used similar CTL generation methodology. A Swedish group, for example, has shown activity by EBV-specific CTLs after their infusion CTLs into patients with an elevated EBV viral load and observed responses in 5 of 6 cases.41 The patient who did not respond received a line lacking discernible EBV-specific activity.41 Comoli et al reported 4 patients with recurrent EBV-LPD after initial rituximab treatment who were rescued permanently by infusion of EBV-specific CTLs.42 The Sloan-Kettering group, in a study that so far has only been mentioned in a review article, used EBV CTLs to treat 14 marrow allograft recipients and 3 recipients of allogeneic organ transplantations who developed monoclonal EBV lymphomas.43 Of these 17 patients, 11 achieved complete remissions and 2 long-term stabilization of their disease. The CTLs were able to mediate regressions of disease not only in lymph nodes, intestine, liver, or lung, but also in the central nervous system.43
One open question concerns the most appropriate role and timing for EBV CTL infusions, given that the CD20 monoclonal antibody rituximab44-47 now offers an alternative prophylactic and therapeutic option. Despite its ready availability and convenience, rituximab does have significant side effects, including an increase in immune compromise and, unlike CTLs, a lack of long-term protection.48 Our current strategy is therefore to manufacture CTLs for the highest-risk patients: those with a diagnosis of immune deficiency or a history of EBV lymphoma or those who redevelop elevated EBV-DNA levels after rituximab therapy. The manufacture and availability of CTLs can be accelerated by selecting virus-reactive cells from donor blood by rapid selection processes, such as tetramer selection or γ-interferon capture38,49 or by novel culture methodologies.50 Alternatively, Haque et al established a bank of polyclonal EBV-CTL lines from unrelated third-party blood donors for use in recipients of predominantly solid organ grafts who develop EBV-related lymphoma.51 They have reported encouraging results with this rapidly available product: an overall response rate of 64% at 5 weeks and 52% at 6 months.51
Obtaining licensure and reimbursement is another issue impeding the wider use of EBV-specific CTL therapy, which we have approached by obtaining an orphan drug designation. Finally, a better understanding of the costs associated with production and testing of EBV-CTL lines would be of immense value in assessing the cost-effectiveness versus other modalities in the prevention and treatment of EBV-LPD in transplantation recipients. Our preliminary calculations indicate that a CTL line can be successfully generated for less than $10 000, a cost that compares favorably with that of monoclonal antibody treatment, with effectiveness even in patients in whom the antibody has failed.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the clinicians involved in the care of these patients, the staff in the Good Manufacturing Practices (GMP) facilities who manufactured cells, the research staff who collected data, and Madeline O'Connor for help with providing long-term follow-up data on the St Jude patients.
This work was supported by the National Institutes of Health (grants RO1CA061384, P01CA094237, and P50CA126752), the Leukemia & Lymphoma Society (SCOR), National Institutes of Health, National Cancer Institute (grant P30-CA21765), the Assisi Foundation, and the American Lebanese Syrian Associated Charities (ALSAC). H.E.H. is supported by a Dan L. Duncan Chair. M.K.B. is supported by a Fayez Sarofim Chair.
National Institutes of Health
Authorship
Contribution: H.E.H., M.K.B., and C.M.R. developed the approach, were Principal Investigators (PIs) on the initial St Jude and Baylor protocols, analyzed the data, and wrote the manuscript; C.M.R. supervised production of cell lines and supervised correlative studies; H.E.H. supervised clinical conduct of the studies and supervised correlative studies; K.S.S. and J.L.H. were PIs of the second St Jude protocol, supervised CTL manufacture and study conduct for that study, and critically reviewed the manuscript; M.A.P., A.R., and C.M.B. performed follow-up studies on patients who received gene-marked products; C.A.S. established CTL generation in the GMP facility and manufactured the first 39 lines; G.A.H. and R.J.R. provided long-term follow-up of St Jude patients; H.L. and M.F.W. performed biostatistical analyses; and P.J.A. was the PI of the Great Ormond St protocol.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helen E. Heslop, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1620, Houston, TX 77030; e-mail: hheslop@bcm.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal