Abstract
Identification of genes involved in in vitro differentiation induction of embryonic stem cells (ESCs) into hematopoietic stem cells (HSCs) has been challenged during last decade. To date, a homeobox transcription factor Hoxb4 has been only demonstrated to possess such an effect in mice. Here, we show that HSC-like cells were efficiently induced from mouse ESCs by enforced expression of Lhx2, a LIM-homeobox transcription factor. Transduction of Lhx2 into ESC-derived mesodermal cells resulted in robust differentiation of c-Kit+/Sca-1+/Lineage− (KSL) cells in vitro. The KSL cell induction frequency was superior to the case of Hoxb4. Furthermore, transplantation of Lhx2-transduced hematopoietic cells into lethally irradiated mice resulted in multilineage repopulation of hematopoietic cells over 4 months. Transduction of Lhx2 into induced pluripotent stem cells (iPSCs) was also effective in generating KSL cells in vitro, as well as HSC-like activities in vivo. These results demonstrate that ectopic expression of Lhx2 confers an in vivo engrafting capacity to ESC/iPSC-derived hematopoietic cells and in vivo behavior of iPSC-derived hematopoietic cells is almost identical to that of ESC-derived cells.
Introduction
Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) can be differentiated into various types of cells in the body.1 Establishment of human iPSCs has led to new clinical strategies using iPSCs as universal sources for regeneration therapy for damaged organs and tissues.2 On the other hand, vigorous self-renewal of iPSCs could pose a risk for uncontrolled cell behavior in vivo, potentially resulting in teratoma formation. Therefore, lineage-directed in vitro differentiation of iPSCs into a desirable cell type is critical for their use in therapeutic applications.
Among the various methods for in vitro differentiation of ESCs/iPSCs, reliable protocols for hematopoietic cell differentiation have already been established, for example, embryoid body formation,3 culture on type-IV collagen-coated plates,4 coculture with OP9 stromal cells,5,6 and combinations of these approaches.7
In mammals, hematopoiesis is supported by a small number of hematopoietic stem cells (HSCs) from which more than 8 distinct lineages of mature blood cells are continuously produced.8 HSCs possess a capacity for self-renewal, which makes it possible to sustain hematopoiesis throughout life. Experimentally, HSCs are defined as those cells exhibiting the long-term repopulating (LTR) activity of multilineage hematopoietic cells when transplanted into lethally irradiated adult mice. LTR-HSCs give rise to short-term repopulating HSCs followed by the production of lineage-restricted hematopoietic progenitor cells (HPCs).
During in vitro ESC differentiation to the hematopoietic lineage, Flk1+ mesodermal cells, including hemangioblasts, are differentiated from ESCs.9 HPCs are then generated from these Flk1+ cells. Although primitive erythrocytes and HPCs are serially emerged, LTR-HSCs are not efficiently generated from ES cells without genetic manipulation, that is, overexpression of Hoxb4.10 To amplify the iPSC-derived HPCs, we focused on the Lhx2 (LH2) gene, which encodes a LIM-homeobox transcription factor, because enforced expression of Lhx2 has been reported to facilitate ex vivo expansion of adult bone marrow HSCs and to immortalize ESC-derived HPCs.11,12 Studies of Lhx2-deficient mice have revealed that Lhx2 is involved in cell fate determination in a wide variety of organs and tissues.13-15 In particular, Lhx2 is required for the development and preservation of hair follicle stem cells.16
Here, we show that the enforced expression of Lhx2 conferred HSC-like ability to mouse ESC/iPSC-derived hematopoietic cells. These HSC-like cells could colonize bone marrow and continuously produce multilineage hematopoietic cells for more than 4 months when transplanted into lethally irradiated recipient mice. These findings suggest that Lhx2 might become a novel molecular tool to amplify HSCs/HPCs derived from ESCs/iPSCs.
Methods
Cell culture
Two mouse ESC lines, C57BL/6-derived RENKA and 129/Ola-derived E14tg2a, were used in this study. RENKA ESCs and iPSCs were maintained on mitomycin C (Sigma-Aldrich)–treated mouse embryonic fibroblasts (MMC-MEFs) in DMEM-based ES-medium that contained DMEM (Sigma-Aldrich) supplemented with 15% FCS (JRH biosciences), 0.1mM nonessential amino acids (Invitrogen), 1mM sodium pyruvate (Invitrogen), 0.1mM 2-mercaptoethanol (Sigma-Aldrich), 50 μg/mL penicillin, 75 μg/mL streptomycin, and ESGRO (Invitrogen). E14tg2a ESCs were cultured on 0.1% gelatin-coated plates in GMEM-based ES-medium in which Glasgow modified essential medium (GMEM; Sigma-Aldrich) and leukemia inhibitory factor (LIF) were used instead of DMEM and ESGRO, respectively. LIF was prepared from the supernatant of CHO-LIF cells expressing exogenous LIF. OP9 stromal cells were maintained in OP9-medium containing α-MEM (Invitrogen) supplemented with 20% FCS, nonessential amino acids, penicillin, and streptomycin. The PLAT-E retrovirus packaging cell line was maintained in DMEM supplemented with 10% FCS, penicillin, and streptomycin. All microphotographic images were obtained using an Olympus Provis microscope with a UPlan APO 40×/0.85 numeric aperture objective lens, an Olympus DP70 camera, and DC controller software ViewFinder 7.1 (all from Olympus) under ambient conditions. The images were processed using Adobe Photoshop element 6 (Adobe Systems).
In vitro hematopoietic differentiation of mouse ESCs/iPSCs
In vitro differentiation of hematopoietic cells from ESCs/iPSCs was performed by coculturing with OP9 stromal cells.17 In some experiments, day-5 differentiated cells were used for retroviral transduction experiments. Thereafter, 1-2 × 105 of day-5 differentiated cells were subcultured on OP9 cells in each well of 6-well plates in the presence of appropriate cytokines. To expand the KSL cells, 10 ng/mL murine IL-6 (Peprotech) and 50 ng/mL murine SCF (kindly provided by Kirin Brewery, Takasaki, Japan) were added to the culture. To induce erythroid cells, megakaryocytes, and myeloid cells, 2 U/mL human erythropoietin (EPO; Kirin), 10 ng/mL human thrombopoietin (TPO; Kirin), 10 ng/mL human macrophage colony stimulating factor (M-CSF; Morinaga milk), 10 ng/mL murine GM-CSF (Peprotech), or murine IL-3 were added, beginning on day 5 of differentiation. IL-3 was produced from the X63 melanoma cell line, a kind gift from Dr H. Karasuyama (Tokyo Medical and Dental University, Tokyo, Japan). Between day 8 and day 10 of the in vitro differentiation, floating and loosely attached cells were collected by vigorous pipetting and reseeded onto OP9 cells.
FACS analyses and colony assays
The in vitro differentiated hematopoietic cells were harvested by gentle pipetting. The cells were stained with various antibodies (supplemental methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After the multicolor staining, the hematopoietic cells were analyzed by FACSCalibur or FACSAria (BD Bioscience). Optical filter sets were installed to detect EGFP and EYFP simultaneously (535AF LP mirror/520DRLP BP filter for EYFP and 510AF LP mirror/502LP BP filter for EGFP). The flowcytometric data were analyzed using FlowJo Version 4.6 software (TreeStar).
The cells (104) were cultured in Methocult M3434 containing IL-6, IL-3, SCF, and EPO (StemCell Technologies) supplemented with TPO for 7 days. For the colony formation assays, Lhx2-transduced hematopoietic cells were sorted from the bone marrow of recipient mice 12 weeks after transplantation, and 2 thousand cells were used in each assay.
The cells were adhered to slides using Cytospin 4 (Thermo Shandon), followed by staining with Diff-Quick solution (Sysmex). Images were analyzed using an inverted microscope (FSX-100; Olympus), equipped with a ×40 objective lens at room temperature in air. The images were processed using Adobe Photoshop element 6.
PCR and Western blotting
Total RNA was recovered using a RNeasy mini kit (QIAGEN) and examined by RT-PCR using the Thermoscript RT-PCR system (Invitrogen). In some experiments, cDNAs were serially diluted and amplified. Gapdh or β-actin were used as a template control. To analyze immunoglobulin rearrangement, 4 × 105 cells were lysed in buffer containing 60 μg/mL proteinase K (Nacalai Tesque), 0.1% Triton X-100, and 10mM Tris-HCl (pH 8.0); incubated at 55°C for 60 minutes; and denatured at 95°C for 12 minutes. Genomic PCR was performed using rTaq polymerase (TOYOBO). The gene fragments for the immunoglobulin heavy chain (IgH) and light chain (IgL) were amplified using primers for VHγ3.8 and JH4 and Vλ1 and Jλ1,3, respectively.18 All of the primer sequences are listed in supplemental Table 1. The amplified DNA were visualized by ethidium bromide staining. Westen blotting was carried out as previously described.19
Plasmids
The retroviral vector for Lhx2 was constructed by inserting mouse Lhx2 cDNA, a kind gift from Dr R. A. Maurer (Oregon Health & Sciences University), into pMY.IRES.EGFP vector (kind gifts from Dr T. Kitamura, University of Tokyo Institute of Medical Science). The FLAG tag was inserted into N-terminal terminus of Lhx2 to construct pMY.FLAG.Lhx2.IRES.EGFP. The retroviral vector pMY.IRES.EYFP was constructed by replacing the EGFP gene of pMY.IRES.EGFP with an EYFP gene purchased from Clontech. Mouse Hoxb4 cDNA, a king gift from Dr A. Iwama (Chiba University), was inserted into pMY.IRES.EYFP. Retroviral vectors for expressing the 4 factors described by the Yamanaka group20 (pMXs-Oct3/4, pMXs-Sox2, pMXs-Klf4, and pMXs-c-Myc) were purchased from Addgene.
Retroviral transduction
The retroviral vectors were transfected into PLAT-E cells using Lipofectamine 2000 (Invitrogen) or FuGene HD (Roche), according to the manufacturers' instructions. ESC/iPSC-derived differentiated cells were inoculated by spin-infection. Five to 10 × 105 of day 5 differentiated cells were suspended in 2 mL of filtered virus supernatant in the presence of 8 μg/mL polybrene (Sigma-Aldrich) and transferred into 1 well of a 24-well plate. The plate was then centrifuged at 1100g for 2 hours at 25°C and the cells were seeded onto OP9 cells.
Establishment of mouse iPSCs
We established 2 new iPSC lines (clone 24 and clone 2) from E13.5 MEFs harvested from C57BL/6 mice (Nihon SLC). Introduction of the 4 factors described by the Yamanaka group was carried out as previously described.20 Alkaline phosphatase staining was carried out using a leukocyte alkaline phosphatase kit (Sigma-Aldrich).
Transplantation experiments
Floating and lightly adherent hematopoietic cells were collected by mild pipetting. The remaining hematopoietic cells underneath the OP9 stromal cells were collected by 0.1% trypsin. The cells were mixed, centrifuged and re-seeded onto gelatin-coated dishes for 30 minutes to remove the OP9 cells. The supernatant was recovered, passed through a cell strainer (BD Bioscience), and resuspended in PBS(-). One to 2 × 106 cells were injected into the lateral tail vein of lethally (10.4 Gy of X-ray) irradiated Ly5.1-C57BL/6 mice (10-16 weeks old). Secondary transplantation experiments were carried out using EGFP+ cells sorted from the bone marrow of primary recipient mice 12 weeks after the transplantation. Cells (3 × 105) were transplanted into each recipient. All animal protocols were approved by the Animal Care and Use Committee of the Tokyo Metropolitan Institute of Medical Science.
Results
In vitro hematopoietic induction from mouse ESCs
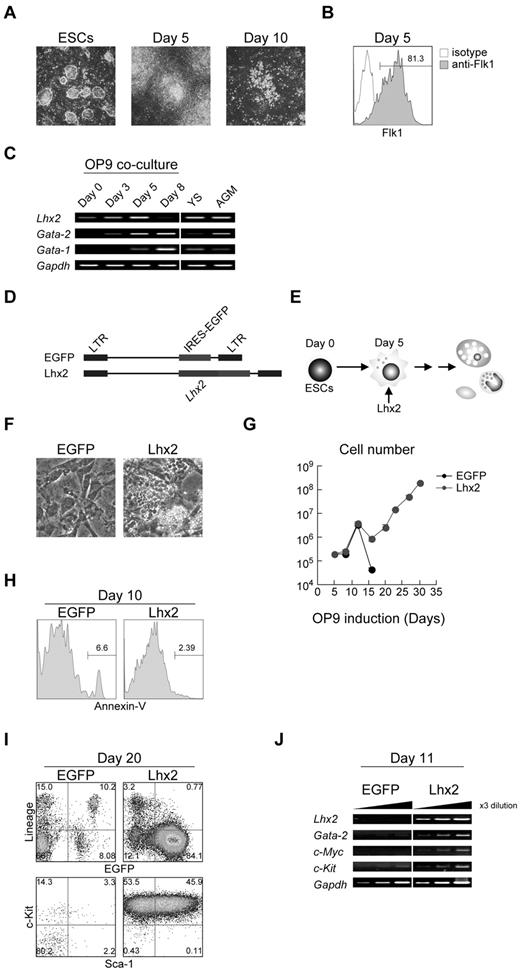
In this study, we have used the OP9 coculture induction method to differentiate mouse ESCs/iPSCs into the hematopoietic lineage.17 This coculture method has been widely used for hematopoietic induction. When ESCs were put onto OP9 stromal layer, mesodermal cell clusters emerged around day 5 of the induction (Figure 1A). Essentially all of the ESC-derived cells on day 5 were Flk1+ (Figure 1B). These cells were harvested and subcultured on fresh OP9 cells. Around days 6 to 8, HPC-like cells emerged. These cells were differentiated into mature hematopoietic cells around days 10-14 (Figure 1A) and subsequently underwent apoptotic cell death.
Robust production of HPCs from mouse ESCs by enforced expression of Lhx2. (A) In vitro hematopoietic differentiation of ESCs by OP9 coculture. Undifferentiated ESCs, day 5–, and day 10–induced cels were shown. Original magnification: ×4. (B) Flk1 expression on day 5. (C) The RT-PCR analysis of Lhx2, Gata-1 and Gata-2 expression during ESC differentiation and at hematopoietic sites. YS and AGM indicate yolk sac and aorta/gonad/mesonephros region at E10.5, respectively. (D) Retroviral vectors used in this study. (E) Study design. Lhx2 was transduced on day 5. (F) Microscopic analysis of EGFP- and Lhx2-transduced cells. Original magnification: ×4. (G) Growth curves of hematopoietic cells from ESCs transduced with EGFP or Lhx2. (H) Annexin-V staining. EGFP+ cells were shown. (I) FACS analysis of KSL cells derived from RENKA ESCs. The relative frequencies (%) are shown in the corners. (J) The RT-PCR analysis Lhx2, Gata-2, c-Myc, and c-Kit.
Robust production of HPCs from mouse ESCs by enforced expression of Lhx2. (A) In vitro hematopoietic differentiation of ESCs by OP9 coculture. Undifferentiated ESCs, day 5–, and day 10–induced cels were shown. Original magnification: ×4. (B) Flk1 expression on day 5. (C) The RT-PCR analysis of Lhx2, Gata-1 and Gata-2 expression during ESC differentiation and at hematopoietic sites. YS and AGM indicate yolk sac and aorta/gonad/mesonephros region at E10.5, respectively. (D) Retroviral vectors used in this study. (E) Study design. Lhx2 was transduced on day 5. (F) Microscopic analysis of EGFP- and Lhx2-transduced cells. Original magnification: ×4. (G) Growth curves of hematopoietic cells from ESCs transduced with EGFP or Lhx2. (H) Annexin-V staining. EGFP+ cells were shown. (I) FACS analysis of KSL cells derived from RENKA ESCs. The relative frequencies (%) are shown in the corners. (J) The RT-PCR analysis Lhx2, Gata-2, c-Myc, and c-Kit.
The expression of Lhx2 mRNA was next examined. The undifferentiated ESCs expressed a lower amount of endogenous Lhx2 mRNA (Figure 1C). The level of Lhx2 mRNA gradually increased as the cells differentiated into mesodermal cells over 5 days, and Lhx2 expression was undetected in hematopoietic cells on day 8 (Figure 1C).
In vitro induction of KSL cells from ESCs by enforced expression of Lhx2
Differentiating ESCs were infected with a retroviral vector carrying Lhx2 (Lhx2) and an empty vector (EGFP) on day 5, and the cells were then further cultured on OP9 in the presence of SCF and IL-6 (Figure 1D-E), since Lhx2-transduced, ESC-derived HPCs have been shown to preferentially grow in the presence of SCF and IL-6.21 In control, almost all hematopoietic cells were fully differentiated and subsequently disappeared by completing terminal differentiation by 2 weeks of the differentiation induction. Consequently, only OP9 cells were present in culture on day 20 (Figure 1F). In contrast, the Lhx2 transduction gave rise to cells that continued to proliferate. Some cells grew underneath OP9 stromal cells, which was known as pseudoemperiopolesis, indicating that these cells were immature hematopoietic cells (Figure 1F). In control, the differentiated cells stopped increasing by day 14 (Figure 1G). In contrast, the Lhx2-transduced cells were continuously increased (Figure 1G). The Lhx2 transduction did not affect cell death (Figure 1H), suggesting that the continued cell growth by Lhx2 would largely result from the enhanced cell proliferation.
Next, the increasing cells were analyzed. FACS analyses revealed that almost all Lhx2-transduced EGFP+ cells were lineage negative (Lin−; Figure 1I). Strikingly, approximately 50% of the Lin− fraction consisted of c-Kit+/Sca-1+ cells (designated KSL cells) and the rest were c-Kit single positive (KL cells; Figure 1I). These results are contradictory, however, to a previous report by Pinto do et al showing that Lhx2-transduced HPC cell lines derived from ESCs expressed c-Kit but not Sca-1.12 Presumably, environmental factors provided by OP9 cells could contribute to this difference, as Pinto do et al used an embryoid body formation protocol.12 Similar results were obtained when E14tg2a ESCs were used (supplemental Figure 1A). The molecular analysis showed that the Lhx2-transduced cells expressed Gata-2, c-Myc, and c-Kit, all of which are known to be expressed in immature hematopoietic cells (Figure 1J).
Establishment of C57BL/6 mouse-derived iPSCs
Because iPSCs are an important cell source for regenerative medicine, we have challenged whether the Lhx2 transduction would be effective in iPSCs. Mouse iPSC lines were newly established from MEFs from C57BL/6 strain using the 4 factors described by Takahashi et al, Oct3/4, Sox2, Klf4, and c-Myc.22 We confirmed that established iPSCs were positive for representative ESC markers (supplemental Figure 2A-B). In addition, the iPSCs were capable of producing various lineages of hematopoietic cells in vitro (supplemental Figure 2C-E). Next, the established iPSCs were differentiated in vitro and transduced with Lhx2. Very similar to the ESCs, on day 20, most of the Lhx2-transduced cells displayed a KSL/KL phenotype (supplemental Figure 2F) and the iPSC-derived KSL cells were morphologically blastic (supplemental Figure 2G). Approximately 107 KSL/KL cells were obtained from 104 iPSCs by Lhx2 transduction and 20 days of the differentiation induction culture.
Comparison between Lhx2 and Hoxb4
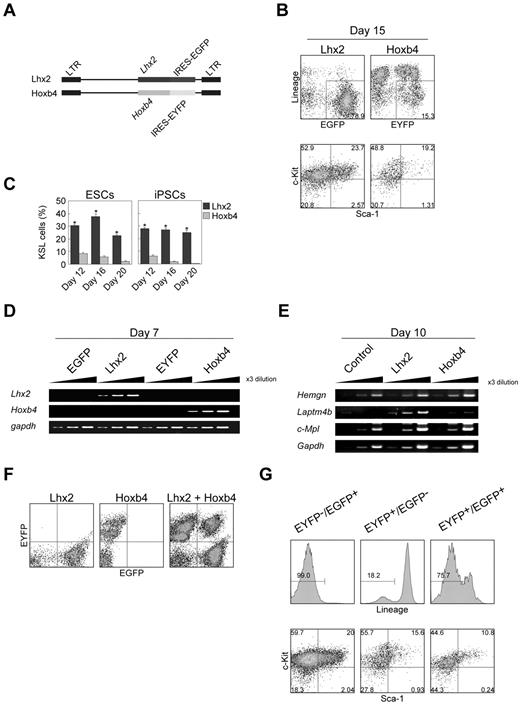
Previously, it has been reported that over-expression of Hoxb4 induced HSCs from ESCs.10 Therefore, we carried out side-by-side comparison between Hoxb4 and Lhx2 under our experimental conditions. The expression of exogenous Lhx2 and Hoxb4 was monitored by EGFP and EYFP fluorescence, respectively (Figure 2A).
Comparison between Lhx2 and Hoxb4. (A) Retroviral vectors. (B-C) Frequencies of KSL cells on day 15 (B) and between day 12 and day 20 (C). The data were shown as mean ± SD (n = 6). Asterisks indicate significant differences (P < .01 by t test). (D-E) The RT-PCR analysis of Lhx2 and Hoxb4 expression (D), and Hoxb4 target genes (E). (F) Verification of the separation of EGFP/EYFP fluorescence. (G) KSL frequency of Lhx2 (EYFP−/EGFP+)–, Hoxb4 (EYFP+/EGFP−)–transduced and cotransduced (EYFP+/EGFP+) cells. Cells within Lin− compartments were shown in bottom panels.
Comparison between Lhx2 and Hoxb4. (A) Retroviral vectors. (B-C) Frequencies of KSL cells on day 15 (B) and between day 12 and day 20 (C). The data were shown as mean ± SD (n = 6). Asterisks indicate significant differences (P < .01 by t test). (D-E) The RT-PCR analysis of Lhx2 and Hoxb4 expression (D), and Hoxb4 target genes (E). (F) Verification of the separation of EGFP/EYFP fluorescence. (G) KSL frequency of Lhx2 (EYFP−/EGFP+)–, Hoxb4 (EYFP+/EGFP−)–transduced and cotransduced (EYFP+/EGFP+) cells. Cells within Lin− compartments were shown in bottom panels.
On day 15, more than half of the hematopoietic cells derived from Hoxb4-transduced cells were Lin+, whereas almost no cells were Lin+ after Lhx2 transduction (Figure 2B). The frequency of KSL cells in Hoxb4-transduced cells was gradually decreased during cultivation (Figure 2C). In contrast, the population of KSL cells was relatively constant in the case of Lhx2 (Figure 2C).
We next evaluated whether Lhx2 would regulate Hoxb4 expression and candidate Hoxb4 target genes or not. The enforced expression of Lhx2 did not up-regulate Hoxb4 mRNA, and Hoxb4 was also ineffective in inducing Lhx2 expression in differentiating ESCs (Figure 2D). Hemgn and Laptm4b, which have been shown to be Hoxb4 target genes in primary murine Lin− bone marrow cells and hematopoietic progenitor cells line EML, respectively,23,24 were not up-regulated by Hoxb4 in the ESC differentiation induction (Figure 2E). The expression of c-Mpl which has been shown to be up-regulated in embryoid bodies with inducible Hoxb4 expression25 was also unaffected (Figure 2E). These data suggest that trans-activation targets of Hoxb4 were cell context dependent. Notably, Laptm4b was up-regulated by Lhx2 (Figure 2E), suggesting some roles of Lhx2 on Laptm4b regulation.
We next examined whether there was synergism between Lhx2 and Hoxb4. Cells cotransduced with the Lhx2 and Hoxb4 retroviral vectors were separated using EGFP and EYFP, respectively (Figure 2F). In the case of cells expressing either Lhx2 or Hoxb4 alone, 99.0% and 18.2% of the ESC-derived hematopoietic cells were Lin−, respectively (Figure 2G). ESCs transduced with both genes exhibited an intermediate phenotype, that is, 75.7% were Lin− (Figure 2G). In addition, the frequency of KSL cells was decreased in the cotransduced cells compared with the Lhx2-transduced cells (Figure 2G). Therefore, the KSL cell induction activities of Lhx2 and Hoxb4 are not synergistic.
Differentiation ability of Lhx2-transduced hematopoietic cells in vitro
Differentiation potentials of Lhx2-transduced hematopoietic cells were next analyzed. It has been reported that bone marrow KL cells can be subdivided into lineage-restricted HPCs, common myeloid progenitors (CMPs), granulocyte/macrophage progenitors (GMPs), and megakaryocyte/erythrocyte progenitors (MEPs).26 The Lhx2-transduced KL cells derived from ESCs contained these HPCs (Figure 3A).
Composition and differentiation of Lhx2-transduced hematopoietic cells. (A) FACS analysis of lineage-restricted HPCs derived from ESCs. (B-C) Morphology of representative colonies (B) and colony forming units per 104 cells (C) of uninfected and Lhx2-transduced cells derived from ESCs. Original magnification: ×4. The data were shown as mean ± SD (n = 5). (D-E) FACS analysis (D) and May-Grünwald-Giemsa staining (E) of the cells differentiated from Lhx2-transduced KSL cells derived from ESCs. Original magnification: ×40. (F) Effects of Lhx2 on erythroid production from ESCs and on β-globin expression.
Composition and differentiation of Lhx2-transduced hematopoietic cells. (A) FACS analysis of lineage-restricted HPCs derived from ESCs. (B-C) Morphology of representative colonies (B) and colony forming units per 104 cells (C) of uninfected and Lhx2-transduced cells derived from ESCs. Original magnification: ×4. The data were shown as mean ± SD (n = 5). (D-E) FACS analysis (D) and May-Grünwald-Giemsa staining (E) of the cells differentiated from Lhx2-transduced KSL cells derived from ESCs. Original magnification: ×40. (F) Effects of Lhx2 on erythroid production from ESCs and on β-globin expression.
The Lhx2-transduced cells were next cultured in semi-solid media supplemented with EPO, TPO, IL-3, IL-6, and SCF. A much larger number of myeloid or mixed colonies were formed by Lhx2 transduction (Figure 3B-C). In addition, the Lhx2-transduced KSL cells were sorted and cultured on OP9 cells in the presence of EPO, TPO, or GM-CSF for 7 days. FACS and morphologic analyses confirmed the successful derivation of erythrocytes, megakaryocytes, and neutrophils (Figure 3D-E). Therefore, Lhx2 transduction did not interfere with the differentiation of these hematopoietic cell lineages.
We next examined whether primitive and definitive erythropoiesis would be affected by Lhx2 or not, since Hoxb4 has been known to affect primitive erythropoiesis. OP9 differentiation induction of ESCs produced primitive erythrocytes (EryP; CD71high/Ter-119−) and definitive erythrocytes (EryD; CD71high/Ter-119+) on day 8 and day 11, respectively (Figure 3F). In the presence of Lhx2, EryP and EryD could be detected, although their frequencies were decreased (Figure 3F). On day 8 and day 11, the cells expressed embryonic globin (βH1) and adult globin (βmaj), respectively (Figure 3F), which confirmed that these erythroid cells were EryP and EryD, respectively. These data showed that EryP and EryD were produced in the presence of Lhx2.
Differentiation and repopulation abilities of Lhx2-transduced hematopoietic cells in vivo
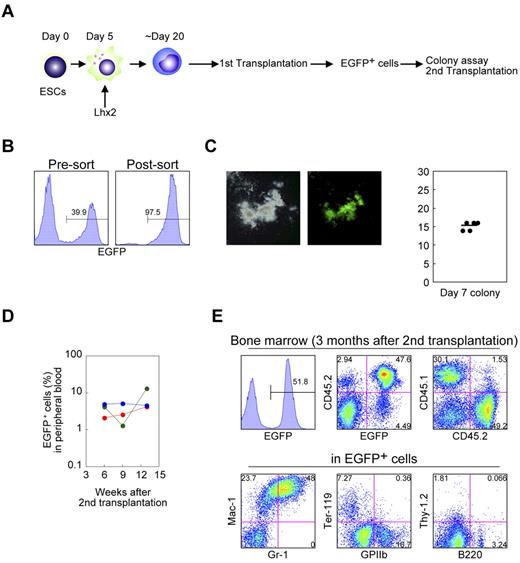
We next evaluated the differentiation potential of the Lhx2-transduced hematopoietic cells by transplantation experiments. The C57BL/6-derived RENKA ESCs were differentiated and transduced with Lhx2 (Figure 4A). Then the Lhx2-transduced cells (1-2 × 106; CD45.2+) were intravenously injected into lethally irradiated congenic CD45.1+ mice. The donor-derived cells were CD45.2+/EGFP+, while recipient hematopoietic cells were CD45.1+/EGFP− (Figure 4B).
In vivo repopulation by Lhx2-transduced hematopoietic cells derived from ESCs. (A) Study design. (B) CD45 isotype expression of donor and recipient cells. (C) Detection of the donor cells in peripheral blood. (D-F) FACS analysis of bone marrow and spleen of recipient mice. Representative data were shown.
In vivo repopulation by Lhx2-transduced hematopoietic cells derived from ESCs. (A) Study design. (B) CD45 isotype expression of donor and recipient cells. (C) Detection of the donor cells in peripheral blood. (D-F) FACS analysis of bone marrow and spleen of recipient mice. Representative data were shown.
Four weeks after transplantation, EGFP+ mononuclear cells were detected at 20% to 34% of the total peripheral blood cells of recipient mice (Figure 4C). In the bone marrow and spleen, EGFP+ cells contributed to the Mac-1+ myeloid cells, Ter-119+ erythroid cells, GPIIb+ megakaryocytes (Figure 4D). B220+ or CD19+ B-lymphoid cells of the donor origin were also detected (Figure 4E). More importantly, donor-derived KSL cells were present in the bone marrow (Figure 4F).
Next, LTR activity was assessed. Sixteen weeks after transplantation, approximately 30% of the hematopoietic cells in the bone marrow and spleen were donor origin in the representative transplanted mouse (supplemental Figure 3A- B). They contributed to myeloid, erythroid, megakaryocyte, and B-lymphoid populations, indicating the multilineage, long-term engraftment capacity of the ESC-derived hematopoietic cells by Lhx2 transduction. On the other hand, no donor cells were detected in the thymus (supplemental Figure 3C). The LTR activities were observed in multiple recipient mice (supplemental Table 2 and supplemental Figure 4A).
It would be predicted that long in vitro culture of cells might exhaust LTR activity. Therefore, we compared LTR activity of Lhx2-transduced cells between days 18 and 31 of the culture. Approximately 100-fold of amplification was seen between days 18 and 31 (supplemental Table 2). Donor cell chimerism in the transplanted mice was analyzed and the results were similar in the both cases (supplemental Figure 4B), indicating that the LTR activity was sustained by Lhx2 at least until day 31 of culture.
Next, we evaluated the functional activity of transplanted hematopoietic cells. EGFP+ cells were sorted from the bone marrow of primary recipient mice 12 weeks after transplantation and subjected to colony formation assays and secondary transplantation experiments (Figures 5A-B). These cells were capable of producing hematopoietic colonies (Figure 5C). Furthermore, EGFP+ cells contained secondary LTR activity (Figure 5D-E) The secondary LTR activity was seen in the multiple primary recipients. These data clearly excluded a possibility that the secondary engraftment ability could be because of retroviral integrative changes of the Lhx2-transduced cells.
Functional analysis of the Lhx2-transduced donor cells derived from ESCs. (A) Study design. (B) Cell sorting of the donor cells from the primary recipients. (C) Colony assays. EGFP+ cells in were cultured in semisolid media for 1 week. Representative colony was shown. The colony numbers per 2000 cells are shown as dots. The bar indicates the mean value. (D) Frequency of donor cells in peripheral blood of secondary recipients. (E) FACS analysis of bone marrow of secondary recipients. Representative data were shown.
Functional analysis of the Lhx2-transduced donor cells derived from ESCs. (A) Study design. (B) Cell sorting of the donor cells from the primary recipients. (C) Colony assays. EGFP+ cells in were cultured in semisolid media for 1 week. Representative colony was shown. The colony numbers per 2000 cells are shown as dots. The bar indicates the mean value. (D) Frequency of donor cells in peripheral blood of secondary recipients. (E) FACS analysis of bone marrow of secondary recipients. Representative data were shown.
No signs of myeloid proliferative disorder were observed in the recipient mice in clear contrast to the results observed in mice transplanted with Lhx2-transduced adult HSCs.27
In vivo nature of iPSC-derived hematopoietic cells
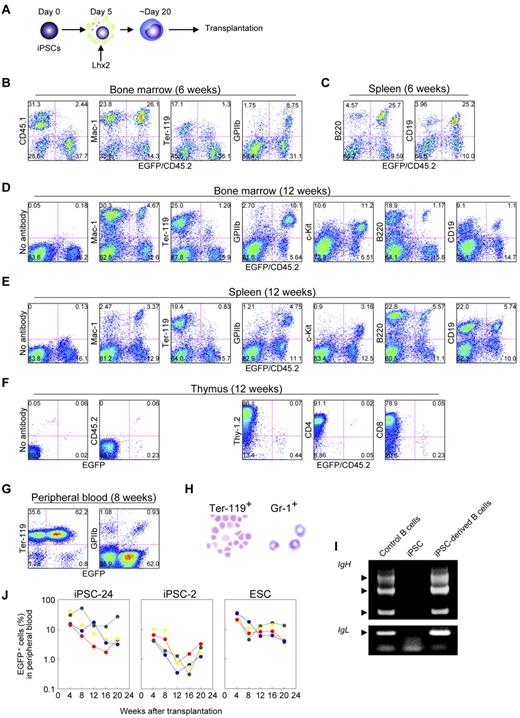
We next carried out the transplantation experiments using iPSCs. iPSCs were differentiated, hematopoietic cells were amplified by Lhx2, and transplanted (Figure 6A). Essentially similar data were obtained; the iPSC-derived hematopoietic cells contributed multilineage of hematopoietic cells at 6 weeks and 12 weeks after transplantation (Figure 6B-F, supplemental Table 2, and supplemental Figure 4A).
Transplantation analysis of iPSC-derived hematopoietic cells amplified by Lhx2. (A) Study design. (B and C) FACS analysis at 6 weeks after transplantation of iPSC-derived hematopoietic cells. (D-F) Long-term hematopoiesis repopulation by Lhx2-transduced hematopoietic cells derived from iPSCs (clone 24). (G) FACS analysis of peripheral blood of recipients transplanted with iPSC-derived cells. (H) May-Grünwald-Giemsa staining EGFP+/Ter-119+ cells and EGFP+/Gr-1+ cells. Original magnification: ×30. (I) Rearrangement of immunoglobulin gene loci in iPSC-derived B-lymphoid cells. EGFP+/B220+ cells were sorted from recipient mice transplanted with iPSC-derived hematopoietic cells and analyzed. (J) Long-term monitoring of chimerism in the peripheral blood of recipient mice transplanted with Lhx2-transduced hematopoietic cells derived from iPSC clone 24, clone 2, and RENKA ESCs.
Transplantation analysis of iPSC-derived hematopoietic cells amplified by Lhx2. (A) Study design. (B and C) FACS analysis at 6 weeks after transplantation of iPSC-derived hematopoietic cells. (D-F) Long-term hematopoiesis repopulation by Lhx2-transduced hematopoietic cells derived from iPSCs (clone 24). (G) FACS analysis of peripheral blood of recipients transplanted with iPSC-derived cells. (H) May-Grünwald-Giemsa staining EGFP+/Ter-119+ cells and EGFP+/Gr-1+ cells. Original magnification: ×30. (I) Rearrangement of immunoglobulin gene loci in iPSC-derived B-lymphoid cells. EGFP+/B220+ cells were sorted from recipient mice transplanted with iPSC-derived hematopoietic cells and analyzed. (J) Long-term monitoring of chimerism in the peripheral blood of recipient mice transplanted with Lhx2-transduced hematopoietic cells derived from iPSC clone 24, clone 2, and RENKA ESCs.
The iPSC-derived mature hematopoietic cells were next analyzed. In the recipients transplanted with iPSC-derived cells, EGFP+ erythrocytes and platelets were abundantly observed (Figure 6G). The EGFP+/Ter-119+ cells in the peripheral blood were enucleated erythrocytes and the EGFP+/Gr-1+ cells in the bone marrow were segmented neutrophils (Figure 6H). Thus, the Lhx2-transduced hematopoietic cells derived from iPSCs underwent terminal differentiation and the mature hematopoietic cells were morphologically normal. Furthermore, EGFP+/B220+ cells in spleen had passed through immunoglobulin gene rearrangement (Figure 6I). Taken together, these results demonstrate that iPSC-derived mature hematopoietic cells could be obtained by the Lhx2 transduction and transplantation.
Comparison between ESC-derived and iPSC-derived hematopoietic cells in vivo
We next compared nature of iPSC-derived cells with that of ESC-derived cells. First, the frequency of KSL cells induced by Lhx2 transduction were compared and no differences were seen (Figure 1I, supplemental Figure 2F). In the case of Hoxb4 transduction, the population of KSL cells from iPSCs was also comparable with that from ESCs (Figure 2C). Next, LTR activity was compared. After transplantation of Lhx2-transduced HSC-like cells derived from iPSC clone 24, donor-derived EGFP+ cells were detected for more than 20 weeks (Figure 6J). Essentially similar results were obtained in the case of ESC-derived HSC-like cells, although ESC-derived hematopoietic cells exhibited slightly higher chimerism (Figure 6J, supplemental Figure 4A). The transplantation of iPSC clone 2-derived HSC-like cells resulted in successful engraftment but the contribution was lower (Figure 6J), suggesting a clonal variation of iPSCs.
We did not detect donor-derived T-lymphoid cells in the recipient mice transplanted with Lhx2-transduced HSC-like cells derived from iPSCs (Figure 6F). The similar results were observed in the case of ESCs (supplemental Figure 3C), indicating that the failure of T-lymphoid contribution was not caused by the nature of iPSCs. It would be expected that Lhx2 might inhibit T-lymphoid differentiation.
Lhx2 expression in vivo
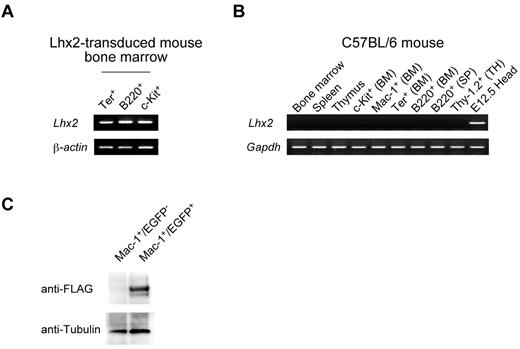
Finally, we confirmed the expression of exogenous Lhx2 in vivo, since it would be postulated that integrative proviral construct itself would act as modulators of transcription factors or epigenetic modulators. The Lhx2 mRNA could be detected in the EGFP+ differentiated cells of transplanted mice (Figure 7A), but not in control mice, indicating that endogenous Lhx2 mRNA was not expressed in any hematopoietic cell fractions examined (Figure 7B). To know protein expression, FLAG-tagged Lhx2 (FLAG.Lhx2) was transduced into ESC-derived cells, and the transduced HSC-like cells were transplanted. The donor-derived differentiated cells were purified, and analyzed by Western blotting (Figure 7C). The FLAG.Lhx2 fusion protein was specifically detected in EGFP+ differentiated hematopoietic cells, indicating that the retrovirally transduced Lhx2 was being translated in vivo.
Expression of Lhx2 in vivo. (A) Expression of Lhx2 mRNA in the donor-derived differentiated cells. The donor-derived erythroid, B-lymphoid and progenitor cells were purified from a recipient transplanted with ESC-derived cells and subjected to RT-PCR analyses. (B) The Lhx2 expression in normal mouse. BM, SP, and TH indicate bone marrow, spleen, and thymus, respectively. Head region dissected from E12.5 mouse embryos was a positive control. (C) Western blot of FLAG.Lhx2 expression in donor-derived differentiated cells. ESC-derived HSC-like cells were amplified by Flag.Lhx2 and transplanted. The donor-derived Mac-1+ cells and control Mac-1+ cells were purified and analyzed.
Expression of Lhx2 in vivo. (A) Expression of Lhx2 mRNA in the donor-derived differentiated cells. The donor-derived erythroid, B-lymphoid and progenitor cells were purified from a recipient transplanted with ESC-derived cells and subjected to RT-PCR analyses. (B) The Lhx2 expression in normal mouse. BM, SP, and TH indicate bone marrow, spleen, and thymus, respectively. Head region dissected from E12.5 mouse embryos was a positive control. (C) Western blot of FLAG.Lhx2 expression in donor-derived differentiated cells. ESC-derived HSC-like cells were amplified by Flag.Lhx2 and transplanted. The donor-derived Mac-1+ cells and control Mac-1+ cells were purified and analyzed.
Discussion
In this study, we demonstrated that Lhx2 conferred LTR capacity to ESC/iPSC-derived hematopoietic cells, with the exception of the T-lymphoid lineage. The new system we developed, that is, the Lhx2 transduction and subculture on OP9 stromal cells, may be useful for investigating the molecular mechanisms by which HSCs develop the pathways underlying lineage specification from pluripotent stem cells.
The multidifferentiation potentials of Lhx2-transduced hematopoietic cells
Our data presented here demonstrated that the Lhx2-transduced HSC-like cells were multipotent for the long term, raising a question of whether the maintenance of HSC-like cells by Lhx2 would be caused by the inhibition of hematopoietic differentiation or not. The data in the literature may provide some clues to the inhibitory actions. Lhx2 binds to Ldb1 (NLI), a LIM-domain binding protein,28 a known member of the Gata-1/2 multiprotein complex, which is composed of Gata-1/2, Ldb1, Scl, E47, and Lmo2.29 We have previously shown that Gata-1 knockout erythroid cells acquired multidifferentiation potentials.30 The data raise a possibility that multipotentials of the Lhx2-transduced HSC-like cells might be related to the function of Gata-1. However, the Lhx2-transduced HSC-like cells could produce mature hematopoietic cells in vitro and in vivo. As revealed by the data assessing the effects of Lhx2 expression on EryD production, the inhibitory action of Lhx2 on hematopoietic differentiation would be partial. Investigation of the interactions between Lhx2 and key hematopoietic regulators may clarify the molecular mechanisms underlying Lhx2-mediated HSC-like cell induction with maintaining their multipotentials.
Comparison between Lhx2 and Hoxb4
Hoxb4 has been known to induce engraftable HSCs from mouse ESCs10 and has also been used to produce iPSC-derived engraftable hematopoietic cells which were effective for treatment of a mouse model of sickle cell anemia.31 Like Hoxb4, Lhx2 also induced HSC-like cells from ESCs/iPSCs, suggesting that Lhx2 might be also theoretically useful for regenerative medicine. However, one study has demonstrated that HOXB4 is dispensable for inducing hematopoiesis from human ESCs.32 Therefore, whether or not LHX2 could induce HSC-like cells from human ESCs/iPSCs remains to be an important issue.
Understanding of the molecular function of Lhx2 as well as Hoxb4 in mice might be helpful for development of effective HSC-like cell induction protocol from human iPSCs. To gain a clue for understanding the Lhx2 and Hoxb4 functions, we carried out side by side comparison between these 2 factors. When Hoxb4 was used, approximately 14.6%, and 3.3% were Lin−−/c-Kit+ and Lin−/Sca-1+ cells on day 15 in the present study. These values were consistent with the data reported by others; Sca-1+ cells were 5.4%,10 c-Kit+ cells and Sca-1+ cells were 4.1% and 1.2%, respectively,33 and c-Kit+/Sca-1+ cells were 12.1%.34 Therefore, we were able to reproduce the Hoxb4 effects. compared with Hoxb4, the KSL cell induction efficiency of Lhx2 was superior. Notably, the Hoxb4 coexpression reduced KSL cell population in the Lhx2-transduced cells. The data raise 2 possibilities; Hoxb4 might inhibit Lhx2 activity or Hoxb4 might induce differentiation. Hoxb4 has been reported to preferentially induce myeloid differentiation from ESC-derived and yolk sac-derived reprogrammed HSCs,10,35 implying that later possibility seems likely.
Failure of T-lymphoid repopulation by Lhx2-transduced hematopoietic cells
Several papers have been reported that the Hoxb4-induced HSCs have low B/T-lymphoid repopulation activity in vivo.34,35 In contrast, the Lhx2-transduced HSC-like cells could be contributed into B-lymphoid cells even in the nonimmunocompetent recipient mice. Therefore, the Lhx2 transduction would be useful for investigating iPSC-derived B-lymphoid cells in vivo. On the other hand, the failure of the Lhx2-transduced HSC-like cells to differentiate into T-lymphoid cells raises the possibility that Lhx2 might disturb T cell differentiation. In Drosophila, Apterous, a Lhx2 homolog, has been shown to inhibit the function of the Gata factor Panier.36 Thus, one could speculate that Lhx2 might inhibit the function of Gata-3, a master regulator of T cell differentiation.37 Interestingly, both Lhx2 and Gata-3 have been implicated in stem cell maintenance in hair follicles16,38 and in eye development,14,39,40 suggesting a functional interaction between Lhx2 and Gata-3.
Investigation of hematopoietic cells derived from iPSCs by comparing with those from ESCs
Using the combination of OP9 differentiation induction and the Lhx2 transduction, we could investigate nature of hematopoietic cells derived from iPSCs by comparing with that from ESCs. All assays we carried out showed that the iPSC-derived hematopoietic cells displayed essentially identical behavior to the ESC-derived cells. The Lhx2-transduced HSC-like cells derived from both of ESCs and iPSCs conferred LTR ability. However, compared with ESC-derived cells, iPSC-derived hematopoietic cells exhibited slightly less contribution. The lower activities of the iPSC-derived cells might be caused by clonal variation rather than innate differences between ESC-derived and iPSC-derived cells, since the RENKA ESC line was selected based on its higher germ line transmission efficiency. In contrast, our iPSC clones were just established based on their ESC-like morphology.
Using Lhx2, we could investigate iPSC-derived cells in vivo. Thus, Lhx2 could be served as a novel tool for analyzing hematopoietic cells derived from various iPSCs established from different tissues, reprogrammed by different defined factors, and derived from genetically modified mice.
Notably, no teratomas have been observed to date in mice transplanted with Lhx2-transduced HSC-like cells derived from ESCs and iPSCs (4 months after transplantation). This suggests that a 2 step in vitro differentiation procedure (lineage-directed differentiation induction and selective amplification) could substantially eliminate the risk of tumorigenesis caused by undifferentiated ESCs/iPSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Authorship
Contribution: K.K. conceived ideas, performed most of the ESC and iPSC experiments, and analyzed the results; K.M. assisted with the transplantation experiments; K.S. provided the C57BL/6-derived ESC system; T.N. supervised the ES experiments; and K.K. and T.H. codirected the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenji Kitajima, PhD, Stem Cell Project Group, The Tokyo Metropolitan Institute of Medical Science, Tokyo Metropolitan Organization for Medical Research, 2–1-6 Kamikitazawa, Setagaya-ku, Tokyo 156-8506, Japan; e-mail: kitajima-kj@igakuken.or.jp; or Takahiko Hara, PhD, Stem Cell Project group, The Tokyo Metropolitan Institute of Medical Science, Tokyo Metropolitan Organization for Medical Research, 2–1-6 Kamikitazawa, Setagaya-ku, Tokyo 156-8506, Japan; e-mail: hara-tk@igakuken.or.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal