Abstract
Antiphospholipid syndrome is characterized by thrombosis, recurrent fetal loss, and the presence of the lupus anticoagulant, anticardiolipin antibodies, or anti–β2-glycoprotein-1 (anti–β2-GP1) antibodies. Although anti–β2-GP1 antibodies have been documented as a biomarker for diagnosis of antiphospholipid syndrome, their direct role in the pathogenesis of thrombosis is unknown. We have demonstrated using intravital microscopy that anti–β2-GP1 autoantibodies purified from the sera of patients with antiphospholipid syndrome complicated by thrombosis greatly amplify thrombus size after laser-induced vessel wall injury in live mice. Anti–β2-GP1 autoantibodies from 3 patients with antiphospholipid syndrome were affinity-purified using human β2-GP1 bound to agarose. The effects of purified anti–β2-GP1 IgG autoantibodies, of anti–β2-GP1–depleted IgG, and of IgG from normal human sera on thrombus formation were measured in mice after arterial injury in the cremaster muscle. Before injury, purified anti–β2-GP1 IgG autoantibodies, anti–β2-GP1 antibody–depleted IgG, or IgG from normal human sera were infused. Increasing amounts of purified anti–β2-GP1 autoantibodies increased thrombus size in a dose-dependent manner, whereas neither anti–β2-GP1 antibody-depleted IgG nor IgG from normal serum affected thrombus size. These results indicate that anti–β2-GP1 IgG autoantibodies in antiphospholipid syndrome patient sera are not only a marker of antiphospholipid syndrome but are directly involved in the pathogenesis of thrombosis.
Introduction
The antiphospholipid syndrome is characterized by thrombosis or fetal loss and the presence of antiphospholipid autoantibodies in patient sera.1 This clinical syndrome is observed in a number of autoimmune disorders, and in particular, systemic lupus erythematosus. Sera from patients with antiphospholipid syndrome contain polyclonal antibodies that bind to various lipids and plasma protein targets, including β2-glycoprotein-1 (β2-GP1), prothrombin, and platelet factor 4.2-4 Serum anti–β2-GP1 antibodies are an independent risk factor for thrombosis,5 and the lupus anticoagulant, cardiolipin antibodies, and anti–β2-GP1 antibodies represent key polyclonal antiphospholipid antibodies assayed in patients suspected of antiphospholipid syndrome.
The mechanism by which antiphospholipid antibodies lead to thromboembolic events is unknown. Hypotheses include platelet activation via the β2-GP1 antibody/β2-GP1 complex binding to the apolipoprotein E2 receptor,6 interaction of anti–β2-GP1 antibody–dimerized β2-GP1 and GPIb, leading to platelet adhesion,7 endothelium activation via the targeting of anti–β2-GP1 antibodies to the β2-GP1-annexin 2 complex,8 the inhibition of activated protein C by β2-GP1/anti–β2-GP1 antibody complex,9 disruption of the potential role of annexin V as an anticoagulant by antiphospholipid antibodies,10 impairment of fibrinolysis by antiphospholipid antibodies associated with endothelial cells,11 and exposure of a cryptic epitope on domain I of β2-GP1 to allow formation of the β2-GP1/anti–β2-GP1 antibody complex.12 These concepts derive from in vitro studies. Animal models of thrombosis in the antiphospholipid syndrome have used whole serum, the immunoglobulin fraction from sera of patients with antiphospholipid syndrome,13,14 or monoclonal antibodies derived from immortalized patient monocytes.15,16 Alternatively, heterologous monoclonal hybridoma antibodies prepared in mice against purified human β2-GP1 have been analyzed.17,18 To date, there has been no direct proof that polyclonal autoantibodies reactive against β2-GP1 that have been isolated from sera of patients with antiphospholipid syndrome cause or enhance thrombosis in an animal model. Nonetheless, there is circumstantial evidence pointing toward anti–β2-GP1 antibodies as possibly being pathogenic in the antiphospholipid syndrome, and there is ample clinical evidence that anti–β2-GP1 autoantibodies are a biomarker for thrombosis and thrombotic risk. We purified anti–β2-GP1 autoantibodies from antiphospholipid syndrome patient sera by affinity chromatography using immobilized human β2-GP1. Using our laser-induced thrombosis model in a living mouse,19,20 we demonstrate that human anti–β2-GP1 autoantibodies are sufficient to enhance thrombus formation.
Methods
Mice
Wild-type C57BL/6J mice were obtained from The Jackson Laboratory. The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee approved all animal care and experimental procedures.
Antibodies and reagents
Rat anti–mouse CD41 antibody (clone MWReg30) was from Emfret. Fab fragments of the anti-CD41 antibody were generated using the ImmunoPure Fab Preparation Kit from Pierce Biotechnology. Rat anti–mouse GPIbβ antibody conjugated to DyLight 649 was obtained from Emfret. A mouse anti–human fibrin monoclonal antibody (clone 59D8) against a synthetic peptide of the human fibrin β chain that cross-reacts with mouse fibrin was produced from the hybridoma. The purified antibody was labeled with Alexa 488. Human serum samples were collected from healthy subjects and from patients with the antiphospholipid syndrome after informed consent. Patients were diagnosed according to the revised criteria for antiphospholipid syndrome.1
Total IgG immunoglobulins were purified from serum samples using A/G protein columns (Pierce Biotechnology). Serum was applied and polyclonal IgG eluted from the agarose matrix with Immunopure Elution buffer. The antibody was dialyzed against phosphate-buffered saline, concentrated, and then dialyzed against 0.1M NaCl. Anti–β2-GP1 antibodies were affinity-purified using human β2-GP1 (Biodesign International) covalently linked to cyanogen bromide-activated agarose beads at a ratio of 5 mg of β2-GP1 to 1 mL of beads. After elution with Tris-glycine, pH 3.0, the antibody was similarly dialyzed, concentrated, and dialyzed against 0.1M NaCl before infusion. Preparations of anti–β2-GP1 antibodies and control IgG contained equivalent amounts of endotoxin (Genscript Toxin Sensor). Fab fragments of anti-CD41 antibody were labeled with Alexa Fluor 647 according to the manufacturer's instructions (Invitrogen). The molar ratio of Alexa Fluor to protein, determined spectrophotometrically, varied from 2.0 to 3.5.
ELISA for measurement of human β2-GP1 IgG and IgM antibodies
Anti–human β2-GP1 antibodies and anticardiolipin antibodies were measured by enzyme-linked immunosorbent assay (ELISA; Inova Diagnostics). A calibration curve was established using reference calibrators supplied by the manufacturer and was linear from 9.4 to 150 standard IgG anti–β2-GP1 units (SGU).
ELISA for the measurement of human IgG in mouse plasma after infusion of human IgG
Human IgG concentrations in mouse plasma were measured in citrated mouse platelet-poor plasma obtained 2.5 hours after infusion of human IgG. No notable cross-reactivity was observed with mouse IgG. ELISA was performed according to the manufacturer's instructions (Bethyl Laboratories) using dilutions from 1/1000 to 1/20 000 on duplicates. A calibration curve was established using reference calibrators.
Intravital microscopy
Intravital videomicroscopy of the cremaster muscle microcirculation was performed as previously described.20 Mice were preanesthetized with intraperitoneal ketamine (125 mg/kg body weight; Abbott Laboratories), xylazine (12.5 mg/kg body weight; Phoenix Pharmaceuticals), and atropine (0.25 mg/kg; American Pharmaceutical Partners). A tracheal tube was inserted, and the mouse was maintained at 37°C on a thermo-controlled rodent blanket. To maintain anesthesia, Nembutal (Abbott Laboratories) was administered through a cannulus placed in the jugular vein. After the scrotum was incised, the testicle and surrounding cremaster muscle were exteriorized onto an intravital microscopy tray. The cremaster preparation was superfused with thermo-controlled (36°C) and aerated (95% N2, 5% CO2) bicarbonate-buffered saline throughout the experiment. Microvessel data were obtained using an Olympus AX microscope with an X60 0.9 NA water-immersion objective. The intravital fluorescence microscopy system has previously been described in detail. Digital images were captured with a Cooke Sensicam CCD camera in 640 × 480 format.
Laser-induced injury
Vessel wall injury was induced with a Micropoint Laser System (Photonics Instruments) focused through the microscope objective, parfocal with the focal plane, and aimed at the vessel wall. Multiple thrombi were studied in a single mouse, with new thrombi formed upstream of earlier thrombi to avoid any contribution from thrombi generated earlier in the animal under study. There were no characteristic trends in thrombus size or thrombus composition in sequential thrombi generated in a single mouse during an experiment. Image analysis was performed using Slidebook (Intelligent Imaging Innovations). Fluorescence data were captured digitally at up to 50 frames/second and analyzed as previously described.20 Typically, wide-field fluorescence images were captured at exposure times of 200 ms, whereas bright-field images were captured with exposure times of 50 ms. Data were collected for 3 to 5 minutes after vessel wall injury. The representative intravital color images were binarized using a thresholding procedure in which the background is defined as the mean fluorescence of a mask upstream of the thrombus. The direction of blood flow is indicated by the arrow. The complete datasets of the representative and multiple identical experiments are presented graphically, plotting the median integrated fluorescence intensity of all pixels in the image, regardless of their intensity, as a function of time. The kinetics of thrombus formation were analyzed by determining median fluorescence values over time in approximately 20 to 30 thrombi.
Statistics
The area under the curve describing the kinetics of platelet or fibrin accumulation as a function of time was used as a quantitative parameter for thrombus size. These data were statistically analyzed by analysis of variance (nonparametric) using GraphPad Prism software Version 5.02. Differences were considered significant at P less than .05.
Results
Sera from patients with antiphospholipid syndrome contain polyclonal autoantibodies that bind to various plasma proteins, including β2-GP1. Anti–β2-GP1 autoantibodies have been well documented as a biomarker for the diagnosis of the antiphospholipid syndrome. Furthermore, anti–β2-GP1 antibodies have also been indirectly implicated in the pathogenesis of thrombosis in in vitro systems. We predicted that, based on the marked sequence homology between human and mouse β2-GP1,21-23 human anti–β2-GP1 autoantibodies would recognize mouse β2-GP1, thus justifying a mouse model of thrombosis. Furthermore, antiphospholipid autoantibodies derived from patients with antiphospholipid syndrome were previously shown to react equivalently with human and mouse β2-GP1.24 In the current study, we have demonstrated using intravital microscopy that purified anti–β2-GP1 autoantibodies isolated from the sera of patients with the antiphospholipid syndrome are sufficient to greatly amplify thrombus size after laser-induced vessel wall injury in mice.
Three unrelated patients were studied. Patient A was a 41-year-old man with systemic lupus erythematosus and the antiphospholipid syndrome complicated by a pulmonary embolism. Patient B was a 30-year-old man with systemic lupus erythematosus and antiphospholipid syndrome complicated by an inferior vena caval thrombosis and bilateral lower extremity arterial thromboses eventually requiring bilateral below-the-knee amputations. Patient C was a 45-year-old woman with systemic lupus erythematosus and antiphospholipid syndrome complicated by recurrent strokes. All patients had anti–β2-GP1 and anticardiolipin autoantibodies (Table 1).
Clinical patient data
| . | Patient A . | Patient B . | Patient C . |
|---|---|---|---|
| Thromboembolic event | Pulmonary embolism | Inferior vena caval thrombosis, bilateral lower extremity arterial thromboses | Stroke |
| ACA, SGU | 149.5 | 61 IgG; 121 IgM | 51 IgG; 5 IgM |
| β2-glycoprotein-1 antibodies, SGU | 150 IgG | 149 IgG; 79 IgM | > 150 IgG; 5 IgM |
| Lupus anticoagulant | Positive | Positive | ND |
| . | Patient A . | Patient B . | Patient C . |
|---|---|---|---|
| Thromboembolic event | Pulmonary embolism | Inferior vena caval thrombosis, bilateral lower extremity arterial thromboses | Stroke |
| ACA, SGU | 149.5 | 61 IgG; 121 IgM | 51 IgG; 5 IgM |
| β2-glycoprotein-1 antibodies, SGU | 150 IgG | 149 IgG; 79 IgM | > 150 IgG; 5 IgM |
| Lupus anticoagulant | Positive | Positive | ND |
Normal values: ACA, < 20 SGU; β2-glycoprotein-1 antibodies, < 20 SGU; and lupus anticoagulant, negative.
ND indicates not done.
IgG was isolated from patient sera by affinity chromatography using Protein A/G-agarose. Serum was applied and polyclonal IgG eluted from the agarose matrix with Immunopure Elution buffer. IgG was purified from patient serum in approximately 70% to 80% yield. To determine whether the polyclonal IgG isolated from patients with antiphospholipid syndrome had prothrombotic activity, these polyclonal antibodies were studied in anesthetized mice using intravital fluorescence microscopy.
In preliminary experiments, thrombi could not be visualized after infusion of purified IgG in the absence of vascular injury. Therefore, all further experiments were conducted using our laser injury model. After infusion of either Fab fragments of rat monoclonal anti–mouse CD41 antibody conjugated to Alexa 647 or anti-CD42 antibody conjugated to Dylight 649 to detect platelet accumulation and polyclonal patient-derived or control IgG antibodies, vessel wall injury was induced with a laser injury directed at the vessel wall. Because of the large size of the thrombi observed in the presence of antiphospholipid syndrome antibody in preliminary experiments, we significantly modified our standard method of thrombus formation20 by lowering the intensity of the laser injury in animals infused with antiphospholipid syndrome antibody and animals infused with control IgG antibody. Data were collected for 3 to 5 minutes after vessel wall injury. The kinetics of thrombus formation were analyzed by determining median fluorescence values over time in approximately 20 to 30 thrombi in 3 mice.
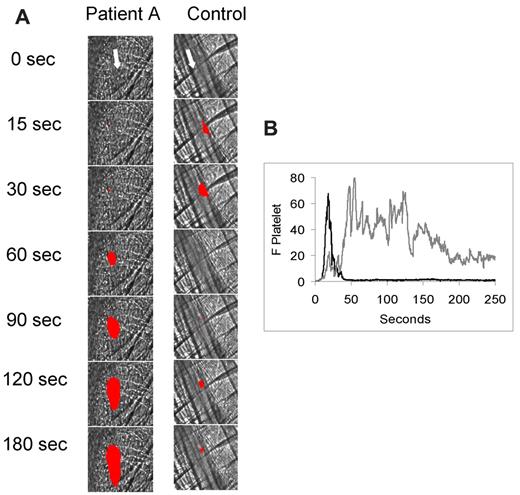
In a representative experiment, infusion of polyclonal IgG from antiphospholipid syndrome in patient A was associated with marked enhancement of thrombus size (Figure 1A). By comparison, polyclonal IgG from a normal subject showed no enhancement of thrombus size; thrombus size was equivalent in the presence or absence of normal polyclonal IgG (images not shown). A lower limit of approximately 400 μg of infused IgG led to detectable thrombus size enhancement. Infusion of 1 mg of IgG into the mouse circulation led to a plasma concentration of 0.43 ± 0.4 mg/mL, a recovery of 86%. Analyses of the kinetics of platelet thrombus development for 25 thrombi generated in 3 mice treated with patient-derived polyclonal IgG demonstrated the formation of large platelet thrombi (Figure 1B). In contrast, 26 thrombi generated in 3 wild-type mice treated with normal polyclonal IgG were small using the same low intensity laser injury. These results confirm earlier observations made in other mouse thrombosis models that polyclonal IgG derived from antiphospholipid syndrome sera significantly increases thrombus size after thrombus formation.13,17
Effect of IgG isolated from antiphospholipid syndrome patient sera on thrombus formation in the mouse laser injury model. Fab fragments of a rat monoclonal anti–mouse CD41 antibody conjugated to Alexa 647 (0.3 μg/g body weight) and either normal IgG (1 mg) or patient A IgG (1 mg) were infused into a wild-type mice 5 minutes before laser-induced arteriolar wall injury. (A) Representative images of the fluorescence signal associated with platelets (red) over 180 seconds after vessel injury are shown within the context of the bright-field histology. (B) The median integrated platelet fluorescence (F Platelet) associated with platelet thrombus formation in 3 wild-type mice after infusion of patient IgG (n = 25 thrombi) or control IgG (n = 25 thrombi) over 250 seconds after vessel wall injury. Gray represents patient A IgG; and black, control IgG.
Effect of IgG isolated from antiphospholipid syndrome patient sera on thrombus formation in the mouse laser injury model. Fab fragments of a rat monoclonal anti–mouse CD41 antibody conjugated to Alexa 647 (0.3 μg/g body weight) and either normal IgG (1 mg) or patient A IgG (1 mg) were infused into a wild-type mice 5 minutes before laser-induced arteriolar wall injury. (A) Representative images of the fluorescence signal associated with platelets (red) over 180 seconds after vessel injury are shown within the context of the bright-field histology. (B) The median integrated platelet fluorescence (F Platelet) associated with platelet thrombus formation in 3 wild-type mice after infusion of patient IgG (n = 25 thrombi) or control IgG (n = 25 thrombi) over 250 seconds after vessel wall injury. Gray represents patient A IgG; and black, control IgG.
Prior studies have indirectly suggested a role for anti–β2-GP1 autoantibodies in thrombosis associated with antiphospholipid syndrome,14 although one study concluded that human anti–β2-GP1 antibodies alone had no effect on thrombus formation.25 Others have demonstrated that anticardiolipin antibodies that do not bind β2-GP1 can cause thrombosis in an animal model.15 We determined whether polyclonal anti–β2-GP1 autoantibodies derived from the sera of patients with antiphospholipid syndrome and a history of thrombosis are sufficient to enhance thrombus formation in a mouse model of thrombosis. Anti–β2-GP1 autoantibodies in the IgG fraction of patient sera were affinity-purified using homogeneous β2-GP1 covalently bound to cyanogen bromide-activated agarose beads. Patient IgG was applied to the column. After extensive washing, purified anti–β2-GP1 autoantibodies were eluted with Tris-glycine buffer at pH 3.0. Patient IgG depleted of anti–β2-GP1 autoantibodies, representing any antibodies that do not bind to anti–β2-GP1, were obtained by repeated adsorption on the β2-GP1 matrix. The antibody populations from the 3 patient sera studied that bound to β2-GP1 also had all anticardiolipin antibody activity. The progress of purification of anti–β2-GP1 antibodies in 3 patients is shown in Table 2.
Purification of anti–β2-glycoprotein-1 autoantibodies from patients and normal serum
| Purification . | Normal . | Patient A . | Patient B . | Patient C . |
|---|---|---|---|---|
| Purified total IgG, mg | 45 | 8.1 | 7.0 | 12 |
| Purified anti–β2-glycoprotein-1, mg | 0.024 | 0.05 | 0.095 | |
| Purified IgG depleted of anti–β2-glycoprotein-1 antibodies, mg | 7.5 | 6.5 | 11 |
| Purification . | Normal . | Patient A . | Patient B . | Patient C . |
|---|---|---|---|---|
| Purified total IgG, mg | 45 | 8.1 | 7.0 | 12 |
| Purified anti–β2-glycoprotein-1, mg | 0.024 | 0.05 | 0.095 | |
| Purified IgG depleted of anti–β2-glycoprotein-1 antibodies, mg | 7.5 | 6.5 | 11 |
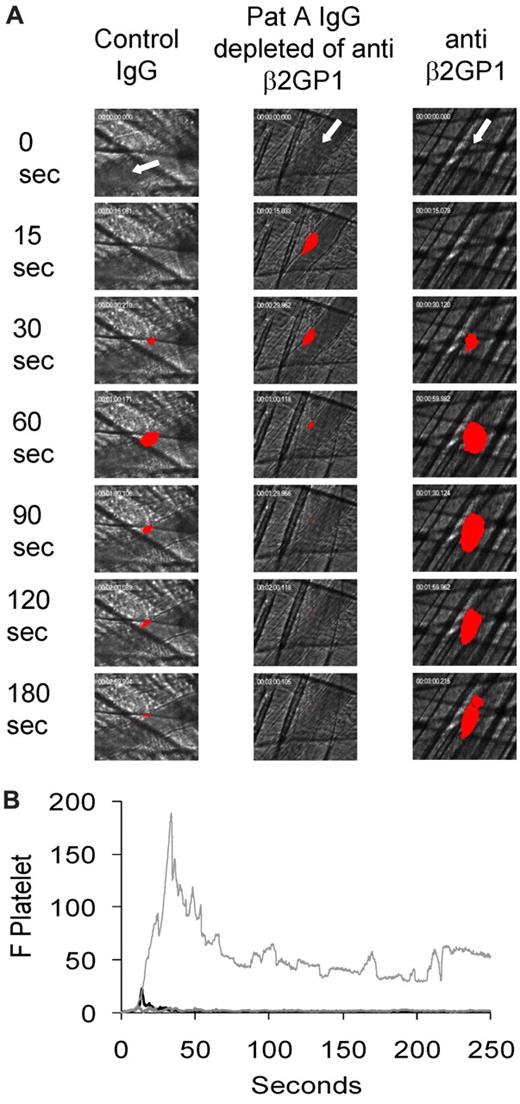
The effects of purified anti–β2-GP1 autoantibodies, anti–β2-GP1 autoantibody-depleted patient IgG, and IgG from normal human sera on thrombus formation were studied quantitatively by intravital microscopy and the laser injury thrombosis model. Five minutes before vessel wall injury, purified anti–β2-GP1 autoantibodies, anti–β2-GP1 autoantibody-depleted patient IgG, or normal human IgG were infused via a jugular catheter. Platelet thrombus size was determined based on fluorescence associated with Alexa 647–conjugated Fab fragments of an anti-CD 41 monoclonal antibody (patient A) or Dylight 649-conjugated anti-CD42 monoclonal antibody (patients B and C). Up to 10 thrombi were generated per mouse, and the median integrated fluorescence for 25 to 30 thrombi was determined. Purified anti–β2-GP1 autoantibodies from patient A enhanced platelet thrombus size (Figure 2A-B). In contrast, anti–β2-GP1 antibody–depleted patient IgG and IgG from normal human sera had no impact on thrombus size (Figure 2B). These results demonstrate that β2-GP1 antibodies isolated from antiphospholipid syndrome serum are capable of greatly enhancing thrombus size. Furthermore, in this patient's serum, other antiphospholipid antibodies of the IgG class make no measurable contribution to thrombus size.
Effect of purified anti–β2-GP1 autoantibodies on thrombus size. Autoantibodies reactive with anti–β2-GP1 were isolated from the purified IgG of patient serum. Anti–β2-GP1-depleted IgG is the IgG from a patient that does not bind to the β2-GP1-agarose column. (A) Anti–β2-GP1 autoantibodies from patient A (10 μg), anti–β2-GP1-depleted IgG from patient A (1 mg), and control normal serum IgG (1 mg) were infused into wild-type mice 5 minutes before laser-induced arteriolar wall injury in addition to Fab fragments of rat monoclonal anti–mouse CD41 antibody conjugated to Alexa 647. Representative images of the fluorescence signal associated with platelets (red) over 180 seconds after vessel injury are shown within the context of the bright-field histology. (B) The median integrated platelet fluorescence (F Platelet) associated with platelet thrombus formation in 3 mice after infusion of anti–β2-GP1 autoantibody (n = 25 thrombi), 3 mice after infusion of IgG depleted of anti–β2-GP1 autoantibodies (n = 36 thrombi), and 4 mice after infusion of control IgG (n = 34 thrombi) is presented over 250 seconds after vessel wall injury. Light gray represents anti–β2-GP1 autoantibodies; gray; IgG-depleted of anti–β2-GP1 autoantibodies; and black, control IgG.
Effect of purified anti–β2-GP1 autoantibodies on thrombus size. Autoantibodies reactive with anti–β2-GP1 were isolated from the purified IgG of patient serum. Anti–β2-GP1-depleted IgG is the IgG from a patient that does not bind to the β2-GP1-agarose column. (A) Anti–β2-GP1 autoantibodies from patient A (10 μg), anti–β2-GP1-depleted IgG from patient A (1 mg), and control normal serum IgG (1 mg) were infused into wild-type mice 5 minutes before laser-induced arteriolar wall injury in addition to Fab fragments of rat monoclonal anti–mouse CD41 antibody conjugated to Alexa 647. Representative images of the fluorescence signal associated with platelets (red) over 180 seconds after vessel injury are shown within the context of the bright-field histology. (B) The median integrated platelet fluorescence (F Platelet) associated with platelet thrombus formation in 3 mice after infusion of anti–β2-GP1 autoantibody (n = 25 thrombi), 3 mice after infusion of IgG depleted of anti–β2-GP1 autoantibodies (n = 36 thrombi), and 4 mice after infusion of control IgG (n = 34 thrombi) is presented over 250 seconds after vessel wall injury. Light gray represents anti–β2-GP1 autoantibodies; gray; IgG-depleted of anti–β2-GP1 autoantibodies; and black, control IgG.
We measured the plasma concentration of human β2-GP1 antibodies infused into the circulation of a donor mouse. At 2.5 hours after infusion of 22 to 25 μg, 10 μg, or 3 μg of human β2-GP1 antibodies into the mouse circulation, the plasma concentration of the human antibody was 7.3 ± 0.3 μg/mL, 3.7 ± 0.7 μg/mL, or 0.9 ± 0.0 μg/mL, a recovery of 59%, 74%, or 60%, respectively. These results suggest that most of the infused antibody remained in the circulation and was not sequestered.
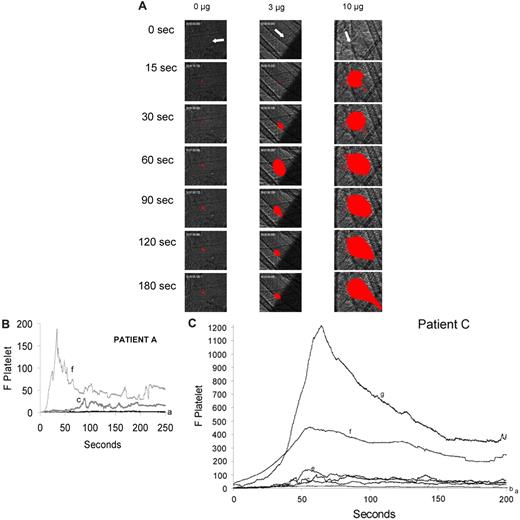
We explored whether increasing the amount of β2-GP1 antibodies infused into the mouse correlated with an increase in thrombus size. Analyses of platelet thrombus size in more than 30 thrombi studied in mice receiving no β2-GP1 antibodies, one receiving 3 μg and another receiving 10 μg indicate that the platelet thrombi are markedly larger in the presence of higher concentrations of antibody. Infusion of purified anti–β2-GP1 antibodies at 3 μg and 10 μg increased thrombus size over time by approximately 18-fold and 122-fold, respectively, compared with thrombi formed in untreated mice, as measured by the area under the curve of integrated median fluorescence over time (Figure 3B).
Amplification of thrombus size as a function of the quantity of anti–β2-GP1 autoantibody infusion. Anti–β2-GP1 autoantibodies from patient A or patient C at various doses were infused into wild-type mice 5 minutes before laser-induced arteriolar wall injury. Fab fragments of rat monoclonal anti–mouse CD41 antibody conjugated to Alexa 647 or rat monoclonal anti–mouse CD42 antibody conjugated to Dylight 649 were also infused to allow fluorescence detection of the platelet thrombus. (A) Representative images of the fluorescence signal associated with platelets (red) within the context of the bright-field histology are presented after vessel injury in mice treated with anti–β2-GP1 autoantibodies from patient A. (B) The median integrated platelet fluorescence (F Platelet) as a function of time in 3 separate mice after infusion of 0 μg (n = 22 thrombi), 3 μg (n = 25 thrombi), or 10 μg (n = 25) of anti–β2-GP1 autoantibodies derived from patient A. (C) The median integrated platelet fluorescence (F Platelet) as a function of time in 3 separate mice after infusion of 0 μg (n = 25 thrombi), 1.5 μg (n = 30 thrombi), 3 μg (n = 28 thrombi), 6 μg (n = 29 thrombi), 10 μg (n = 29 thrombi), and 65 μg (n = 24 thrombi) of anti–β2-GP1 autoantibodies derived from patient C. a indicates 0 μg; b, 1.5 μg; c, 3 μg; d, 6 μg; e, 7 μg; f, 10 μg; and g, 65 μg.
Amplification of thrombus size as a function of the quantity of anti–β2-GP1 autoantibody infusion. Anti–β2-GP1 autoantibodies from patient A or patient C at various doses were infused into wild-type mice 5 minutes before laser-induced arteriolar wall injury. Fab fragments of rat monoclonal anti–mouse CD41 antibody conjugated to Alexa 647 or rat monoclonal anti–mouse CD42 antibody conjugated to Dylight 649 were also infused to allow fluorescence detection of the platelet thrombus. (A) Representative images of the fluorescence signal associated with platelets (red) within the context of the bright-field histology are presented after vessel injury in mice treated with anti–β2-GP1 autoantibodies from patient A. (B) The median integrated platelet fluorescence (F Platelet) as a function of time in 3 separate mice after infusion of 0 μg (n = 22 thrombi), 3 μg (n = 25 thrombi), or 10 μg (n = 25) of anti–β2-GP1 autoantibodies derived from patient A. (C) The median integrated platelet fluorescence (F Platelet) as a function of time in 3 separate mice after infusion of 0 μg (n = 25 thrombi), 1.5 μg (n = 30 thrombi), 3 μg (n = 28 thrombi), 6 μg (n = 29 thrombi), 10 μg (n = 29 thrombi), and 65 μg (n = 24 thrombi) of anti–β2-GP1 autoantibodies derived from patient C. a indicates 0 μg; b, 1.5 μg; c, 3 μg; d, 6 μg; e, 7 μg; f, 10 μg; and g, 65 μg.
Similarly, infusion of anti–β2-GP1 antibodies from patient C increased thrombus size in a dose-dependent manner. Infusion of purified anti–β2-GP1 antibodies at 1.5, 3, 5, 10, and 62 μg increased thrombus size by approximately 11-, 18-, 20-, 95-, and 172-fold, respectively, compared with thrombi formed in mice treated with saline (Figure 3C).
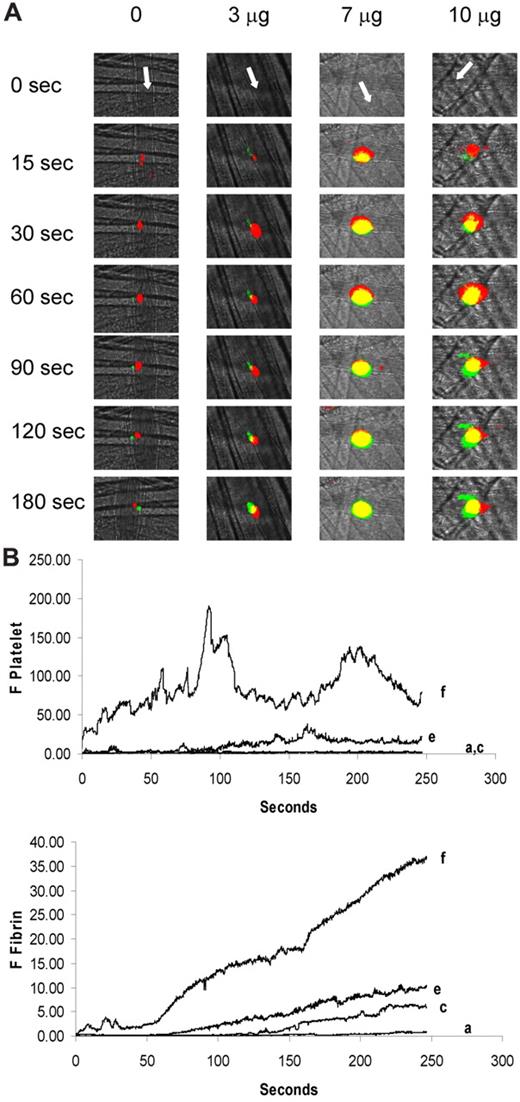
To determine whether fibrin generation paralleled enhanced platelet accumulation, we examined both fibrin and platelet accumulation during thrombus formation after infusion of purified anti–β2-GP1 antibodies at 3, 7, and 10 μg. As shown in the representative images in Figure 4A, anti–β2-GP1 antibodies from patient B increased contributions of both platelets and fibrin to thrombus size in a dose-dependent manner. The kinetics of platelet and fibrin accumulation demonstrate that 3, 7, and 10 μg of antibody increased fibrin generation by approximately 8-, 18-, and 70-fold, and platelet accumulation by approximately 2-, 11-, and 66-fold, respectively, compared with thrombi formed in mice treated with saline (Figure 4B).
Amplification of fibrin generation and platelet thrombus accumulation as a function of the quantity of anti–β2-GP1 autoantibody infusion. Anti–β2-GP1 autoantibodies from patient B at various doses were infused into wild-type mice 5-15 minutes before laser-induced arteriolar wall injury. Fab fragments of rat monoclonal anti–mouse CD42 antibody conjugated to Dylight 649 and mouse anti–human fibrin monoclonal antibodies labeled with Alexa 488 were also infused to allow fluorescence detection of the platelet thrombus and fibrin. (A) Representative images of the fluorescence signal associated with platelets (red) and fibrin (green) over 180 seconds after vessel injury are for mice treated with anti–β2-GP1 autoantibodies from patient B. Yellow represents merge. (B) The median integrated platelet fluorescence (F Platelet; top panel) and the median integrated fibrin fluorescence (F Fibrin; bottom panel) as a function of time in 3 to 5 mice after infusion of 0 μg (n = 25 thrombi), 3 μg (n = 23 thrombi), 7 μg (n = 19), or 10 μg (n = 24) of anti–β2-GP1 autoantibodies derived from patient B. a indicates 0 μg; c, 3 μg; e, 7 μg; and f, 10 μg.
Amplification of fibrin generation and platelet thrombus accumulation as a function of the quantity of anti–β2-GP1 autoantibody infusion. Anti–β2-GP1 autoantibodies from patient B at various doses were infused into wild-type mice 5-15 minutes before laser-induced arteriolar wall injury. Fab fragments of rat monoclonal anti–mouse CD42 antibody conjugated to Dylight 649 and mouse anti–human fibrin monoclonal antibodies labeled with Alexa 488 were also infused to allow fluorescence detection of the platelet thrombus and fibrin. (A) Representative images of the fluorescence signal associated with platelets (red) and fibrin (green) over 180 seconds after vessel injury are for mice treated with anti–β2-GP1 autoantibodies from patient B. Yellow represents merge. (B) The median integrated platelet fluorescence (F Platelet; top panel) and the median integrated fibrin fluorescence (F Fibrin; bottom panel) as a function of time in 3 to 5 mice after infusion of 0 μg (n = 25 thrombi), 3 μg (n = 23 thrombi), 7 μg (n = 19), or 10 μg (n = 24) of anti–β2-GP1 autoantibodies derived from patient B. a indicates 0 μg; c, 3 μg; e, 7 μg; and f, 10 μg.
Discussion
β2-GP1, with a molecular weight of approximately 50 000, is an abundant plasma protein that circulates at a concentration of approximately 170 μg/mL. Its function is unknown, and humans with hereditary deficiency of β2-GP1 have no phenotype.26,27 Similarly, mice lacking β2-GP1 have no obvious abnormalities, although an in vitro defect in thrombin generation has been suggested.28 Autoantibodies reactive against this protein have proven useful as clinical biomarkers of the antiphospholipid syndrome, and prior in vitro and in vivo studies have suggested that antiphospholipid antibodies, including antibodies to β2-GP1, may play an important role in the pathogenesis of thrombosis.
Our results demonstrated that small amounts of naturally occurring anti–β2-GP1 autoantibodies of the IgG class derived from antiphospholipid syndrome sera of patients with prior thromboembolic events are sufficient, in the absence of additional antibodies, to markedly potentiate thrombus size in a laser-induced vascular injury model. This provides, for the first time, a direct demonstration of the ability of this naturally occurring polyclonal autoantibody to enhance thrombus propagation in an in vivo model. In the absence of vascular injury, this autoantibody has no acute effect on inducing thrombosis. Although we have only studied the sera from 3 patients, it would appear that, in these sera, IgG autoantibodies other than anti–β2-GP1 autoantibodies are not required for enhanced thrombus formation.
Previous studies of thrombosis associated with antiphospholipid syndrome in animal models have taken 2 separate approaches. In the first approach, total IgG or IgM from patients with antiphospholipid syndrome or monoclonal antibodies derived from subpopulations of patient monocytes were used. Because the polyclonal autoantibodies contain multiple antiphospholipid antibodies, it is not possible to specifically define the role of anti–β2-GP1 autoantibodies. In the second approach, antibodies prepared in mice immunized with human β2-GP1 were used. These polyclonal or monoclonal antibodies, developed by immunizing mice with pure human β2-GP1, are not autoantibodies and may have specificities for epitopes that differ considerably from those recognized by naturally occurring human anti–β2-GP1 autoantibodies. Therefore, they may not possess the pathogenic potential of those anti–β2-GP1 autoantibodies derived from humans with antiphospholipid syndrome.
In prior studies, polyspecific antibodies against human β2-GP1 have been passively introduced into a live mouse and found to increase the incidence of fetal demise without an increased risk of thrombosis.29 Immunization of mice with human β2-GP1 resulted in elevated levels of antibodies directed against negatively charged phospholipids29,30 and prolongation of the activated partial thromboplastin time, thrombocytopenia, and fetal resorption. However, immunization of mice with human β2-GP1 led to less than a 2-fold increase in thrombus size in a pinch model of venous thrombosis.17 Jankowski et al, using a mouse monoclonal antibody against human β2-GP1, showed in a photochemically induced thrombosis model in the hamster carotid artery that this antibody and its Fab′2 amplified thrombus size, in contrast to the monomeric Fab fragment.18
IgG fractions of antiphospholipid patient sera have been isolated and used in animal thrombosis models. Infusion of purified IgG and IgM from a patient with antiphospholipid syndrome into a mouse resulted in increased fetal loss but no evidence of thrombosis.31 In contrast, some studies showed the thrombogenicity of IgG and IgM purified from sera of patients with antiphospholipid syndrome,13 whereas others demonstrated thrombocytopenia but not thrombosis.32 Human monoclonal antibodies derived from immortalized monocytes from a patient with antiphospholipid syndrome were reactive with cardiolipin but not with β2-GP1.15 One of these monoclonal antibodies, introduced intraperitoneally at high concentration into a mouse, led to increased thrombus size after pinch injury to the femoral vein. In vitro studies showed that this antibody, which bound to cardiolipin, required a serum factor found in bovine serum that could not be substituted by β2-GP1. Additional monoclonal antibodies reactive with cardiolipin as well as β2-GP1 were thrombogenic in this model.16 The IgG fraction of antiphospholipid sera injected intra-arterially into a rat treated with lipopolysaccharide led to thrombi formation in the microvasculature, but removal of β2-GP1 antibodies blocked this effect.14 Domain I33 and domain V34 of β2-GP1 have been shown to inhibit thrombus formation in a mouse model.
The current work demonstrates that purified autoantibodies against β2-GP1 from patients with antiphospholipid syndrome have the capacity to greatly enhance thrombus formation that is triggered by an initiating event. The molecular mechanism by which anti–β2-GP1 autoantibodies potentiate thrombus formation is unknown. This quantitative in vivo model using a purified autoantibody offers an approach to identification of the target antigen in antiphospholipid syndrome and discovery of an inhibitory agent that will block thrombus potentiation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.A. and V.P. designed and performed the experiments, analyzed the results, and edited the manuscript; R.A.F. analyzed the results and edited the manuscript; and B.F. and B.C.F. designed the experiments, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Furie, Department of Medicine, Harvard Medical School, Division of Hemostasis-Thrombosis Beth Israel Deaconess Medical Center, Center For Life Science, Rm 903, 3 Blackfan Cir, Boston, MA 02115; e-mail: bfurie@bidmc.harvard.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal