Abstract
CD8-positive/T-cell receptor–negative (CD8+/TCR−) graft facilitating cells (FCs) are a novel cell population in bone marrow that potently enhance engraftment of hemopoietic stem cells (HSCs). Previously, we showed that the CD11c+/B220+/CD11b− plasmacytoid-precursor dendritic cell (p-preDC) FC subpopulation plays a critical but nonredundant role in facilitation. In the present study, we investigated the mechanism of FC function. We report that FCs induce antigen-specific CD4+/CD25+/FoxP3+ regulatory T cells (Tregs) in vivo. The majority of chimeric Tregs were recipient derived. Chimeric Tregs harvested at ≥ 4 weeks after transplantation significantly enhanced engraftment of donor- and recipient-derived HSCs, but not third-party HSCs, in conditioned secondary recipients, demonstrating antigen specificity. Although Tregs were present 2 and 3 weeks after transplantation, they did not enhance engraftment. In contrast, week 5 and greater Tregs potently enhanced engraftment. The function of chimeric Tregs was directly correlated with the development of FoxP3 expression. Chimeric Tregs also induced significantly stronger suppression of T-cell proliferation to donor antigen in vitro. Removal of p-preDC FCs resulted in impaired engraftment of allogeneic HSCs and failure to produce chimeric Tregs, suggesting that the CD8α+ p-preDC subpopulation is critical in the mechanism of facilitation. These data suggest that FCs induce the production of antigen-specific Tregs in vivo, which potently enhance engraftment of allogeneic HSCs. FCs hold clinical potential because of their ability to remain tolerogenic in vivo.
Introduction
Recently, a great deal of interest has focused on the therapeutic potential of cell-based therapies to induce tolerance. Of greatest interest is the subpopulation of bone marrow–derived plasmacytoid-precursor dendritic cells (p-preDCs) and the regulatory T cells (Tregs) that they induce. A major limitation to the use of p-preDCs and Tregs in vivo has been the failure to identify an approach to prevent them from losing their tolerogenic properties and becoming immunogenic after transplantation. We recently demonstrated that CD8α-positive/T-cell receptor–negative (CD8α+/TCR−) graft facilitating cells (FCs) enhance the engraftment of hematopoietic stem cells (HSCs) and tolerance induction in allogeneic recipients.1-3 FCs suppress graft-versus-host disease (GVHD) in vivo by producing CD4+/CD25+/FoxP3+ Tregs4 and induce Tregs in vitro in the presence of CpG.5 The majority of CD8α+/TCR− FCs share the B220+/CD11c+/CD11b− p-preDC phenotype, and we have demonstrated the first in vivo engraftment-enhancing and tolerance-promoting effect of the p-preDC FC subpopulation.2 Although removal of p-preDC FCs from total FCs completely abrogates facilitation, p-preDC FCs alone do not replace FCs to provide the full in vivo biologic effect of facilitation. The mechanism of FC function has yet to be precisely characterized.
CD4+/CD25+/FoxP3+ Tregs play a critical role in the maintenance of self-tolerance.6 Defects in Treg development or homeostasis result in systemic autoimmunity,7 whereas adoptive transfer of Tregs as a therapeutic method can control ongoing autoimmune diseases.8-10 Recently, several studies have demonstrated an important role for Tregs in mediating transplantation tolerance in animal models,11-14 but little is known about the mechanism of Treg development and homeostasis.15-17 p-preDCs may be important in the generation of Tregs, as evidenced by their potential to facilitate engraftment of HSCs2,18 and to prolong heart allograft survival.19,20 In addition, in vitro activation of p-preDCs with CpG-oligodeoxynucleotides (CpG-ODNs) induces the production of Tregs in vitro.5,21 We therefore evaluated whether the mechanism of FC function in vivo is to induce Tregs.
In the present study, we first evaluated whether FCs enhance allogeneic HSC engraftment in diabetes-prone nonobese diabetic (NOD) mice. Second, we investigated whether FCs induce the production of Tregs and examined their function using in vivo transplantation models and in vitro suppressor assays. We found that B6 FCs enhance engraftment of B6 HSCs in NOD mice and induce the production of donor (B6)– and recipient (NOD)–derived Tregs (chimeric Tregs). The majority of chimeric Tregs were recipient (NOD)–derived. In contrast to naive B6 Tregs, chimeric Tregs are significantly more effective in suppressing the proliferation of effector T cells in vitro. Strikingly, chimeric Tregs enhance donor-specific B6 HSC engraftment in secondary recipients, but do not facilitate engraftment of major histocompatibility complex (MHC)–disparate third-party B10.BR HSCs. Removal of p-preDCs from FCs before transplantation resulted in a failure to produce functional chimeric Tregs in vivo, suggesting that p-preDC FCs are a critical component in inducing Treg generation. A better understanding of the effects of FCs in enhancing HSC engraftment may contribute to the development of cell-based strategies to establish tolerance and address concerns regarding the need to prevent DCs from converting from tolerogenic to immunogenic after transplantation.
Methods
Mice
Four- to 6-week-old NOD (H-2g7; Taconic Laboratories) and C57BL/6 (B6; H-2b) and B10.BR (H-2k; The Jackson Laboratory) female mice were used. Animals were housed in the barrier animal facility at the Institute for Cellular Therapeutics (Louisville, KY) and cared for according to National Institutes of Health animal care guidelines. All research was approved by the University of Louisville institutional animal care and use committee.
HSC, FC, and Treg sorting
All monoclonal antibodies were purchased from Pharmingen. HSCs (c-Kit+/Sca-1+/Lin−) sorting experiments used the following monoclonal antibodies (mAbs): stem cell antigen 1 (Sca-1) phycoerythrin (PE; E13-161.7; rat immunoglobulin G2a [IgG2a]), c-Kit allophycocyanin (APC; 2B8; IgG2b), and the lineage panel consisting of CD8α fluorescein isothiocyanate (FITC; 53-6.7; rat IgG2a), Mac-1 FITC (M1/70; IgG2b), B220 FITC (RA3-6B2; rat IgG2a), Gr-1 FITC (RB6-8C5; rat IgG2b), γδ-TCR FITC (GL3; Armenian hamster IgG), and β-TCR FITC (H57-597; Armenian hamster IgG). CD8+TCR− FC sorting experiments used β-TCR FITC, γδ-TCR FITC, and CD8α PE (53-6.7; IgG2a). p-preDC FCs were sorted using β-TCR FITC, γδ-TCR FITC, CD8α APC (53-6.7; rat IgG2a), CD11b FITC, CD11c PE (HL3; Armenian hamster IgG), and B220 APC-Cy7 (RA3-6B2; rat IgG2a). CD8−CD4+CD25bright Tregs were sorted using CD4 APC (RM4-5; rat IgG2a), CD25 PE (PC61; rat IgG1), and CD8α FITC.
HSCs and FCs were isolated from bone marrow by multiparameter, live sterile cell sorting (FACSVantage SE and FACSAria; Becton Dickinson), as described previously.2 Briefly, bone marrow was isolated and resuspended in a single cell suspension at a concentration of 100 × 106 cells/mL in sterile cell-sort medium containing sterile 1× Hanks balanced salt solution without phenol red (GIBCO), 2% heat-inactivated fetal calf serum (GIBCO), 10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (GIBCO), and 0.5% gentamicin (GIBCO). Directly labeled mAbs were added at saturating concentrations and the cells were incubated for 30 minutes on ice and washed twice. Cells were resuspended in cell-sort medium at 2.5 × 106 cells/mL. CD8−/CD4+/CD25bright Tregs were sorted from spleens of donor B6 or B6 → NOD chimeric mice.
HSC and/or FC, Treg transplantation
In the HSC + FC allogeneic model, NOD recipients were conditioned with 950 cGy or 1050 cGy of total body irradiation (TBI) from a cesium source (Nordion), and transplanted with 4000, 5000, or 10 000 B6 HSCs with or without 30 000 FCs or 45 000 B6 FCs via lateral tail-vein injection at least 6 hours after irradiation. The HSCs and FCs were mixed before injection. A group of irradiated untransplanted mice served as controls.
In the HSC + Treg allogeneic model, CD8−/CD4+/CD25bright Tregs were sorted from spleens of naive B6 or B6 → NOD chimeric mice. Various doses of Tregs + B6 or B10.BR HSCs were transplanted into NOD recipients conditioned with 950 cGy of TBI.
Treg generation in vivo
Recipient NOD mice were conditioned with 950 cGy of TBI and reconstituted with 1000 syngeneic NOD HSCs and 10 000 allogeneic B6 HSCs with 45 000 CD8+/TCR− FCs or 45 000 FCs without B220+/CD11c+/CD11b− p-preDCs by tail-vein injection. Recipients were euthanized at 2, 3, 4, and 5 weeks after transplantation. The thymus, spleen, and bone marrow were harvested, and donor (B6)- and recipient (NOD)-origin CD8−/CD4+/CD25bright/FoxP3+ Tregs were analyzed by flow cytometry using CELLQuest software (Becton Dickinson).
Assessment of chimerism
Donor engraftment was evaluated by peripheral blood lymphocyte (PBL) typing using 4-color flow cytometry, as described previously.22 Briefly, whole blood from recipients was collected in heparinized tubes, and 100-μL aliquots were stained with donor-specific anti–H-2Kb FITC (AF6-88.5; mouse IgG2a), along with a combination of the following mAbs (Pharmingen): CD8α peridinin chlorophyll protein (PerCP; 53-6.7; rat IgG2a), CD4 PerCP (RM4-5; rat IgG2a), β-TCR APC (H57-597; Armenian hamster IgG), Pan–natural killer (Pan-NK) cell PE (DX5; rat IgM), NK1.1 PE (PK136; mouse IgG2a), B220 PerCP (RA3-6B2; rat IgG2a), CD11c PE, Gr-1 PE (RB6-8C5; rat IgG2b), and CD11b APC (M1/70; IgG2b).
Treg suppression assays
The in vitro suppression assay was carried out as described previously.23 Briefly, stimulator splenocytes were isolated from donor-specific B6, third-party B10.BR, and NOD mice. Cells were reconstituted in mixed lymphocyte reaction (MLR) medium containing Dulbecco modified Eagle medium with 5% fetal bovine serum, 1mM sodium pyruvate, 2mM glutamine, 100 U/mL of penicillin, 100 U/mL of streptomycin, 10mM HEPES, 0.05mM 2-mercaptoethanol, 100mM N-methyl-L-arginine, 05mM L-arginine, 0.3mM L-asparagine, 0.01mM folic acid, and 1% NOD-responder mouse serum. Splenocytes were then incubated overnight in a humidified chamber at 37°C with 5% CO2. Lymph node–responder cells (1 × 105) were isolated from naive NOD animals, reconstituted in MLR medium, and cultured with 1 × 105 irradiated (2000 cGy) B6, B10.BR, or NOD-stimulator splenocytes in triplicate in 96-well round-bottomed plates. Sorted CD8−/CD4+/CD25bright Tregs from splenocytes of chimeras (B6 → NOD) or naive B6 mice were added to the stimulator/responder mix at 1:1, 1:0.25, and 1:0.125 responder/Treg ratios for 4 days in a humidified chamber at 37°C with 5% CO2. The cell mixture was pulsed on day 4 for an additional 18 hours with 10 μCi of [3H] thymidine (Perkin Elmer). The cell mixture was harvested on day 5 with an automated cell harvester (Tomtec Harvester 96; Wallac), and the radionucleotide uptake determined by scintillation counting (1205 BetaPlate; Wallac). The data are expressed as a stimulation index, the ratio of counts per million generated by the host responder cells in response to a given stimulator relative to the autoresponse of the host. Each value represents the mean of triplicate determinations ± SEM.
Statistical analysis
Experimental data were presented as the means ± SEM. Statistical significance was assessed using the Student t test; P < .05 was considered significant. Graft survival was calculated according to the Kaplan-Meier method.2
Results
CD8+/TCR− FCs enhance allogeneic B6 HSC engraftment in NOD recipients
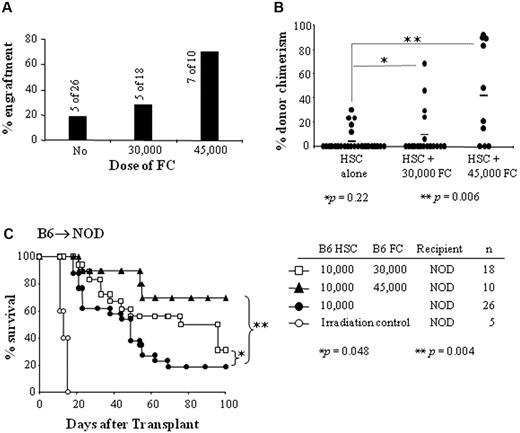
We previously reported that CD8+/TCR− FCs potently enhance engraftment of allogeneic HSCs in diabetes-resistant recipients.1-3 In the present study, we evaluated whether FCs have a similar effect in prediabetic NOD mice. NOD mice are relatively radioresistant, and require a higher bone marrow–cell dose and higher levels of conditioning to establish allogeneic engraftment compared with wild-type mice.24 They also are deficient in Tregs.25 We first performed titrations of HSC dose and TBI dose to establish the model. HSCs (c-Kit+/Sca-1+/Lin−) were sorted from the bone marrow of B6 donors, and 4000, 5000, or 10 000 HSCs were transplanted into NOD recipients conditioned with 950 or 1050 cGy of TBI. In the 950-cGy group, 0% (0 of 5), 11% (1 of 9), and 19% (5 of 26) of recipients of 4000, 5000, or 10 000 HSCs engrafted, respectively (Figure 1A). Only 20%-22% of recipients survived up to 100 days (Figure 1C). In the 1050-cGy group, 0% (0 of 5), 0% (0 of 9), or 50% (5 of 10) of recipients of 4000, 5000, or 10 000 HSCs engrafted, respectively (Figure 1A). Only 10% of recipients survived up to 100 days (Figure 1D). The percentage of donor chimerism was not significantly different between the 2 groups that received 10 000 HSCs (P = .212; Figure 1B).
HSCs and TBI dose titration. (A) Percent engraftment of NOD mice given 4000, 5000, or 10 000 B6 HSCs and conditioned with 950 or 1050 cGy of TBI. (B) Percentage of donor chimerism in engrafted NOD recipient mice. (C-D) Survival of NOD recipients conditioned with 950 or 1050 cGy of TBI and transplanted with various B6 HSC doses in an allogeneic model (B6 → NOD). Results are from 3-5 separate transplant experiments.
HSCs and TBI dose titration. (A) Percent engraftment of NOD mice given 4000, 5000, or 10 000 B6 HSCs and conditioned with 950 or 1050 cGy of TBI. (B) Percentage of donor chimerism in engrafted NOD recipient mice. (C-D) Survival of NOD recipients conditioned with 950 or 1050 cGy of TBI and transplanted with various B6 HSC doses in an allogeneic model (B6 → NOD). Results are from 3-5 separate transplant experiments.
We then tested whether FCs facilitate B6 HSC engraftment in allogeneic NOD mice. HSCs and CD8α+/TCR− FCs were sorted from donor B6 mice, and 10 000 HSCs + 30 000 or 45 000 FCs were mixed with the HSCs and transplanted into NOD recipients conditioned with 950 cGy of TBI. Only 19% (5 of 26) of recipients transplanted with HSCs alone engrafted and survived up to 100 days (Figure 2A-C). Five of 18 (28%) recipients transplanted with HSCs + 30 000 FCs engrafted (Figure 2A) and survived up to 100 days (Figure 2C). The percentage of donor chimerism in recipients of 30 000 FCs + 10 000 HSCs was not significantly different compared with the group that received HSCs alone (P = .22; Figure 2B). The survival of these mice was significantly longer than that of mice that received HSCs alone (P = .048; Figure 2C). In contrast, 70% (7 of 10) of recipients given HSCs + 45 000 FCs showed long-term engraftment, with survival for >100 days (Figure 2A-C). This was a significant difference compared with recipients of HSCs alone (P = .004). The percentage of donor chimerism in NOD recipients transplanted with HSCs + 45 000 FCs was significantly higher than recipients of HSCs + 30 000 FCs or HSCs alone (P = .006; Figure 2B). Our previous studies showed that 30 000 FCs are sufficient to significantly enhance engraftment of HSCs in diabetes-resistant allogeneic recipients.1-3,22 The present data suggest that FCs enhance engraftment of allogeneic HSCs in NOD recipients, but that higher numbers of FCs are required compared with disease-resistant controls.
CD8+/TCR− FCs enhance HSC engraftment in NOD mice (B6 → NOD). The ability of B6 FCs to promote B6 HSC engraftment in allogeneic NOD recipients and long-term survival were evaluated. (A) Percent engraftment of NOD mice that received 10 000 B6 HSCs + 30 000 B6 FCs or 45 000 B6 FCs. (B) Percentage of donor chimerism in NOD recipient mice with engraftment. Dot plots represent percentage of donor chimerism in individual animal. Lines indicate median percentages. *P = .22; **P = .006. (C) Survival of NOD recipients conditioned with 950 cGy of TBI and transplanted with 10 000 B6 HSCs + 30 000 FCs (□), 10 000 B6 HSCs + 45 000 B6 FCs (▲), or 10 000 B6 HSCs alone (●). Results are shown for survival data from 3-5 separate transplant experiments.
CD8+/TCR− FCs enhance HSC engraftment in NOD mice (B6 → NOD). The ability of B6 FCs to promote B6 HSC engraftment in allogeneic NOD recipients and long-term survival were evaluated. (A) Percent engraftment of NOD mice that received 10 000 B6 HSCs + 30 000 B6 FCs or 45 000 B6 FCs. (B) Percentage of donor chimerism in NOD recipient mice with engraftment. Dot plots represent percentage of donor chimerism in individual animal. Lines indicate median percentages. *P = .22; **P = .006. (C) Survival of NOD recipients conditioned with 950 cGy of TBI and transplanted with 10 000 B6 HSCs + 30 000 FCs (□), 10 000 B6 HSCs + 45 000 B6 FCs (▲), or 10 000 B6 HSCs alone (●). Results are shown for survival data from 3-5 separate transplant experiments.
FCs induce CD8−/CD4+/CD25brightFoxP3+ Tregs in vivo
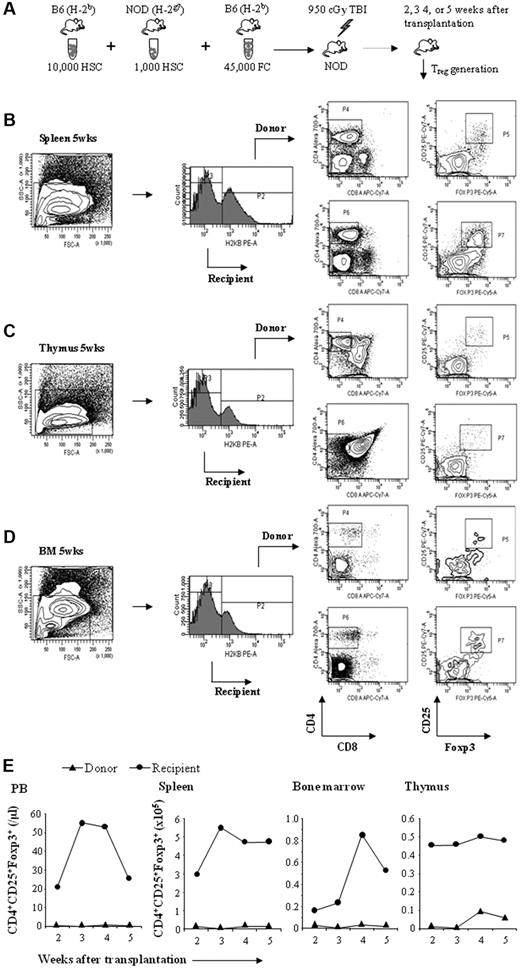
To determine whether FC-mediated facilitation of allogeneic HSC engraftment and tolerance occurs by induction of Tregs in vivo, we evaluated the production of Tregs after HSC + FC transplantation. CD8+/TCR− FCs were sorted from the bone marrow of donor B6 mice and HSCs from the bone marrow of donor B6 and host NOD mice. 10 000 B6 HSCs + 1000 NOD HSCs with or without 45 000 CD8+/TCR− B6 FCs were transplanted into recipient NOD mice conditioned with 950 cGy of TBI in competitive repopulation assays (Figure 3A). Donor chimerism ranged from 9.9%-53.3%. At 2, 3, 4, and 5 weeks after transplantation, thymus, spleen, and bone marrow were harvested from NOD recipients and the absolute numbers of donor (B6) or recipient (NOD) Tregs were determined by flow cytometry (Figure 3B-D). As shown in Figure 3E, donor- and recipient-derived CD4+/CD25+ FoxP3+ Tregs (chimeric Tregs) were detectable in thymus, spleen, and bone marrow at 2 weeks after transplantation. At 2 weeks, the highest numbers of Tregs were present in spleen and thymus, with absolute numbers increasing in the peripheral blood, spleen, and bone marrow over time. The majority of Tregs were recipient derived (88%-92%), with only 8%-12% being donor–derived (supplemental Table 1 and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
FCs induce Treg generation in vivo. Purified B6 HSCs and NOD HSCs were mixed with B6 FCs, administered to ablatively conditioned NOD recipients, and Treg generation was evaluated. (A) Experimental design for the induction of in vivo Treg generation. (B-D) Representative analysis of donor or recipient CD8−/CD4+/CD25+/FoxP3+ Tregs in chimeric spleen, thymus, and bone marrow at 5 weeks after transplantation. (E) Kinetics of absolute number of donor or recipient Tregs in chimeric spleen, thymus, peripheral blood, and bone marrow at 2, 3, 4, and 5 weeks after transplantation. Results are from 4 separate experiments (n = 4).
FCs induce Treg generation in vivo. Purified B6 HSCs and NOD HSCs were mixed with B6 FCs, administered to ablatively conditioned NOD recipients, and Treg generation was evaluated. (A) Experimental design for the induction of in vivo Treg generation. (B-D) Representative analysis of donor or recipient CD8−/CD4+/CD25+/FoxP3+ Tregs in chimeric spleen, thymus, and bone marrow at 5 weeks after transplantation. (E) Kinetics of absolute number of donor or recipient Tregs in chimeric spleen, thymus, peripheral blood, and bone marrow at 2, 3, 4, and 5 weeks after transplantation. Results are from 4 separate experiments (n = 4).
Previous studies have shown that the main subpopulation of FCs comprises B220+/CD11c+/CD11b− p-preDC FCs.2,26 p-preDC FCs and p-preDCs share many phenotypic, morphologic, and functional features.2 p-preDC FCs produce interferon-α and tumor necrosis factor-α in response to Toll-like receptor-9 (TLR-9) ligand stimulation with CpG-ODNs.2,26 FCs also produce several cytokines also made by p-preDCs, including macrophage inflammatory protein-1α/CC-motif chemokine ligand 3, interleukin-6 (IL-6), RANTES (regulated on activation normal T-cell expressed and secreted)/CC-motif chemokine ligand 5, and IL-12. We therefore examined p-preDC FCs for TLR-9 expression and found that they expressed high levels of TLR-9 (data not shown).
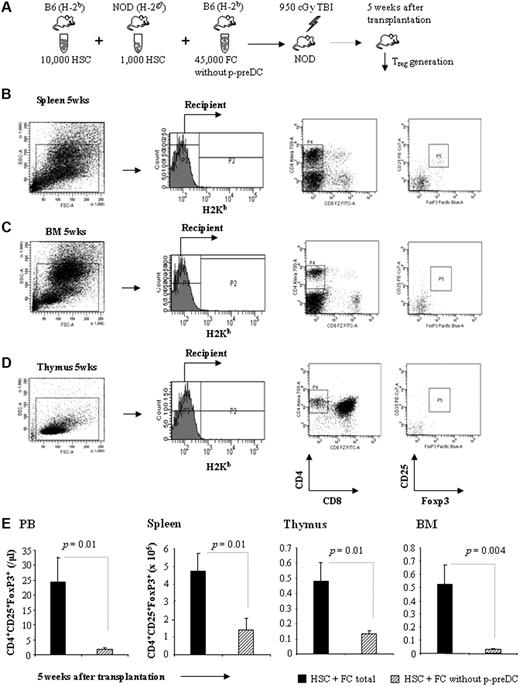
To test whether the p-preDC FC subpopulation plays an important role in inducing Treg production, 45 000 B6 FCs from which the B220+/CD11c+/CD11b− p-preDC subpopulation had been removed were transplanted with 10 000 B6 HSCs and 1000 NOD HSCs into conditioned NOD recipients. At 5 weeks after transplantation, thymus, spleen, and bone marrow were harvested from NOD recipients, and the numbers of donor (B6) or recipient (NOD) Tregs were determined by flow cytometry. All animals (n = 4) engrafted exclusively with only recipient HSCs; they failed to engraft donor B6 HSCs and did not produce chimeric Tregs (Figure 4A-D). The absolute number of recipient-derived Tregs in peripheral blood, thymus, spleen, and bone marrow was significantly decreased compared with mice that received total FCs (Figure 4E). These data suggest that the p-preDC subpopulation in FCs is a critical component in the mechanism of FC function in inducing the generation of chimeric Tregs in vivo.
Removal of p-preDCs from FCs abrogates the induction of chimeric Treg generation. (A) Sorted 45 000 FCs, from which the p-preDC subpopulation had been removed, were transplanted with 10 000 B6 HSCs + 1000 NOD HSCs into conditioned NOD recipients. (B-D) Representative analysis of recipient CD8−/CD4+/CD25+/FoxP3+ Tregs in spleen, thymus, and bone marrow at 5 weeks after transplantation. (E) The absolute number of recipient-derived Tregs in peripheral blood, spleen, thymus, and bone marrow from mice that received HSC + FC (n = 4) and HSC + FC without p-preDCs (n = 4) 5 weeks after transplantation. Results are means ± SEM.
Removal of p-preDCs from FCs abrogates the induction of chimeric Treg generation. (A) Sorted 45 000 FCs, from which the p-preDC subpopulation had been removed, were transplanted with 10 000 B6 HSCs + 1000 NOD HSCs into conditioned NOD recipients. (B-D) Representative analysis of recipient CD8−/CD4+/CD25+/FoxP3+ Tregs in spleen, thymus, and bone marrow at 5 weeks after transplantation. (E) The absolute number of recipient-derived Tregs in peripheral blood, spleen, thymus, and bone marrow from mice that received HSC + FC (n = 4) and HSC + FC without p-preDCs (n = 4) 5 weeks after transplantation. Results are means ± SEM.
Chimeric Tregs induced by FCs prevent rejection and potently increase long-term donor chimerism
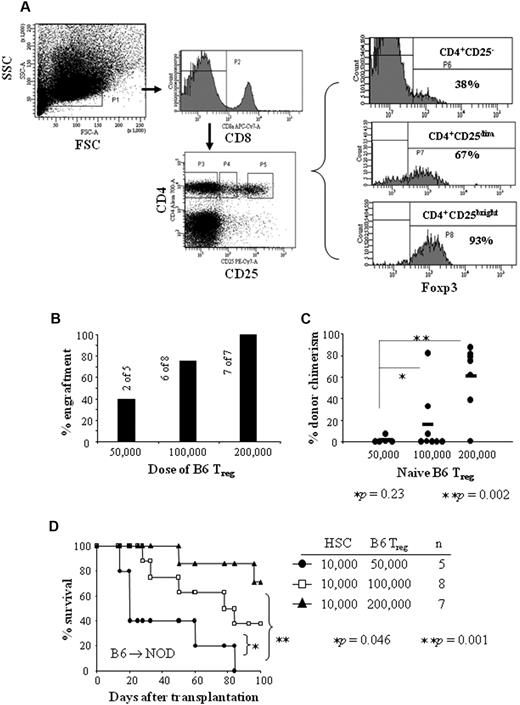
It has been shown that donor-derived CD4+/CD25+ Tregs inhibit lethal GVHD after allogeneic bone marrow transplantation across major histocompatibility complex class I and II barriers in mice.22,27,28 B6 Tregs enhanced engraftment of T-cell–depleted bone marrow cells in an MHC-mismatched B6 → BALB/c animal model.29 FoxP3 is crucial in the development and function of natural CD4+/CD25+ Tregs.30-32 We observed a significantly higher level of FoxP3 expression in the CD4+/CD25bright fraction compared with the CD4+/CD25dim Treg fraction (Figure 5A). To investigate whether Tregs are involved in the enhancement of engraftment of allogeneic HSCs, we tested CD8−/CD4+/CD25bright naive Treg function in an allogeneic model for facilitation (B6 → NOD). CD8−/CD4+/CD25bright Tregs were sorted from spleens of naive B6 mice. 10 000 B6 HSCs + 50 000, 100 000, or 200 000 Tregs were transplanted into NOD recipients conditioned with 950 cGy of TBI. Only 40% (2 of 5) recipients of HSCs + 50 000 Tregs engrafted. These recipients exhibited low levels of donor chimerism (range: 0.5%-7.5%) and all survived less than 90 days (Figure 5B-D). Seventy-five percent (6 of 8) of recipients transplanted with HSCs + 100 000 Tregs engrafted (average percentage of donor chimerism: 15%; range: 0.6%-82%) and 40% of the recipients survived up to 100 days (Figure 5B-D). In contrast, 100% (7 of 7) recipients of HSCs + 200 000 Tregs engrafted and exhibited high levels of donor chimerism (average: 60%; range: 1%-87%); 71% survived more than 100 days (Figure 5B-D). These data suggest that naive Tregs enhance engraftment of allogeneic HSCs in a cell dose–dependent fashion.
Naive B6 Tregs enhance engraftment of HSCs in a cell-dose–dependent manner. CD8−/CD4+/CD25bright Tregs were sorted from naive B6 spleens. Purified naive B6 Tregs + B6 HSCs were transplanted into NOD recipients conditioned with 950 cGy of TBI. (A) Splenocytes were stained (CD8−/CD4+/CD25bright) and gated for CD8− (top middle panel) and CD4+/CD25−, CD4+/CD25dim or CD4+/CD25bright (bottom middle panel). The level of FoxP3 expression in these cell fractions was analyzed (right panel). (B) Percent engraftment in NOD recipients given 10 000 B6 HSCs + 50 000, 100 000, or 200 000 Tregs from spleens of naive B6 mice. (C) Percentage of donor chimerism in engrafted NOD recipients. (D) Survival of NOD recipients conditioned 950 cGy of TBI and given 10 000 B6 HSCs + 50 000 B6 Tregs (●), 100 000 B6 Tregs (□), or 200 000 B6 Tregs (▲). Results are from 3 separate transplant experiments.
Naive B6 Tregs enhance engraftment of HSCs in a cell-dose–dependent manner. CD8−/CD4+/CD25bright Tregs were sorted from naive B6 spleens. Purified naive B6 Tregs + B6 HSCs were transplanted into NOD recipients conditioned with 950 cGy of TBI. (A) Splenocytes were stained (CD8−/CD4+/CD25bright) and gated for CD8− (top middle panel) and CD4+/CD25−, CD4+/CD25dim or CD4+/CD25bright (bottom middle panel). The level of FoxP3 expression in these cell fractions was analyzed (right panel). (B) Percent engraftment in NOD recipients given 10 000 B6 HSCs + 50 000, 100 000, or 200 000 Tregs from spleens of naive B6 mice. (C) Percentage of donor chimerism in engrafted NOD recipients. (D) Survival of NOD recipients conditioned 950 cGy of TBI and given 10 000 B6 HSCs + 50 000 B6 Tregs (●), 100 000 B6 Tregs (□), or 200 000 B6 Tregs (▲). Results are from 3 separate transplant experiments.
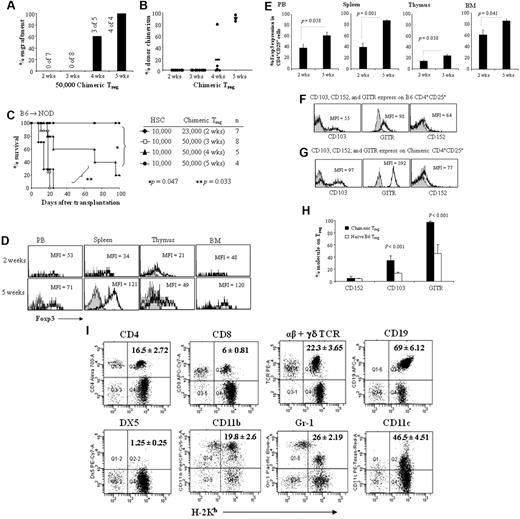
To evaluate the function of FC-induced chimeric Tregs, we tested the ability of chimeric Tregs to enhance engraftment and donor chimerism after transplantation. Spleens were harvested from mixed chimeras 2, 3, 4, and 5 weeks after HSC + FC transplantation. CD8−/CD4+/CD25bright chimeric Tregs were sorted from the spleens of chimeras, and 50 000 chimeric Tregs + 10 000 B6 HSCs were transplanted into NOD recipients conditioned with 950 cGy of TBI. All secondary recipients of 2-week (n = 7) or 3-week (n = 8) chimeric Tregs + HSCs died before 30 days after transplantation, suggesting that at these time points the Tregs were not functional (Figure 6A-C). Three of 5 recipients of 4-week chimeric Tregs engrafted, with an average of 18% donor chimerism (range: 1.7%-79%; Figure 6A-B). Only 1 of 5 (20%) NOD mice receiving 4-week chimeric Tregs survived up to 100 days (Figure 6C). In striking contrast, chimeric Treg function was significantly enhanced by week 5, because 100% of recipients (n = 4) of chimeric Tregs + HSCs engrafted and all survived more than 100 days (Figure 6A-C; P = .047). All of the recipients showed donor-cell chimerism in excess of 90% (range: 84%-95%; Figure 6B). Analysis of chimeric Tregs at 5 weeks after transplantation showed that 78% of Tregs were recipient derived (supplemental Table 2 and supplemental Figure 2). These data suggest that the FC-induced chimeric Tregs acquired function over at least 5 weeks after transplantation.
Chimeric Tregs potently enhance HSC engraftment. CD8−/CD4+/CD25bright Tregs were sorted from the spleens of mixed chimeras (B6 → NOD). Sorted 23 000 to 50 000 chimeric Tregs + 10 000 B6 HSCs were transplanted into ablatively conditioned NOD recipients. The chimeric Tregs were harvested 2-5 weeks after transplantation. (A) Percent engraftment in NOD recipients of 10 000 B6 HSCs + chimeric Tregs at 1 month. (B) Percentage of donor chimerism at 1 month in transplanted NOD mice. (C) Facilitative ability of chimeric Tregs administered to NOD mice. CD4+/CD25+ Tregs were sorted at selected time points: 2-week chimeric Tregs (♦) + 3-week (□), 4-week (▲), or 5-week (●) chimeric Tregs. (D) Representative analysis of FoxP3 expression in CD4+/CD25+ Tregs in chimeric spleen, thymus, bone marrow, and peripheral blood at 2 or 5 weeks after transplantation. Overlay histogram plots display FoxP3 mean fluorescence intensity (MFI) of the CD4+/CD25+ cells (black line) in comparison with the isotype control stain (shaded histogram). (E) The percentage of total CD4+/CD25bright cells with FoxP3 expression in 2-week (n = 4) or 5-week (n = 4) chimeric Tregs of spleen, thymus, peripheral blood, and bone marrow. (F) Levels of CD152, CD103, and GITR expression on Tregs. Overlay histogram plots display CD103, GITR, and CD152 MFI of B6 CD4+/CD25+ cells (black line) in comparison with the isotype control stain (shaded histogram). (G) Overlay histogram plots display CD103, GITR, and CD152 MFI of 5-week chimeric CD4+/CD25+ cells. (H) Percentage of CD103, GITR, and CD152 expression on B6 CD4+CD25+ cells or chimeric CD4+/CD25+ cells. (I) Multilineage PBL typing of NOD recipients of B6 HSCs + 5-week chimeric Tregs. The data are from one representative recipient 3 months after transplantation and were analyzed based on the lymphoid and myeloid gate. Results are means ± SEM.
Chimeric Tregs potently enhance HSC engraftment. CD8−/CD4+/CD25bright Tregs were sorted from the spleens of mixed chimeras (B6 → NOD). Sorted 23 000 to 50 000 chimeric Tregs + 10 000 B6 HSCs were transplanted into ablatively conditioned NOD recipients. The chimeric Tregs were harvested 2-5 weeks after transplantation. (A) Percent engraftment in NOD recipients of 10 000 B6 HSCs + chimeric Tregs at 1 month. (B) Percentage of donor chimerism at 1 month in transplanted NOD mice. (C) Facilitative ability of chimeric Tregs administered to NOD mice. CD4+/CD25+ Tregs were sorted at selected time points: 2-week chimeric Tregs (♦) + 3-week (□), 4-week (▲), or 5-week (●) chimeric Tregs. (D) Representative analysis of FoxP3 expression in CD4+/CD25+ Tregs in chimeric spleen, thymus, bone marrow, and peripheral blood at 2 or 5 weeks after transplantation. Overlay histogram plots display FoxP3 mean fluorescence intensity (MFI) of the CD4+/CD25+ cells (black line) in comparison with the isotype control stain (shaded histogram). (E) The percentage of total CD4+/CD25bright cells with FoxP3 expression in 2-week (n = 4) or 5-week (n = 4) chimeric Tregs of spleen, thymus, peripheral blood, and bone marrow. (F) Levels of CD152, CD103, and GITR expression on Tregs. Overlay histogram plots display CD103, GITR, and CD152 MFI of B6 CD4+/CD25+ cells (black line) in comparison with the isotype control stain (shaded histogram). (G) Overlay histogram plots display CD103, GITR, and CD152 MFI of 5-week chimeric CD4+/CD25+ cells. (H) Percentage of CD103, GITR, and CD152 expression on B6 CD4+CD25+ cells or chimeric CD4+/CD25+ cells. (I) Multilineage PBL typing of NOD recipients of B6 HSCs + 5-week chimeric Tregs. The data are from one representative recipient 3 months after transplantation and were analyzed based on the lymphoid and myeloid gate. Results are means ± SEM.
Treg function is correlated with acquisition of FoxP3
The level of FoxP3 expression was recently reported to be correlated with the suppressive function of Tregs.30-32 We therefore compared the expression of FoxP3 in 2-week vs 5-week chimeric CD4+/CD25+ Tregs from mouse spleen, peripheral blood, thymus, and bone marrow (Figure 6D-E). There was a significant increase in the level of FoxP3 expression in 5-week chimeric Tregs of spleen compared with 2-week chimeric Tregs (86.9% ± 1.8% vs 38.9% ± 7.6%; P = .001). There was also a significant increase in the level of FoxP3 expression in 5-week chimeric Tregs of thymus compared with 2-week chimeric Tregs (23.7% ± 3.1% vs 14.1% ± 1.8%; P = .038) and bone marrow (86.1% ± 3.3% vs 61.0% ± 9.1%; P = .041). However, there was no significant difference in 5-week chimeric Tregs of peripheral blood compared with 2-week chimeric Tregs (60.9% ± 6.6% vs 38.5% ± 6.9%; P = .058). The majority of FoxP3+ cells were CD4+/CD25+ cells (supplemental Figures 4-5). These results indicate that FoxP3 gene expression is associated with the suppressive capacity of CD4+/CD25+ Tregs in vivo. In addition, we analyzed the expression of CD152, CD103, and glucocorticoid-induced tumor necrosis factor receptor family related gene (GITR) on 5-week chimeric Tregs. The level of CD103 and GITR expression on chimeric Tregs was significantly increased compared with naive B6 Tregs (Figure 6F-H), indicating that CD103 and GITR expression is correlated with Treg function.
These mixed chimeras exhibited durable engraftment and showed the presence of multilineage donor cells, including T cells (CD8, CD4, β-TCR), NK cells (NK1.1DX5), B cells (CD19), DCs (CD11c), macrophage (Mac-1), and granulocytes (Gr-1; Figure 6I). These data suggest that 5-week chimeric Tregs are more efficient in enhancing engraftment of allogeneic B6 HSCs in NOD recipients compared with naive B6 Tregs (Figure 5B, D).
Chimeric Tregs potently suppress proliferation of T cells in vitro in an antigen-specific fashion
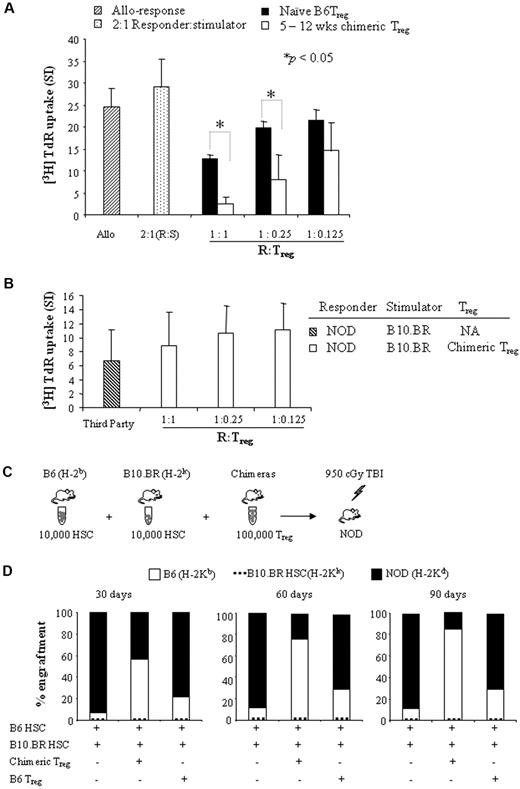
We compared the suppressive function of chimeric Tregs vs naive Tregs in vitro using MLR suppressor cell assays. CD8−/CD4+/CD25bright chimeric Tregs were sorted from spleens of chimeras 5 to 12 weeks after HSC + FC transplantation. As shown in Figure 7A, Tregs from naive B6 mice resulted in 1.9-fold, 1.3-fold, and 1.1-fold inhibition of proliferation at responder/Treg ratios of 1:1, 1:0.25, and 1:0.125 (n = 3), respectively. In contrast, chimeric Tregs potently suppressed T-cell proliferation by 10.5-fold, 3.2-fold, and 1.7-fold at responder/Treg ratios of 1:1, 1:0.25, and 1:0.125 (n = 4), respectively. Chimeric Tregs significantly suppressed T-cell proliferation at responder/Treg ratios of 1:1 and 1:0.25 compared with naive B6 Tregs (P < .05). NOD lymph node–responder cells remained hypoproliferative in response to B6 stimulator + chimeric Tregs compared with stimulator + naive B6 Tregs, suggesting that chimeric Tregs are significantly more potent than naive B6 Tregs in suppressing effector T-cell proliferation in vitro. In a second experiment, we tested whether chimeric Tregs suppress T-cell proliferation in an antigen-specific fashion. Third-party B10.BR splenocytes served as the stimulator. Our results show that chimeric Tregs failed to suppress T-cell proliferation at responder/Treg ratios of 1:1, 1:0.25, and 1:0.125 (n = 4; Figure 7B; supplemental Figure 3), demonstrating antigen-specific function.
The function of chimeric Tregs is antigen-specific. (A) CD8−/CD4+/CD25bright cells were sorted from the spleens of mixed chimeras (B6 → NOD) or naive B6 mice. Sorted Tregs were mixed with NOD lymph node–responder cells in decreasing ratios (1:1, 1:0.25, and 1:0.125) and stimulated with irradiated B6 or NOD-stimulator splenocytes. T-cell proliferation was measured at 5 days. Results are means ± SEM of 3-4 independent experiments. (B) Sorted chimeric Tregs were mixed with NOD lymph node–responder cells in decreasing ratios (1:1, 1:0.25, and 1:0.125) and stimulated with irradiated B10.BR (third-party) stimulator splenocytes. Irradiated B6 stimulator splenocytes serves as a control (supplemental Figure 3). T-cell proliferation was measured at 5 days. Results are means ± SEM of 4 independent experiments. (C) Experimental design for the evaluation of the function of chimeric Tregs in vivo. (D) 10 000 B6 HSCs + 10 000 B10.BR HSCs with or without 100 000 sorted chimeric Tregs or naive B6 Tregs were transplanted into NOD recipient mice conditioned with 950 cGy of TBI in competitive repopulation assays (n = 4-9). PBL typing was performed by staining with anti–H-2Kb, H-2Kk, and H-2Kd mAbs at 30, 60, and 90 days. Analysis of donor (B6 or B10.BR) origin and recipient (NOD) origin were based on lymphoid gate. The bar represents the mean.
The function of chimeric Tregs is antigen-specific. (A) CD8−/CD4+/CD25bright cells were sorted from the spleens of mixed chimeras (B6 → NOD) or naive B6 mice. Sorted Tregs were mixed with NOD lymph node–responder cells in decreasing ratios (1:1, 1:0.25, and 1:0.125) and stimulated with irradiated B6 or NOD-stimulator splenocytes. T-cell proliferation was measured at 5 days. Results are means ± SEM of 3-4 independent experiments. (B) Sorted chimeric Tregs were mixed with NOD lymph node–responder cells in decreasing ratios (1:1, 1:0.25, and 1:0.125) and stimulated with irradiated B10.BR (third-party) stimulator splenocytes. Irradiated B6 stimulator splenocytes serves as a control (supplemental Figure 3). T-cell proliferation was measured at 5 days. Results are means ± SEM of 4 independent experiments. (C) Experimental design for the evaluation of the function of chimeric Tregs in vivo. (D) 10 000 B6 HSCs + 10 000 B10.BR HSCs with or without 100 000 sorted chimeric Tregs or naive B6 Tregs were transplanted into NOD recipient mice conditioned with 950 cGy of TBI in competitive repopulation assays (n = 4-9). PBL typing was performed by staining with anti–H-2Kb, H-2Kk, and H-2Kd mAbs at 30, 60, and 90 days. Analysis of donor (B6 or B10.BR) origin and recipient (NOD) origin were based on lymphoid gate. The bar represents the mean.
Chimeric Tregs enhance HSC engraftment in an antigen-specific fashion
We evaluated whether chimeric Tregs enhance engraftment of HSCs in an antigen-specific manner using an in vivo competitive repopulation assay. Five-week chimeric Tregs were sorted from spleens of mixed chimeras (B6 → NOD). 100 000 chimeric Tregs were then mixed with 10 000 B6 HSCs (donor-specific) + 10 000 B10.BR HSCs (third-party) and transplanted into conditioned NOD recipients (Figure 7C). NOD mice given HSCs + B6 Tregs or HSCs alone served as controls. Two of the 4 animals that received HSCs alone engrafted and exhibited an average of 6.7% donor B6 chimerism at 30 days, 11.2% at 60 days, and 10.6% at 90 days (Figure 7D). Three of 5 animals transplanted with HSCs + B6 Tregs engrafted, with 21.3% donor B6 chimerism at 30 days, 28.8% at 60 days, and 28.9% at 90 days. In striking contrast, 8 of 9 recipients of HSCs + chimeric Tregs engrafted and exhibited significantly higher levels of donor B6 chimerism, ranging from 56.3% at 30 days and 75.4% at 60 days to 85% at 90 days (P = .031 compared with B6 Tregs). None of the recipients exhibited engraftment of MHC-disparate third-party B10.BR HSCs. These data show that chimeric Tregs enhance donor HSC engraftment but not third-party HSCs, demonstrating that chimeric Tregs function in an antigen-specific fashion in vivo.
Discussion
The use of Tregs as a cell-based therapy to induce graft/host tolerance in vivo holds great therapeutic potential. However, a major challenge to the clinical use of Treg therapies has been obtaining sufficient numbers of cells for transplantation and maintaining their tolerogenic properties in vivo after in vitro expansion and transplantation.33 Most attempts at in vitro expansion have been limited by a loss of regulatory function and a concomitant loss of FoxP3 expression.34 We previously reported that CD8+/TCR− FCs significantly enhance HSC engraftment and tolerance induction in vivo and that the p-preDC FC subpopulation plays a critical but nonredundant role in facilitation.2 We show here for the first time that FCs function to induce antigen-specific Tregs in vivo, thereby promoting chimerism and tolerance.
Several reports have suggested that CD4+/CD25+/FoxP3+ Tregs are generated in the thymus35-37 and that FoxP3 is a critical regulator of their development and suppressive function.31 Evidence also suggests that FoxP3+ Tregs can develop extrathymically under certain conditions.38-40 Our present findings show that phenotypic chimeric Tregs are generated beginning at 2 weeks after HSC + FC transplantation, but they are not fully functional until 5 weeks after transplantation. The percentage of CD4+/CD25+ Tregs and the level of FoxP3 expression in 5-week chimeric Tregs of spleen was significantly increased compared with 2- and 3-week chimeric Tregs (P = .001), suggesting that FoxP3 is essential for the suppressive function of chimeric Tregs. These results support previous studies showing a direct correlation between up-regulation of FoxP3 and Treg function.34 It was previously reported that mice receiving ablative irradiation exhibited severe thymic atrophy associated with peripheral T-cell hypoplasia.41 The recovery of function of thymocytes occurred 3-5 weeks after syngeneic bone marrow transplantation, while splenic function resumed 2-3 weeks later.42 A recent study showed that the recovery of functional donor-derived CD4+/CD25+/FoxP3+ Tregs occurred in the recipient's thymus and lymph nodes 6 weeks after bone marrow transplantation.31 These results provide evidence for the FC-induced generation of chimeric Tregs in the thymic and splenic environments after FC + HSC transplantation in recipient animals.
In the present study, we found that CD8α+ p-preDC FCs are critical to the generation of antigen-specific Tregs in vivo. Removal of the CD8α p-preDC FC subpopulation from total FCs abrogated the generation of Tregs in vivo. Mature p-preDCs activated by IL-3 + CD40 ligand or by the TLR-9 ligand have been shown to up-regulate the expression of inducible costimulator ligand and the generation of IL-10–producing Tregs.43 Ochando et al demonstrated that pDCs as phagocytic antigen-presenting cells mediate tolerance to vascularized allografts by inducing Treg development in vivo.19 Their data also demonstrated that the generation of Tregs depends on direct interaction between CD4+ T cells and pDCs in the lymph nodes of allograft recipients.19 A recent report demonstrated that liver pDCs prevented oral T-cell priming and induced systemic tolerance to CD4+ and CD8+ T-cell–mediated delayed-type hypersensitivity.44 B220+/CD11c+/CD11b− p-preDC FCs display characteristic plasmacytoid morphology; low expression of MHC class II, CD80, and CD86; and produce interferon-α, tumor necrosis factor-α, and other cytokines in response to CpG-ODNs.2,26 p-preDC FCs stimulated with CpG-ODNs promote the differentiation of CD4+/CD25− T cells into CD4+/CD25+/FoxP3+ Tregs in vitro.5 In the present study, we found that FCs express significant levels of TLR-9 and induce the generation of both donor- and host-derived CD4+/CD25+/FoxP3+ Tregs (chimeric Tregs) in vivo. The majority of chimeric Tregs were recipient derived. In contrast to naive Tregs, in vivo FC-induced chimeric Tregs potently enhanced engraftment of allogeneic HSCs in ablatively conditioned secondary NOD recipients. They were also significantly more potent in suppressing T-cell proliferation in MLR suppressor cells assays in vitro compared with naive Tregs.
Strategies for the production of antigen-specific Tregs for use in transplantation are being pursued. Most models have used in vitro manipulation for expansion of Tregs to obtain sufficient numbers. However, a major limitation has been identifying an approach to achieve efficient expansion yet retain suppressive function. Joffre et al reported that recipient CD4+/CD25+/FoxP3+ Tregs stimulated in vitro with alloantigens induced antigen-specific tolerance to bone marrow and subsequent skin and cardiac allografts.11 Another study found that in vitro–expanded Tregs exhibited reduced levels of FoxP3 expression, which significantly impaired their immune suppressive function.34 Alternatively, potent antigen-specific Tregs can be induced in vivo by targeting antigens to DCs under certain circumstances. For example, maternal cells have been shown to cross the placenta and engraft into human fetal tissues in utero, resulting in “maternal microchimerism” and inducing the development of antigen-specific CD4+/CD25+/FoxP3+ Tregs.45
A recent study showed that donor-specific Tregs of recipient origin are recruited, whereas donor antigens are present in low-intensity conditioning transplantation models, and that these cells may play a critical role in the establishment of host-versus-graft tolerance.46 We found that FC-induced chimeric Tregs enhanced donor-specific but not MHC-disparate third-party HSC engraftment in NOD recipients, suggesting that the function of chimeric Tregs is highly antigen specific. Similarly, suppression of proliferative responses by chimeric Tregs in vitro was also antigen specific. The effect is very potent and ex vivo expansion of the FC population is not required. Chimeric Tregs express significantly higher levels of CD103 and GITR compared with naive Tregs. Most importantly, our studies confirm that FCs maintain their tolerogenic properties in vivo after transplantation. The antigen specificity of chimeric Tregs induced by FCs address one other major concern regarding the use of naive Tregs in clinical trials, because the naive Tregs exhibited nonspecific suppression. FCs therefore offer an attractive cell-based approach for tolerance induction in vivo.
p-preDCs play important regulatory roles in allogeneic HSC and organ transplant outcomes.47 A recent study showed that depletion of p-preDCs from bone marrow grafts resulted in an acceleration of mortality from GVHD, while the depletion of mature p-preDCs from granulocyte colony-stimulating factor–mobilized splenic grafts had no effect. These data suggest that donor bone marrow p-preDCs, but not mature p-preDCs, attenuate acute GVHD.48 A significantly higher ratio of p-preDCs to monocytoid DCs in the peripheral blood is correlated with successful withdrawal of immunosuppression after liver transplantation.49 In addition, a high ratio of coinhibitory programmed death ligand-1 to costimulatory CD86 on circulating pDCs is associated with elevated levels of Tregs in human liver transplant tolerance.50 We previously demonstrated that FCs facilitate engraftment of HSCs in allogeneic recipients without causing GVHD.1,2 Removal of the p-preDC FC subpopulation completely abrogated facilitation. However, p-preDC FCs and p-preDCs did not replace CD8+/TCR− total FCs in in vivo FC function. In the present study, we found that removal of the p-preDC subpopulation from FC grafts resulted in significantly decreased frequencies of CD4+/CD25+ FoxP3+ Tregs in thymus, spleen, bone marrow, and peripheral blood compared with mice that received total FCs. Moreover, the phenotypic Tregs that were generated in the FCs from which the p-preDC subpopulation had been removed were not functional in that they did not facilitate. These data provide further evidence that p-preDC FCs play an important role in the induction of Treg generation in vivo.
We provide here the first in vivo evidence of a role for FCs in inducing antigen-specific chimeric Tregs. This represents an advantage to naive Tregs, which suppress alloreactivity nonspecifically. The fact that the p-preDC subpopulation represents the majority of FCs and plays a critical role in facilitation2,26 suggests that p-preDC FCs could represent a key component in the induction of antigen-specific Tregs. These findings may provide a novel cell-based approach to induce graft/host tolerance and treat autoimmune disorders through immunomodulation and mixed chimerism.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Deborah M. Ramsey, Haval Shirwan, and Jun Yan for review of the manuscript and helpful comments; Michael Tanner for cell sorting and technical assistance; Carolyn DeLautre for manuscript preparation; and the staff of the University of Louisville animal facility for outstanding animal care.
This work was supported in part by National Institutes of Health grants R01 DK069766 and 5RO1 HL063442; by Juvenile Diabetes Research Foundation grants (JDRF 1-2005-1037 and JDRF 1-2006-146); by the American Diabetes Association; by the Commonwealth of Kentucky Research Challenge Trust Fund; by the W. M. Keck Foundation; and by The Jewish Hospital Foundation.
Research was conducted in compliance with the Animal Welfare Act Regulations and other federal statues relating to animals and experiments involving animals, and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996.
This work is dedicated to the memory of Michael Tanner.
National Institutes of Health
Authorship
Contribution: Y.H. and L.D.B. designed and executed experiments, analyzed data, and wrote the manuscript; T.M. executed the experiments and analyzed data; H.X. designed and executed experiments; L.H. executed the experiments and analyzed data; and S.T.I designed experiments, analyzed data, and wrote and finalized the manuscript.
Conflict-of-interest disclosure: S.T.I. is the founding scientist and director of Regenerex, a biotech start-up company; it has not been capitalized. L.D.B. is a research scientist and employee of Regenerex. The remaining authors declare no competing financial interests.
Correspondence: Suzanne T. Ildstad, MD, Director, Institute for Cellular Therapeutics, The Jewish Hospital Distinguished Professor of Transplantation, Distinguished University Scholar, Professor of Surgery, Microbiology and Immunology, and Physiology, University of Louisville, 570 S Preston St, Ste 404, Louisville, KY 40202-1760; e-mail: suzanne.ildstad@louisville.edu.
References
Author notes
Y.H. and L.D.B. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal