Abstract
We have investigated combining adoptive immunotherapy with cytokine-induced killer (CIK) cells and anti-CD20 monoclonal antibodies (mAb) GA101 or rituximab to optimize B-cell non-Hodgkin lymphoma (B-NHL) therapy. CIK cultures alone demonstrated significant cytotoxic activity against B-NHL cell lines or freshly isolated samples in either an autologous or allogeneic combination. This natural cytotoxicity (NC) was mainly due to the predominating CD3+CD56+ CIK population (40%–75%) present in the cultures. The addition of anti-CD20 mAb GA101 or rituximab further increased cytotoxicity by 35% and 15%, respectively. This enhancement was mainly due to antibody-dependent cytotoxicity (ADCC) mediated by the 1%–10% NK cells contaminating CIK cultures. The addition of human serum (HS) inhibited NK-cell activation induced by rituximab, but not activation induced by GA101.Overall lysis in presence of serum, even of a resistant B-NHL cell line, was significantly increased by 100 μg/mL of rituximab, but even more so by GA101, with respect to CIK cultures alone. This was due to the combined action of complement-mediated cytotoxicity (CDC), ADCC, and CIK-mediated NC. These data suggest that rituximab, and even more so GA101, could be used in vivo to enhance CIK therapeutic activity in B-NHL.
Introduction
Cytokine-induced killer (CIK) cells are activated T cells with natural killer (NK) properties that can be expanded in vitro in presence of recombinant human interleukin-2 (rhIL-2) starting from peripheral blood mononuclear cells stimulated by interferon-γ and anti-CD3 antibody.1 They express CD3 and CD56 as well as the NKG2D antigen and show major histocompatibility complex (MHC)–unrestricted cytotoxicity toward neoplastic but not normal targets.2-5 CIK cells express several chemokine receptors, and in vivo models suggest that they can migrate to the site of tumors after intravenous administration,6-10 there carrying out their cytotoxic potential and helping to control tumor growth.
The ease of CIK cell production in vitro and the cells' antitumor potential have made them suitable candidates for cell therapy programs in solid and hematopoietic tumor treatment. Indeed, both autologous and allogeneic CIK cells have been used in phase I and II clinical trials for the treatment of different tumor types. In these trials, they have shown limited toxicity in vivo, and evidence of antitumor activity has been seen.11-18 CIK cells have shown cytotoxic activity in vitro and in vivo against hematopoietic neoplastic cells, including AML (acute myeloid leukemia), CML (chronic myelogenous leukemia), and CLL (chronic lymphocytic leukemia).9,19-22 Their cytotoxicity against B-NHL (B-cell non-Hodgkin lymphoma), however, has not been fully investigated in vitro, although in vivo murine models suggest they may be active.1,23 Other biologic treatments available for B-NHL are the anti-CD20 antibodies, for instance, rituximab, and a new-generation glycoengineered type II GA101 antibody. These antibodies are thought to act through immune-mediated mechanisms.24-26 Rituximab induces complement-mediated cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and phagocytosis.24,27 In contrast, GA101, which is glycoengineered and defucosylated in its Fc portion, has diminished CDC, but enhanced ADCC compared with rituximab.25 ADCC mediated by NK cells is very effectively induced by GA101, because these cells express the FcγRIIIA receptor (CD16A), and GA101 binds with higher affinity to this receptor regardless of FcγRIIIA polymorphism at residue 158.25,28 Furthermore, recent data suggest that GA101 binding to CD16A is not inhibited by complement, in contrast to that of rituximab.29
In this report, we investigate whether CIK cells alone have cytotoxic potential against B-NHL targets in vitro and whether addition of rituximab or GA101 could increase the killing activity of CIK cell cultures toward these cellular targets. We show that both antibodies enhance killing, and that GA101 has superior cytotoxicity compared with rituximab.
Methods
Cells
Diffuse large B-cell lymphoma (SU-DHL4, WSU-NHL), follicular lymphoma (KARPAS 422, DOHH2), and Burkitt lymphoma (BJAB, DAUDI, RAJI, NAMALWA) cell lines were maintained in a combination of RPMI-1640 medium (Cambrex Bio Science) supplemented with 10% fetal bovine serum (Euroclone), 2mM l-glutamine (Euroclone), and 110μM gentamycin (PHT Pharma), hereafter called complete medium.
To generate Epstein-Barr virus–transformed lymphoblastoid cell lines (EBV-LCL), 5 × 106 peripheral blood mononuclear cells (PBMCs) from normal donors were exposed overnight to B95-8 virus supernatant and 1μg/mL cyclosporin A and expanded in complete medium.
PBMCs were acquired from B-NHL patients. Waldenström macroglobulinemia, follicular lymphoma, and mantle cell lymphoma cells were obtained by Ficoll Hypaque (Lympholyte-H; Cedarlane) gradient centrifugation after informed consent. CD19+ cells were immunoselected by passage through a separation column (MS MidiMacs; Miltenyi Biotec) with anti-CD19 microbeads (Miltenyi Biotec) according to the manufacturer instructions. Positive fractions of more than 90% CD19+ and CD20+ were cryopreserved and subsequently thawed to be used in the experiments, and negative fractions were used to generate CIK cultures.
Generation of CIK cells
CIK cells were prepared as described previously.30 Briefly, PBMCs were cultured in serum-free X-VIVO 15 medium (BioWhittaker) with 1000 U/mL interferon-γ (IFN-γ; Gammakine; Boehringer Ingelheim) added on day 0, 50 ng/mL anti-CD3 (OKT-3, Janssen-Cilag SpA, Italy) added on day 1, and 500 U/mL rhIL-2 (Proleukin, Chiron BV) included in the medium from day 1 onwards. Expansion was performed for 21 to 28 days. In some experiments, NK cells were removed at the beginning of the culture by CD56 immunodepletion.
Calcein release cytotoxicity assay
Cell lysis was evaluated using the calcein release assay as previously described.31 Briefly, lymphoma cell lines or patient derived lymphoma cells were labeled for 30 minutes at 37°C with 3.5μM calcein–acetoxymethyl ester (Calcein-AM; Fluka, Sigma-Aldrich Company). After washing, labeled target cells were distributed in 96-well plates at 5 × 103 cells per well. Cells of CIK culture were harvested, washed, and added at different effector-to-target (E/T) ratios in the presence or absence of different concentrations of rituximab, GA101, or the control irrelevant antibody trastuzumab (Roche). After 4 hours, the cells were sedimented by centrifugation, 100 μL of supernatant were collected and calcein release was determined using a fluorescence microplate reader (GENios; TECAN, Austria GmbH) with excitation at 485nm and emission at 535nm. The percentage of specific Calcein release was calculated using the following formula: percent specific lysis = (test release minus spontaneous release) times 100 divided by (maximal release minus spontaneous release). Maximal lysis was achieved with 1% Triton X-100. In some experiments, CD5+ CIK cells immunoselected at term of the expansion were used as effector cells.
FACS cytotoxicity assay
Cytotoxicity against the WSU-NHL cell line was measured using carboxyfluorescein diacetate succinimidyl ester (CFSE)–based cytotoxicity assays as previously described.32 Target cells were labeled with 5μM CFSE (Molecular Probes Europe). 20 × 103 labeled target cells were plated with 60 × 104 CIK cells (30:1 E/T ratio) in presence or absence of mAbs or 20% human serum (HS). After 4 hours of co-culture, fluorescence-activated cell sorting (FACS) analysis was performed. Cell death was measured as a decrease in the CFSE+/ 7-aminoactinomycin D (7-AAD)− population. In some experiments, a fixed volume of calibration beads was added to each sample before FACS analysis to measure the decrease in absolute number of CD19+/7AAD− cells. Equivalent results were obtained by measuring the relative or absolute decrease in WSU-NHL cells (data not shown). Analyses were performed using a FACScan instrument (BD Biosciences).
CD107a mobilization assay
NK-cell activation and degranulation were evaluated using the lysosomal marker CD107a as previously described.33,34 CIK cultures from normal donors or lymphoma patients were incubated with lymphoma cell lines or autologous primary lymphoma cells at a 1:1 effector-target (E/T) ratio in the presence of increasing concentrations of rituximab, GA101, and trastuzumab. After 3 hours, cells were washed with phosphate buffer solution (PBS) and stained with phycoerythrin anti-CD107a (BD Pharmingen), peridinin-chlorophyll-protein complex–conjugated anti-CD3, and allophycocyanin-conjugated anti-CD56 (BD Biosciences) to gate the different populations: CD3−CD56+ (NK), CD3+CD56+ (CIK) and CD3+CD56− (T). Percent CD107a positive cells for each population was evaluated using a FACSCantoI instrument (BD Biosciences). In some experiments, incubations were performed in presence of 20% HS with or without 200 μg/mL blocking anti-C5 mAb eculizumab (Soliris; Alexion Pharmaceuticals), 20% heat inactivated HS, or 400 μg/mL intravenous immunoglobulin (IVIG).
Immunofluorescence analyses
For complement deposition measurement, lymphoma cells were incubated in complete medium supplemented with 20% HS and increasing concentrations of mAbs for 1 hour at 37°C and then stained with Alexa488-labeled anti-C3 mAbs 1H8 specific for C3b/iC3b/C3dg and 7C12 specific for C3b/IC3b (a kind gift of Prof R. P. Taylor, University of Virginia School of Medicine, Charlottesville, VA).
Statistical analysis
The data were analyzed using paired or unpaired Student t tests, as appropriate.
Results
CIK cultures are cytotoxic for B lymphoma cells
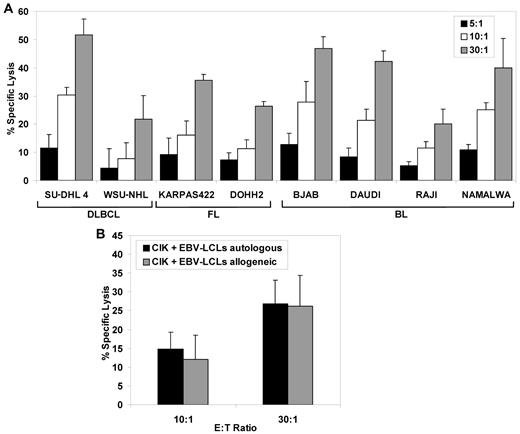
To investigate whether cells of CIK culture were cytotoxic against B-NHL targets, B-NHL target cell lines derived from patients with diffuse large B-cell lymphomas (DLBCLs), follicular lymphomas (FLs), or Burkitt lymphomas (BLs) were put in contact with cells of CIK culture at different E/T ratios and target cell killing measured after 4 hours by calcein release assays. As shown in Figure 1A, the cells of CIK cultures quite efficiently killed all B-NHL target cell lines tested, with 20% to 52% specific cell death observed in different cell lines at the 30:1 E/T ratio. In several experiments, SU-DHL4 was consistently the most responsive and WSU-NHL the least responsive cell line, respectively (Figure 1A and data not shown).
Cytotoxic activity of CIK cultures against malignant human B-cell lines. (A) Cytotoxicity of CIK against B lymphoma cell lines. A panel of diffuse large B-cell lymphoma (SU-DHL4 and WSU-NHL), follicular lymphoma (KARPAS422 and DOHH2), and Burkitt lymphoma (BJAB, DAUDI, RAJI and NAMALWA) cell lines was used as the target. Calcein-AM labeled target cells were co-incubated for 4 hours with CIK effector cells at effector-to-target ratios of 30:1, 10:1, and 5:1; the mean percentage of specific lysis ± SD from 3 independent experiments is shown. (B) Cytotoxicity of CIK cells against autologous and allogeneic lymphoblastoid B-cell lines. Three CIK effector cultures derived from normal donors, each against an autologous and allogeneic EBV-transformed B-cell line (EBV-LCL), were tested in standard 4 hours calcein-AM release assay at effector-to-target ratios of 30:1 and 10:1. Data are expressed as mean percentage ± SD of 3 independent experiments.
Cytotoxic activity of CIK cultures against malignant human B-cell lines. (A) Cytotoxicity of CIK against B lymphoma cell lines. A panel of diffuse large B-cell lymphoma (SU-DHL4 and WSU-NHL), follicular lymphoma (KARPAS422 and DOHH2), and Burkitt lymphoma (BJAB, DAUDI, RAJI and NAMALWA) cell lines was used as the target. Calcein-AM labeled target cells were co-incubated for 4 hours with CIK effector cells at effector-to-target ratios of 30:1, 10:1, and 5:1; the mean percentage of specific lysis ± SD from 3 independent experiments is shown. (B) Cytotoxicity of CIK cells against autologous and allogeneic lymphoblastoid B-cell lines. Three CIK effector cultures derived from normal donors, each against an autologous and allogeneic EBV-transformed B-cell line (EBV-LCL), were tested in standard 4 hours calcein-AM release assay at effector-to-target ratios of 30:1 and 10:1. Data are expressed as mean percentage ± SD of 3 independent experiments.
To determine whether allogeneic or autologous CIK cells differed in activity against transformed B-cell targets, we generated CIK cells and EBV-immortalized B-cell lines from several healthy donors. We then measured the cytotoxic potential of these CIK cultures against EBV-LCL in either the autologous or allogeneic combination (ie, derived from a unrelated donor). As shown in Figure 1B, both autologous and allogeneic EBV-LCL targets were killed by CIK cells to a similar extent, with 26% to 27% killing observed at the 30:1 ratio in both cases.
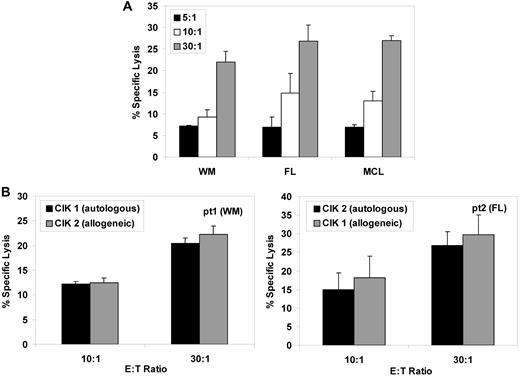
We then investigated whether freshly isolated primary B lymphoma targets could be killed by CIK cells. Primary neoplastic cells purified from 3 B-NHL patients were lysed by 22% to 27% at a 30:1 E/T ratio by CIK cells cultured from healthy normal donors (Figure 2A). CIK cultures were also be generated from CD19-depleted peripheral blood mononuclear cells from the same patients and were equally cytotoxic against autologous or allogeneic primary lymphoma cells (Figure 2B).
Expanded CIK cells from lymphoma patients present comparable cytotoxicity against autologous or allogeneic freshly isolated lymphoma cells. (A) CIK cultures from healthy normal donors were used as effectors. The patient-derived purified lymphoma cells were labeled with calcein-AM and incubated 4 hours with CIK cells at effector-to-target ratios of 30:1, 10:1, and 5:1. The mean percentage of specific lysis ± SD from 3 independent experiments is shown. (B) CIK cells were expanded from PBMCs from 2 patients (with Waldenström macroglobulinemia and follicular lymphoma, respectively) and were used as effector cells against their own purified blasts as targets (patient1 target, left panel; patient 2 target, right panel), testing them in both the autologous combination (CIK1 vs patient 1 or CIK2 vs patient 2, black columns) or allogeneic combination (CIK1 vs patient 2 and CIK2 vs patient 1, gray columns), at the indicated effector-to-target ratios. Data are expressed as mean percentage ± SD of 3 independent experiments.
Expanded CIK cells from lymphoma patients present comparable cytotoxicity against autologous or allogeneic freshly isolated lymphoma cells. (A) CIK cultures from healthy normal donors were used as effectors. The patient-derived purified lymphoma cells were labeled with calcein-AM and incubated 4 hours with CIK cells at effector-to-target ratios of 30:1, 10:1, and 5:1. The mean percentage of specific lysis ± SD from 3 independent experiments is shown. (B) CIK cells were expanded from PBMCs from 2 patients (with Waldenström macroglobulinemia and follicular lymphoma, respectively) and were used as effector cells against their own purified blasts as targets (patient1 target, left panel; patient 2 target, right panel), testing them in both the autologous combination (CIK1 vs patient 1 or CIK2 vs patient 2, black columns) or allogeneic combination (CIK1 vs patient 2 and CIK2 vs patient 1, gray columns), at the indicated effector-to-target ratios. Data are expressed as mean percentage ± SD of 3 independent experiments.
We conclude that autologous or allogeneic CIK cell cultures have significant cytotoxic activity against both established cell lines and primary freshly isolated B lymphoma cells in vitro.
B lymphoma target cell killing by CIK cultures is enhanced by anti-CD20 antibodies, a process mediated by the NK-cell subpopulation
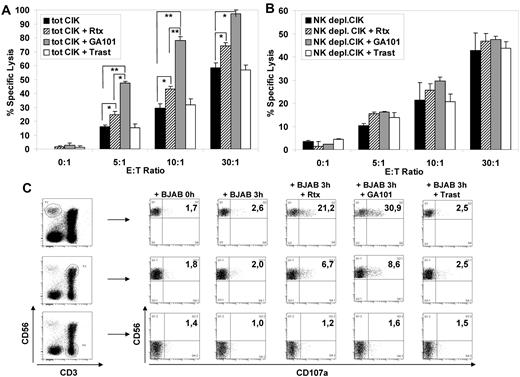
We investigated whether the addition of anti-CD20 monoclonal antibodies rituximab or GA101 could enhance CIK cell culture-mediated cytotoxicity. As shown in Figure 3A, rituximab increased BJAB cell killing by 8.6%, 14%, and 15% at 5:1, 10:1, and 30:1 E/T ratios, respectively. This was statistically significant (P < .05). Much more strikingly, GA101 anti-CD20 antibodies enhanced target cell killing by 32%, 49%, and 39% more than controls without antibodies, at the same E/T ratios (P < .05). The effect of GA101 was significantly greater than that of rituximab (P < .01). These data show that anti-CD20 mAbs can enhance the cytotoxic activity of CIK cell cultures against B-NHL cells. Importantly, this additivity is specific, because the addition of the irrelevant antibody trastuzumab, which binds to the Her2 antigen absent on B-NHL cells, did not significantly modify the cytotoxicity (Figure 3A).
Cytotoxicity of CIK cultures against B lymphoma cell line BJAB was increased in presence of anti CD20 monoclonal antibody. (A-B) Cytotoxicity of CIK cells against B lymphoma cell line BJAB in the presence of mAbs. Target cells were labeled with calcein-AM and incubated 4 hours with effector cells at effector-to-target ratios of 30:1, 10:1, 5:1, and 0:1. The killing assay was done with no added antibody (black columns), 1 μg/mL rituximab (striped columns), 1 μg/mL GA101 (gray columns), or 1 μg/mL trastuzumab control mAb (open columns). Total CIK cultures (A) and NK-depleted CIK cultures (B) were used as effector cells. Data are expressed as mean percentage ± SD of 3 independent experiments. (C) CD107a mobilization in CIK cell cultures on interaction with BJAB. CIK cultures and BJAB were mixed at a 1:1 ratio (0 hours) or co-incubated for 3 hours in presence of 10 μg/mL of rituximab, GA101, or the irrelevant antibody trastuzumab. Cells were harvested and stained with phycoerythrin anti-CD107a, peridinin-chlorophyll-protein complex –conjugated anti-CD3, and allophycocyanin–conjugated anti-CD56 mAbs. To determine the percentage of CD107a+ NK, CIK, and T lymphocytes, gates defining, respectively, CD3−/CD56+, CD3+/CD56,+ and CD3+/CD56− lymphocytes were carried out (left dot plots). The percentage of CD107+ lymphocytes for each gate was evaluated under different conditions (right dot plots). One representative of 3 experiment is shown.
Cytotoxicity of CIK cultures against B lymphoma cell line BJAB was increased in presence of anti CD20 monoclonal antibody. (A-B) Cytotoxicity of CIK cells against B lymphoma cell line BJAB in the presence of mAbs. Target cells were labeled with calcein-AM and incubated 4 hours with effector cells at effector-to-target ratios of 30:1, 10:1, 5:1, and 0:1. The killing assay was done with no added antibody (black columns), 1 μg/mL rituximab (striped columns), 1 μg/mL GA101 (gray columns), or 1 μg/mL trastuzumab control mAb (open columns). Total CIK cultures (A) and NK-depleted CIK cultures (B) were used as effector cells. Data are expressed as mean percentage ± SD of 3 independent experiments. (C) CD107a mobilization in CIK cell cultures on interaction with BJAB. CIK cultures and BJAB were mixed at a 1:1 ratio (0 hours) or co-incubated for 3 hours in presence of 10 μg/mL of rituximab, GA101, or the irrelevant antibody trastuzumab. Cells were harvested and stained with phycoerythrin anti-CD107a, peridinin-chlorophyll-protein complex –conjugated anti-CD3, and allophycocyanin–conjugated anti-CD56 mAbs. To determine the percentage of CD107a+ NK, CIK, and T lymphocytes, gates defining, respectively, CD3−/CD56+, CD3+/CD56,+ and CD3+/CD56− lymphocytes were carried out (left dot plots). The percentage of CD107+ lymphocytes for each gate was evaluated under different conditions (right dot plots). One representative of 3 experiment is shown.
CIK cultures at the end of in vitro expansion contained a mean of 47% ± 8% (range 40%–75%) CD3+CD56+ “true” CIK cells, 49% ± 9% (22%–59%) CD3+CD56− cells, and 4% ± 3% (1%-10%) CD3−CD56+ NK cells (data not shown and30 ). We and others have demonstrated that the CD3+CD56+ population has NK-like natural cytotoxic activity against tumor cells, whereas the CD3+CD56− fraction represents CIK precursors.23,30 Because NK cells are CD16 (FcγRIII)–positive whereas CIK cells do not express significant levels of all FcγRs (CD16, CD32 and CD64),30 we considered whether the enhancement of cytotoxicity by rituximab and GA101 mAbs was due to CIK cells themselves or rather to the NK cells present in CIK cultures. CIK cultures were therefore depleted of NK cells by CD5 immunoselection at the end of the expansion and cytotoxicity experiments repeated in the presence or absence of anti-CD20 mAbs. As shown in Figure 3B, NK-cell depletion did not abolish the basal natural cytotoxicity of CIK cell cultures against the B lymphoma target, with 22% lysis observed at a 10:1 E/T ratio (Figure 3B black bars). However, in these conditions, no significant enhancement of killing could be observed by either rituximab or GA101. These data suggest that basal natural cytotoxicity toward B lymphoma targets is predominantly mediated by CD3+CD56+ “true” CIK cells, whereas enhanced killing in presence of anti-CD20 mAbs is mediated by the small fraction of NK cells present in CIK cultures.
NK and CD8+ T cell–mediated cytotoxicity is accompanied by the release of cytotoxic granules, which can be followed by the appearance of CD107a on the surface of the NK cells33,34 and is used as a surrogate marker of NK-mediated ADCC.35 We therefore directly identified the cell population degranulation in the presence of B lymphoma target cells, rituximab, and GA101 antibodies by measuring CD107a induction in the different subpopulations present in CIK cultures, using triple immunofluorescence analysis. As shown in Figure 3C, addition to CIK cultures of rituximab or GA101, together with BJAB targets, led to CD107a induction on CD3−CD56+ NK cells by nearly 20% and 30% more than controls, respectively. In contrast, CD107a was induced much more weakly, and by only 5%-7% above background levels, on CD3+CD56+ CIK cells, and not at all on CD3+CD56− T cells. These data confirm that antibody-mediated cytotoxicity in CIK cell cultures is due mostly to the activated NK cells present in the final cell product that degranulate on addition of anti-CD20 antibodies. Finally, the data show that degranulation is more efficient with GA101 compared with rituximab (28% ± 6% vs 19 ± 5%, respectively; P < .005).
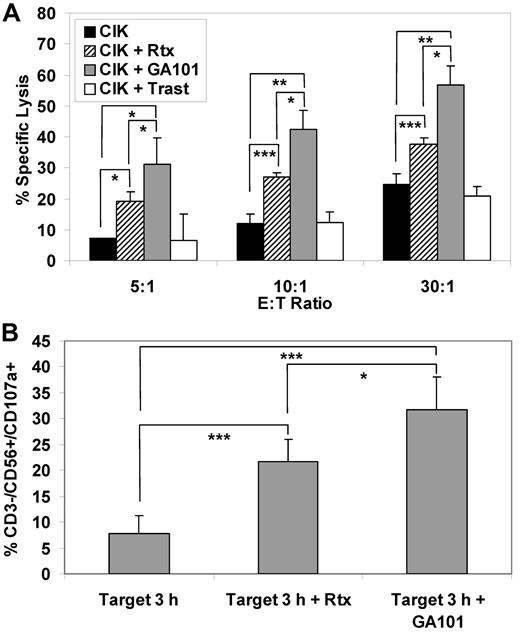
Similar experiments were performed using primary lymphoma cells as targets. Three independent experiments were performed on CIK cell cultures expanded from the peripheral blood of the patients and tested against the autologous freshly purified tumor cells. Addition of anti-CD20 monoclonal antibodies increased CIK-mediated cytotoxicity. Again, more killing was observed with the addition of GA101 compared with rituximab, with approximately 34% versus 16% increased lysis, respectively, at both the 30:1 or 10:1 E/T ratios (P < .05; Figure 4A). This correlated with higher CD107a induction on CD3−CD56+ NK cells by GA101 compared with rituximab (24% vs 14%, P < .05, Figure 4B).
Cytotoxicity of expanded CIK cells from lymphoma patients against autologous lymphoma cell in presence of anti CD20 antibody. (A) Cytotoxicity of patient-derived CIK cultures against autologous lymphoma cells in the presence of mAbs. Target cells were labeled with calcein-AM and incubated for 4 hours with effector cells at effector-to-target ratios of 30:1, 10:1, and 5:1. The killing assay was done with no added antibody (black columns), 1 μg/mL rituximab (striped columns), 1 μg/mL GA101 (gray columns), or 1 μg/mL trastuzumab (open columns). Data are expressed as mean percentage ± SD of 3 independent experiments. (B) CD107a mobilization: 3 expanded CIK cultures from lymphoma patients and each autologous patient-derived lymphoma's cells were mixed at a 1:1 ratio for 3 hours with 1 μg/mL of rituximab or GA101. Surface CD107a marker expression was determined using flow cytometry with gating on CD3−/CD56+ NK lymphocytes. Mean percentage of CD107a+/CD3−/CD56+ cells and SDs are shown (n = 3).
Cytotoxicity of expanded CIK cells from lymphoma patients against autologous lymphoma cell in presence of anti CD20 antibody. (A) Cytotoxicity of patient-derived CIK cultures against autologous lymphoma cells in the presence of mAbs. Target cells were labeled with calcein-AM and incubated for 4 hours with effector cells at effector-to-target ratios of 30:1, 10:1, and 5:1. The killing assay was done with no added antibody (black columns), 1 μg/mL rituximab (striped columns), 1 μg/mL GA101 (gray columns), or 1 μg/mL trastuzumab (open columns). Data are expressed as mean percentage ± SD of 3 independent experiments. (B) CD107a mobilization: 3 expanded CIK cultures from lymphoma patients and each autologous patient-derived lymphoma's cells were mixed at a 1:1 ratio for 3 hours with 1 μg/mL of rituximab or GA101. Surface CD107a marker expression was determined using flow cytometry with gating on CD3−/CD56+ NK lymphocytes. Mean percentage of CD107a+/CD3−/CD56+ cells and SDs are shown (n = 3).
Effect of complement activation on anti-CD20 antibody–mediated degranulation
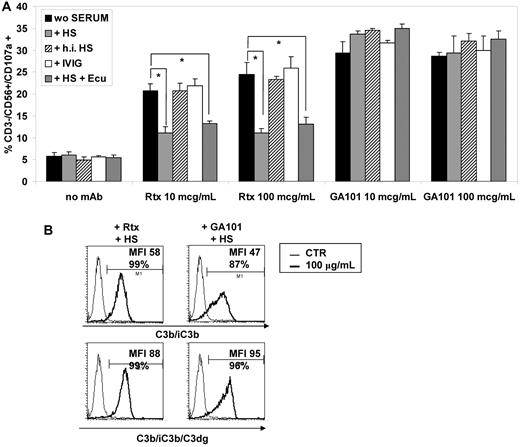
Complement activation in vivo is the most rapid effect of rituximab administration in patients.27 In vitro, complement activation by rituximab has been shown to inhibit ADCC,36 presumably because deposited C3 fragments interfere with FcγR binding. We therefore wished to investigate the effect of complement on the activation of NK cells present in the CIK cultures. For this purpose, we performed degranulation assays in presence of BJAB target, 10 or 100 μg/mL rituximab or GA101, and one of the following: complete human serum (HS), heat-inactivated HS, IVIG, or HS and the blocking anti-C5 antibody eculizumab. Addition of HS inhibited NK-cell degranulation induced by 10 or 100 μg/mL rituximab by 65% and 70%, respectively (Figure 5A). Inhibition of NK-cell activation by HS was due to complement activation, because heat inactivation (thick striped bars) abrogated it completely. In contrast, human immunoglobulins (open bars) had no effect, confirming lack of inhibitory effect due to competition by excess serum immunglobulins. Finally, blocking complement activation downstream of C5 by the anti-C5 antibody eculizumab (thin striped bars) did not modify the inhibitory effect of HS, showing that a step upstream from C5 was involved in the complement-mediated inhibition of NK-cell degranulation by rituximab and serum (Figure 5A).
Complement inhibits rituximab-mediated but not GA101-mediated NK-cell degranulation. (A) Expanded CIK cultures from normal donors and BJAB were mixed at 1:1 ratio for 3 hours with 10 or 100 μg/mL of rituximab or GA101 in complete medium (black columns), in the presence of 20% human serum (HS) with (thin striped columns) or without (open columns) 200 μg/mL blocking anti-C5 mAb eculizumab, heat-inactivated HS (thick striped columns), or 400 μg/mL IVIG (gray columns). Surface CD107a marker expression was determined using flow cytometry with gating on CD3−/CD56+ NK lymphocytes. Mean percentage of CD107a+/CD3−/CD56+ cells and SDs are shown (n = 2). (B) C3b/iC3b (top panels) or C3b/iC3b/C3dg deposition (bottom panels) on BJAB cell line was measured by direct immunofluorescence after addition of 100 μg/mL rituximab (left) or GA101 (right).
Complement inhibits rituximab-mediated but not GA101-mediated NK-cell degranulation. (A) Expanded CIK cultures from normal donors and BJAB were mixed at 1:1 ratio for 3 hours with 10 or 100 μg/mL of rituximab or GA101 in complete medium (black columns), in the presence of 20% human serum (HS) with (thin striped columns) or without (open columns) 200 μg/mL blocking anti-C5 mAb eculizumab, heat-inactivated HS (thick striped columns), or 400 μg/mL IVIG (gray columns). Surface CD107a marker expression was determined using flow cytometry with gating on CD3−/CD56+ NK lymphocytes. Mean percentage of CD107a+/CD3−/CD56+ cells and SDs are shown (n = 2). (B) C3b/iC3b (top panels) or C3b/iC3b/C3dg deposition (bottom panels) on BJAB cell line was measured by direct immunofluorescence after addition of 100 μg/mL rituximab (left) or GA101 (right).
Interestingly, in contrast to what was observed with rituximab, GA101-induced NK-cell degranulation (that is, ADCC) was not at all affected by HS, whether heat inactivated or not (Figure 5A). Similarly, excess human immunoglobulins or the presence of eculizumab did not modify degranulation (Figure 5A). Together, these data show that activation by rituximab of NK cells in CIK cultures is inhibited by complement, whereas that of GA101 is not. This was true of concentrations of 10 and 100 μg/mL GA101. This lack of inhibition of GA101-mediated NK-cell degranulation may have been due to the lower C3 activation reported for this type II CD20 antibody compared with the type I CD20 antibody rituximab, or to a higher-affinity binding of GA101 to CD16. To test this hypothesis, we verified C3 deposition induced by GA101. We observed equivalent C3b/iC3b and C3dg deposition with 100 μg/mL rituximab or GA101 by FACS analysis (Figure 5B). We conclude that lack of inhibition by GA101 and serum is not due to lack of C3 deposition.
Cell-killing by CIK cultures and anti-CD20 mAbs in presence of complement
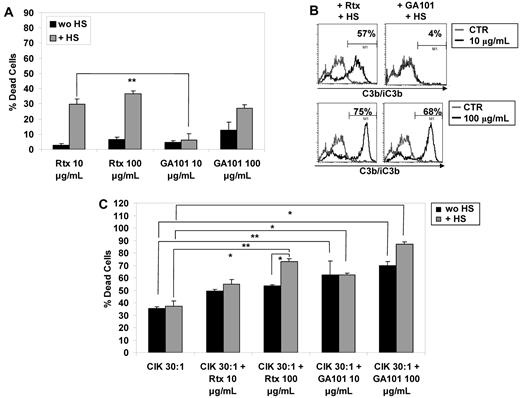
All the data in the previous paragraph indicate that anti-CD20 mAbs activate the NK cells present in the CIK cultures and may therefore increase the cytotoxic potential of the entire cultures through induction of ADCC. On the other hand, complement activation in the presence of serum impairs NK-cell activation, and should therefore also impair ADCC by rituximab, but not GA101. We wanted therefore to measure directly global cell lysis induced by CIK cultures in the presence of serum and rituximab or GA101. In these conditions, overall target cell lysis may include CIK cell-mediated natural cytotoxicity (NC) and NK-mediated and mAb-mediated ADCC, as well as mAb-mediated and complement-mediated cytotoxicity (CDC). For these experiments, we chose the WSU-NHL cell line, which is partially resistant to rituximab and GA101 and is not very responsive to CIK-mediated cytotoxicity (Figure 1A and data not shown).
We first investigated CDC only, that is, lysis in the presence of HS without addition of CIK cells. We observed that rituximab induced CDC at both 10 and 100 μg/mL (mean 27% and 37% target lysis, respectively; Figure 6A). In contrast, significant CDC with GA101 was observed only at the higher 100 μg/mL concentration (27%; Figure 6A). Cell lysis correlated with C3b/iC3b deposition, which was observed with 10 μg/mL rituximab (57%) and increased with 100 μg/mL of this antibody (75%), and was observed at levels of 4% and 68%, respectively, with the same doses of GA101 (Figure 6B). We then investigated the effect on cell lysis of adding CIK cells to the same targets in the presence or absence of antibodies and HS. Addition of CIK alone at a 30:1 E/T ratio led to a mean of 36% lysis from natural cytotoxicity; this was not significantly modified by HS (Figure 6C). Further addition of 10 or 100 μg/mL rituximab in the absence of serum increased lysis to 49% and 53%, respectively (Figure 6C). This increase was greater when GA101 was used (62% and 70% lysis at 10 and 100 μg/mL, respectively), presumably because of ADCC; this observation agrees with the higher NK-cell activation seen with GA101 than with rituximab (Figure 5A). The further addition of serum did not significantly modify the amount of cell lysis with either antibody at 10 μg/mL (55% vs 49% for rituximab and 63% vs 62% for GA101; Figure 6C). However, with antibody concentrations of 100 μg/mL, serum significantly increased lysis, which reached 73% for rituximab and 87% for GA101. Based also on the CDC (Figure 6A) and NK-cell activation observations, these data suggest that in the presence of HS, killing by rituximab is mainly due to the combined effect of NC and CDC, whereas killing by GA101 is the additive effect of NC, CDC, and ADCC.
Cell killing by CIK cultures and anti-CD20 mAbs in presence of human serum. (A) CDC of WSU-NHL cell line in the presence of 10 or 100 μg/mL rituximab or GA101 and 20% HS. (B) Deposition of C3b/iC3b on WSU-NHL cell line was measured by direct immunofluorescence after addition of 10 μg/mL (top panels) or 100 μg/mL (bottom panels) rituximab (left) or GA101 (right). (C) CFSE-labeled WSU-NHL cells were incubated for 4 hours with 10 μg/mL or 100 μg/mL of rituximab or GA101 and CIK cells at effector-to-target ratios of 30:1 in the presence (gray bars) or absence (black bars) of 20% HS. Cell death was measured as a decrease in CFSE+/ 7-AAD− cells relative to untreated control. The results are the mean and SD of duplicate wells and are representative of at least 2 independent experiments.
Cell killing by CIK cultures and anti-CD20 mAbs in presence of human serum. (A) CDC of WSU-NHL cell line in the presence of 10 or 100 μg/mL rituximab or GA101 and 20% HS. (B) Deposition of C3b/iC3b on WSU-NHL cell line was measured by direct immunofluorescence after addition of 10 μg/mL (top panels) or 100 μg/mL (bottom panels) rituximab (left) or GA101 (right). (C) CFSE-labeled WSU-NHL cells were incubated for 4 hours with 10 μg/mL or 100 μg/mL of rituximab or GA101 and CIK cells at effector-to-target ratios of 30:1 in the presence (gray bars) or absence (black bars) of 20% HS. Cell death was measured as a decrease in CFSE+/ 7-AAD− cells relative to untreated control. The results are the mean and SD of duplicate wells and are representative of at least 2 independent experiments.
Discussion
In this report, we investigated the cytotoxic capacity of CIK cultures against B-NHL targets in the presence or absence of anti-CD20 antibodies and human serum. We show that CIK cultures alone have significant cytotoxic activity in vitro against B lymphoma cell lines and freshly isolated samples from patients in either the autologous or allogeneic combination. Most of this cytotoxicity can be attributed to the CD3+/CD56+ CIK cell subpopulation, which kills target cells by an MHC-unrestricted, NK-like mechanism (natural cytotoxicity).29 Furthermore, rituximab, and more markedly, GA101, enhance this effect via ADCC. Finally, for GA101, an additive effect of complement-mediated lysis was observed, leading to very high killing also of resistant lymphoma targets.
The basal cytotoxic activity of CIK cells against both autologous and allogeneic B-cell lymphoma, both from cell lines and patients samples, is of interest because CIK cultures are easily expanded in clinically useful numbers in good manufacturing practice conditions. Furthermore, in previous experiences, these cells, in an allogeneic or autologous setting, have been shown to be relatively safe in vivo and to have antitumor activity in vitro and in vivo against either solid tumors or hematopoietic neoplasms, including AML, CLL, and HD.11-18
Cytotoxicity in the absence of anti-CD20 antibodies was shown to be mediated predominantly by CD3+CD56+ bone fide CIK cells, which represent 40%-70% of cells at the end of culture. In contrast, killing enhancement mediated by anti-CD20 antibodies in the absence of serum was mediated by the NK-cell fraction present in CIK cultures, as shown by its absence from NK-depleted CIK cultures and correlation with CD107a surface induction on NK cells only. This fraction represents only 5%–10% of total cells in final CIK culture products. We have previously demonstrated that the third population present in culture, the CD3+CD56− cells, are proliferating CIK precursor cells without cytotoxic potential.30
Interestingly, the activation of NK cells in CIK cultures, measured both as CD107a expression as well as target cell lysis, was more effective using GA101 than rituximab. Indeed our data demonstrate that activation by the GA101 antibody of even a small subpopulation within CIK cultures can approximately double the cytotoxic potential of these cells. Cytotoxicity was enhanced by 15%–20% from doses of 10 or 100 μg/mL rituximab, and by 26%–34% from the same doses of GA101. The presumed reason is that the NK cells present in CIK cultures are preactivated because they are cultured in presence of rhIL-2. Such cells are known to be very active in mediating ADCC.36 Furthermore, GA101 itself is a very strong activator of NK cells, because it has an approximately 10-fold higher affinity for CD16 than rituximab due to the glycoengineering of its Fc-portion.25,28 From the present data, we conclude that GA101 induces stronger NK-cell activation and ADCC than does rituximab.
We also show that not only is the type II CD20 antibody GA101 more effective than rituximab in activating NK cells, but simultaneous complement activation did not inhibit this activation, as occurs with rituximab antibody-induced inhibition. Enhancement of NK-cell activation by GA101 was fully maintained even in the presence of human serum and complement activation. This was true for low and high concentrations of GA101, up to 100 μg/mL; at the latter concentration, GA101 is nearly as efficient as rituximab at activating complement and C3 deposition. In contrast, activation of NK cells by rituximab at all doses was inhibited by serum. This inhibition was presumably due to complement C3b deposition, because it was not observed with heat-inactivated serum, nor with serum in the presence of the blocking C5 antibody eculizumab, which blocks the complement cascade downstream from C3. Finally, we showed that excess immunoglobulin is not responsible for the inhibitory effect of serum on rituximab-mediated NK-cell activation. Together, these data suggest that C3 deposition blocks rituximab-induced but not GA101-induced NK-cell activation and ADCC, as previously suggested using resting peripheral blood NK cells.29,37,38
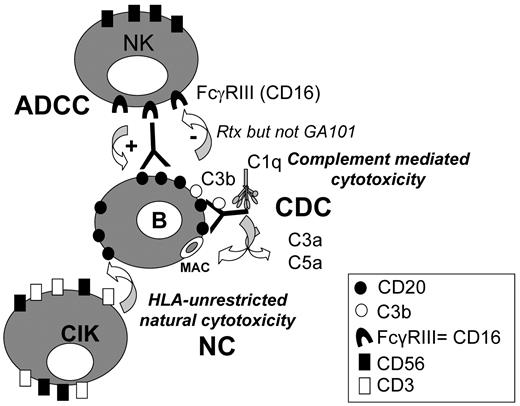
Measurement of global target cell lysis showed that addition of serum and rituximab to CIK led to a significant increase in lysis with 100 μg/mL antibody. Lysis in this case was presumably the sum of natural cytotoxicity by CIK cells (36% lysis with CIK alone) and CDC induced by this antibody (37% with antibody and serum alone), leading to the 73% global lysis observed in the presence of CIK, antibody and serum. ADCC is unlikely to play a role in this case because of the demonstrated inhibition of NK-cell activation by complement activation (summarized in Figure 7). GA101 also increased lysis of target cells in the presence of serum when added at doses of 100 μg/mL. Killing in this case was probably due to natural cytotoxicity combined with both ADCC and CDC, because GA101-mediated NK-cell activation is not blocked by complement. Thus, the 87% observed overall target cell killing at this mAb concentration may be the approximately additive effect of 36% NC by CIK cells, 34% ADCC by NK cells, and 27% CDC. GA101 may therefore have the advantage over rituximab of more effective ADCC and a potentially additive effect of CDC, ADCC, and NC (Figure 7).
Multiple mechanisms of B lymphoma cell killing by CIK cultures in the presence of anti-CD20 mAbs and human serum. CIK cells kill B lymphoma target by an HLA-unrestricted mechanism (natural cytotoxicity, NC). In the presence of anti-CD20 mAbs, complement-dependent cytotoxicity (CDC) may take place, as well as antibody-dependent cellular cytotoxicity (ADCC), by activated NK cells present in CIK cultures. NK activation and ADCC induced by rituximab, but not by GA101 coated target cells, are inhibited by complement fragments deposited on the antibody.
Multiple mechanisms of B lymphoma cell killing by CIK cultures in the presence of anti-CD20 mAbs and human serum. CIK cells kill B lymphoma target by an HLA-unrestricted mechanism (natural cytotoxicity, NC). In the presence of anti-CD20 mAbs, complement-dependent cytotoxicity (CDC) may take place, as well as antibody-dependent cellular cytotoxicity (ADCC), by activated NK cells present in CIK cultures. NK activation and ADCC induced by rituximab, but not by GA101 coated target cells, are inhibited by complement fragments deposited on the antibody.
Together, these data suggest that CIK cultures could be used to treat B-NHL patients in both an autologous or allogeneic setting. Furthermore, rituximab, and even more so GA101, could be used to enhance the antitumor activity of CIK cells. Clearly, the potential in vivo toxicity of combination treatments of CIK cultures and anti-CD20 mAbs, in particular toxicity from the release of complement anaphylatoxins or cytokines, will need to be investigated before clinical use in humans.39 We have generated CIK cultures from CD19-depleted peripheral blood mononuclear cells from B-NHL patients, and no significant differences were observed in terms of expansion and phenotype compared with CIK preparations from normal donors (data not shown). We have also attempted to expand CIK cells in clinically useful numbers in good manufacturing practice conditions starting from unmanipulated leukapheresis sample of one B-NHL patient in remission and demonstrated that this is feasible. Furthermore, we could show that contaminating lymphoma cells present at the start are not detectable by flow cytometry at the end of culture (G. M. Borleri, unpublished observations, February 2010). Thus, CIK cells could be used for the treatment of B-NHL at relapse, either in an autologous or allogeneic context.
We conclude that autologous CIK cells may be combined with third-generation anti-CD20 GA101 antibodies to offer novel immunotherapy protocols for the treatment of chemotherapy-resistant and rituximab-resistant B-cell lymphoma patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by research funding from the Italian Association for Cancer Research (AIRC; Investigator Grant to J.G., Regional Grant Lombardia and Special Program in Molecular Clinical Oncology 5x1000 “Innate Immunity in Cancer” to M.I.), by the Italian Ministry of Health (Progetto Ordinario, Provincia Autonoma di Bolzano), and the Associazione Italiana contro le Leucemie, Linfomi e Mieloma (AIL), Bergamo-Sezione Paolo Belli (Bergamo, Italy).
Authorship
Contribution: A.P. designed and performed research, analyzed data, and wrote the paper; C.B. performed research and analyzed data; C.K. provided GA101 and edited the paper; A.R. provided clinical samples and edited the paper; J.G. designed research, interpreted data, and wrote the paper; and M.I. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: M.I. and J.G. have received research grants and honoraria from Roche Italia (Monza, Italy), the company selling rituximab and GA101, in the past 5 years. C.K. is an employee of Roche. The remaining authors declare no competing financial interests.
Correspondence: Martino Introna, Laboratory of Cellular Therapy G. Lanzani, Ospedali Riuniti, c/o Presidio Matteo Rota, Via Garibaldi 11-13, 24128 Bergamo, Italy; e-mail: mintrona@ospedaliriuniti.bergamo.it.