Abstract
Graft-versus-host disease (GVHD) remains the major barrier to the success of allogeneic hematopoietic stem cell transplantation (HSCT). GVHD is caused by donor T cells that mediate host tissue injury through multiple inflammatory mechanisms. Blockade of individual effector molecules has limited efficacy in controlling GVHD. Here, we report that Notch signaling is a potent regulator of T-cell activation, differentiation, and function during acute GVHD. Inhibition of canonical Notch signaling in donor T cells markedly reduced GVHD severity and mortality in mouse models of allogeneic HSCT. Although Notch-deprived T cells proliferated and expanded in response to alloantigens in vivo, their ability to produce interleukin-2 and inflammatory cytokines was defective, and both CD4+ and CD8+ T cells failed to up-regulate selected effector molecules. Notch inhibition decreased the accumulation of alloreactive T cells in the intestine, a key GVHD target organ. However, Notch-deprived alloreactive CD4+ T cells retained significant cytotoxic potential and antileukemic activity, leading to improved overall survival of the recipients. These results identify Notch as a novel essential regulator of pathogenic CD4+ T-cell responses during acute GVHD and suggest that Notch signaling in T cells should be investigated as a therapeutic target after allogeneic HSCT.
Introduction
Graft-versus-host disease (GVHD) is a life-threatening complication that limits the efficacy of allogeneic hematopoietic stem cell transplantation (HSCT).1-4 Despite prophylaxis, GVHD still occurs in many allogeneic HSCT patients. Furthermore, standard immunosuppressive therapy for acute GVHD gives rise to disappointing sustained response rates (< 50%) and impairs graft-versus-tumor (GVT) activity, increasing the risk of tumor relapse.4,5 GVHD is caused by donor T cells attacking normal host tissues, involving complex interactions of immune cells and inflammatory mechanisms mediating target organ injury.1-4 In particular, multiple T-cell effector differentiation pathways can induce GVHD.6-12 Novel strategies that inhibit GVHD while preserving GVT could markedly improve allogeneic HSCT.
Notch signaling controls cell fate and tissue homeostasis in numerous contexts.13 Notch1-4 receptors interact with Notch ligands of the Jagged and δ-like families. Ligand-receptor interaction leads to proteolytic cleavage of the receptor by γ-secretase, followed by nuclear translocation of intracellular Notch. Notch target gene activation is mediated by a multiprotein complex including intracellular Notch, the transcription factor CSL/RBP-Jk and a member of the Mastermind-like (MAML) family.13,14 Although initially identified for its role during early T-cell development, Notch can influence mature T cells during antigen-specific immune responses.15-18 For example, Notch regulates Th2 CD4+ T-cell differentiation through effects on Il4 and Gata3 transcription.19-22 Notch may also regulate Th1, Th17, and regulatory T cells.19,23-25 Recently, Notch was described to control the effector program of CD8+ cytotoxic T cells.26,27 However, some T-cell responses are unaffected by Notch inhibition.16,20,21 Thus, Notch can be an important regulator of antigen-driven T-cell differentiation and function, but with context-dependent effects. Several factors could influence the effects of Notch signaling in distinct T-cell responses, including the nature of the Notch ligand-receptor interactions, the intensity and duration of Notch signals, and the crosstalk of Notch with other signaling pathways.16,17 Whether Notch signaling is critical to allogeneic T-cell responses and GVHD remains unknown.
Here, we report that Notch inactivation in donor CD4+ T cells inhibits their capacity to mediate acute GVHD, but preserves antileukemic activity in mouse models of allogeneic HSCT. Notch-deprived T cells expanded in response to alloantigens in vivo, but displayed a reduced accumulation in the gut and failed to produce a broad range of effector cytokines. Furthermore, both CD4+ and CD8+ lymphocytes showed defective expression of several effector molecules, although the master transcription factor genes Tbx21 and Eomes were induced and some effector pathways were not affected. These findings differ from past observations of Notch signaling in mature CD4+ and CD8+ T cells.15,16,18,25-28 Our results indicate that Notch inhibition in alloreactive T cells may be a promising strategy to control GVHD while preserving significant GVT effects after allogeneic HSCT.
Methods
Mice
BALB/c (H-2d) mice were from Harlan; B6xDBA/2 F1 (BDF1) mice (H-2b/d) from The Jackson Laboratory; C57BL/6.Ptprca (B6-SJL, H-2b, CD45.1+) from National Cancer Institute. ROSA26DNMAMLf/+ mice generated as described20,29 were crossed to Cd4-Cre transgenic mice, before backcrossing to the B6 background (> 8 generations). Rbpjf/f mice were kindly provided by Tasuku Honjo (Kyoto, Japan).21 Because no effect of Cre expression was observed in alloreactive T cells (data not shown), Cd4-Cre+ or Cd4-Cre– controls were used. Protocols were approved by the University of Pennsylvania's Office of Regulatory Affairs and the University of Michigan's Committee on Use and Care of Animals.
Antibodies and cell lines
The following antibodies were from eBioscience, BioLegend, or BD Biosciences: anti-CD3, CD8α, CD4, T-cell receptor β (TCRβ), CD25, CD44, CD69, CD45.2, CD45.1, H-2Kb, H-2Kd, interferonγ (IFNγ), interleukin-2 (IL-2), tumor necrosis factorα (TNFα), FoxP3. For restimulation, we used anti-CD3 (145-2C11) and anti-CD28 (37.51; 2.5 μg/mL each; Biolegend) or phorbol myristate acetate (PMA; 50 ng/mL) and ionomycin (500 ng/mL; Sigma-Aldrich). A20 (BALB/c, H-2d) lymphoma/leukemia cells expressing luciferase were kindly provided by Marcel van den Brink (Memorial Sloan Kettering Cancer Center, New York, NY).30
Cell preparations
T cell–depleted bone marrow (TCD BM) was prepared with microbead-conjugated anti-CD4/CD8 antibodies.31 CD4+ and CD8+ lymphocytes were isolated from spleens and lymph nodes using microbead-conjugated antibodies (MiniMACS; Miltenyi Biotech). Purity was consistently > 92%. In selected experiments, T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) or eFluor670 (eBioscience).
Induction of GVHD and GVT, in vivo bioluminescence
For the B6 anti-BALB/c model, we irradiated BALB/c recipients using 2 × 500 rads from a 137Cs source, 4 hours apart. We transplanted donor B6 TCD BM (5.0 × 106) with B6 or B6-DNMAML CD4+ T cells (2.0 × 106) into irradiated BALB/c recipients (4-8 mice/group per experiment). In selected experiments, 2.0 × 106 B6 or B6-DNMAML cells were compared with 0.5 × 106 B6 and 5.0 × 106 B6-DNMAML cells. In experiments studying GVHD target organs or using bioluminescence imaging, 850 rads were used. The GVHD score was assessed as described.32 GVHD severity was also assessed by histopathological analysis.33,34 Images were obtained with an Olympus BX41 microscope (10×/0.3 NA lens, 100× magnification, digital DP70 camera). In GVT experiments, we determined bioluminescent signal intensity at days 14-46 after transplantation after intraperitoneal injection of 4.5 mg of Firefly D-Luciferin (Biosynth) using the IVIS 200 system (Xenogen). We determined the cause of death by necropsy.35
Isolation of intestinal lymphocytes
Intraepithelial and lamina propria lymphocytes (IELs, LPLs) were isolated from small intestines as described, with slight modifications.36 Briefly, intestines were washed, and Peyer patches removed. Fragments (0.5-1 cm) of intestine were incubated in phosphate-buffered saline with 1mM EDTA and 1mM DTT for 45′ while shaking at 37°C. Supernatant was passed through nylon wool columns and IELs were isolated after centrifugation on a Percoll (Sigma-Aldrich) gradient. For LPL isolation, the remaining tissue was incubated in RPMI/fetal bovine serum 5% with 200 U/mL collagenase (Invitrogen) for 100′ while shaking at 37°C. Supernatant was filtered, and LPLs were isolated after centrifugation on a Percoll gradient.
Flow cytometry
Stained cells were sorted on a FACSAria (Becton Dickinson). Analysis was performed on a FACSCanto or LSRII (Becton Dickinson). Intra-cellular staining was performed after addition of Monensin for > 2 hours (Becton Dickinson). For BrdU incorporation, mice were pulsed with 1 mg BrdU intraperitoneally 6 hours before euthanasia. Staining was performed using an APC BrdU detection kit (Becton Dickinson), followed by DNA counterstaining (DAPI [4′,6-diamidino-2-phenylindole]). Files were analyzed in FlowJo Version 8.8.4 (TreeStar).
Cytokine measurements
Protein levels of cytokines were quantified using a Bio-Plex bead-based (Luminex) cytokine assay purchased from Bio-Rad Laboratories.
Quantitative reverse-transcription PCR
RNA was isolated using the RNEasy Micro kit (QIAGEN). cDNA was prepared with Superscript II (Invitrogen). Real-time polymerase chain reaction (PCR) was performed with TaqMan Master Mix on a Mastercycler realplex (Eppendorf). Transcript abundance was calculated using the ΔΔCt method (normalization with Hprt1). Primer sequences were from PrimerBank (http://pga.mgh.harvard.edu/primerbank/): Dtx1 (11611467a1), Prf1 (6755042a3), Gzmb (7305123a1), Il12rb2 (6680401a1), Fasl (6753818a1), Tnfsf10 (6678431a1), Eomes (5738950a2), FoxP3 (16905075a1). Additional primers were: Il4 (5′-AGATCATCGGCATTTTGAACG-3′; 5′-TTTGGCACATCCATCTCCG-3′), Ifng (5′-GGATGCATTCATGAGTATTGC-3′; 5′-CCTTTTCCGCTTCCTGAGG-3′), Tbx21 (5′-CAACAACCCCTTTGCCAAAG-3′; 5′-TCCCCCAAGCAGTTGACAGT-3′), Hprt1 (5′-CTCCTCAGACCGCTTTTTGC-3′; 5′-TAACCTGGTTCATCATC-GCTAATC-3′).
Ex vivo cytotoxicity assays
CFSE-based cytotoxicity assays were as described.37 CD4+ T cells were isolated from wild-type (WT) B6 mice (unstimulated) or recovered from spleens and livers of BALB/c recipients of WT or DNMAML CD4+ T cells 7-14 days after transplantation. In selected experiments, we used N2B2 anti-Trail and MFL3 anti-FasL antibodies (12.0 μg/mL; Biolegend).
In vivo cytotoxicity assay
BALB/c allogeneic target splenocytes were labeled with 2.5μM CFSE. Control targets were B6/SJL splenocytes labeled at a low CFSE concentration (0.25μM). On day 13, we infused a 1:1 mixture of CFSE-labeled BALB/c and B6/SJL splenocytes (107) into BALB/c recipients of TCD BM, TCD BM and WT CD4+ T cells, or TCD BM and DNMAML CD4+ T cells. Spleens were harvested 18 hours later to assess killing of BALB/c-derived CFSEhiI-Ad+ target B cells.
Statistical analysis
Survival in different groups was compared using the log-rank test. Comparison of 2 means was analyzed using the 2-tailed unpaired Student t test.
Results
Inactivation of Notch signaling by DNMAML in donor T cells inhibits acute GVHD
To inactivate Notch in mature T cells, we conditionally expressed the pan-Notch inhibitor DNMAML. DNMAML contains the MAML1 Notch-binding domain fused to green fluorescent protein (GFP) and blocks transcriptional activation downstream of all Notch receptors.14,20 We crossed Cd4-Cre transgenic mice with C57BL/6 (B6) ROSA26DNMAMLf/+ mice.20,29 This resulted in Cre-mediated DNMAML activation in CD4+CD8+ thymocytes, followed by stable expression in CD4+ and CD8+ lymphocytes. This strategy does not interfere with the requirement for Notch signaling during early T-cell development and produces normal numbers of T cells without baseline abnormalities.20 All mature CD4+ and CD8+ T cells in these mice cannot respond to Notch signaling, an excellent model to study the role of the Notch pathway during immune responses in vivo.
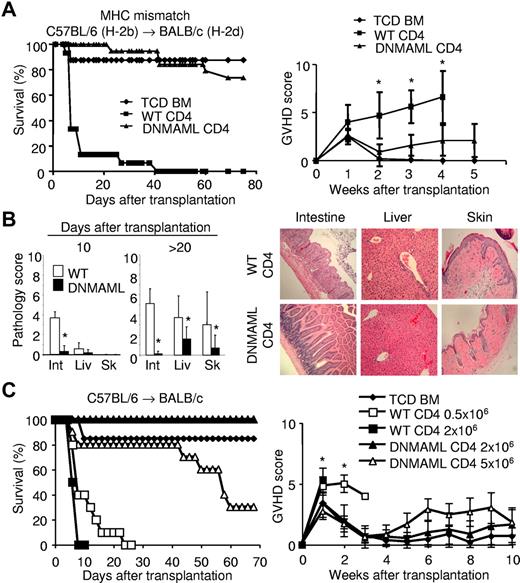
We first tested the impact of Notch deprivation in allore-active CD4+ T cells using the major histocompatibility complex (MHC)–mismatched B6 anti-BALB/c model (Figure 1). WT or DNMAML CD4+ B6 T cells (CD45.2, H-2b) were transplanted with TCD BM from B6-SJL mice (CD45.1) into lethally irradiated BALB/c mice (H-2d). Control mice receiving TCD BM remained free of GVHD. All WT CD4+ T-cell recipients developed severe acute GVHD with > 50% dying around day 10 and all by day 40 (Figure 1A). In contrast, BALB/c mice receiving DNMAML CD4+ T cells did not develop significant clinical signs of acute GVHD, with 75% surviving > 80 days after transplantation. The survival rate of DNMAML T-cell recipients was similar to that of TCD BM recipients. Assessment of the clinical GVHD score indicated markedly decreased acute GVHD severity in DNMAML compared with WT T-cell recipients (Figure 1A). This suggested that DNMAML CD4+ cells only induced mild disease. Histological examination of GVHD target organs showed markedly reduced inflammation in the skin, liver, and intestine of DNMAML compared with WT CD4+ T-cell recipients (Figure 1B). To further quantify the protection provided by Notch inhibition, we titrated up the number of donor DNMAML CD4+ T cells and down the number of WT CD4+ T cells (Figure 1C). Mice receiving a very high dose of DNMAML CD4+ T cells (5.0 × 106) remained significantly better protected against GVHD lethality and morbidity than mice receiving only 0.5 × 106 WT CD4+ T cells. Thus, DNMAML expression had more protective effects than a 10-fold reduction in donor T cells. Together, these results demonstrate that inactivation of Notch signaling in donor CD4+ T cells prevents acute lethal GVHD in mice.
Inactivation of Notch signaling in donor CD4+ T cells inhibits acute GVHD in irradiated MHC-mismatched hosts. (A) Lethally irradiated BALB/c mice (1000 rads) were transplanted with 5 × 106 TCD BM from B6-SJL mice (n = 10, ♦), with or without 2 × 106 WT B6 CD4+ T cells (WT CD4, n = 15, ■) or B6 DNMAML CD4+ T cells (DNMAML CD4, n = 19, ▴). Survival was assessed over time (P < .001, ■ versus ▴). Data shown are pooled from 3 independent experiments (left panel). Right panel, clinical GVHD score (*P < .05, ■ versus ▴), determined as described.32 Representative data from 1 of 2 independent experiments are presented. (B) Histological GVHD score.33,34 Tissues were collected at day 10 (n = 4 for each group) or > 20 days (range 20-40; WT, n = 8; DNMAML, n = 9) after transplantation (850 rads; left). Int, intestine; Liv, liver; Sk, skin. Right panel, histological analysis of intestine, liver, and skin after transplantation of WT (n = 8) or DNMAML (n = 9) CD4+ B6 T cells (×100),33,34 which is representative of 2 independent experiments. (C) Dose-response ex-periment. Lethally irradiated BALB/c mice were transplanted with 5.0 × 106 TCD BM from B6-SJL mice (n = 13, ♦), or TCD BM with 0.5 × 106 (□) or 2.0 × 106 (■) WT B6 CD4+ T cells (WT CD4, n =10/group) versus 2.0 × 106 (▴) or 5.0 × 106 B6 DNMAML (r) CD4+ T cells (DNMAML CD4, n = 10/group). Survival (left panel) and clinical GVHD score (right panel) were assessed over time, as described.32 Survival (P = .012) and GVHD severity (P < .01) were worse after administration of 0.5 × 106 WT CD4+ than 5.0 × 106 DNMAML CD4+ T cells (□ versus ▵).
Inactivation of Notch signaling in donor CD4+ T cells inhibits acute GVHD in irradiated MHC-mismatched hosts. (A) Lethally irradiated BALB/c mice (1000 rads) were transplanted with 5 × 106 TCD BM from B6-SJL mice (n = 10, ♦), with or without 2 × 106 WT B6 CD4+ T cells (WT CD4, n = 15, ■) or B6 DNMAML CD4+ T cells (DNMAML CD4, n = 19, ▴). Survival was assessed over time (P < .001, ■ versus ▴). Data shown are pooled from 3 independent experiments (left panel). Right panel, clinical GVHD score (*P < .05, ■ versus ▴), determined as described.32 Representative data from 1 of 2 independent experiments are presented. (B) Histological GVHD score.33,34 Tissues were collected at day 10 (n = 4 for each group) or > 20 days (range 20-40; WT, n = 8; DNMAML, n = 9) after transplantation (850 rads; left). Int, intestine; Liv, liver; Sk, skin. Right panel, histological analysis of intestine, liver, and skin after transplantation of WT (n = 8) or DNMAML (n = 9) CD4+ B6 T cells (×100),33,34 which is representative of 2 independent experiments. (C) Dose-response ex-periment. Lethally irradiated BALB/c mice were transplanted with 5.0 × 106 TCD BM from B6-SJL mice (n = 13, ♦), or TCD BM with 0.5 × 106 (□) or 2.0 × 106 (■) WT B6 CD4+ T cells (WT CD4, n =10/group) versus 2.0 × 106 (▴) or 5.0 × 106 B6 DNMAML (r) CD4+ T cells (DNMAML CD4, n = 10/group). Survival (left panel) and clinical GVHD score (right panel) were assessed over time, as described.32 Survival (P = .012) and GVHD severity (P < .01) were worse after administration of 0.5 × 106 WT CD4+ than 5.0 × 106 DNMAML CD4+ T cells (□ versus ▵).
DNMAML preserves donor T cell–mediated GVT effects
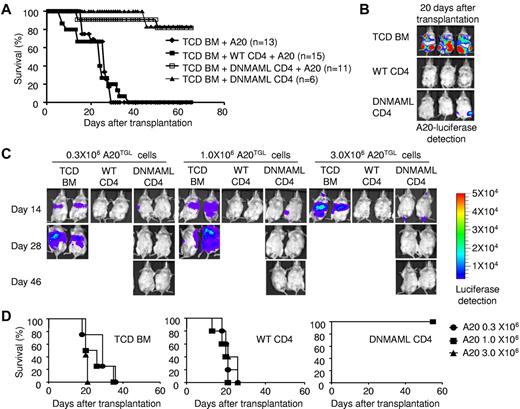
To evaluate whether Notch-deprived CD4+ T cells retained GVT activity, we challenged recipient BALB/c mice with host-type A20 B lineage leukemia/lymphoma cells (H-2d), mimicking residual leukemia in patients receiving allogeneic HSCT. We tracked luciferase-expressing A20 cells through bioluminescence imaging.30 Mice transplanted with TCD BM and challenged with A20 cells rapidly died of leukemia (Figure 2A-B). No leukemia growth was observed in mice receiving WT CD4+ cells, indicating potent GVT activity. However, all WT CD4+ cell recipients succumbed to GVHD within 40 days after transplantation (Figure 2A-B). In contrast, recipients of DNMAML CD4+ T cells effectively controlled leukemia without clinical signs of acute GVHD. As a result, > 80% of DNMAML CD4+ T-cell recipients survived free of leukemia and GVHD.
Preserved GVT activity of DNMAML-expressing T cells. (A) Lethally irradiated BALB/c mice (1000 rads) were transplanted with TCD BM and 5 × 105 A20 B cell leukemia/lymphoma cells (H-2d), with or without 2 × 106 WT or DNMAML CD4+ T cells from B6 mice. Overall survival after transplantation. (B) In vivo detection of luciferase activity at day 20 after transplantation and injection of 5 × 105 A20 luciferase cells (A20TGL). (C) Lethally irradiated BALB/c mice (850 rads) were transplanted with TCD BM and 0.3, 1.0, or 3.0 × 106 A20TGL cells, with or without 2.0 × 106 B6 WT or DNMAML CD4+ T cells. A representative example is shown for in vivo detection of luciferase activity at day 14, 28, and 46 after transplantation (5 mice/group). (D) Overall survival after transplantation for each dose of A20-TGL cells (n = 5 in each group). Recipients of TCD BM died of tumor progression. Recipients of WT CD4+ T cells controlled leukemia growth but died of severe GVHD. Recipients of DNMAML CD4+ T cells had markedly decreased GVHD, but were still able to control leukemia progression, resulting in improved overall survival.
Preserved GVT activity of DNMAML-expressing T cells. (A) Lethally irradiated BALB/c mice (1000 rads) were transplanted with TCD BM and 5 × 105 A20 B cell leukemia/lymphoma cells (H-2d), with or without 2 × 106 WT or DNMAML CD4+ T cells from B6 mice. Overall survival after transplantation. (B) In vivo detection of luciferase activity at day 20 after transplantation and injection of 5 × 105 A20 luciferase cells (A20TGL). (C) Lethally irradiated BALB/c mice (850 rads) were transplanted with TCD BM and 0.3, 1.0, or 3.0 × 106 A20TGL cells, with or without 2.0 × 106 B6 WT or DNMAML CD4+ T cells. A representative example is shown for in vivo detection of luciferase activity at day 14, 28, and 46 after transplantation (5 mice/group). (D) Overall survival after transplantation for each dose of A20-TGL cells (n = 5 in each group). Recipients of TCD BM died of tumor progression. Recipients of WT CD4+ T cells controlled leukemia growth but died of severe GVHD. Recipients of DNMAML CD4+ T cells had markedly decreased GVHD, but were still able to control leukemia progression, resulting in improved overall survival.
We further determined the potency of DNMAML CD4+ T cells to control leukemia growth in BALB/c recipients by challenging mice with increasing doses of A20 cells (0.3 × 106, 1.0 × 106, and 3.0 × 106 cells/mouse). Despite the increased tumor burden, DNMAML T cell recipients completely eradicated the leukemia by 28 days after infusion (Figure 2C), and all survived > 65 days (Figure 2D). WT T cell recipients rapidly controlled leukemia by day 14, but all died from GVHD (Figure 2C-D). These results suggest that DNMAML CD4+ T cells can efficiently control leukemia without causing severe GVHD.
Extensive proliferation and expansion of donor DNMAML CD4+ T cells in irradiated MHC-mismatched hosts
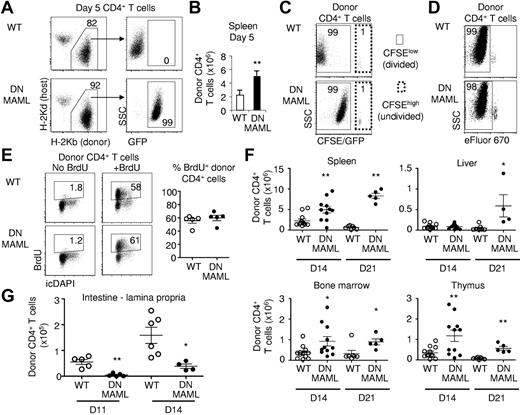
To determine the mechanisms by which Notch inhibition in CD4+ T cells prevents GVHD while preserving GVT activity, we first assessed engraftment and expansion of Notch-deprived donor T cells. We tracked DNMAML CD4+ T cells in irradiated BALB/c recipients at day 5 after transplantation (Figure 3A-E). We found modestly but significantly increased donor-derived CD4+ T-cell numbers in spleens from BALB/c recipients of DNMAML compared with WT T cells (Figure 3A-B). When CFSE was used to track cell division, both groups had a similar percentage of donor CFSElow T cells (approximately 99%), consistent with extensive proliferation, although the full division history could not be compared due to fluorescence of the DNMAML-GFP fusion protein (Figure 3C). To assess proliferation of DNMAML-expressing CD4+ T cells without GFP interference, we labeled donor T cells with the tracking dye eFluor670 (Figure 3D). This revealed a similar eFluor670 dilution profile in day 5 WT and DNMAML B6 CD4+ T cells. Furthermore, we pulsed BALB/c recipient mice with BrdU and assessed BrdU incorporation by donor-derived CD4+ T cells (Figure 3E). A high percentage (approximately 60%) of both WT B6 and B6 CD4+ DNMAML T cells were found to be BrdU+, consistent with a similar rate of cell cycle entry. To evaluate if Notch inhibition affected the expansion of alloreactive T cells at later stages, we tracked donor-derived CD45.2+ B6 and B6-DNMAML CD4+ T cells in the spleen, BM, liver, and thymus of recipient BALB/c mice at days 14 and 21 (Figure 3F). Higher numbers of donor-derived CD4+ T cells were recovered from these organs in B6-DNMAML compared with WT B6 recipients, most prominently at day 21 after transplantation. Given their similar proliferation rate, this increased expansion could be related to decreased activation-induced cell death of the DNMAML CD4+ T cells.
Preserved proliferation and in vivo expansion of DNMAML alloreactive CD4+ T cells. (A) Identification of donor-derived CD4+ T cells in the spleen on day 5 after transplantation. DNMAML T cells expressed the DNMAML-GFP fusion protein. Data are representative of 5 experiments. (B) Absolute number of donor-derived CD4+ T cells in the spleen at day 5 (mean ± SD, n = 4). (C) Tracking of CFSE-labeled donor CD4+ T cells at day 5. Both WT and DNMAML CD4+ T cells had undergone extensive proliferation, as shown by the high percentage of CFSElow cells. Fluorescence levels appear higher in the DNMAML-expressing cells due to fluorescence of the covalently fused GFP protein. This effect is absent in Rbpj knockout mice (see supplemental Figures 3-4). (D) Tracking of eFluor670-labeled donor WT and DNMAML CD4+ T cells at day 5, showing extensive proliferation in both (% eFluor670low cells). (E) BrdU uptake by donor WT and DNMAML CD4+ T cells at day 5. Mice were pulsed with BrdU 6 hours before harvest (n = 5 per group). (F) Absolute number of CD45.2+ donor-derived WT and DNMAML CD4+ T cells in spleen, liver, BM, and thymus on day 14 (WT, n = 12; DNMAML, n = 11) and 21 (WT, n = 7; DNMAML, n = 5) posttransplantation (pool of 2 independent experiments). (G) Decreased number of donor-derived DNMAML compared with WT CD4+ T cells in the lamina propria of the small intestine on days 11 (WT, n = 5; DNMAML, n = 5) and 14 after transplantation (WT, n = 6; DNMAML, n = 4). *P < .05, **P < .01 (2-tailed unpaired Student t test).
Preserved proliferation and in vivo expansion of DNMAML alloreactive CD4+ T cells. (A) Identification of donor-derived CD4+ T cells in the spleen on day 5 after transplantation. DNMAML T cells expressed the DNMAML-GFP fusion protein. Data are representative of 5 experiments. (B) Absolute number of donor-derived CD4+ T cells in the spleen at day 5 (mean ± SD, n = 4). (C) Tracking of CFSE-labeled donor CD4+ T cells at day 5. Both WT and DNMAML CD4+ T cells had undergone extensive proliferation, as shown by the high percentage of CFSElow cells. Fluorescence levels appear higher in the DNMAML-expressing cells due to fluorescence of the covalently fused GFP protein. This effect is absent in Rbpj knockout mice (see supplemental Figures 3-4). (D) Tracking of eFluor670-labeled donor WT and DNMAML CD4+ T cells at day 5, showing extensive proliferation in both (% eFluor670low cells). (E) BrdU uptake by donor WT and DNMAML CD4+ T cells at day 5. Mice were pulsed with BrdU 6 hours before harvest (n = 5 per group). (F) Absolute number of CD45.2+ donor-derived WT and DNMAML CD4+ T cells in spleen, liver, BM, and thymus on day 14 (WT, n = 12; DNMAML, n = 11) and 21 (WT, n = 7; DNMAML, n = 5) posttransplantation (pool of 2 independent experiments). (G) Decreased number of donor-derived DNMAML compared with WT CD4+ T cells in the lamina propria of the small intestine on days 11 (WT, n = 5; DNMAML, n = 5) and 14 after transplantation (WT, n = 6; DNMAML, n = 4). *P < .05, **P < .01 (2-tailed unpaired Student t test).
Because immune-mediated intestinal damage is a prominent cause of mortality in the B6 anti-BALB/c GVHD model, we assessed accumulation of WT or DNMAML donor-derived T cells in the small intestine at days 11 and 14 after transplantation (Figure 3G and supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Few IELs were recovered at these time points in both groups (data not shown). In contrast, a significant number of donor WT T cells were recovered from the lamina propria. We observed a significant decrease (4- to 10-fold) in the number of infiltrating DNMAML T cells at both time points (Figure 3G). To assess if this was related to a cell-autonomous effect of Notch inhibition, we coinjected equal numbers of WT and DNMAML CD4+ T cells. In this context, DNMAML CD4+ T cells were not excluded from the intestine (supplemental Figure 1B), indicating that Notch does not directly control their homing or retention in the gut. Instead, the decreased accumulation of donor T cells in the gut of DNMAML compared with WT recipients may result from a reduced inflammatory response initiated by the alloreactive T cells.
Altogether, Notch-deficient donor CD4+ T cells were able to proliferate and accumulate in lymphoid and hematopoietic tissues of allogeneic HSCT recipients, but showed decreased accumulation in the gut, a key GVHD target organ. This might account at least in part for the decreased GVHD severity and mortality in DNMAML T-cell recipients.
Impaired activation and effector functions of alloreactive DNMAML CD4+ T cells
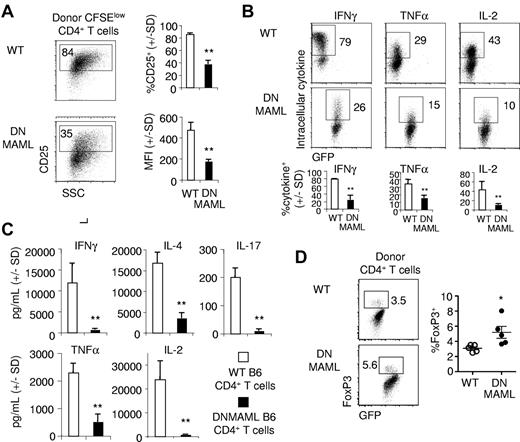
We next assessed the in vivo effect of Notch inhibition on the activation and effector functions of alloantigen-stimulated CD4+ T cells. Donor T cells were recovered 5 days after transplantation. CD25 expression was reduced in donor DNMAML CD4+ T cells, suggesting decreased activation (Figure 4A). Intracellular staining showed that production of the inflammatory cytokines IFNγ and TNFα, as well as IL-2, was markedly decreased in DNMAML CD4+ T cells (Figure 4B). Further analysis revealed that DNMAML CD4+ cells displayed impaired production of cytokines associated with Th1 (IFNγ, TNFα), Th2 (IL-4), and Th17 differentiation (IL-17), in addition to IL-2 (Figure 4C). These data suggest that DNMAML-expressing alloreactive T cells had a markedly decreased ability to produce a broad range of cytokines. This included cytokines from multiple T helper cell subsets as well as IL-2, an effect of Notch inhibition in mature T cells that has not previously been reported.
Impaired activation and cytokine response in alloreactive DNMAML CD4+ T cells. WT or DNMAML B6 CD4+ T cells were tracked after transplantation into lethally irradiated MHC-mismatched BALB/c mice (900 rads). (A) Decreased expression of CD25 (IL-2Rα) in DNMAML CFSElow donor CD4+ T cells. Bar graphs show the percentage of cells expressing CD25 and the CD25 mean fluorescence intensity (MFI; mean ± SD, n = 3). (B) Intracellular staining for IFNγ, TNFα, and IL-2 after ex vivo restimulation with plate-bound anti-CD3 and anti-CD28 antibodies. Data are representative of 5 experiments. Bar graphs show mean ± SD (n = 3). (C) Cytokine production by 3 × 104 donor-derived CD4+ T cells sort-purified 5 days after transplantation and cultured for 6 hours in the presence of anti-CD3/anti-CD28 antibodies (mean ± SD). (D) Intracellular staining for FoxP3 in donor WT and DNMAML CD4+ T cells on day 5. *P < .05, **P < .01 (2-tailed unpaired Student t test).
Impaired activation and cytokine response in alloreactive DNMAML CD4+ T cells. WT or DNMAML B6 CD4+ T cells were tracked after transplantation into lethally irradiated MHC-mismatched BALB/c mice (900 rads). (A) Decreased expression of CD25 (IL-2Rα) in DNMAML CFSElow donor CD4+ T cells. Bar graphs show the percentage of cells expressing CD25 and the CD25 mean fluorescence intensity (MFI; mean ± SD, n = 3). (B) Intracellular staining for IFNγ, TNFα, and IL-2 after ex vivo restimulation with plate-bound anti-CD3 and anti-CD28 antibodies. Data are representative of 5 experiments. Bar graphs show mean ± SD (n = 3). (C) Cytokine production by 3 × 104 donor-derived CD4+ T cells sort-purified 5 days after transplantation and cultured for 6 hours in the presence of anti-CD3/anti-CD28 antibodies (mean ± SD). (D) Intracellular staining for FoxP3 in donor WT and DNMAML CD4+ T cells on day 5. *P < .05, **P < .01 (2-tailed unpaired Student t test).
Next, we assessed whether DNMAML impaired in vivo expansion of FoxP3-expressing CD4+ T cells (Figure 4D). In contrast to decreased cytokine production and CD25 expression, we found a slightly but significantly increased percentage of FoxP3+ cells among day 5 donor-derived B6 DNMAML compared with WT B6 CD4+ T cells. To evaluate if increased T cell–mediated suppression could account for all the effects of DNMAML in preventing acute GVHD, we cotransferred equal numbers of WT and DNMAML B6 CD4+ T cells into irradiated BALB/c mice (supplemental Figure 2A). Comparable expansion of both populations was observed in vivo (supplemental Figure 2B). Recipients of mixed WT and DNMAML CD4+ T cells still developed severe lethal GVHD, similarly to mice receiving only WT cells (supplemental Figure 2C). Thus, it is unlikely that an increased suppressive activity explains by itself the inability of DNMAML CD4+ T cells to induce acute GVHD.
Loss of CSL/RBP-Jk impairs the effector functions of alloreactive CD4+ T cells
Notch-mediated transcriptional activation requires CSL/RBP-Jk, encoded by Rbpj.13,21 To rule out off-target effects of DNMAML, we studied T cells with conditional Rbpj inactivation (supplemental Figure 3).21 This also allowed tracking of CFSE-labeled T cells without interference from DNMAML-GFP fluorescence. Five days after transfer into BALB/c recipients, Rbpjf/f Cd4-Cre+ T cells had proliferated extensively, with most cells having divided > 8 times, similar to control cells (supplemental Figure 3A). The expansion of CSL/RBP-Jk-deficient CD4+ cells was preserved. As with DNMAML, CD25 expression (supplemental Figure 3B) and production of IFNγ, TNFα, and IL-2 were reduced in CSL/RBP-Jk–deficient CD4+ T cells (supplemental Figure 3C-D). When assessed around the onset of proliferation, CSL/RBP-Jk-deficient CD4+ T cells displayed normal up-regulation of the activation markers CD69, CD44, and CD25 (supplemental Figure 3E). The equivalent effects of CSL/RBP-Jk loss and DNMAML expression independently validate the critical role of Notch in alloreactive T cells.
Hyporesponsiveness of Notch-deficient alloreactive T cells
Our results demonstrate that Notch blockade inhibits effector differentiation of alloantigen-activated T cells, in particular Th1 effector cells. Previous studies have reported that Notch can regulate expression of T-bet, the transcription factor that is essential for Th1 differentiation.25,28 To test whether Notch inhibition impairs T-bet induction in alloreactive T cells, we purified donor T cells 5 days after transplantation from BALB/c mice receiving WT or DNMAML CD4+ T cells. Upon ex vivo stimulation with anti-CD3/CD28 antibodies, DNAMML CD4+ T cells expressed similar levels of Tbx21 mRNA (encoding T-bet) as WT CD4+ T cells despite defective Ifng and Il2 induction (Figure 5A). Stimulation of DNMAML CD4+ cells with PMA/ionomycin, which can bypass proximal steps of signaling downstream of the T-cell receptor (TCR), did not affect Tbx21 transcription, but restored induction of Ifng and to a lesser extent Il2 mRNA in DNMAML T cells (Figure 5A). These findings differed from previous reports,25,28 suggesting that inactivation of Notch signaling in alloreactive T cells impaired Th1 differentiation regardless of Tbx21 transcription.
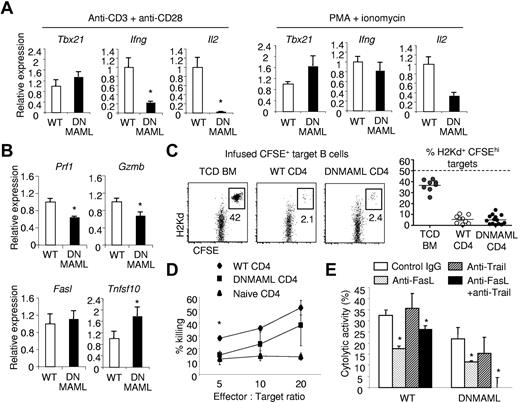
Hyporesponsiveness of alloreactive DNMAML CD4+ T cells to CD3/CD28-mediated signals, but significant preservation of cytotoxic potential. CFSElow donor-derived WT or DNMAML CD4+ T cells were sort-purified at day 5 after transplantation into lethally irradiated (900 rads) BALB/c hosts. The cells were restimulated ex vivo during 6 hours with plate-bound anti-CD3/anti-CD28, or with PMA and ionomycin. (A) Decreased Il2 transcripts and impaired expression of Ifng despite normal induction of Tbx21 (encoding T-bet) in DNMAML CD4+ T cells after stimulation with anti-CD3/anti-CD28 antibodies. PMA and ionomycin could partially rescue Il2 and fully induce Ifng expression. (B) Modest decrease in Prf1 and Gzmb transcripts, but preserved induction of Fasl and Tnfsf10 mRNA (encoding Trail) in DNMAML CD4+ T cells. (C) In vivo cytotoxic activity of WT or DNMAML CD4+ T cells against infused CFSEhi host-type BALB/c (H-2d) B cell targets, compared with control CFSElo B6-SJL cells (H-2b). Analysis was performed on day 14, 18 hours after infusion of CFSE-labeled targets at a 1:1 ratio (107 each). Data are shown as percent residual CFSEhiH-2Kd+ cells among all infused CFSE-labeled B cells. The dotted line represents expected recovery in the absence of cytotoxicity. (D) In vitro cytotoxic activity of WT or DNMAML CD4+ T cells against A20 leukemia cells. *P < .05 (2-tailed unpaired Student t test). (E) Inhibition of in vitro cytotoxic activity of DNMAML CD4+ T cells by anti-FasL and anti-Trail antibodies. *P < .05 (2-tailed unpaired Student t test).
Hyporesponsiveness of alloreactive DNMAML CD4+ T cells to CD3/CD28-mediated signals, but significant preservation of cytotoxic potential. CFSElow donor-derived WT or DNMAML CD4+ T cells were sort-purified at day 5 after transplantation into lethally irradiated (900 rads) BALB/c hosts. The cells were restimulated ex vivo during 6 hours with plate-bound anti-CD3/anti-CD28, or with PMA and ionomycin. (A) Decreased Il2 transcripts and impaired expression of Ifng despite normal induction of Tbx21 (encoding T-bet) in DNMAML CD4+ T cells after stimulation with anti-CD3/anti-CD28 antibodies. PMA and ionomycin could partially rescue Il2 and fully induce Ifng expression. (B) Modest decrease in Prf1 and Gzmb transcripts, but preserved induction of Fasl and Tnfsf10 mRNA (encoding Trail) in DNMAML CD4+ T cells. (C) In vivo cytotoxic activity of WT or DNMAML CD4+ T cells against infused CFSEhi host-type BALB/c (H-2d) B cell targets, compared with control CFSElo B6-SJL cells (H-2b). Analysis was performed on day 14, 18 hours after infusion of CFSE-labeled targets at a 1:1 ratio (107 each). Data are shown as percent residual CFSEhiH-2Kd+ cells among all infused CFSE-labeled B cells. The dotted line represents expected recovery in the absence of cytotoxicity. (D) In vitro cytotoxic activity of WT or DNMAML CD4+ T cells against A20 leukemia cells. *P < .05 (2-tailed unpaired Student t test). (E) Inhibition of in vitro cytotoxic activity of DNMAML CD4+ T cells by anti-FasL and anti-Trail antibodies. *P < .05 (2-tailed unpaired Student t test).
To further understand the mechanisms underlying the preservation of GVT in Notch-deprived T cells, we studied the production of other effector molecules in alloantigen-stimulated DNMAML CD4+ T cells. DNMAML alloreactive CD4+ cells expressed modestly decreased Prf1 and Gzmb transcripts (encoding Perforin and Granzyme B), but normal or marginally increased Fasl and Tnfsf10 mRNA (encoding Fas ligand and Trail; Figure 5B). To assess the global impact of DNMAML expression on CD4+ T cell–mediated cytotoxicity in vivo, we quantified the ability of BALB/c recipients of WT B6 or DNMAML CD4+ T cells to eliminate CFSE-labeled allogeneic I-Ad+ BALB/c targets (Figure 5C). Compared with TCD BM recipients, only a small fraction of infused BALB/c allogeneic targets was recovered from both WT and DNMAML CD4+ T-cell recipients, indicating similar and efficient in vivo cytotoxic activity. Furthermore, DNMAML alloreactive CD4+ T cells effectively killed A20 leukemic cells in an in vitro assay, at least at high effector:target ratios, despite their modestly reduced killing capability compared with WT alloreactive T cells (Figure 5D). Addition of neutralizing anti-FasL but not anti-Trail antibodies significantly reduced the killing activity of DNMAML alloreactive CD4+ T cells (Figure 5E). Notably, blockade of both FasL and Trail abrogated the capability of DNMAML to kill A20 cells (Figure 5E). This suggested that intact expression of FasL and Trail in DNMAML T cells is crucial to their potent antitumor activity. In comparison, blockade of FasL but not Trail partially reduced the cytotoxic effect of WT alloreactive T cells against A20 cells, indicating the involvement of other mechanisms for them to eliminate leukemic cells. Altogether, Notch blockade profoundly impaired the ability of alloreactive T cells to produce effector cytokines, with less drastic effects or preserved expression of other effector molecules.
Notch inhibition prevents acute GVHD in a nonirradiation mouse model mediated by CD4+ and CD8+ T cells
A limitation of GVHD models involving irradiation is that proliferation of alloreactive T cells occurs in parallel to homeostatic proliferation in lymphopenic hosts. To avoid this limitation, we extended our observations to the B6 anti-B6xDBA/2 F1 (BDF1) model, a GVHD model that does not require irradiation (Figure 6).38 It has previously been established that alloantigen-specific T cells are the only cells that proliferate in this model.38 Thus, tracking donor-derived CFSElow cells in BDF1 recipient mice allowed us to study the impact of Notch signaling in a polyclonal population of alloantigen-specific T cells.
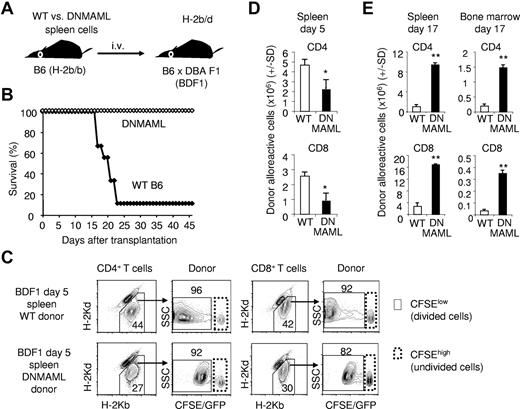
Notch inhibition prevents lethality in a nonirradiation semiallogeneic transplantation model. (A) Experimental design. Splenocytes (4 × 107) from WT or DNMAML B6 mice were transplanted into unirradiated B6xDBA F1 (BDF1) recipients. BDF1 recipients (H-2b/d) are tolerant to the infused B6 cells (H-2b), but donor T cells mount a potent alloreactive response against host H-2d antigens. (B) Improved overall survival of BDF1 recipients receiving DNMAML B6 T cells (n =9 in each group; P < .01). (C) Tracking of CFSE-labeled donor-derived T cells at day 5 after transplantation into BDF1 recipients. CFSElow cells are alloantigen-specific in this model. (D) Modest decrease in the expansion of alloreactive CD4+ and CD8+ DNMAML T cells at day 5. (E) Enhanced accumulation of DNMAML CD4+ and CD8+ alloreactive T cells in the spleen and target BM at day 17 after transplantation. *P < .05, **P < .01 (2-tailed unpaired Student t test).
Notch inhibition prevents lethality in a nonirradiation semiallogeneic transplantation model. (A) Experimental design. Splenocytes (4 × 107) from WT or DNMAML B6 mice were transplanted into unirradiated B6xDBA F1 (BDF1) recipients. BDF1 recipients (H-2b/d) are tolerant to the infused B6 cells (H-2b), but donor T cells mount a potent alloreactive response against host H-2d antigens. (B) Improved overall survival of BDF1 recipients receiving DNMAML B6 T cells (n =9 in each group; P < .01). (C) Tracking of CFSE-labeled donor-derived T cells at day 5 after transplantation into BDF1 recipients. CFSElow cells are alloantigen-specific in this model. (D) Modest decrease in the expansion of alloreactive CD4+ and CD8+ DNMAML T cells at day 5. (E) Enhanced accumulation of DNMAML CD4+ and CD8+ alloreactive T cells in the spleen and target BM at day 17 after transplantation. *P < .05, **P < .01 (2-tailed unpaired Student t test).
CFSE-labeled WT or DNMAML B6 splenocytes (H-2b) were infused into BDF1 recipients (H-2b/d; Figure 6A). WT T cells caused high-grade lethality by 3-4 weeks after transplantation (Figure 6B). In contrast, all DNMAML T-cell recipients survived the procedure. Immune-mediated BM failure was the dominant cause of death in this model (data not shown). By flow cytometric analysis, we tracked populations of donor-derived CD4+ and CD8+ lymphocytes (Figure 6C). The overall expansion of CFSElow alloreactive DNMAML CD4+ and CD8+ cells was moderately reduced at early time points (2- to 3-fold at day 5; Figure 6D). This differed from our observations in the B6 anti-BALB/c irradiation model of GVHD, in which expansion of DNMAML CD4+ T cells was preserved or slightly enhanced at day 5 (Figure 3), suggesting that the inflammatory environment and the effects of irradiation in BALB/c recipients can compensate for a modest early expansion defect of DNMAML T cells. However, expansion of donor-derived DNMAML T cells also appeared to increase at later stages in BDF1 recipients (day 17), giving rise to higher number of donor-derived alloreactive T cells both in lymphoid organs and in the BM of DNMAML compared with WT recipient mice (Figure 6E). The preserved proliferation of Notch-deprived alloreactive T cells was confirmed again by tracking CFSE-labeled CSL/RBP-Jk–deficient T cells in BDF1 recipients, a strategy avoiding DNMAML-GFP fluorescence (supplemental Figure 4). Thus, the inability of Notch-deprived donor T cells to cause acute GVHD is not due to defective expansion or impaired homing in this model in which lethality is mostly driven by immune-mediated BM damage.
DNMAML impairs the effector differentiation of alloreactive CD4+ and CD8+ T cells
To evaluate the effects of Notch inhibition on effector functions in CD4+ and CD8+ T cells, we purified donor-derived CFSElow T cells from unirradiated BDF1 recipients 5 days after transplantation (Figure 7). Dtx1 Notch target gene transcripts were less abundant in DNMAML than WT CD4+ and CD8+ cells. DNMAML T cells decreased expression of Th1-related Ifng and Il12rb2 genes, with a similar pattern in DNMAML CD4+ and CD8+ cells, while also affecting Th2-related Il4 mRNA in CD4+ cells (Figure 7A). While DNMAML T cells decreased Prf1 and Gzmb, most prominently in CD8+ cells, expression of Fasl and Tnfsf10 was maintained (Figure 7B). Again, Tbx21 mRNA was preserved in alloreactive DNMAML T cells, while Eomes transcripts were significantly elevated. This was in contrast with previous reports suggesting that Notch controlled the expression of these genes.25-28 We also observed up-regulated Foxp3 mRNA in alloreactive DNMAML compared with WT B6 CD4+ T cells in this model (Figure 7C). Up-regulated expression was confirmed at the protein level among CFSElow alloreactive CD4+ T cells, consistent with the emergence of peripheral Tregs (supplemental Figure 5). Thus, DNMAML did not inhibit, but rather slightly enhanced expansion of FoxP3+CD4+ cells both in the B6 anti-BDF1 and B6 anti-BALB/c models of GVHD (Figures 4, 7C, and supplemental Figure 5).
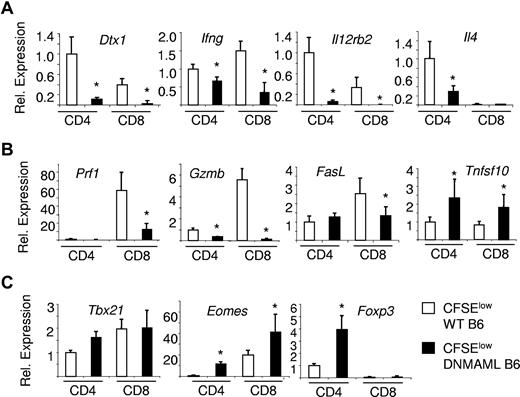
DNMAML-expressing alloreactive CD4+ and CD8+ T cells show impaired activation of many but not all effector differentiation pathways. Quantitative reverse-transcription PCR was performed with RNA purified from alloreactive CFSElow donor T cells at day 5 after transplantation of WT or Cd4-cre x ROSA26DNMAMLf/+ (DNMAML) B6 splenocytes into unirradiated B6xDBA F1 (BDF1) recipients (Figure 6). Results are shown for alloreactive CD4+ and CD8+ T cells isolated from the spleen, after normalization with Hprt1. A relative value of 1 was assigned to WT CD4+ T cells. Error bars indicate the 95% confidence interval. (A) Expression of Notch target genes and effector cytokines; (B) expression of cytolytic molecules; and (C) expression of the master transcription factors of Th1, CD8+ effector T cell, and Treg differentiation. *P < .05.
DNMAML-expressing alloreactive CD4+ and CD8+ T cells show impaired activation of many but not all effector differentiation pathways. Quantitative reverse-transcription PCR was performed with RNA purified from alloreactive CFSElow donor T cells at day 5 after transplantation of WT or Cd4-cre x ROSA26DNMAMLf/+ (DNMAML) B6 splenocytes into unirradiated B6xDBA F1 (BDF1) recipients (Figure 6). Results are shown for alloreactive CD4+ and CD8+ T cells isolated from the spleen, after normalization with Hprt1. A relative value of 1 was assigned to WT CD4+ T cells. Error bars indicate the 95% confidence interval. (A) Expression of Notch target genes and effector cytokines; (B) expression of cytolytic molecules; and (C) expression of the master transcription factors of Th1, CD8+ effector T cell, and Treg differentiation. *P < .05.
Altogether, Notch was essential for the normal functional differentiation of CD4+ Th1, CD4+ Th2 and CD8+ alloreactive effector T cells despite preserved induction of the master regulators Tbx21 and Eomes.
Discussion
Identifying the critical signals controlling the complex features of alloimmunity is essential for regulating the balance between harmful and beneficial effects of allogeneic HSCT.3,5 Our findings demonstrate a critical role for Notch signaling in alloreactive CD4+ T-cell activation, differentiation, and function during acute GVHD. Notch inactivation in donor T cells preserved their ability to engraft and proliferate, but inhibited their accumulation in the gut and acquisition of selected effector functions during the acute GVH reaction. Notch inhibition decreased the production of multiple cytokines by alloantigen-activated CD4+ T cells, including IL-2, and prevented severe GVHD through mechanisms that differed from past observations of Notch function in mature T cells. However, Notch inhibition preserved significant antitumor activity in alloreactive CD4+ T cells. Thus, modulation of Notch signaling may identify novel approaches to prevent GVHD while preserving useful GVT effects.
Multiple studies have shown that inhibition of single effector pathways provides limited therapeutic benefit in GVHD.6-12 For example, inhibition of single cytokines or inactivation of the master transcription factors controlling individual effector T-cell lineages have not translated into a marked survival advantage. In many cases, organ injury mediated by a specific T-cell lineage (eg, Th1 cells) is replaced by another pattern of target organ injury mediated by other lineages (eg, Th2 or Th17 cells). In contrast, Notch inhibition had functional consequences on multiple T helper lineages and on both CD4+ and CD8+ alloreactive T cells. As a result, Notch inhibition had a dramatic impact on survival even in otherwise uniformly lethal models of GVHD. However, Notch inhibition did not merely cause immunosuppression, because in vivo expansion of alloreactive CD4+ T cells and significant antitumor activity were preserved. Thus, we suggest that Notch signaling in T cells should be explored as a novel therapeutic target after allo-HSCT.
Past evidence suggests that Notch can play multiple roles in T-cell differentiation, although these effects depend on the immune context.15-18 For example, Notch regulates CD4+ Th2 cell differentiation through transcriptional activation of Il4 and Gata3 genes.19-22 Other studies suggest that Notch can affect certain types of CD4+ Th1 responses.16,19,25 Recently, Notch was also described to regulate CD4+ Th17 and CD8+ lymphocytes.24,26,27 In alloimmunity, Notch inhibition decreased the acquisition of effector functions, including elements of CD4+ Th1, Th2, and Th17, as well as CD8+ T-cell differentiation. Previous observations have described direct effects of Notch on master transcription factor genes (such as Tbx21, Gata3, and Eomes) and on genes encoding effector molecules (such as Il4, Il17, or Gzmb).19,21,22,24-27 Although some of these mechanisms could have contributed to the defects of Notch-deficient alloreactive T cells, our findings extended to both CD4+ and CD8+ lymphocytes and could not be characterized as a single helper or cytotoxic T cell differentiation defect. For example, we observed normal Tbx21 and increased Eomes transcripts in DNMAML T cells, indicating that transcriptional activation of these genes by Notch did not account for the powerful effects of Notch in alloreactive T cells. Instead, Notch deficiency blunted the response of alloreactive lymphocytes to TCR and CD28 engagement. This differs from past findings and suggests additional effects of Notch on T cell responsiveness and induction of the effector program in alloreactive cells. Because we observed similar consequences from DNMAML expression and Rbpj inactivation, 2 independent genetic loss-of-function systems, our observations suggest an essential function of Notch-mediated transcriptional activation in alloreactive T cells.
Past reports using overexpression of Jagged Notch ligands have shown that Jagged can modulate the reactivity of T cells to alloantigens, inducing expansion of Tregs and T cell hyporesponsiveness.23 This differs from our observations, because we observed a significant trend for enhanced in vivo Treg expansion and decreased production of effector T cell cytokines. Thus, in vitro gain-of-function assays do not predict the in vivo function of Notch signaling in alloimmunity. In addition, it is possible that the effects of Jagged ligands in these studies could differ from and even antagonize those of δ like ligands, as reported in other experimental systems.39 In addition, our results also differ from past reports in which γ-secretase inhibitors and anti-Notch1 antibodies were shown to inhibit Treg function in vitro and in a mouse model of allergic airway inflammation.40,41 Although the reasons underlying these differences remain to be explored, this also highlights the value of assessing the role of Notch signaling in vivo in specific types of immune response using genetic strategies of pan-Notch inhibition.
Although Notch-deficient alloreactive T cells had multiple functional defects, their initial activation, proliferation, and expansion appeared generally normal. Subsequently, significant features of Notch deprivation included decreased responsiveness to TCR/CD28-mediated signals and decreased expression of CD25 (IL-2Rα). Notch signaling was reported to regulate CD25 at least in developing T cells.29,42 It is possible that decreased CD25 expression could contribute to impaired activation of Notch-deprived T cells through decreased IL-2 sensitivity. However, we did not observe decreased CD25 expression at all time points and in all models of allogeneic HSCT. For example, decreased CD25 expression was not observed in unirradiated semiallogeneic recipients, despite multiple changes in DNMAML T-cell differentiation. Thus, reduced CD25 expression cannot account for all functional defects in Notch-deprived T cells. Furthermore, preserved proliferation despite impaired IL-2 production is consistent with past studies of alloreactive T cells showing that other cytokines such as IL-15, but not IL-2, are most important to sustain their proliferation in vivo.43
Several studies have suggested that effector mechanisms mediating GVHD are also important for GVT activity, although not completely overlapping.44 Therefore, preventing GVHD without impairing GVT activity is an important but difficult task.5,11,45-47 Our observations provide an unexpected approach to this problem, because Notch-deprived CD4+ T cells preserved significant antitumor activity despite their inability to trigger severe GVHD. One possibility to explain these findings is that reduced alloreactivity in Notch-deficient T cells brought their pathogenicity below the threshold required to trigger severe GVHD, while GVT activity was less sensitive to this effect. However, DNMAML expression conferred more protection than a > 10-fold decrease in the number of donor CD4+ T cells, while in vivo elimination of allogeneic targets was similar in WT and DNMAML CD4+ T cell recipients. In addition, a 10-fold increase in leukemic cell dose did not overcome the GVT activity of DNMAML CD4+ T-cells recipients. Thus, a purely quantitative effect of DNMAML appears unlikely. As an alternative, effector mechanisms involved in GVHD and GVT may be differentially affected by Notch deprivation. In support of this idea, we observed preserved FasL and Trail expression in DNMAML T cells, while cytokine production was profoundly inhibited. Furthermore, combined inhibition of FasL and Trail blocked the cytotoxic activity of DNMAML CD4+ T cells against tumor targets in vitro. Thus, Notch-deficient T cells may be particularly dependent on these molecules for efficient killing, while WT CD4+ T cells may use a broader spectrum of effector functions. Finally, decreased accumulation of Notch-deficient T cells in the gut may provide protection against life-threatening damage to this key GVHD target organ, while allowing preserved cytotoxic activity against allogeneic targets at other sites, especially in the lymphohematopoietic system.
Our work presents the first example of a disease model in which Notch inhibition prevents otherwise uniform lethality. Although systemic Notch inhibition can cause significant side effects, in particular gastrointestinal toxicity due to combined Notch1/Notch2 inhibition, multiple strategies are being developed to overcome this important limitation. This includes targeting of specific Notch receptors and ligands, intermittent administration of Notch inhibitors, or pharmacological strategies that counteract the effects of Notch inhibition in the gut.17,48-50 In allogeneic HSCT, creative approaches to bypass systemic side effects of Notch inhibition could include manipulation of the cellular product administered to patients so that Notch is inhibited only in infused donor T cells.
In the future, it will be important to determine whether Notch is important only at the onset of allogeneic responses or remains active later. If its main effects are restricted to priming and early differentiation of T cells, it may be desirable to inhibit Notch transiently after allogeneic HSCT, which would avoid consequences of Notch inhibition on extrahematopoietic tissues or de novo T-cell generation. Although our observations were made after allogeneic HSCT, they may extend to other types of immune responses with exposure to persistent antigens. For example, Notch may regulate organ rejection and autoimmune diseases. From a therapeutic perspective, our work points to the Notch pathway as an attractive target to achieve beneficial context-specific immunomodulation in T cell–mediated disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Marcel van den Brink for providing A20-luciferase cells, Tasuku Honjo for Rbpj conditional knockout mice, and Pavan Reddy, James Ferrara, and Sean Morrison for critical reading of the manuscript.
This work was supported by a Damon Runyon-Rachleff Innovation Award (to Y.Z. and I.M.), the American Society of Hematology (to I.M.), the University of Michigan Biological Sciences Scholar Program (to I.M.), a SCOR Award from the Leukemia & Lymphoma Society (to S.G.E. and W.S.P.), and the National Institutes of Health (to Y.Z., S.G.E., and W.S.P.; grant nos. R01 CA102464 and R01 AI047833). This research is supported in part by a grant from the National Institutes of Health through the University of Michigan's Cancer Center Support Grant (5P30CA46592) and the University of Pennsylvania's Cancer Center Support Grant. A.S. was supported by T32A1007413 from the National Institute of Allergy and Infectious Diseases. V.R. and G.T.S. were supported by a training award from the American Society of Hematology.
National Institutes of Health
Authorship
Contribution: Y.Z. and I.M. designed the study, performed experiments, analyzed data, and wrote the paper; A.R.S., J.W., V.R., G.T.S., I.T.T., A.F., K.K., S.H., S.C., E.H., and D.M.F. performed experiments and analyzed data; S.G.E. and W.S.P. participated in study design and edited the manuscript; and all authors reviewed and approved the final draft of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zhang, 6219 Cancer Center, 1500 E Medical Dr, University of Michigan, Ann Arbor, MI 48109; e-mail: yizha@med.umich.edu; or Ivan Maillard, Life Sciences Institute, Rm 6382A, 210 Washtenaw Ave, University of Michigan, Ann Arbor, MI 48109; e-mail: imaillar@umich.edu.