Abstract
Abstract 3513
Based on donor versus no-donor studies, allogeneic stem cell transplantation (SCT) is the best treatment option for younger patients with intermediate- or adverse-risk AML in first CR (Cornelissen et al. Blood 2007; Koreth et al. JAMA 2009). However, in these intent-to-treat studies, the benefit associated with SCT seems often small in the adverse-risk subgroup, probably due to a higher incidence of early relapse. In addition, the definition of the donor group has evolved with the increasing use of alternative SC sources. To assess the role of current SCT procedures in adverse-risk patients specifically, we thus performed the present real-life transplant versus no-transplant analysis.
A total of 205 adults aged 50 years or less with adverse-risk AML in first CR (CR1) were included. All have reached CR1 after induction therapy within the ALFA-9000 (N=69; period 1990–1996) or ALFA-9802 (N=136; period 1999–2006) trial. Adverse-risk AML was defined as: 1) unfavorable cytogenetics, including 3q26 or 11q23 anomalies, del(7q)/-7, del(5q)/-5, and complex karyotypes (N=106); 2) initial WBC>100.109/L (N=72); 3) late CR1 achievement (N=47); and more recently 4) FLT3-ITD, after protocol amendment in 2002 (N=35). Patients with CBF-AML were excluded. Among these 205 eligible patients (median age, 38y), 102 (50%) received SCT, including 74 patients in CR1 (36%). In CR1 patients, the median time between CR and SCT (60 sibling and 14 unrelated donors, including 4 cord blood; 65 myeloablative and 9 reduced intensity [RIC] conditioning) was 88 days (5%-95% percentiles, 24–245). In patients allografted after relapse, the median time between relapse and SCT was 109 days (5%-95% percentiles, 8–205). Outcomes of transplanted versus non-transplanted patients were compared using Mantel-Byar estimates. The median follow-up was 7 years.
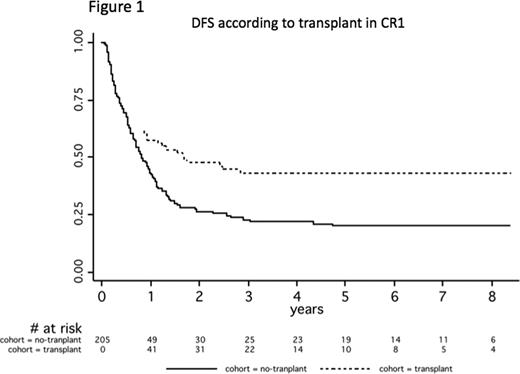
According to SCT in CR1, both transplant and no-transplant groups were comparable in terms of age, WBC, cytogenetics, and time to CR. Transplant rate in CR1 was higher in the latter 9802 trial (42% versus 25%, P=0.02), due to the increasing use of unrelated donors over time (24% versus 0%, P=0.03). Overall, Mantel-Byar estimates revealed a better outcome of patients transplanted in CR1 compared to those not transplanted in CR1. At 5 years, estimates were 43% (95%CI, 31–54) versus 20% (95%CI, 13–28) (Figure 1; P=0.05) and 41% (95%CI, 29–53) versus 26% (95%CI, 19–34) (P=0.17) for DFS and OS from CR in the transplant and no-transplant groups, respectively. Notably, SCT was associated with an impressive decrease of relapses (5-year cumulative incidence, 30% [95%CI, 21–43] versus 77% [95%CI, 69–84]; P<0.0001), attesting that a strong and long-lasting anti-AML effect can be achieved with SCT even in these patients at very high risk of relapse. Similar results were observed in various adverse-risk subsets (unfavorable, complex, or monosomal karyotypes; high WBC). In patients not transplanted in CR1, almost 20% of relapses occurred within the first 3-month period (i.e. the median time to SCT in transplanted patients). In patients relapsing without having been transplanted in CR1, SCT after relapse was, however, not able to prolong OS which was as poor as in patients not transplanted after relapse (10% at 5 years).
Even if SCT results remain unsatisfactory, this real-life study confirms its superiority over chemotherapy in patients with adverse-risk AML in CR1. With a very high short-term relapse incidence and evidence of anti-AML allogeneic effect, the study also provides a strong rationale to propose upfront early sequential regimens to these patients, combining innovative chemotherapy and RIC-SCT (Schmid et al. Blood 2006). This option will be prospectively evaluated in the next younger AML French ALFA-GOELAMS Intergroup trial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal