Abstract
The balance between survival and death in many cell types is regulated by small changes in the intracellular content of bioactive sphingolipids. Enzymes that either produce or degrade these sphingolipids control this equilibrium. The findings here described indicate that the lysosomal galactocerebrosidase (GALC) enzyme, defective in globoid cell leukodystrophy, is involved in the maintenance of a functional hematopoietic stem/progenitor cell (HSPC) niche by contributing to the control of the intracellular content of key sphingolipids. Indeed, we show that both insufficient and supraphysiologic GALC activity—by inherited genetic deficiency or forced gene expression in patients' cells and in the disease model—induce alterations of the intracellular content of the bioactive GALC downstream products ceramide and sphingosine, and thus affect HSPC survival and function and the functionality of the stem cell niche. Therefore, GALC and, possibly, other enzymes for the maintenance of niche functionality and health tightly control the concentration of these sphingolipids within HSPCs.
Introduction
The lysosomal galactocerebrosidase (GALC) enzyme, whose inherited deficiency results in the lysosomal storage disorder (LSD) globoid cell leukodystrophy (GLD), catalyzes the hydrolysis of galactose from several glycosphingolipids, including galactosylceramide (Gal-Cer) and galactosylsphingosine (psychosine; Psy), which are important for myelination in the nervous system. In GLD, pathology is characterized by accumulation of nonmetabolized substrates leading to widespread demyelination and patients' early death1 in the absence of overt abnormalities affecting the hematopoietic and immune systems. Alterations in the immune system have been recently described in the Twitcher mouse, a naturally occurring Galc mutant carrying a stop codon that abrogates enzyme activity. This defect was attributed to a progressive autonomic denervation of the lymphoid organs of affected mice.2 The sympathetic nervous system also regulates hematopoietic stem cell (HSC) egress from the bone marrow (BM) niche upon granulocyte colony-stimulating factor (GCSF) stimulation, as shown in mice defective in galactosyltransferase (CGT),3 an enzyme that synthesizes Gal-Cer by the addition of UDP-galactose to ceramide (Cer). Interestingly, these 2 lipids, as well as other molecules found in the GALC metabolic pathway, such as sphingosine (Sph) and sphingosine-1-phosphate (S1P), have been reported to regulate cell growth, differentiation, senescence, and apoptosis in several cell types and are comprised among “bioactive sphingolipids,”4 small quantitative changes of which can result in functional consequences in most cell types.
Compared with the other lysosomal enzymes, which are constitutively expressed in most tissues, GALC protein is found at low levels in all tested tissue samples.5 When the human GALC cDNA was cloned, very low GALC expression was obtained following transfection.6 Moreover, a suboptimal initiation start site and inhibitory sequences in the 5′ regulatory region characterize the human GALC gene.7,8 Overall, these features suggest the need for tight physiologic regulation of GALC expression and activity. We obtained indication of a requirement for such a regulation of GALC expression within the hematopoietic stem/progenitor cell (HSPC) compartment in the context of gene therapy studies (B.G., I.V., H. Hiramatsu, E. Lechman, S.U., A. Giustacchini, G. Schira, M. Amendola, S.M., A.O., J. E. Dick, L.N., and A.B., Identification of HSC-specific miRNAs enables tight regulation of gene expression and effective gene therapy of globoid leukodystrophy, manuscript submitted).
We here investigate the biochemical and functional consequences of alterations in the physiologic GALC expression levels in HSPCs and show that both insufficient and supraphysiologic GALC activity is associated to an altered content of bioactive sphingolipids and to functional abnormalities affecting HSPCs and their niche. Thus, these data suggest a novel role for GALC in the maintenance of a healthy hematopoietic niche. Regulation of the intracellular content of bioactive sphingolipids such as Cer may represent the mechanism by which the enzyme exerts this activity.
Methods
Flow cytometric analysis
For immunostaining, cells were blocked in phosphate-buffered saline (PBS), 5% rat serum, 2% fetal bovine serum (FBS) for 15 minutes at 4°C, stained with specific antibody for 20 minutes at 4°C, and washed. Cells were resuspended in PBS 2% FBS and read on a Becton Dickinson next generation flow cytometer equipped with 3 lasers (Canto II; BD Biosciences). Fluorochrome compensation was performed manually based on single-color marked samples and/or compensation beads (BD Biosciences) when appropriate. DIVA software was used for acquisition (BD Biosciences), and data files were exported in the FCS 3.0 format. FlowJo version 8.5 software (TreeStar) was used for the analysis. All gates were set based on specific fluorescence minus one (FMO) controls. The gating strategy is described in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Lentiviral vector production and titration
Vesicular stomatitis virus (VSV), pseudotyped lentiviral vectors (LV) were produced by transient cotransfection of the transfer constructs (pRRLsin.cPPT.humanPosphoGlycerateKinase.murineGalc.Wpre [PGK.Galc]; pRRLsin.cPPT.humanPGK.GreenFluorescentProtein.Wpre [PGK.GFP]; pRRLsin.cPPT.humanPGK.humanArylsulfataseA.Wpre [PGK.ARSA]; pRRLsin.cPPT.murineGalc.minimalCitomegaloVirus-PGK.9 humanAcidCeramidase.Wpre [Galc&AC]; pRRLsin.cPPT.humanGALC.murineGalc.Wpre [GALC.Galc]; pRRLsin.cPPT.humanPGK.DeletedNerveGrowthFactorReceptor.Wpre [PGK.DeltaNGFR]) and the packaging constructs in 293T cells and titrated by end point integration in HeLa cells.10
Mouse studies
Procedures were performed according to protocols approved by the Animal Care and Use Committee of the Fondazione San Raffaele (IACUC #325) and communicated to the Ministry of Health and local authorities. Mice genotyping was performed on DNA from tail biopsies as described.11
Isolation, transduction, and transplantation of murine hematopoietic cells
Five-week-old mice were killed with CO2, and the bone marrow (BM) was harvested by flushing the femurs and tibias. Different populations were isolated and transplanted into 8-day-old lethally irradiated (900 rad) mice: (1) total BM to be transplanted as a whole (2 × 106 cells/mouse); (2) HSPCs, which were purified by lineage negative (lineage−) selection using the Stem Sep Separation kit (StemCell Technologies Inc.) and transplanted intraperitoneally (1 × 106 cells/mouse) or intravenously (5 × 105 cells/mouse); (3) c-Kit+, Lineage−, Sca1+ (KLS) cells, which were isolated as c-Kit+, Sca1+ cells from lineage− isolated HSPCs by Cell Activated Sorting (Vantage Diva apparatus; Becton Dickinson). 5 × 104 KLS cells/mouse were transplanted intravenously; and (4) Sca1+ cells were depleted from whole BM by Sca1+ selection kit (MACS; Miltenyi Biotec) and the negative fraction was collected. Aliquots of 2 × 107 Sca1− cells/mouse were transplanted.
All these hematopoietic populations (except for Sca1− cells) were tracked in vivo by green fluorescent protein (GFP) expression. HSPCs were transduced with PGK.GFP LV at multiplicity of infection (MOI) 100 in the absence of serum and with cytokines (100 ng/mL murine stem cell factor [SCF], 100 ng/mL human FMS-like tyrosine kinase 3 ligand [hFlt3L], 100 ng/mL mouse interleukin 3 [IL-3], 200 ng/mL human IL-6) for 12 hours, as described.12 Untransplanted cells were kept in culture as described.12 Total BM, HSPCs, and KLS cells were retrieved from GFP transgenic mice, which were generated as described9 and screened for GFP expression in peripheral blood mononuclear cells by fluorescence-activated cell sorting (FACS) analysis before use; only animals showing GFP expression in at least 90% of the cells were used.
Engraftment was analyzed on peripheral blood and/or bone marrow by FACS analysis (as percentage of GFP+ cells) and/or by quantitative polymerase chain reaction (PCR) at +120 days after transplantation (and +20, in selected cases) or, in case of earlier death of transplanted animals, at mouse death.
Isolation and transduction of human HSPCs
Human HSPCs were isolated by positive selection of CD34-expressing cells (CD34 progenitor cell isolation kit; MACS) from cord blood (CB) or GLD BM (as approved by the Ethical Committee of the Fondazione San Raffaele and of the San Gerardo Hospital, Monza, and collection upon informed consent in accordance with the Declaration of Helsinki) and transduced with LV at MOI 100 as described.13 BM HSPCs were transduced using a different cytokine cocktail (IL-3 60 ng/μL, thrombopoietin [TPO] 100 ng/μL, SCF 300 ng/μL, Flt3-L 300 ng/μL) in CellGro medium (Cell Genics).
Homing assay
Lineage− HSPCs were isolated from 4-week-old mice and transduced with PGK.GFP LV, as described above. After transduction, cells were washed and transplanted into 8-day-old lethally irradiated mice. Mice were euthanized 20 hours after transplantation, and BM was harvested for cytofluorimetric analysis.
Competitive repopulation assay
Lineage− HSPCs were isolated from 4-week-old CD45.2 Twitcher mice and from CD45.1 wild-type donors. After overnight transduction with PGK.GFP LV (see above for transduction protocol), cells were washed, mixed in 1:1 proportion and transplanted intravenously (1.6 × 105 total cells/mouse for the low dose group or 3 × 105 total cells/mouse for the high dose group) in 2-month-old lethally irradiated CD45.1 recipients. Engraftment was analyzed on peripheral blood by FACS analysis (CD45 miss-match and GFP expression) at 8 and 16 weeks after the transplant.
GALC activity
GALC activity was determined as described.14
Quantitative PCR
Genomic DNA was extracted from liquid culture samples with QIAamp DNA Blood Mini kit (QIAGEN). Quantitative PCR analysis was performed on at least 50 ng of total genomic DNA as described.13,15 The number of LV copies per cell (vector copy number [VCN]) was calculated by the following equation: (ng LV/ng endogenous DNA) × (n° of LV integrations in the standard curve).
Colony-forming cell assay
After transduction, HSPCs were washed, counted, and seeded at a density of 1000 cells/mL (human) or 5000 cells/mL (murine) in semisolid medium (MethoCult). After 10 days, colonies were scored and counted.
TUNEL assay
HSPCs were washed twice with PBS, stained with TUNEL In Situ Cell Death Detection kit, TMR red (Roche), and analyzed by confocal microscopy.
Antiapoptotic treatment
HSPCs, washed after LV transduction, were treated in vitro with insulin-like growth factor 1 (IGF1) 50 ng/mL in RPMI without serum at room temperature (RT) for 40 minutes.
Immunofluorescence
Cells were seeded on Matrigel-coated coverslips, fixed with PBS 4% paraformaldehyde, blocked with PBS 1% bovine serum albumin (BSA), 5% goat serum for 1 hour at RT, and incubated with the primary antibody in PBS 10% goat serum, 0.3% Triton for 2 hours at RT or overnight at 4°C (Ceramide, Alexis Biochemicals; and Lamp1, Abcam). After washing, samples were incubated for 1 hour at RT with the conjugated secondary antibody (Alexa Fluor; Molecular Probes) 1:500 in PBS 0.1% Triton. Nuclei were labeled with ToProIII (Molecular Probes).
Sphingolipid analysis
Quantification of sphingolipids was conducted by liquid chromatography, electrospray tandem mass spectrometry as described.16
Culture and transduction of U937 cells
U937 cells were kept in culture in RPMI medium 10% FBS (Hyclone), penicillin 100 U/mL, and streptomycin 100 μg/mL, l-glutamine 2 mM. Transduction was performed at MOI 50 and 100 for 12 hours.
Western blot analysis
Western blot analysis was performed as described17 using the following primary antibodies: anti-Cathepsin D, anti-PAR4, and anti-b-actin (Santa Cruz Biotechnology), anti-Caspase-9, and anti-Caspase-3 (Cell Signaling Technology). Immunostaining was performed using the enhanced chemiluminescence (ECL) kit (GE Healthcare, United Kingdom).
Annexin V staining
To detect early apoptotic cells the annexin V–PE apoptosis detection kit (Becton Dickinson) was used. After staining with specific antibodies for surface markers, cells were washed with PBS and resuspended in Binding Buffer at the concentration of 106 cells/mL and labeled following manufacturer's protocol. 7-Amino-actinomycin D (7-AAD) was also added to the cells to exclude from the analysis dead cells. Samples were analyzed by flow cytometry (Canto II) within 1 hour, and results were analyzed by the Flow-Jo 8.3 software.
Hoechst 33 342 proliferation assay
After staining with specific antibodies for surface markers, cells were washed with PBS and incubated in the presence of 5 μg/mL Hoescht 33 342 for 20 minutes at 37°C in the dark. 7-AAD was then added, and samples were analyzed using Canto II.
Statistical analysis
Analyses were made by 1-way analysis of variance (ANOVA) for repeated measurements using Bonferroni's test for post-hoc analysis and by unpaired t test (confidence interval 95%).
Results
GALC deficiency affects the compositions of the hematopoietic stem and progenitor cell compartment
To assess whether GALC deficiency could account for defects in hematopoiesis, we studied the BM of the Galc natural mutant Twitcher mouse in the FVB background (FVB/Twi).18 Differently from what was observed in the thymus, spleen, and liver,19 the BM cellular content was equal between Galc mutant (−/−) and wild-type (+/+) animals (not shown). However, KLS cells, which contain HSC activity in mice, were significantly reduced in frequency and absolute counts in −/− as respect to +/+ controls (Figure 1A); further, within mutant KLS, multipotent progenitors type 1 (MPP1) and lineage restricted/granulocyte-monocyte-lymphocyte progenitors (GMLP) were less abundant than in unaffected controls (Figure 1B), as analyzed by multicolor cytofluorimetry.20,21 The characterization of committed progenitors (Figure 1C) and of more mature cells (Figure 1D-F) revealed a low frequency of granulocytes and an expansion of orthochromatic erythroblasts in −/− mice.
Phenotype and clonogenic potential of Galc−/− HSPCs. (A-F) Immunophenotypic characterization of the BM of Galc−/− mice. Frequency and absolute cell number (#) of c-kit+ Sca1+ cells within lineage− (Lin)− BM cells (KLS; A), and of long-term HSC (LT-HSC), MPP1, MPP2, and GMLP (within the KLS fraction) of −/− and +/+ mice (B). Frequency of lymphoid and myeloid progenitors within Lin− cells (C) of wild-type and mutant mice. Lymphoid (D), erythroid (E), and myeloid (F) differentiation in −/− and +/+ BM. Each dot represents a pool of 3 mice in panels A through C and a single mouse in panels D through F; means are shown. For details on the gating strategy, please see supplemental Table 1. (G-H) Frequency of proliferating cells at Hoechst staining (G) and of apoptotic annexin V positive cells (H) within KLS, LT-HSC, MPP1, MPP2, and GMLP from +/+ and −/− mice. Each dot represents a pool of 3 mice; means are shown. (I-J) Number (#) of colonies retrieved from colony-forming cell assays performed with murine (I) and human (J) HSPCs from wild-type (Galc+/+ and Arsa+/+), Galc−/− and Arsa −/− mice, and from normal donors' (nd) and GLD/MLD patients' BM. Before plating, murine HSPCs were (w) or were not (w/o) prestimulated with a standard cytokine cocktail for 12 hours.12 n ≥ 10 (murine) and n ≥ 3 (human). *P < .05, **P < .01 on 1-way ANOVA.
Phenotype and clonogenic potential of Galc−/− HSPCs. (A-F) Immunophenotypic characterization of the BM of Galc−/− mice. Frequency and absolute cell number (#) of c-kit+ Sca1+ cells within lineage− (Lin)− BM cells (KLS; A), and of long-term HSC (LT-HSC), MPP1, MPP2, and GMLP (within the KLS fraction) of −/− and +/+ mice (B). Frequency of lymphoid and myeloid progenitors within Lin− cells (C) of wild-type and mutant mice. Lymphoid (D), erythroid (E), and myeloid (F) differentiation in −/− and +/+ BM. Each dot represents a pool of 3 mice in panels A through C and a single mouse in panels D through F; means are shown. For details on the gating strategy, please see supplemental Table 1. (G-H) Frequency of proliferating cells at Hoechst staining (G) and of apoptotic annexin V positive cells (H) within KLS, LT-HSC, MPP1, MPP2, and GMLP from +/+ and −/− mice. Each dot represents a pool of 3 mice; means are shown. (I-J) Number (#) of colonies retrieved from colony-forming cell assays performed with murine (I) and human (J) HSPCs from wild-type (Galc+/+ and Arsa+/+), Galc−/− and Arsa −/− mice, and from normal donors' (nd) and GLD/MLD patients' BM. Before plating, murine HSPCs were (w) or were not (w/o) prestimulated with a standard cytokine cocktail for 12 hours.12 n ≥ 10 (murine) and n ≥ 3 (human). *P < .05, **P < .01 on 1-way ANOVA.
Having observed a reduction in the frequency of mutant KLS cells, and in particular of the more committed fractions within this population, we wanted to assess whether these cells exhibited an altered proliferation rate or were more prone to apoptosis compared with their +/+ counterpart. Interestingly, cytofluorimetric analysis using the Hoechst 33 342 dye, which binds DNA during cells division, demonstrated that bona fide CD150+ CD48− long-term HSC (LT-HSC, the more immature KLS fraction) retrieved from Galc−/− mice proliferated less than their wild-type counterpart (Figure 1G). Conversely, no increase in the frequency of annexin V+ cells was observed in any of the −/− fractions analyzed, and rather a lower incidence of apoptosis in −/− LT-HSC compared with +/+ cells was noticed (Figure 1H). These 2 observations indicate that the reduced frequency of KLS in the BM of mutant mice might be due to a below-normal proliferation of early progenitors, resulting in a defect in the frequency and overall number of multipotent progenitors.
Interestingly, and in agreement with these findings, when clonogenic assays were performed on mutant HSPCs retrieved from the BM of GLD mice, a significantly lower number of colonies was obtained from Galc−/− compared with +/+ HSPCs, both in the case of cytokine prestimulation and of its absence (Figure 1I), with a normal proportion of erythroid versus myeloid colonies (not shown). A similar clonogenic impairment was also detected in human CD34+ isolated from GLD patients (Figure 1J). HSPCs obtained from mice and patients affected by metachromatic leukodystrophy (MLD), a LSD caused by the deficiency of the ARSA enzyme that acts upstream GALC in the sulfatide catabolic pathway, were instead intact in their clonogenic potential (Figure 1I-J).
GALC deficiency results in HSPC functional impairment
To unravel whether these alterations in the composition of −/− stem and progenitor cells could have functional consequences, we performed in vivo functional studies (Figure 2A). Galc−/− and +/+ murine lineage− HSPCs were efficiently transduced with GFP-encoding LV (PGK.GFP LV; 95% ± 4% GFP+ cells) for donor cell tracking (Figure 2B). The transduced cells were then transplanted intraperitoneally into heterozygous (+/−) or Galc−/−, 8-day-old, lethally irradiated syngeneic recipients. The short-term homing of the transplanted cells to the BM (within 20 hours from transplant) was similar in all the transplant pairs (Figure 2C). In the longer term, however, death due to myeloablation (in between +14 and +21 days posttransplantation, thus before 28 postnatal days, similar to what observed in irradiation controls; data not shown) and elevated frequency of engraftment failure (defined as death at ≤ 21 days postirradiation, and/or ≤ 5% donor cells in the BM at death or at +120 postnatal days) were observed in −/− animals receiving Galc−/− HSPCs (Figure 2D-F). Heterozygous mice transplanted with Galc−/− cells survived long-term, but in the presence of autologous reconstitution (namely in the presence of ≤ 5% of GFP+ cells in the BM, being the donor cells GFP+ at a frequency equal to 95% ± 4%; Figure 2D-F). Conversely, transplantation of +/+ HSPCs into both Galc−/− and wild-type recipients resulted in survival after myeloablation, low engraftment failure frequency, and good donor chimerism (Figure 2D-F), suggesting that Galc−/− HSPCs are defective in their repopulation ability compared with Galc+/+ cells. Interestingly, the lower donor chimerism observed when wild-type HSPCs were transplanted into Galc−/− recipients as respect to +/− mice suggests that in Galc−/− mice a niche defect could also be present.
In vivo functional characterization of Galc−/− HSPCs. (A) Experimental scheme for panels D through F. (B) Representative cytofluorimetric dot plots of GFP-transduced HSPCs and their negative control. (C) Frequency of GFP positive cells in the BM of FVB/Twi Galc−/− and +/+ recipients 20 hours after the transplantation of GFP+ Galc−/− and +/+ HSPCs (n = 4 for each group). (D) Survival curve of Galc−/− and +/− recipient mice (rec.) after the intraperitoneal transplantation of Galc +/+ or −/− HSPCs. (E-F) Engraftment failure frequency (E; defined as percent of animals showing a survival less than or equal to 21 days postirradiation, and/or less than or equal to 1% donor cells in the BM at death or at 120 postnatal days) and donor chimerism (% of GFP+ cell in the BM) at 120 days after transplantation or at mice death (F), measured in the same experimental groups as in panel D. Average values ± SD are reported. (G) Experimental scheme for competitive transplantation experiments (H-I). (H) Engraftment of Galc+/+ CD45.1+ and of Galc−/− CD45.2+ cells in peripheral blood of wild-type CD45.1 mice transplanted with HSPCs measured at 8 and 16 weeks (ws) after the transplantation (high or the low dose, see text for details; n = 6 for each group). Average values ± SD are reported. (I) Representative plots from cytofluorimetric analysis showing engraftment of CD45.1 and CD45.2 cells on peripheral blood of mice receiving competitive transplantation and GFP expression in each of the 2 populations.
In vivo functional characterization of Galc−/− HSPCs. (A) Experimental scheme for panels D through F. (B) Representative cytofluorimetric dot plots of GFP-transduced HSPCs and their negative control. (C) Frequency of GFP positive cells in the BM of FVB/Twi Galc−/− and +/+ recipients 20 hours after the transplantation of GFP+ Galc−/− and +/+ HSPCs (n = 4 for each group). (D) Survival curve of Galc−/− and +/− recipient mice (rec.) after the intraperitoneal transplantation of Galc +/+ or −/− HSPCs. (E-F) Engraftment failure frequency (E; defined as percent of animals showing a survival less than or equal to 21 days postirradiation, and/or less than or equal to 1% donor cells in the BM at death or at 120 postnatal days) and donor chimerism (% of GFP+ cell in the BM) at 120 days after transplantation or at mice death (F), measured in the same experimental groups as in panel D. Average values ± SD are reported. (G) Experimental scheme for competitive transplantation experiments (H-I). (H) Engraftment of Galc+/+ CD45.1+ and of Galc−/− CD45.2+ cells in peripheral blood of wild-type CD45.1 mice transplanted with HSPCs measured at 8 and 16 weeks (ws) after the transplantation (high or the low dose, see text for details; n = 6 for each group). Average values ± SD are reported. (I) Representative plots from cytofluorimetric analysis showing engraftment of CD45.1 and CD45.2 cells on peripheral blood of mice receiving competitive transplantation and GFP expression in each of the 2 populations.
To confirm the functional defect observed in Galc−/− HSPCs, competitive repopulation experiments were performed in a congenic reporter setting. Galc−/− HSPCs retrieved from Twitcher mice (in C57Bl/6 background and expressing the CD45.2 antigen on cell surface) and HSPCs from wild-type donors carrying the CD45.1 allele were transduced with the PGK.GFP LV at high efficiency and then transplanted into lethally irradiated CD45.1 adult recipients by intravenous administration at 1:1 ratio; 2 different cell doses were used (1.6 and 3 × 105 total cells; Figure 2G). Frequency of the CD45.1+ and CD45.2+ cells and GFP expression within each subset were measured 8 and 16 weeks after the transplantation on peripheral blood mononuclear cells. Interestingly, a significantly lower frequency of Galc−/− CD45.2 cells as respect to Galc+/+ CD45.1 cells was observed at both cell doses, with much lower CD45.2 cell engraftment at the lowest dose (Figure 2H). Of note, the frequency of GFP+ cells within each of the 2 antigen miss-matched populations was similar and above 95%, confirming that lethal myeloablation occurred after irradiation and that the detected CD45.1 cells were of donor origin (Figure 2I).
GALC deficiency causes a microenvironmental defect that reduces the repopulating activity of wild-type HSPCs
To further assess the functional integrity of the BM hematopoietic niche of GLD mice, different GFP+ hematopoietic populations from Galc+/+ donors were transplanted into lethally irradiated 8-day-old Galc−/− syngeneic animals (Figure 3A,D). The different populations to be transplanted were distinguished according to a progressive enrichment of the stem cell fraction and to depletion of committed cells (Figure 3A,D). In particular, the following populations were used: (1) total BM from GFP transgenic mice (generated by LV transgenesis using the PGK.GFP LV used for HSPC transduction, and having > 90% of their hematopoietic cells expressing GFP); (2) lineage− HSPCs from GFP transgenic mice plus the Sca1− fraction of the BM of wild-type syngeneic mice (GFP negative); (3) HSPCs from GFP transgenic mice; and (4) KLS from the same GFP transgenic animals. These populations were administered either intraperitoneally or intravenously, as indicated in Figure 3A,D. FVB/Twi mice were also transplanted intravenously to favor homing of the cells to the BM, whereas Twitcher mice could only be transplanted intraperitoneally.
Functional characterization of the Galc−/− niche. (A,D) Experimental schemes. (B,E) Survival curves of Galc−/− mice (FVB/Twi in panel B and Twi in panel E) after the intravenous or intraperitoneal transplantation of GFP+Galc+/+ HSPC or KLS or total BM (TBM) or HSPC plus GFP− Sca1− BM cells, as indicated. KLS intravenous (WT): KLS transplanted intravenously in wild-type recipients. n ≥ 3 in each group. (C,F) Engraftment failure frequency and donor chimerism measured in the same experimental groups as in panels B and E. Average values ± SD are reported. *P < .05, **P < .01 on 1-way ANOVA.
Functional characterization of the Galc−/− niche. (A,D) Experimental schemes. (B,E) Survival curves of Galc−/− mice (FVB/Twi in panel B and Twi in panel E) after the intravenous or intraperitoneal transplantation of GFP+Galc+/+ HSPC or KLS or total BM (TBM) or HSPC plus GFP− Sca1− BM cells, as indicated. KLS intravenous (WT): KLS transplanted intravenously in wild-type recipients. n ≥ 3 in each group. (C,F) Engraftment failure frequency and donor chimerism measured in the same experimental groups as in panels B and E. Average values ± SD are reported. *P < .05, **P < .01 on 1-way ANOVA.
Prolonged survival above untreated controls, high chimerism of donor cells, and low frequency of engraftment failure were observed in FVB/Twi mice only upon intravenous transplantation of HSPCs (Figure 3B-C). KLS cells transplanted intravenously and HSPCs transplanted intraperitoneally gave rise to engraftment failure at high frequency and to low donor chimerism (with autologous reconstitution), resulting in early death likely due to myeloablation in the first case and death around 50 days due to the disease in the latter (Figure 3B-C). KLS were unable to repopulate Twitcher mice (not shown), in which sustained donor chimerism and long-term survival were obtained only upon transplantation of populations also comprising committed progenitors (Figure 3E-F). Importantly, all tested Galc+/+ populations repopulated with equal efficiency Galc+/+ (Figure 3B-C for KLS cells and Figure 2D-F for HSPCs) and Arsa−/− mice (data not shown, please refer to our previous studies12,15 ), indicating that the BM of Galc−/− mice is poorly permissive to syngeneic HSPC engraftment.
Physiologic, but not supranormal GALC expression restores HSPC function
To assess whether restoration of GALC activity in HSPCs by means of gene transfer could rescue the functional defect of Galc−/− cells, HSPCs from +/+ and −/− mice were transduced with LV encoding the murine Galc cDNA under the control of either the human GALC promoter (GALC.Galc LV; kindly provided by Dr. P. Luzi) or the human PGK promoter (PGK.Galc LV; Figure 4A-B). ARSA was chosen as control lysosomal enzyme that does not affect HSPC function.13 Upon similar efficient transduction (measured as number of LV copies integrated per cell, VCN), partial reconstitution of GALC activity was observed in the culture progeny of −/− HSPCs transduced with GALC.Galc LV (35% of wild-type values), while enzyme supraphysiologic expression was measured in the progeny of both −/− and +/+ cells transduced with the PGK.Galc LV (up to approximately 2-fold the values of +/+ mock transduced cells; Figure 4B). Interestingly, −/− HSPCs expressing Galc from the GALC promoter showed restoration of a normal clonogenic potential compared with mock-transduced +/+ and −/− cells (Figure 4C). Conversely, GALC overexpression driven by the PGK promoter caused a significant impairment of the clonogenic potential of both Galc−/− and +/+ cells compared with ARSA transduced, genotypically identical cells (Figure 4C). The functional impairment of PGK.Galc transduced −/− and +/+ HSPCs was confirmed in vivo upon intraperitoneal transplantation into −/− and +/− FVB/Twi recipients (Figure 4D-G) and was likely due to the widespread occurrence of apoptosis of the GALC overexpressing cells (Figure 4H). Comparable results were obtained upon PGK.Galc transduction and transplantation of Galc−/− HSPCs in another GLD mouse model that, differently from the knock outs FVB/Twi and Twitcher mice, is characterized by residual GALC activity (Trs mice,22 and as reported in B.G., I.V., H. Hiramatsu, E. Lechman, S.U., A. Giustacchini, G. Schira, M. Amendola, S.M., A.O., J. E. Dick, L.N., and A.B., Identification of HSC-specific miRNAs enables tight regulation of gene expression and effective gene therapy of globoid leukodystrophy, manuscript submitted). Conversely, moderate enzyme expression corrected the functional deficit of mutant HSPCs also in vivo. Indeed, mutant HSPCs transduced with the GALC.Galc LV efficiently engrafted in the BM of +/− FVB/Twi mice (Figure 4D-G). Thus, while GALC expression driven by its own promoter restores a normal function of mutant HSPCs, enzyme supraphysiologic expression further worsens the functional impairment of −/− HSPCs, causes a functional impairment of normal cells and apoptotic cell death of both cell types. Similar findings were also obtained on CD34+ HSPCs obtained from GLD BM and normal donors' CB (Figure 4I and B.G., I.V., et al, manuscript submitted).
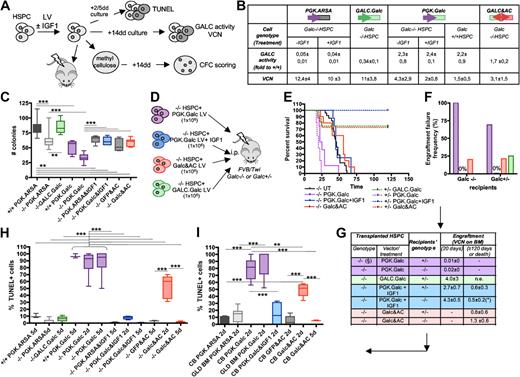
Characterization of the diverse phenotypes associated to alterations of GALC expression in murine and human HSPCs. (A,D) Experimental schemes. (B) GALC activity (measured as fold to mock-transduced Galc+/+ cells) and LV copy number (VCN) measured on the in vitro progeny of murine Galc−/− and +/+ HSPCs transduced with the indicated LV and exposed or not to IGF1 after transduction. n ≥ 3 in each group. (C) Number (#) of colonies retrieved from colony-forming cell assays performed with murine HSPCs obtained from Galc−/− and +/+ mice after transduction with the specified LV and exposed to IGF1 after transduction, if indicated. n ≥ 3 in each group. (E) Survival curves of FVB/Twi Galc−/− and +/− mice after the intraperitoneal transplantation of Galc−/− HSPCs transduced with the indicated vectors and exposed to IGF1 after transduction, if indicated. n ≥ 3 in each group. (F-G) Engraftment failure frequency (F) and transduced cell engraftment (VCN in the BM; G) at 20 and at 120 days after transplantation or at death as in (*), measured in the same experimental groups as in panel E. (§) Similar results were obtained with +/+HSPCs. (H-I) TUNEL assay performed on murine (H) and human (I) HSPCs obtained from Galc−/− and +/+ mice and from normal donors (nd) CB and GLD patients' BM, 2 and 5 days (d) after transduction with the specified LV and exposed to IGF1 after transduction, if indicated. Percentage of TUNEL+ nuclei over the total number of nucleated cells is reported (≥ 8 fields and ≥ 100 cells were counted per condition). n ≥ 5 individual experiments per condition, average values ± SD are reported. *P < .05, **P < .01, ***P < .001 on 1-way ANOVA.
Characterization of the diverse phenotypes associated to alterations of GALC expression in murine and human HSPCs. (A,D) Experimental schemes. (B) GALC activity (measured as fold to mock-transduced Galc+/+ cells) and LV copy number (VCN) measured on the in vitro progeny of murine Galc−/− and +/+ HSPCs transduced with the indicated LV and exposed or not to IGF1 after transduction. n ≥ 3 in each group. (C) Number (#) of colonies retrieved from colony-forming cell assays performed with murine HSPCs obtained from Galc−/− and +/+ mice after transduction with the specified LV and exposed to IGF1 after transduction, if indicated. n ≥ 3 in each group. (E) Survival curves of FVB/Twi Galc−/− and +/− mice after the intraperitoneal transplantation of Galc−/− HSPCs transduced with the indicated vectors and exposed to IGF1 after transduction, if indicated. n ≥ 3 in each group. (F-G) Engraftment failure frequency (F) and transduced cell engraftment (VCN in the BM; G) at 20 and at 120 days after transplantation or at death as in (*), measured in the same experimental groups as in panel E. (§) Similar results were obtained with +/+HSPCs. (H-I) TUNEL assay performed on murine (H) and human (I) HSPCs obtained from Galc−/− and +/+ mice and from normal donors (nd) CB and GLD patients' BM, 2 and 5 days (d) after transduction with the specified LV and exposed to IGF1 after transduction, if indicated. Percentage of TUNEL+ nuclei over the total number of nucleated cells is reported (≥ 8 fields and ≥ 100 cells were counted per condition). n ≥ 5 individual experiments per condition, average values ± SD are reported. *P < .05, **P < .01, ***P < .001 on 1-way ANOVA.
IGF1 rescues GALC overexpressing HSPCs from apoptosis and functional impairment
Several studies have implicated IGF1 in preventing cell death due to the activation of the phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway,23 which is also required for the survival of hematopoietic cells.24 Cer acts on this same pathway by inhibiting the phosphorylation of Akt and Erk1/Erk2.25,26 After transduction with PGK.Galc or PGK.ARSA LV, Galc−/− murine HSPCs and CB CD34+ cells were briefly (40 minutes) incubated with IGF1. The treatment rescued PGK.Galc-transduced HSPCs from toxicity; a significant increase in the number of the colonies (Figure 4C) and protection of GALC overexpressing cells from apoptosis (Figure 4H-I) were observed. When Galc−/− HSPCs transduced with PGK.Galc and treated with IGF1 were transplanted into irradiated Galc+/− and −/− mice, they were able to prevent recipient animals from early death and to engraft at sustained levels (Figure 3D-G), confirming that apoptotic cell death of GALC overexpressing HSPCs was responsible for the observed functional impairment. In the long-term, however, transduced cells were no longer detectable in the BM of the recipients, which died due to GLD manifestations (−/− mice) or survived long-term due to reconstitution by donor nontransduced or autologous cells (+/− mice; Figure 4E-G), because of a likely exhaustion of the effect of the brief in vitro treatment with IGF1, which was thus unable to protect the transduced HSPCs from GALC toxicity long term.
Functional impairment is associated to variations in the intracellular content of bioactive GALC products
An intracellular sphingolipid profile was then performed on control vector and PGK.Galc-transduced murine HSPCs before and at different intervals from gene transfer. Sphingomyelin (SM), Cer, Sph, S1P, Psy, and sulfatides (Sulf) were detected in +/+ and −/− cells (Figure 5A). SM was the most abundant sphingolipid, and the C:16 fractions of SM and Cer were the most represented among the measured isoforms. Gal-Cer was poorly detectable, being in the range of 0 to 2 pmol/106 cells.
Sphingolipid profile of murine HSPCs. (A) Total sphingolipid content (pmol/106 cells) of Galc−/− and +/+ murine HSPCs. Each dot represents an individual sample; means are shown. (B) Intracellular content of C:16 Cer (left panel), Sph (middle panel), and S1P (right panel), in GALC or GFP LV transduced −/− HSPCs at 12 to 24 hours (h), 2 and 7 days (d) after gene transfer (n ≥ 3). *P < .05, **P < .01, ***P < .001 on 1-way ANOVA. (C) Representative images obtained after immunofluorescence staining for ceramide and for the lysosomal marker Lamp1 performed on human monocytes (U937 cells) transduced with GALC and control DeltaNGFR (DNGFR) LV 2 and 7 days after transduction. Magnification 100× in the 2 top rows and 200× in the bottom row.
Sphingolipid profile of murine HSPCs. (A) Total sphingolipid content (pmol/106 cells) of Galc−/− and +/+ murine HSPCs. Each dot represents an individual sample; means are shown. (B) Intracellular content of C:16 Cer (left panel), Sph (middle panel), and S1P (right panel), in GALC or GFP LV transduced −/− HSPCs at 12 to 24 hours (h), 2 and 7 days (d) after gene transfer (n ≥ 3). *P < .05, **P < .01, ***P < .001 on 1-way ANOVA. (C) Representative images obtained after immunofluorescence staining for ceramide and for the lysosomal marker Lamp1 performed on human monocytes (U937 cells) transduced with GALC and control DeltaNGFR (DNGFR) LV 2 and 7 days after transduction. Magnification 100× in the 2 top rows and 200× in the bottom row.
At the beginning of the culture, the intracellular content of Cer and Sph was significantly lower (approximately 60%) in Galc−/− as respect to +/+ HSPCs, suggesting that GALC-dependent metabolism could represent one of the pathways regulating intracellular levels of these sphingolipids in HSPCs. Moreover, a higher Psy intracellular content was observed in −/− HSPCs, despite not reaching statistical significance.
Two to 7 days after PGK.Galc transduction, we detected a progressive accumulation of Cer, Sph, and of S1P both in −/− and +/+ HSPCs, compared with basal levels and to GFP controls (up to 8- and 2-fold increase in respect to the values measured in freshly isolated −/− cells and GFP control cells at 7 days after transduction, respectively, in the case of C:16 Cer; Figure 5B and data not shown for +/+ cells). Further, Psy showed a tendency to decrease upon GALC gene transfer (not shown). Immunofluorescence confirmed the presence of Cer in hematopoietic cells (Figure 5C and data not shown). In GALC transduced cells, Cer signal colocalized more abundantly with the lysosomal marker Lamp1, compared with cells transduced with a control vector, suggesting that the intracellular Cer increase could be due to an increase of the lysosomally generated Cer.
Interestingly, when murine and human HSPCs were transduced with a bidirectional LV,9 which allowed the simultaneous expression of GALC (Figure 4B) and of the Cer-degrading enzyme acid ceramidase (GALC&AC LV), rescue from GALC toxicity was seen (Figure 4C,H-I). The progressive reduction of TUNEL+ cells by 2 to 5 days after gene transfer suggests an active and progressive degradation of accumulated Cer by acid ceramidase (Figure 4H-I). Importantly, when Galc−/− HSPCs transduced with the GALC&AC LV were transplanted in lethally irradiated +/− and −/− recipients, mice were rescued from early lethality in the presence of transduced cell engraftment (Figure 4D-G).
Discussion
Here we describe a defect in the frequency, proliferation, clonogenic potential, and engraftment capability of early hematopoietic progenitors retrieved from Galc mutant mice and a similar defect in the clonogenic ability of HSPCs from GLD patients. This defect was rescued by low-level reconstitution of GALC activity, while unregulated, supraphysiologic GALC expression rather worsened the preexisting defect and induced apoptotic death also of wild-type HSPCs. The 2 dysfunctional phenotypes were associated to alterations of the intracellular content of the bioactive sphingolipid Cer; functionally defective Galc−/− cells had a lower Cer content compared with normal cells, whereas enzyme overexpressing cells accumulated Cer, which localized within the lysosomal compartment. A similar pattern applies also to Sph. The hydrolysis of Cer upon coexpression, together with GALC, of acid ceramidase rescued HSPC from apoptosis and functional impairment, confirming the key role of this sphingolipid in affecting HSPC function and survival. In addition, we here also show a microenvironmental defect of the hematopoietic niche of Galc−/− animals that reduces the repopulating activity of wild-type HSPCs.
LSDs are genetically inherited diseases characterized by the accumulation of disease-specific metabolic intermediates within the lysosome. The importance of the lysosomal compartment to normal cell function is demonstrated by the various pathologies that arise in LSDs. These disorders are invariably fatal, and many display profound neurologic impairment. Recent studies have revealed that LSDs are also characterized by irregularities in the function of the immune system.27 Further, in some LSDs the practice of allogeneic HSC transplantation (HCT) revealed a high frequency of mixed donor chimerism and of engraftment failure.28 A recent report suggests that lymphomonocytes from GLD patients could have a basal proinflammatory pattern likely related to Psy accumulation,29 but no specific abnormalities of HSC have been investigated or described thus far in GLD patients. Moreover, the limited experience in HCT in GLD patients has not revealed low efficiency of donor cell engraftment.30
Most of the mutations of the human GALC gene are responsible for a reduction of enzyme activity but do not abrogate enzyme production. The residual, low enzyme activity present in most of the patients but not in Twitcher and FVB/Twi mice, which are natural knockouts, could be responsible for the lack of an overt phenotype in the patients. This could be consistent with the low level of GALC protein detected in tissues from normal subjects and the very strict therapeutic window for GALC activity shown by our gene transfer studies in HSPCs. Very tiny amounts of GALC are likely required and sufficient for the maintenance of a healthy hematopoietic niche. Otherwise, the very short life span and the neurologic symptoms, which prevail in the patients, could have masked this phenotype.
Our mass spectrometry data obtained in −/− cells, together with the functional studies using IGF1 and acid ceramidase indicate that Cer may play a central role in the events consequent to alterations of GALC activity within HSPCs and, possibly, their niche. Recently, a novel role of Cer and other sphingolipids in regulating cell survival, senescence, and apoptosis has been demonstrated.4 Cer can be considered a metabolic hub because it occupies a central position in sphingolipid biosynthesis and catabolism. Interestingly, our data suggest that GALC, beside other relevant pathways,31 may contribute to the regulation of the intracellular levels of lysosomally generated Cer in HSPCs. Similar variations of intracellular levels of Sph, which appears to act in a similar fashion as Cer, occurred. On the contrary, S1P, which increases in the transduced HSPCs but remains at very low concentration, appears to have opposite effects as Cer in many of the pathways in which it is involved, particularly in those relating to cell growth and survival. It has been suggested that the balance between survival and death in many cell types may be affected by the equilibrium between intracellular levels of each of these interconvertible sphingolipids. Enzymes that either produce or degrade the sphingolipids control this equilibrium. Thus, our findings suggest that an alteration of the intracellular content of Cer and, possibly, of other bioactive sphingolipids in HSPCs following either abrogation of GALC activity (because of a severe mutation in the gene) or gene transfer and supraphysiologic GALC expression, may substantially affect this balance. Indeed, the increase in Cer and Sph, despite being modest when considering levels detected in other tissues or cell types, might exceed intracellular antiapoptotic signals, like that of S1P, thus triggering apoptosis of HSPCs. On the contrary a reduction of Cer and Sph content in HSPC due to GALC deficiency could affect the survival and function of these cells and the tropism of the hematopoietic niche. The downstream molecular pathways activated by the imbalance of Cer, Sph, and S1P relative ratios in HSPCs are not yet completely understood (supplemental Figure 1).
Interestingly, differentiated hematopoietic cells are not affected by GALC overexpression (B.G., I.V., H. Hiramatsu, E. Lechman, S.U., A. Giustacchini, G. Schira, M. Amendola, S.M., A.O., J. E. Dick, L.N., and A.B., Identification of HSC-specific miRNAs enables tight regulation of gene expression and effective gene therapy of globoid leukodystrophy, manuscript submitted), suggesting a unique sensitivity of HSPCs to GALC- and sphingolipid-mediated control of cell survival. Further studies will elucidate the actual mechanisms responsible for this peculiar sensitivity.
The defect in the mutant hematopoietic stem cell niche here described could be interpreted in light of the previous reports in the Twitcher mouse thymus2 and in CGT mutant mice3 as a results of a defective sympathetic innervation of the niche. However, the role of the described spingolipid imbalances in contributing to the niche defect or of the altered niche in aggravating the cell-intrinsic defect of mutant HSPCs remains to be determined. Interestingly, recent literature has demonstrated that S1P regulates the SDF-1/CXCR4 axis and, consequently, the interaction of the HSPCs with their niche as well as critical events like HSPC chemotaxis, in vivo homing, and recirculation.32,33 Moreover, S1P mobilizes osteoclast precursors and regulates bone homeostasis.34,35 Interestingly, since in our experimental model GALC, besides Cer, is also critically affecting S1P concentration in HSPCs, we could hypothesize that GALC deficiency and overexpression affects the SDF-1/CXCR4 axis and/or the trophism of the endosteal niche.
Overall, our findings demonstrate that both GALC deficiency and forced GALC expression affect HSPC survival by altering a delicate intracellular bioactive sphingolipid balance, showing a possible novel regulatory role for this enzyme.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to A. Merrill for tandem mass spectrometry analyses; J. Medin for the acid ceramidase cDNA; P. Luzi and D. Wenger for the GALC promoter sequence; S. Marchesini, I. di Girolamo, and R. Tiribuzi for biochemical studies; and A. Rovelli for GLD patients' BM.
This research was supported by grants from the Italian Telethon to A.B. and L.N., the European Leukodystrophy Association and EU (FP7 LEUKOTREAT 241622) to A.B., the Italian Ministry of Health (PS NEURO ex 56-05-16) and the Cariplo Foundation (Cariplo NOBEL) to L.N., and the National Institutes of Health (GM069338) to A. Merrill.
National Institutes of Health
Authorship
Contribution: I.V. performed research and contributed to experiment design; S.U., S.M., H.P., M.C., and A.O. performed research and analyzed the data; B.G. provided protocols; L.S.S. provided reagents; L.N. interpreted the data; and A.B. designed experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandra Biffi, San Raffaele Telethon Institute for Gene Therapy, San Raffaele Scientific Institute, Via Olgettina 58, Milan, Italy; e-mail: biffi.alessandra@hsr.it.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal