Abstract
Abstract 1080
The role of arsenic trioxide (ATO) in the frontline treatment of patients with acute promyelocytic leukemia (APL) remains unclear with a number of studies reporting high and durable responses with single agent ATO. We have conducted a trial combining all-trans-retinoic acid (ATRA) with ATO with or without gemtuzumab ozogamicin (GO) in patients with previously untreated APL.
From July 2002 to June 2010, 104 patients with newly diagnosed APL were treated with a combination of ATRA plus ATO in two studies. The first cohort of 47 patients received ATRA (45 mg/m2 daily) and ATO (0.15 mg/kg daily beginning on day 10 of ATRA). High-risk patients (White blood cell count [WBC] > 10 × 109/L) received GO 9 mg/m2 on the first day of induction. From July 2007, the second cohort of 57 patients received ATRA (45 mg/m2 daily) and ATO (0.15 mg/kg daily) concomitantly on day one of induction. They also received GO 9 mg/m2 on day 1, if high risk, and any time during induction if the WBC rose to > 30 × 109/L (and more recently if > 10 × 109/L). Monitoring for PML-RARA fusion gene using reverse transcriptase-polymerase chain reaction (RT-PCR) was conducted after induction and throughout consolidation and follow up. The median age for the 104 patients was 46 years (range, 14–81). Their median presenting WBC was 2.7 × 109/L (0.4-131.4 × 109/L) and their median platelet count was 36 × 109/L (range, 7–261 × 109/L). Seventy three (70%) had low risk and 31 (30%) high risk disease (based only on the presentation WBC ≤ or > 10.0 × 109/L).
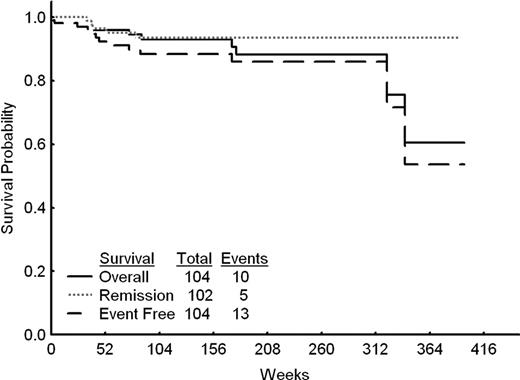
Overall, 102 patients (98%) achieved complete remission (CR) and 2 died during induction. With a median follow-up of 115 weeks (range, 4 to 397 weeks), 94 patients remain alive. The estimated 5-year survival rate was 88% and event-free survival 86%; only 5 of the patients achieving a CR (5%) have relapsed. The median overall survival, remission duration and event-free survival have not been reached (Figure). Thirty six patients have been alive and in remission for more than 3 years and 21 for more than 5 years. Two late deaths (beyond 300 weeks) occurred in CR from unrelated causes.
The combination of ATRA and ATO (with or without GO) as initial therapy for APL is highly effective and safe; it can potentially substitute for chemotherapy containing regimens in high and low risk patients.
Ravandi:Cephalon: Honoraria, Research Funding, Speakers Bureau. Off Label Use: Off-label use of arsenic trioxide in frontline therapy of APL; off label use of gemtuzumab ozogamicin in APL. Verstovsek:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal