Abstract
Heme binds to proteins to modulate their function, thereby functioning as a signaling molecule in a variety of biologic events. We found that heme bound to Bach2, a transcription factor essential for humoral immunity, including antibody class switch. Heme inhibited the DNA binding activity of Bach2 in vitro and reduced its half-life in B cells. When added to B-cell primary cultures, heme enhanced the transcription of Blimp-1, the master regulator of plasma cells, and skewed plasma cell differentiation toward the IgM isotype, decreasing the IgG levels in vitro. Intraperitoneal injection of heme in mice inhibited the production of antigen-specific IgM when heme was administered simultaneously with the antigen but not when it was administered after antigen exposure, suggesting that heme also modulates the early phase of B-cell responses to antigen. Heme oxygenase-1, which is known to be regulated by heme, was repressed by both Bach2 and Bach1 in B cells. Furthermore, the expression of genes for heme uptake changed in response to B-cell activation and heme administration. Our results reveal a new function for heme as a ligand of Bach2 and as a modulatory signal involved in plasma cell differentiation.
Introduction
Heme is an essential molecule for diverse living organisms, including human beings. It plays a variety of roles in oxygen transport, electron transfer, and catalytic reactions.1 In addition to these well-established functions as a prosthetic group, recent reports have revealed that heme regulates several transcription factors, including Bach1,2-5 NPAS2,6 and REV-ERBs,7 and modulates gene expression as an inter- and intracellular signaling molecule in mammals. Several observations have suggested a regulatory role for heme in the immune system. For example, heme oxygenase-1 (HO-1), an enzyme that degrades heme, inhibits dendritic cell maturation.8 Greater serum immunoglobulin M levels are observed in HO-1–deficient mice compared with wild-type (WT) mice,9 suggesting that intracellular heme levels may regulate antibody production. Moreover, suppression of heme synthesis by iron depletion impairs the blastogenic response of splenic lymphocytes.10 These observations collectively suggest that heme may play a critical regulatory role in the immune system. However, little is known about the mechanism of the heme-mediated responses or changes in heme metabolism in the immune system.
Bach1 and Bach2 constitute a subfamily of the basic region-leucine zipper (bZip) family.11 They form heterodimers with small Maf proteins, bind to the Maf recognition element (MARE), and repress target gene expression. Bach2 is specifically expressed in hematopoietic cells of the B-cell lineage, where it is expressed from pro-B to mature-B cell stages.12
Resting mature-B cells resume proliferation and then differentiate into antibody-secreting plasma cells in response to antigen stimulation or polyclonal stimulation. A default pathway for plasma cells is to become IgM-secreting plasma cell. An alternative pathway is to undergo class switch recombination (CSR) and somatic hypermutation (SHM) to become plasma cells, secreting isotypes of Igs such as IgG or IgA with greater affinity for a specific antigen.13 Bach2 plays 2 critical roles in the process of plasma cell differentiation. First, it is required for both CSR and SHM of immunoglobulin genes.14,15 Bach2-deficient (Bach2−/−) B cells preferentially differentiate into IgM-secreting plasma cells, compared with control WT cells.15 Second, Bach2 represses the expression of the transcription factor B lymphocyte-induced maturation protein 1 (Blimp-1),16,17 which is expressed in plasma cells but not in mature-B cells,18 and is essential for plasmacytic differentiation.18,19 Bach2 postpones terminal differentiation of B cells to plasma cells by inhibiting Blimp-1 and hence secures enough time for CSR to occur (a delay-driven diversity model for CSR).15 Such critical roles of Bach2 suggest that Bach2 would be precisely regulated during B-cell activation and plasma cell differentiation, but little is known about the control of Bach2.20
A salient feature of Bach1 is its regulation by heme. Bach1 possesses at least 4 Cys-Pro motifs (CP motifs)21,22 involved in heme-binding. Heme binds to Bach1 to inhibit its DNA binding, to induce its nuclear export, and to induce its polyubiquitination and proteasome-dependent degradation.2-5,23,24 As a net effect, heme achieves derepression of Bach1 target genes, including globin and Hmox1, which encodes HO-1, and allows their induction by competing transcription activators such as NF-E2 and Nrf2.3,25-29 Bach1 deficiency in mice results in derepression of HO-1 in multiple tissues and cell types.23 Although we have previously reported that Bach2 might be a repressor of HO-1 in transformed B cells,30 this regulation has not been proven in normal B cells. Noting that Bach2 also possesses CP motifs, we explored the possibility that heme may control the Bach2 function in B cells. We also examined the expression of genes involved in the heme metabolism in B cells. The results suggest a cross-regulation of heme metabolism and B-cell activation by Bach2.
Methods
Plasmids
A Bach2 cDNA fragment encoding residues 331-761 was amplified from the pBSF69-J plasmid.11 template by PCR with the following primers (Invitrogen): 5′-CTTAGGATCCTCCAGGAGTGTGTCCTCGCC-3′, which also introduced a BamHI site; and 5′-CCTAGCGGCCGCTTATCACTATCCTACTGCACACGGGGAGG-3′, which contained a NotI site next to the termination codon. The amplified fragment was then digested with BamHI and NotI and cloned in pGEX6P-1 (GE Healthcare). The GST-fusion Bach2 protein was named GST-Bach2. Mutations were created with Bach2 cDNA cloned into the pAlter1 vector (Promega) as described by the manufacturer. A Prdm1 reporter plasmid (promoter-MARE-luc) and pCMVBach2 have been described previously.11,16
Expression and purification of GST-Bach2 and GST-Bach2mCP
Escherichia coli Rosetta (DE3) cells (Merck) transformed with the expression constructs were grown in LB media at 37°C, and the recombinant proteins were induced as described previously.5 Soluble protein fractions were purified with glutathione-sepharose 4B (GE Healthcare) and heparin-sepharose CL-6B (Amersham Biosciences) columns. Proteins were finally concentrated with the use of an Amicon Ultra-15 membrane (Millipore).
Spectroscopic analysis for the heme-binding assay
The heme-binding was estimated by optical titration in 50mM Tris-HCl at pH 8.5 and 50mM NaCl at 20°C in the presence of 20μM BSA as described previously5 using heme (hemin; Sigma-Aldrich) and a PerkinElmer Lambda 45 spectrophotometer.
EMSA
An oligonucleotide designed based on previously reports (5′-GAGTAATCGTGAGTCATCAATTCCGAGC-3′)11,26 was labeled with [γ-32P] ATP (PerkinElmer) with T4 polynucleotide kinase (Takara). GST-Bach2 protein or MBP-MafK (60 ng) was incubated with the probe in 10 μL of gel shift buffer.11,26 After the addition of heme, the reaction mixtures were left on ice for 10 minutes. Electrophoresis and image analysis were performed as described previously.11,26,31
Luciferase reporter assay
Mice
C57BL/6J mice were purchased from Charles River Laboratories. The Bach2−/− and Blimp-1-enhanced green fluorescent protein (EGFP) transgenic mice used in this study were described previously.14,33 Mice lacking both the Bach1 and Bach2 genes were generated by crossing respective heterozygous mutant mice.14,23 Detailed phenotypic evaluations of the double deficient mice will be reported elsewhere (A.I.N., A.M., K.I., manuscript in preparation). All experiments involving mice were approved by Tohoku University.
Cell lines, preparation of primary B cells, and heme treatment
The 18-81 pre-B cells were maintained in IMDM medium (Invitrogen). B cells were purified from the single-cell suspensions of spleens as described previously.15 The cells were plated at 1 × 106 cells/mL in 24-well plates containing RPMI 1640 medium and 20 μg/mL LPS with or without 20μM heme. Deferoxamine mesylate salt (Sigma-Aldrich) was used as an iron chelator. ELISA and ELISPOT were performed as described previously.14
Bach2 protein stability
The 18-81 pre-B cells were centrifuged and resuspended in medium containing 20 μg/mL cycloheximide (Chx) with or without 5μM heme. Primary B cells were incubated with 20 μg/mL LPS with or without 20μM heme for 4 days. The Bach2 protein was detected by a Western blotting assay with the use of MafK and α-tubulin as internal controls. The band intensities of Western blots were measured by a densitometric analysis (National Institutes of Health imaging analyzer). The primary antibodies used were anti-Bach2 antiserum (F69-1)11 and anti-MafK antiserum (A-1).34
Quantitative PCR
RNA isolation and qPCR were carried out as described previously.15 The sequences of the qPCR primers for Blimp-1, ALAS-N, AID, HO-1, Tfrc, CD91, CD163, ferroportin-1, and β-actin are available from the authors on request.
FACS analysis
The FACS analyses were performed with EGFP and antibodies against mouse CD45R/B220 and CD138 (BD). The cells were analyzed on a FACScalibur instrument with the CellQuest software package (BD).14
Intraperitoneal injection of heme
Heme (hemin; Frontier Scientific) was dissolved in 10% ammonium hydroxide in 0.15M NaCl to prepare a stock solution of 100 mg/mL, then was further diluted 1:40 with sterile 0.15M NaCl and injected intraperitoneally into mice (10 μL/g) as described.35 Vehicle-injected mice received an identical NH4OH-containing solution lacking heme. 2,4-dinitrophenyl-conjugated Ficoll (DNP-Ficoll) was injected intraperitoneally into mice (100 μg/mouse).
Results
Heme directly binds to Bach2
Bach2 is structurally related to Bach1 and possesses 5 CP motifs (Figure 1A). To investigate the possibility that Bach2 is a heme-binding protein, we over-expressed a Bach2 fragment spanning residues 331-761, (called GST-Bach), containing the 5 CP motifs and the bZip domain (DNA binding domain) in E coli. GST-Bach2 was purified to > 85% purity as judged by SDS-PAGE (data not shown). The pale brown color of the purified protein solution (λmax: ∼ 420 nm) was suggestive of substoichiometric heme-binding to the Bach2 fragment expressed in E coli.
Bach2 is a heme-binding protein. (A) A schematic representation of the Bach1 and Bach2 domain structure. The bZip domain, BTB domain (Broad complex-Tramtrack-Bric-a-brac domain), known as a protein-protein interaction motif, and CP motifs are indicated with boxes and filled circles. GST-Bach2 and GST-Bach2mCP illustrate the recombinant Bach2 fragments examined in this study. GST-Bach2mCP contains Cys→Ala mutations in the 5 CP motifs. (B) The heme titration of GST-Bach2 (1μM) was monitored by the differences in absorption spectra. Each arrow represents the directions of the absorbance changes with increasing heme concentrations. (C) The titration curve observed at 401 nm for GST-Bach2 with heme. (D) The heme titration of GST-Bach2mCP as monitored by the differences in the absorption spectra. (E) EMSA was performed by the use of 60 ng of each recombinant GST-Bach2 and a MARE DNA probe. The heme concentrations were 0.05μM (lane 3), 0.1μM (lane 4), 1μM (lane 5), 3μM (lane 6), and 10μM (lane7). Representative EMSA data are shown from 3 independent experiments. (F) EMSA was carried out with 50 ng of recombinant MBP-MafK. The heme concentrations were the same as in panel E. Representative EMSA data are shown from 2 independent experiments. (G) The band intensities in panels E and F were measured by a densitometry analysis. The open circle with a dashed line, closed circle with a line, and closed gray circle with a gray line indicate 3 independent experiments of EMSA with GST-Bach2 proteins. The open box with the dashed line and filled box with the line indicate 2 independent EMSA experiment with MBP-MafK proteins.
Bach2 is a heme-binding protein. (A) A schematic representation of the Bach1 and Bach2 domain structure. The bZip domain, BTB domain (Broad complex-Tramtrack-Bric-a-brac domain), known as a protein-protein interaction motif, and CP motifs are indicated with boxes and filled circles. GST-Bach2 and GST-Bach2mCP illustrate the recombinant Bach2 fragments examined in this study. GST-Bach2mCP contains Cys→Ala mutations in the 5 CP motifs. (B) The heme titration of GST-Bach2 (1μM) was monitored by the differences in absorption spectra. Each arrow represents the directions of the absorbance changes with increasing heme concentrations. (C) The titration curve observed at 401 nm for GST-Bach2 with heme. (D) The heme titration of GST-Bach2mCP as monitored by the differences in the absorption spectra. (E) EMSA was performed by the use of 60 ng of each recombinant GST-Bach2 and a MARE DNA probe. The heme concentrations were 0.05μM (lane 3), 0.1μM (lane 4), 1μM (lane 5), 3μM (lane 6), and 10μM (lane7). Representative EMSA data are shown from 3 independent experiments. (F) EMSA was carried out with 50 ng of recombinant MBP-MafK. The heme concentrations were the same as in panel E. Representative EMSA data are shown from 2 independent experiments. (G) The band intensities in panels E and F were measured by a densitometry analysis. The open circle with a dashed line, closed circle with a line, and closed gray circle with a gray line indicate 3 independent experiments of EMSA with GST-Bach2 proteins. The open box with the dashed line and filled box with the line indicate 2 independent EMSA experiment with MBP-MafK proteins.
The heme titration of GST-Bach2 was carried out in the presence of excess BSA, which is known to bind heme weakly,32 to suppress nonspecific binding of heme to Bach2. Because the spectroscopic features of heme are sensitive to its environment, the heme-binding to Bach2 can be assessed directly by spectrometric measurements. Absorption spectra during the heme titration were recorded in the presence and absence of the GST-Bach2 protein to calculate the differences in the absorption spectra, as shown in Figure 1B. The positive peaks in both the Soret and visible regions represent the formation of a Bach2-heme complex, and 2 intense Soret peaks at 366 and 432 nm are indicative of at least 2 distinct heme-binding modes in the GST-Bach2 protein. The negative peaks at 401 and 615 nm are because of the decreased accumulation of the BSA-heme complex in the presence of GST-Bach2. The titration curve observed at 401 nm reveals that Bach2 can bind 4 or 5 mole equivalents of heme (Figure 1C). These spectral features and the heme-binding capacity of Bach2 are essentially the same as those observed for Bach1.5
The far blue-shifted positive peak at 366 nm is typical of a 5-coordinate ferric high-spin heme with coordination of a thiolate ligand in the CP motif cysteine.5 In fact, substitution of all of the cysteine residues in the 5 CP motifs of Bach2 by alanine (GST-Bach2mCP) resulted in the disappearance of the 366-nm peak (Figure 1A,D). The peak at 432 nm shifted to 426 nm with these mutations, but it was not lost, suggesting that the 4 CP motifs were not essential for this mode of heme binding. Similar results were obtained by the use of at least 3 independent preparations of the recombinant proteins. These findings unequivocally show that heme binds Bach2 with 2 modes, similar to Bach1.
Heme regulates both the DNA binding and protein stability of Bach2
The effect of heme on the DNA binding activity of Bach2 was examined by EMSA. A homodimer of Bach2, as well as its heterodimer with Maf proteins, can bind to DNA containing a MARE sequence.11 In the presence of various concentrations of heme, a MARE-containing DNA probe11,26 was incubated with GST-Bach2 or maltose binding protein-MafK (MBP-MafK)2 (Figure 1E-F). The DNA-binding activity of the GST-Bach2 was markedly inhibited by heme (Figure 1E lanes 6 and 7, G). This finding is in contrast to the marginal inhibition of the DNA-binding activity of the MBP-MafK by heme (Figure 1F-G). These results clearly indicate that heme regulates the DNA binding activity of Bach2. The DNA binding activity of GST-Bach2mCP was still inhibited by heme in vitro (data not shown), thus suggesting that the remaining heme binding mode was also involved in the regulation by heme.
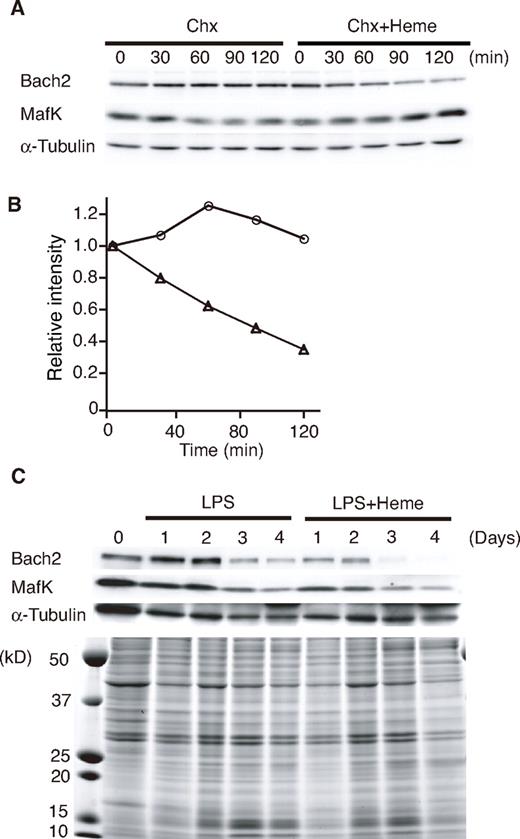
Heme induces Bach2 degradation
To examine the effects of heme on endogenous Bach2 in B cells, we determined the levels of Bach2 in the 18-81 pre-B cell line treated with heme. Chx was added to the culture medium to inhibit de novo protein synthesis. The 18-81 pre-B cells were incubated in medium containing Chx with or without heme. Endogenous Bach2 was relatively stable in the absence of heme, but it disappeared more rapidly in the presence of heme (Figure 2A-B). In contrast to Bach2, the half-lives of MafK and α-tubulin were not markedly affected by heme (Figure 2B). We obtained similar results from 3 independent experiments.
Heme induces Bach2 degradation. (A) Heme-induced degradation of endogenous Bach2. The 18-81 pre-B cells were treated with 5μM heme or without heme for the indicated periods (minutes) in the presence of Chx. A Western blotting analysis of whole cell extracts was performed with antibodies against the indicated proteins. (B) Determination of the half-life of Bach2. The band intensities in panel A were measured by a densitometric analysis (National Institutes of Health imaging analysis). Open circle indicate treatment with Chx, and open triangles indicate treatment with Chx and 5μM heme. (C) Heme-induced degradation of endogenous Bach2 in mouse splenic B cells. Purified mouse splenic B220-positive B cells were stimulated with 20 μg/mL LPS in the presence or absence of 20μM heme for the indicated periods. A Western blotting analysis of whole-cell extracts was performed with antibodies against the indicated proteins (top). Coomassie blue staining of gels indicated that heme did not cause any gross change in the overall protein levels (bottom).
Heme induces Bach2 degradation. (A) Heme-induced degradation of endogenous Bach2. The 18-81 pre-B cells were treated with 5μM heme or without heme for the indicated periods (minutes) in the presence of Chx. A Western blotting analysis of whole cell extracts was performed with antibodies against the indicated proteins. (B) Determination of the half-life of Bach2. The band intensities in panel A were measured by a densitometric analysis (National Institutes of Health imaging analysis). Open circle indicate treatment with Chx, and open triangles indicate treatment with Chx and 5μM heme. (C) Heme-induced degradation of endogenous Bach2 in mouse splenic B cells. Purified mouse splenic B220-positive B cells were stimulated with 20 μg/mL LPS in the presence or absence of 20μM heme for the indicated periods. A Western blotting analysis of whole-cell extracts was performed with antibodies against the indicated proteins (top). Coomassie blue staining of gels indicated that heme did not cause any gross change in the overall protein levels (bottom).
We next examined the effect of heme on the protein level of Bach2 in primary B cells, which can be induced to differentiate to plasma cells in vitro with lipopolysaccharide (LPS). Splenic B220-positive B cells were stimulated with LPS in the presence or absence of heme for up to 4 days, as indicated in Figure 2C. The levels of Bach2 protein remained high for 2 days, and then decreased between 2 to 4 days in response to LPS (Figure 2C). In contrast, in the presence of heme, the levels of Bach2 started to decrease at 1 and 2 days after LPS stimulation (Figure 2C). Although the MafK protein level remained high for the first 2 days, it decreased on days 3 and 4. The addition of heme resulted in a reduction of MafK on days 1 and 2, suggesting that heme may also affect the MafK protein via the change in the Bach2 level in primary B cells. Heme did not cause any gross change in other protein levels, as revealed by Coomassie blue staining of the gel (Figure 2C bottom panel). Similar results were obtained in 3 independent experiments. On the basis of these observations, we concluded that heme induced the degradation of Bach2 in B cells.
Heme increases the population of Blimp-1–expressing cells through Bach2
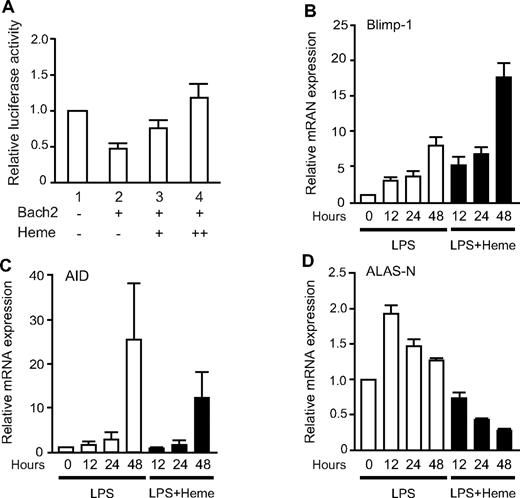
To explore the regulatory significance of the effects of heme on Bach2 in B cells, we examined the effect of heme on the transcriptional repression activity of Bach2 in the 18-81 pre-B cell line (Figure 3A). We used a luciferase reporter plasmid with a single MARE sequence derived from the Prdm1 gene encoding Blimp-1. As reported previously,16 Bach2 repressed the Prdm1 MARE reporter in untreated cells (Figure 3A). Bach2-mediated repression of the reporter was alleviated by adding heme in the medium for the last 4 hours of cell culture (Figure 3A). These results suggested that heme inhibited the repressor activity of Bach2 in the 18-81 pre-B cells.
Effects of heme on the Bach2 transcription network. (A) The 18-81 cells were transiently transfected with the Prdm1 promoter-MARE-luc reporter and pCMV-Bach2 as described previously.16 Concentrations of 1μM (lane 3) and 5μM heme (lane 4) were added to each cell culture for 4 hours at the end of culture. The data are presented as the mean ± SD of triplicate determinations. (B-D) The relative expression levels of Blimp-1, AID, and ALAS-N mRNA, respectively, in mouse splenic B220-positive B cells. B cells were stimulated with 20 μg/mL LPS (open bars) or LPS and 20 μM heme (filled bars) for the indicated periods. The transcription levels were analyzed by qPCR. The data are presented as the means ± SD of triplicate determinations.
Effects of heme on the Bach2 transcription network. (A) The 18-81 cells were transiently transfected with the Prdm1 promoter-MARE-luc reporter and pCMV-Bach2 as described previously.16 Concentrations of 1μM (lane 3) and 5μM heme (lane 4) were added to each cell culture for 4 hours at the end of culture. The data are presented as the mean ± SD of triplicate determinations. (B-D) The relative expression levels of Blimp-1, AID, and ALAS-N mRNA, respectively, in mouse splenic B220-positive B cells. B cells were stimulated with 20 μg/mL LPS (open bars) or LPS and 20 μM heme (filled bars) for the indicated periods. The transcription levels were analyzed by qPCR. The data are presented as the means ± SD of triplicate determinations.
To investigate the effect of heme on gene expression during the plasma cell differentiation, we isolated B220-positive B cells and then stimulated them with LPS alone or with LPS and heme. We measured the time course of mRNAs for Blimp-1 and activation-induced cytidine deaminase (AID) by using qPCR. Blimp-1 is not only essential for plasma cell differentiation, but it also suppresses CSR by repressing AID,36 an enzyme essential for CSR.13 The level of Blimp-1 mRNA increased between day 0 and day 2 after LPS stimulation. In the presence of heme, its induction became more prominent (Figure 3B), consistent with the results of the reporter assays. Although the levels of AID mRNA were increased in response to LPS treatment, the level was decreased in the presence of heme to ∼ 50% (Figure 3C).
Nonspecific 5-aminolevulinate synthase (ALAS1 or ALAS-N) is the rate-limiting enzyme of heme synthesis in diverse types of cells, and its expression correlates well with the intracellular levels of heme.37,38 The expression of ALAS-N was induced on B-cell activation by LPS in vitro (Figure 3D), thus suggesting that the synthesis of heme was induced on B-cell activation by LPS. However, it was repressed when B cells were treated with both LPS and heme (Figure 3D), confirming that the heme treatment resulted in an increase in the intracellular heme levels. Heme is known to inhibit ALAS-N expression.37 These results suggest that the increased levels of heme in B cells affect the expression of the genes involved in CSR and plasma cell differentiation, and that heme synthesis increases during B-cell activation because of increased ALAS-N expression.
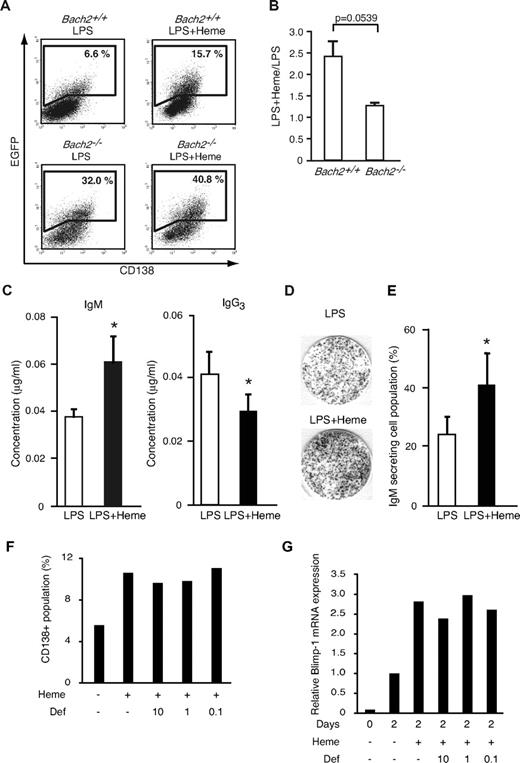
To examine the effect of heme on Blimp-1 expression in B cells at the single-cell level, we used an EGFP reporter transgenic mouse for the Prdm1 locus.33 We isolated splenic B220-positive B cells, stimulated them with LPS for 2 days to induce plasma cell differentiation, and determined the percentage of the Blimp-1-EGFP–positive cell population by using FACS.15 In the WT background, 6.6% ± 1.57% of cells became EGFP-positive in response to LPS (Figure 4A). Additional treatment with heme increased the frequency of EGFP-positive cells by 2.4-fold (15.7% ± 2.66%) compared with cells treated with LPS alone (Figure 4A-B). Most of the EGFP-positive cells expressed CD138, which is a surface marker of plasma cells (Figure 4A). We confirmed that the effect of heme on the frequency of EGFP-positive cells was concentration dependent (data not shown). These results suggested that heme acted as a costimulatory factor for Blimp-1 expression, regulating the frequency of its expression in activated B cells.
Effects of heme on plasma cell differentiation. (A) The expression of the Blimp-1-EGFP reporter gene determined by a FACS analysis. B220-positive cells from WT (Bach2+/+) Blimp-1-EGFP mice and Bach2-deficient Blimp-1-EGFP (Bach2−/−) mice were stimulated with 20 μg/mL LPS in the presence or absence of 20μM heme on day 2. Each gate shows the percentage of EGFP-positive cells. (B) The ratio of EGFP-positive cells cultured with LPS + heme or LPS alone in Bach2+/+ (left) or Bach2−/− (right) cells. The data are presented as the means ± SD of triplicate determinations. The statistical analyses were performed by the use of the Student t test. (C) The IgM and IgG3 secretion from splenic B220-positive B cells was analyzed by ELISA. B220-positive B cells were cultured with 20 μg/mL LPS alone (open bars) or LPS and 20 μM heme (filled bars) for 7 days, and the secreted immunoglobulin levels were measured. The data are presented as the means ± SD of triplicate determinations. Groups of 3-5 mice were used for the statistical analysis. P values (*P < .05) were calculated by use of the Student t test. (D) The differentiation of IgM-producing plasma cells in mouse splenic B220-positive B cells stimulated with 20 μg/mL LPS in the presence or absence of 20μM heme for 2 days. IgM-producing cells were detected by ELISPOT assay. (E) The percentage of IgM-secreting cells within the B-cell population. Each bar indicates the average percentage of IgM-secreting cells in 8 × 102 B cells (open bars: stimulation with LPS alone, filled bars: stimulation with LPS and heme). The data are presented as the means ± SD of triplicate determinations. Groups of 3-5 mice were used for the statistical analysis. *P values were calculated with use of the Student t test. (F-G) The percentages of cells expressing CD138 (F) and relative expression levels of Blimp-1 mRNA (G) in splenic B cells from in vitro culture treated with LPS for 2 days. Heme and deferroxamine (μM) were added as indicated. Mean of 2 independent experiments are shown.
Effects of heme on plasma cell differentiation. (A) The expression of the Blimp-1-EGFP reporter gene determined by a FACS analysis. B220-positive cells from WT (Bach2+/+) Blimp-1-EGFP mice and Bach2-deficient Blimp-1-EGFP (Bach2−/−) mice were stimulated with 20 μg/mL LPS in the presence or absence of 20μM heme on day 2. Each gate shows the percentage of EGFP-positive cells. (B) The ratio of EGFP-positive cells cultured with LPS + heme or LPS alone in Bach2+/+ (left) or Bach2−/− (right) cells. The data are presented as the means ± SD of triplicate determinations. The statistical analyses were performed by the use of the Student t test. (C) The IgM and IgG3 secretion from splenic B220-positive B cells was analyzed by ELISA. B220-positive B cells were cultured with 20 μg/mL LPS alone (open bars) or LPS and 20 μM heme (filled bars) for 7 days, and the secreted immunoglobulin levels were measured. The data are presented as the means ± SD of triplicate determinations. Groups of 3-5 mice were used for the statistical analysis. P values (*P < .05) were calculated by use of the Student t test. (D) The differentiation of IgM-producing plasma cells in mouse splenic B220-positive B cells stimulated with 20 μg/mL LPS in the presence or absence of 20μM heme for 2 days. IgM-producing cells were detected by ELISPOT assay. (E) The percentage of IgM-secreting cells within the B-cell population. Each bar indicates the average percentage of IgM-secreting cells in 8 × 102 B cells (open bars: stimulation with LPS alone, filled bars: stimulation with LPS and heme). The data are presented as the means ± SD of triplicate determinations. Groups of 3-5 mice were used for the statistical analysis. *P values were calculated with use of the Student t test. (F-G) The percentages of cells expressing CD138 (F) and relative expression levels of Blimp-1 mRNA (G) in splenic B cells from in vitro culture treated with LPS for 2 days. Heme and deferroxamine (μM) were added as indicated. Mean of 2 independent experiments are shown.
To investigate whether the heme effects involved Bach2, we next performed the same experiments by using Bach2−/− B cells. We interbred Bach2-deficient (Bach2−/−) and Blimp-1-EGFP reporter mice to generate Bach2−/−/Blimp-1-EGFP mice. We first confirmed that Bach2−/− B cells stimulated with LPS alone showed a greater EGFP-positive population (32.0% ± 6.78%) compared with Bach2+/+ B cells stimulated with LPS alone (6.6% ± 1.57%; Figure 4A). This observation reiterates that Bach2 is important for the inhibition of Prdm1 gene.14-16
In the Bach2−/− B cells, additional treatment with heme showed only a marginal effect on the percentage of EGFP-positive cells (40.8% ± 7.33% and 1.3-fold; Figure 4A-B). These results suggested that the enhancement of Blimp-1-EGFP expression by heme involved the inactivation of Bach2. The residual effect of heme in Bach2−/− B cells may reflect the derepression of Blimp-1 by the inactivation of Bach1 because we have recently found that Bach1 binds to a Prdm1 gene regulatory region and represses its expression (unpublished observation).
Heme inhibits CSR in activated B cells
Heme is expected to affect CSR because Bach2 is required for CSR. To examine the possibility, mouse splenic B220-positive B cells were stimulated with LPS and measured for Ig secretion by isotype-specific ELISA. Heme augmented the IgM secretion induced by LPS (Figure 4C). In contrast, heme inhibited IgG3 secretion (Figure 4C). To detect and quantify individual antibody-secreting B cells, we performed an ELISPOT assay (Figure 4D). When B cells were stimulated with LPS and heme, the number of IgM-secreting cells increased roughly 2-fold compared with cells treated with LPS alone (Figure 4E). Taken together, these results indicate that heme promotes plasma cell differentiation toward IgM-secreting plasma cell differentiation rather than CSR.
Heme-derived iron is not involved in the regulation of B cells
It is possible that iron, a product of heme degradation by HO-1 and/or HO-2, but rather than heme itself, might have affected Bach2 and its downstream effects on gene expression. Indeed, we found that heme treatment of splenic B cells resulted in decreases in transferrin receptor mRNA levels (data not shown), indicating an increase in the intracellular iron level on the heme treatment. To examine the possibility that iron was responsible for the effects of heme, we stimulated splenic B cells from WT mice with LPS and heme in the presence or absence of an iron chelator deferoxamine, and the cells were analyzed by FACS for CD138 expression (Figure 4F). Although heme enhanced the plasma cell differentiation, the additional treatment with deferoxamine did not reverse the stimulatory effect of heme. Blimp-1 induction was not affected by the deferoxamine treatment (Figure 4G). These results support the interpretation that heme, but not iron, stimulated the plasma cell differentiation.
The effects of heme on antigen stimulation in vivo
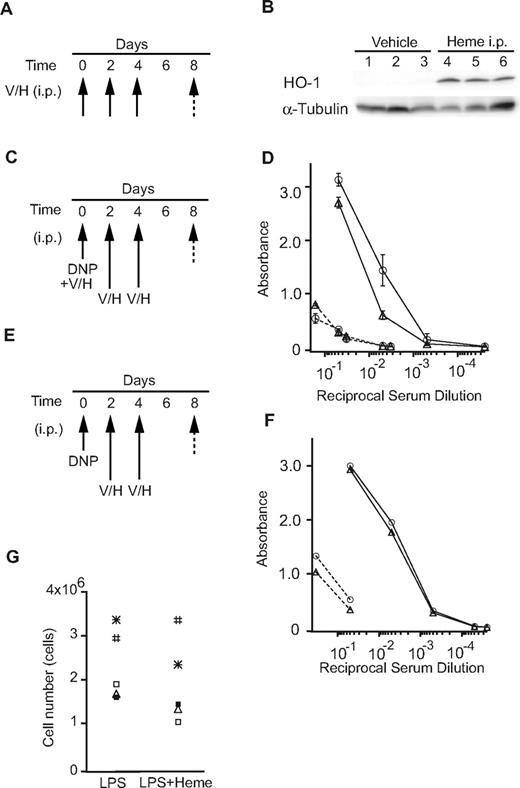
To examine the effects of heme on antibody production in vivo, we performed intraperitoneal administration of heme in mice. We first confirmed that heme induced the HO-1 levels in the liver (Figure 5A-B), indicating that exogenous heme was taken up by surrounding cells and organs, and that it induced HO-1 expression. WT mice were immunized with the T cell–independent antigen DNP-Ficoll with or without heme (Figure 5C-D). Heme was also injected intraperitoneally 2 additional times (Figure 5C). The production of antigen-specific IgM was reduced in the presence of heme (Figure 5D). In contrast, when heme was administered only after the immunization of DNP-Ficoll, the production of specific IgM antibodies remained unchanged (Figure 5E-F). These observations suggest that heme may affect some of the initial processes of B-cell responses to antigen. One possibility was that heme inhibited B-cell proliferation. However, heme did not show any significant inhibition of proliferation of primary B cells activated with LPS in vitro (Figure 5G). Thus, heme may affect the early events driven by antigen stimulation. These results suggest that heme modulates the production of antibodies in mice depending on the timing of the B-cell encounter.
The effects of heme on the immune response. (A-B) Mice were injected intraperitoneally with heme (H) or vehicle (V; 3 mice/group) every other day (3 times; arrow). The total liver homogenates were obtained from these mice and tested for protein expression by a Western blotting analysis using antibodies to HO-1. (C-D) Mice were immunized with DNP-Ficoll with or without heme (Day 0), followed by intraperitoneal injection of heme (H; n = 6) or vehicle (V; n = 6) every other day (Days 2, 4: 2 times; arrow). Sera were collected on days 0 (dashed line) and 8 (solid line) and were assayed for DNP-specific antibodies (open circles, vehicle; open triangles, heme). The data are presented as the means ± SD (E-F) Mice were immunized with DNP-Ficoll (Day 0) and treated with heme as described previously. The amounts of DNP-specific antibodies (open circles, vehicle; open triangles, heme) are shown as described previously. (G) The proliferation of primary B cells stimulated with LPS in the presence or absence of heme for 2 days. Each symbol shows data from the same mouse.
The effects of heme on the immune response. (A-B) Mice were injected intraperitoneally with heme (H) or vehicle (V; 3 mice/group) every other day (3 times; arrow). The total liver homogenates were obtained from these mice and tested for protein expression by a Western blotting analysis using antibodies to HO-1. (C-D) Mice were immunized with DNP-Ficoll with or without heme (Day 0), followed by intraperitoneal injection of heme (H; n = 6) or vehicle (V; n = 6) every other day (Days 2, 4: 2 times; arrow). Sera were collected on days 0 (dashed line) and 8 (solid line) and were assayed for DNP-specific antibodies (open circles, vehicle; open triangles, heme). The data are presented as the means ± SD (E-F) Mice were immunized with DNP-Ficoll (Day 0) and treated with heme as described previously. The amounts of DNP-specific antibodies (open circles, vehicle; open triangles, heme) are shown as described previously. (G) The proliferation of primary B cells stimulated with LPS in the presence or absence of heme for 2 days. Each symbol shows data from the same mouse.
Regulation Hmox1 by Bach2 and Bach1 in B cells
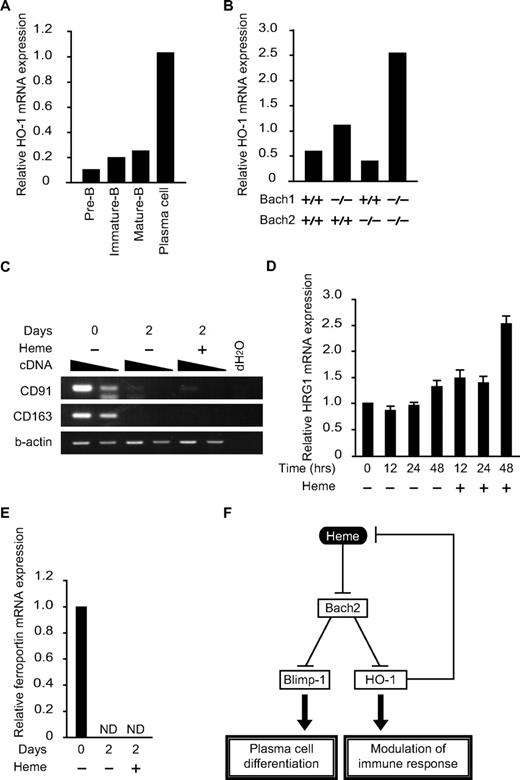
Although HO-1 is critical for proper antibody production,9 little is known about its expression or regulation in B cells. To examine the correlation between HO-1 expression and B-cell differentiation, pre-B cells (B220low, CD43−IgM− cells in BM), immature-B cells (B220low, IgM+ cells in BM), mature-B cells (B220hi, IgM+ cells in BM), and plasma cells (B220low/+, CD138hi cells in spleen) were sorted and purified, and the HO-1 mRNA expression was analyzed (Figure 6A). Compared with B cells in immature stages of differentiation, HO-1 expression was increased dramatically in plasma cells. Because the expression of HO-1 is induced by heme, these results also suggested that there was an increase in the intracellular heme levels in plasma cells.
Expression of genes for heme metabolism in B and plasma cells. (A and B) The relative expression levels of HO-1 mRNA were determined at indicated developmental stages (A) or in B220low pro-B and pre-B cells of indicated genotypes (B) by qPCR. (C-E) Expression of indicated mRNAs (C, CD91 and CD163; D, HRG-1; E, ferroportin) in splenic B cells was determined. Cells were treated with LPS for indicated periods with or without heme. Two-fold dilutions of cDNA were compared in panel C. ND, not detectable. (F) A model for the function of heme in B cells. Heme regulates plasma cell differentiation and HO-1 expression by binding to Bach2. Because HO-1 degrades heme, the regulatory loop maintains intracellular heme levels within a certain range.
Expression of genes for heme metabolism in B and plasma cells. (A and B) The relative expression levels of HO-1 mRNA were determined at indicated developmental stages (A) or in B220low pro-B and pre-B cells of indicated genotypes (B) by qPCR. (C-E) Expression of indicated mRNAs (C, CD91 and CD163; D, HRG-1; E, ferroportin) in splenic B cells was determined. Cells were treated with LPS for indicated periods with or without heme. Two-fold dilutions of cDNA were compared in panel C. ND, not detectable. (F) A model for the function of heme in B cells. Heme regulates plasma cell differentiation and HO-1 expression by binding to Bach2. Because HO-1 degrades heme, the regulatory loop maintains intracellular heme levels within a certain range.
The expression profile of HO-1 in B and plasma cells is opposite that of Bach2,12 raising the possibility that Bach2 may be involved in the repression of HO-1 in B cells. To investigate this possibility, we isolated a B220low population containing both pro-B and pre-B cells from the BM of control WT or Bach2−/− mice. We also examined the B220low population from Bach1−/− mice and Bach1/Bach2 double-deficient mice, considering their possible redundancy (Figure 6B). The expression of HO-1 was increased 2-fold by the Bach1 deficiency but not by the Bach2 deficiency. HO-1 expression was further increased in the double-deficient B cells (Figure 6B). We concluded that Bach1 and Bach2 redundantly repressed the expression of HO-1 in B cells.
Dynamic expression of genes for heme and iron transport in B cells
The aforementioned results revealed 2 unique aspects of heme in B cells: heme regulates B-cell responses, whereas B-cell activation regulates heme metabolism. As indicated in Figures 3D and 6A, the expression of ALAS-N and HO-1 was increased on B-cell activation, suggesting a dynamic change in heme metabolism. Consistent with this idea, the mRNA expression levels of hemopexin receptor CD91 and haptoglobin receptor CD163, both involved in protein-bound heme uptake,39 were reduced dramatically on B-cell activation (Figure 6C). In contrast, the mRNA expression of the HRG1 heme transporter40 remained unchanged on B-cell activation (Figure 6D). Therefore, B cells appear to use distinct mechanisms for heme uptake before and after activation. Interestingly, HRG1 expression was increased on heme treatment (Figure 6D), suggesting that extracellular heme stimulates heme uptake by activated B cells. Ferroportin mRNA was reduced on B-cell activation by LPS (Figure 6E). This change may also contribute to the changes in heme and iron metabolism in activated B cells.
Discussion
To the best of our knowledge, this study is the first report suggesting a molecular mechanism by which heme regulates humoral immunity. Together with our recent report,15 our present results provide strong evidence that heme augments plasma cell differentiation by inhibiting Bach2, and thus increasing the population of Blimp-1–expressing cells. Because Blimp-1 is the master regulator of plasma cell differentiation,19,41 its derepression by heme through Bach2 inactivation may fine-tune the response of humoral immunity. In addition, heme may regulate the B-cell responses by inducing the expression of HO-1, which possesses antioxidant and immunomodulatory functions.9
In the spectral analyses, the 432-nm absorption band (6-coordinate heme-binding mode) and the 366-nm absorption band (5-coordinate heme-binding mode) both became apparent on addition of heme (Figure 1B). These results suggested that Bach2 bound to heme directly and had at least 2 distinct binding modes. Moreover, the spectral changes of GST-Bach2mCP showed that the 366-nm absorption band was because of heme-binding by the CP motifs (Figure 1D). The presence of 2 heme-binding modes is similar to Bach1.5 Although amino acid residues involved in the heme binding represented by the 432-nm peak remain to be identified, our observation clearly indicates that Bach2 is an intracellular heme receptor in B cells.
We have previously reported that Bach2−/− B cells show profound up-regulation of Blimp-1 and a severe reduction in the CSR and SHM of immunoglobulin genes.14-16 When WT B cells carrying the Blimp-1-EGFP reporter gene were stimulated with LPS and heme, we found that the frequency of EGFP-positive cells was increased compared with cells treated with LPS alone (Figure 4A-B). Hence, heme may increase the population of Blimp-1-expressing cells by inactivating Bach2. Interestingly, the exposure of B cells to heme resulted in a 2-fold increase in IgM secretion and decreased IgG secretion (Figure 4C). Concomitantly, the AID mRNA level decreased in the presence of heme to half of the level present in cells with LPS stimulation alone (Figure 3C). Considering the fact that Blimp-1 inhibits AID expression and promotes plasma cell differentiation,15,36 our data suggest that heme-mediated induction of Blimp-1 resulted in reduced CSR and enhanced plasma cell differentiation. In addition, we found a reduction in the production of antigen-specific IgM in vivo, only when heme was administered concomitantly with the antigen (Figure 5). Because Blimp-1 may be induced more rapidly in the presence of heme, premature plasma cell differentiation may ensue after heme administration, resulting in a reduction in the amount of IgM produced. Taken together with the fact that Bach2 is required for SHM,14 heme appears to inhibit SHM, resulting in less production of antigen-specific antibodies. Although further studies are required to address these possibilities, our present observations strongly suggest a regulatory function for heme in B cells to inhibit CSR and to reduce the total amounts of specific immunoglobulins produced in response to antigens.
The expression pattern of HO-1 in B and plasma cells (Figure 6A) is interesting in 2 respects. First, the pattern strongly suggests that the intracellular heme levels increase on terminal differentiation to plasma cells because its expression is primarily regulated by heme.1,29 Second, because the expression pattern of HO-1 is shaped in part by Bach2 and Bach1 (Figure 6B), heme and HO-1 constitute a negative feedback loop via Bach2 and Bach1 (Figure 6F). In this loop, heme modulates humoral immunity by inactivating Bach2 and Bach1 and thereby inducing not only Blimp-1 but also HO-1. In addition to its immunomodulatory functions, HO-1 may also keep the heme in B cells between certain levels to avoid a prooxidant effect by heme.
On the basis of these results, we propose that heme modulates B-cell differentiation by binding to Bach2. There are potentially 2 sources of heme that regulate Bach2 in B cells. The intracellular heme synthesized by ALAS may regulate Bach2. Alternatively, considering that heme is a ubiquitous molecule, it may serve as a component of endogenous damage-associated molecular patterns.42,43 In the first model, the heme-Bach2 pathway may couple the metabolic status and gene expression in activated B cells. Supporting this possibility, when B cells were activated by LPS, ALAS-N mRNA was induced in B cells (Figure 3D), which is the rate-limiting enzyme of heme synthesis.44 The biochemical resources required for B-cell activation and plasma cell differentiation are likely synthesized in part by heme-containing enzymes. Bach2 may monitor the changes in intracellular metabolic activities using heme. To test this hypothesis, genetic manipulation of heme synthesis and degradation enzymes in B cells will be informative. Because ALAS-N−/− mice are early embryonic lethal45 and not available for analysis of B cells, we examined heterozygous ALAS-N+/− mice. We did not find any gross change in B-cell development or function (O.N., M.Y., unpublished observation), but homeostatic compensation of ALAS-N function may be responsible for this lack of effect. To further explore the role of ALAS-N, an inducible knockout system will be required.
Alternatively, B cells may uptake heme from the microenvironment of lymphoid organs. The dynamic changes in the expression of genes involved in heme transport in B cells (Figure 6) are consistent with this hypothesis. Heme could be released in large amounts from dying cells as a result of many pathologic conditions such as hemoglobinopathies and infection.46 In addition, macrophages uptake hemoglobin and secrete heme to the microenvironment.47 Thus, heme trafficking at the site of infection and lymphoid tissues may reveal a novel communication network between macrophages and B cells mediated by heme.
The identification of Bach2 as an intracellular heme receptor will help in investigation of the regulation of the immune system by heme. The metabolism and transport of heme in B cells are interesting issues for future studies. Elucidating the entire picture of regulation by heme in plasma cell differentiation will provide valuable information for our understanding of the complex immune system and its dysfunction, including the initiation and development of various diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs. T. Nakano (Osaka University), S. Shibahara, K. Furuyama, and T. Shiraki (Tohoku University) for discussions about the manuscript. We thank Dr M. Saitou (RIKEN) for the Blimp-1-EGFP transgenic mice. We also thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for technical support.
This work was supported by Grants-in-aid and the Network Medicine Global-COE Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan and grants from the Uehara Foundation, the Takeda Foundation, the NOVARTIS Foundation (Japan) for the Promotion of Science, and the Astellas Foundation for Research on Metabolic Disorders.
Authorship
Contribution: M.W.-M., A.M., and A.I.-N. performed the experiments; T.M. and K.M. assisted with the experiments; K.I. designed and conceptualized the study; O.N. and M.Y. generated unpublished materials; M.W.-M., A.M., T.M., M.I.-S., and K.I. interpreted the data; and M.W.-M., A.M., T.M., M.I.-S., and K.I. wrote the manuscript. All authors made comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kazuhiko Igarashi, Seiryo-machi 2-1, Sendai 980-8575, Japan; e-mail: igarashi@med.tohoku.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal