Abstract
The Blood and Marrow Transplant Clinical Trials Network conducted 2 parallel multicenter phase 2 trials for individuals with leukemia or lymphoma and no suitable related donor. Reduced intensity conditioning (RIC) was used with either unrelated double umbilical cord blood (dUCB) or HLA-haploidentical related donor bone marrow (Haplo-marrow) transplantation. For both trials, the transplantation conditioning regimen incorporated cyclophosphamide, fludarabine, and 200 cGy of total body irradiation. The 1-year probabilities of overall and progression-free survival were 54% and 46%, respectively, after dUCB transplantation (n = 50) and 62% and 48%, respectively, after Haplo-marrow transplantation (n = 50). The day +56 cumulative incidence of neutrophil recovery was 94% after dUCB and 96% after Haplo-marrow transplantation. The 100-day cumulative incidence of grade II-IV acute GVHD was 40% after dUCB and 32% after Haplo-marrow transplantation. The 1-year cumulative incidences of nonrelapse mortality and relapse after dUCB transplantation were 24% and 31%, respectively, with corresponding results of 7% and 45%, respectively, after Haplo-marrow transplantation. These multicenter studies confirm the utility of dUCB and Haplo-marrow as alternative donor sources and set the stage for a multicenter randomized clinical trial to assess the relative efficacy of these 2 strategies. The trials are registered at www.clinicaltrials.gov under NCT00864227 (BMT CTN 0604) and NCT00849147 (BMT CTN 0603).
Introduction
Reduced intensity conditioning (RIC) has allowed older and less clinically fit patients with high-risk hematologic malignancies to proceed to potentially curative treatment with allogeneic hematopoietic cell transplantation (HCT).1-5 For patients lacking an HLA-matched sibling, it is routine to initiate an unrelated donor search. However, a suitably matched unrelated donor cannot be identified for as many as one-third of patients, even more for members of ethnic minorities. Even when an unrelated donor is identified, the interval between search initiation and transplantation can be as long as 4 months. Consequently, some patients, specially ethnic minorities, experience disease progression while awaiting identification of a suitably HLA-matched donor.6
Single-center studies have shown that unrelated double umbilical cord blood (dUCB)7,8 and partially HLA-mismatched related bone marrow (Haplo-marrow)9,10 are valuable alternative sources of donor cells for RIC HCT, thus extending this treatment modality to patients who lack a matched sibling or suitably matched unrelated donor. To study the reproducibility, and thus the wider applicability, of the 2 alternative donor strategies (dUCB and Haplo-marrow), the Blood and Marrow Transplantation Clinical Trials Network (BMT CTN) conducted 2 parallel phase 2 trials. Using identical inclusion and exclusion criteria and a common study design, these trials evaluated the efficacy of dUCB trans-plantation (BMT CTN 0604) and Haplo-marrow transplantation (BMT CTN 0603) after RIC regimens at 27 transplantation centers in the United States. The goal of these studies was to generate pilot multicenter data to support a future phase 3 randomized clinical trial.
Methods
Patients
Eligible patients were ≤ 70 years of age with an advanced or high-risk leukemia or lymphoma and lacked a suitable matched related donor. Acute leukemia was required to be in morphologic complete remission. Large-cell, mantle-cell, and Hodgkin lymphomas were required to have achieved at least a partial remission with treatment before allogeneic HCT. Low-grade lymphoma patients were required to have failed 2 prior chemotherapy regimens, but were not required to demonstrate chemotherapy sensitivity. A suitably matched related donor was defined as 1 match or 1-locus mismatch at HLA-A, HLA-B, or HLA-DRB1. A formal unrelated adult donor search was not a prerequisite for eligibility in either trial. Patients were required to have adequate organ function defined as: left ventricular ejection fraction > 35%; carbon monoxide corrected diffusion lung capacity, forced expiratory volume in the first second, or functional vital capacity > 50% of predicted; total bilirubin ≤ 2.5 mg/dL and aspartate and alanine aminotransferases and alkaline phosphatase < 5 × the upper limit of normal; and serum creatinine within the normal range for age or measured creatinine clearance or calculated glomerular filtration rate > 40 mL/min/1.73 m.2 A Karnofsky performance score of 60-100 was required, and patients who had undergone prior autologous transplantation were not excluded as long as 3 months had elapsed since the procedure. Ten transplantation centers enrolled patients only in the dUCB trial, 11 centers enrolled patients only in the Haplo-marrow trial, and 6 centers enrolled in both trials. Centers that enrolled patients in both trials provided a document to the data coordinating center indicating the patient/disease types that they would enroll in each trial, and the coordinating center ensured adherence. Targeted toxicity was monitored by the Common Toxicity Criteria for Adverse Events version 3.
Trials were approved by the National Heart, Lung and Blood Institute Protocol Review Committee, the Data and Safety Monitoring Board for the BMT CTN, and the institutional review board of each participating institution. Patients gave informed consent according to the principles of the Declaration of Helsinki. The trials were registered at www.clinicaltrials.gov under NCT00864227 (BMT CTN 0604) and NCT00849147 (BMT CTN 0603)
Graft and donor selection
dUCB transplantation (BMT CTN 0604).
In protocol 0604, all patients received 2 partially HLA-matched UCB units. The maximum allowable mismatch between the cord blood unit and the recipient or between cord blood units was 2 of 6 HLA loci (HLA-A and HLA-B at the antigen level and HLA-DRB1 at the allele level). Each unit was required to contain a minimum pre-cryopreserved, nucleated cell dose of 1.5 × 107/kg of recipient weight. For UCB units that were not red cell depleted, the minimum cryopreserved nucleated cell dose was 2.0 × 107/kg of recipient body weight. Units were first selected for the best donor-recipient match and thereafter the total nucleated cell dose per kilogram of recipient body weight. Anti-donor HLA antibody screen was not required for UCB unit selection. Units were thawed and infused per institutional practice after validation using methods reviewed and approved by the BMT CTN. The protocol-recommended UCB unit thawing and processing procedure used was described previously by Rubinstein et al.11 After 2 patients had severe infusional reactions, this method was required for all units containing > 20 mL of erythrocytes; however, transplantation centers were allowed to use a validated alternative processing technique.
Haplo-marrow transplantation (BMT CTN 0603).
In protocol 0603, Haplo-marrow donors were required to be first-degree relatives of the patient, which was defined as biologic parents, siblings, children, or half-siblings. Donors and recipients were typed at HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 at the allele level. Donor-recipient pairs were considered HLA-haploidentical if they were genotypically identical for at least one allele of each of these loci. Donors were required to be ≥ 18 years of age and were screened per the American Association of Blood Banks guidelines. Donors were excluded if the recipient's serum contained anti-donor HLA antibodies. If 2 or more eligible donors were identified, donor selection hierarchy was: (1) donor-recipient matching of cytomegalovirus serology and (2) donor-recipient red blood cell compatibility. An earlier report did not show a detrimental effect of increasing donor-recipient HLA-mismatch on outcomes after nonmyeloablative HLA-haploidentical BMT with posttransplantation cyclophosphamide.12 Therefore, the number of mismatched HLA alleles between donor and recipient was not considered as a criterion for donor selection.
Conditioning regimens and immunosuppressive therapies
dUCB transplantation (BMT CTN 0604).
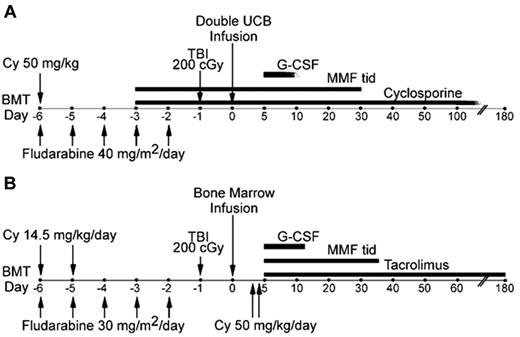
Recipients of cord blood grafts were conditioned with fludarabine 40 mg/m2/d IV from days −6 to −2 (total dose of 200 mg/m2), cyclophosphamide 50 mg/kg IV on day −6, and 2 Gy total body irradiation in a single dose on day −1 (Figure 1A). The dose of fludarabine was adjusted for creatinine clearance as clinically indicated per the manufacturer's recommendations. For patients with actual body weight > 125% of ideal body weight (IBW), cyclophosphamide was dosed based on adjusted ideal body weight (AIBW), calculated as AIBW = IBW + [(0.25) × (actual body weight − IBW)], with MESNA and vigorous IV hydration for uroprotection. GVHD prophylaxis included mycophenolate mofetil (MMF) given at 1 g every 8 hours for patients > 50 kg of body weight; 15 mg/kg every 8 hours for those < 50 kg, beginning on day −3 and continuing until day +30 or 7 days after engraftment, whichever was later. In addition, patients received cyclosporine A to achieve a target trough level of 200-400 ng/mL until day +100; in the absence of GVHD, taper was instituted at 10% of the dose per week beginning on day +101 and discontinued at approximately day +180-200. Tacrolimus dosed to achieve a target trough level of 5-10 ng/mL could be substituted for cyclosporine. Filgrastim was initiated on day +1 at 5 μg/kg/d and was continued until the absolute neutrophil count was ≥ 2000/μL for 3 consecutive days.
Treatment plans. Treatment schemas for (A) RIC and dUCB transplantation and (B) haplo-marrow transplantation.
Treatment plans. Treatment schemas for (A) RIC and dUCB transplantation and (B) haplo-marrow transplantation.
Haplo-marrow transplantation (BMT CTN 0603).
Recipients of HLA-haploidentical related donor grafts were conditioned with fludarabine 30 mg/m2/d IV daily from days −6 to −2 (total dose of 150 mg/m2), cyclophosphamide 14.5 mg/kg IV on days −6 and −5, and 2 Gy total body irradiation in a single dose on day −1 (Figure 1B). The dose of fludarabine was adjusted for creatinine clearance as clinically indicated. For patients with actual body weight > 125% of IBW, cyclophosphamide was dosed based on AIBW as in the preceding paragraph with MESNA and vigorous IV hydration for uroprotection. GVHD prophylaxis consisted of cyclophosphamide 50 mg/kg IBW by IV infusion over 1-2 hours on days +3 (between 60 and 72 hours after marrow infusion) and +4 after transplantation. In addition, patients received tacrolimus and MMF beginning on day +5 after transplantation. MMF was given at a dose of 15 mg/kg every 8 hours with the maximum total daily dose not to exceed 3 g. MMF prophylaxis was discontinued on day +35 or continued at the discretion of the treating center if active GVHD was present. Tacrolimus was dosed to achieve a target trough level of 5-10 ng/mL with the goal of discontinuing by day +180 after transplantation. Filgrastim was initiated on posttransplantation day +5 at a dose of 5 μg/kg/d and continued until the absolute neutrophil count was ≥ 1000/μL for 3 consecutive days.
Supportive care
Supportive care, including blood product administration, prophylaxis, therapy for infection, and treatment of GVHD, was at the discretion of treating physicians and transplantation center practices.
Statistical considerations
The primary end point of both trials was overall survival (OS) at 180 days after transplantation, based on the hypothesis that the probability of OS would not be inferior to that reported after RIC adult unrelated donor transplantation (60% at day +180).1 The target sample size of 50 in each trial would provide 84% power to reject the null hypothesis if the true OS were < 40%. Nonrelapse mortality (NRM) and graft failure were monitored monthly against prespecified safety boundaries with the sequential probability ratio test, and no boundaries for safety end points were triggered during the trial. Secondary end points included PFS and cumulative incidences of hematopoietic recovery, grades II-IV and III-IV acute GVHD, chronic GVHD, relapse, and NRM. Time to neutrophil recovery was defined as the interval between transplantation and the first of 3 consecutive neutrophil counts ≥ 500/μL. Time to platelet recovery was defined as the interval between transplantation and the first of 3 consecutive platelet counts > 20 000/μL or > 50 000/μL without a platelet transfusion in the preceding 7 days. Donor cell engraftment was defined as > 5% donor chimerism on day +56 or beyond after transplantation, and graft failure was defined as < 5% donor chimerism. Patients who did not achieve neutrophil recovery and died within 28 days after transplantation were also scored as graft failures. Chimerism was evaluated at days +28, +56, +180, and +365 after transplantation in whole blood or marrow and/or T cells according to institutional practice by either variable nucleotide tandem repeats or single tandem repeats. For the purpose of the dUCB study, the chimerism of both donor units was combined.
The probabilities of OS and PFS were calculated using the Kaplan-Meier estimator.12 For OS, death from any cause was considered an event and for PFS, the first occurrence of relapse after transplantation, disease progression, or death was considered an event. Patients without an event were censored at last follow-up. Neutrophil and platelet recovery were calculated using the cumulative incidence function,12 with death before recovery as the competing risk. The incidences of acute and chronic GVHD were calculated using the cumulative incidence function, with death, relapse, disease progression, and graft failure as competing risks. The incidences of NRM and relapse were calculated using the cumulative incidence function; for NRM, relapse was the competing risk and for relapse or disease progression, NRM was the competing risk. All analyses were done using SAS software Version 9.2.
Results
Patient, donor, and graft characteristics
Characteristics of the patients in the 2 clinical trials are summarized in Table 1, and of the donors and grafts in Table 2. Fifty-four patients were registered in the dUCB trial. Four were not treated according to protocol and were excluded from the analysis: 1 withdrew consent and 3 were found to have disease relapsed/progression. Accrual occurred between January 2009 and March 2010. Among recipients of dUCB transplantation, the median age was 58 (range 16-69) and median weight, 79 kg. One patient was < 18 years of age at transplantation. Eighty percent of patients had Karnofsky performance scores ≥ 90%. The indications for transplantation were acute leukemia for 36 patients and lymphoma for 14 patients. The median combined nucleated cell dose at the time of cryopreservation was 5.0 × 107/kg and at infusion, 4.2 × 107/kg. Donor-recipient HLA disparity was assigned based on the highest mismatch of the dUCB unit; 33 donor-recipient pairs were assigned 4 of 6; 14 pairs 5 of 6; and 3 pairs 6 of 6 HLA-matched. Cord blood units for 35 donor-recipient pairs (70%) were mismatched to each other at 2 HLA loci.
Patient and disease characteristics
| . | CTN 0604 . | CTN 0603 . |
|---|---|---|
| dUCB | Haplo-marrow | |
| Number of patients | 50 | 50 |
| Age, y | ||
| Median | 58 | 48 |
| Range | 16-69 | 7-70 |
| Weight, kg | ||
| Median | 79 | 78 |
| Range | 46-119 | 21-184 |
| Performance status | ||
| ≥ 90 | 40 (80%) | 38 (76%) |
| < 90 | 10 (20%) | 12 (24%) |
| Primary disease | ||
| Acute lymphoblastic leukemia | 6 (12%) | 6 (12%) |
| Acute myelogeneous leukemia | 29 (58%) | 22 (44%) |
| Biphenotypic/undifferentiated leukemia | 1 (2%) | 3 (6%) |
| Burkitt lymphoma | 1 (2%) | 0 |
| Hodgkin lymphoma | 5 (10%) | 7 (14%) |
| Large-cell lymphoma | 3 (6%) | 8 (16%) |
| Marginal zone B-cell lymphoma | 1 (2%) | 1 (2%) |
| Follicular non-Hodgkin lymphoma | 4 (8%) | 3 (6%) |
| Disease stage | ||
| Acute leukemia | ||
| First complete remission | 23 (64%) | 15 (48%) |
| Second complete remission | 10 (28%) | 12 (39%) |
| Third or subsequent complete remission | 3 (8%) | 4 (13%) |
| Lymphomas | ||
| Complete remission | 5 (36%) | 7 (37%) |
| Partial response | 8 (57%) | 12 (63%) |
| Resistant | 1 (7%) | 0 |
| Number of prior chemotherapy regimens (for lymphoma patients) | ||
| 2 | 1 (7%) | 4 (21%) |
| 3 | 5 (36%) | 6 (32%) |
| > 3 | 8 (57%) | 9 (47%) |
| Prior autologous transplantation | ||
| Yes | 6 (12%) | 11 (22%) |
| . | CTN 0604 . | CTN 0603 . |
|---|---|---|
| dUCB | Haplo-marrow | |
| Number of patients | 50 | 50 |
| Age, y | ||
| Median | 58 | 48 |
| Range | 16-69 | 7-70 |
| Weight, kg | ||
| Median | 79 | 78 |
| Range | 46-119 | 21-184 |
| Performance status | ||
| ≥ 90 | 40 (80%) | 38 (76%) |
| < 90 | 10 (20%) | 12 (24%) |
| Primary disease | ||
| Acute lymphoblastic leukemia | 6 (12%) | 6 (12%) |
| Acute myelogeneous leukemia | 29 (58%) | 22 (44%) |
| Biphenotypic/undifferentiated leukemia | 1 (2%) | 3 (6%) |
| Burkitt lymphoma | 1 (2%) | 0 |
| Hodgkin lymphoma | 5 (10%) | 7 (14%) |
| Large-cell lymphoma | 3 (6%) | 8 (16%) |
| Marginal zone B-cell lymphoma | 1 (2%) | 1 (2%) |
| Follicular non-Hodgkin lymphoma | 4 (8%) | 3 (6%) |
| Disease stage | ||
| Acute leukemia | ||
| First complete remission | 23 (64%) | 15 (48%) |
| Second complete remission | 10 (28%) | 12 (39%) |
| Third or subsequent complete remission | 3 (8%) | 4 (13%) |
| Lymphomas | ||
| Complete remission | 5 (36%) | 7 (37%) |
| Partial response | 8 (57%) | 12 (63%) |
| Resistant | 1 (7%) | 0 |
| Number of prior chemotherapy regimens (for lymphoma patients) | ||
| 2 | 1 (7%) | 4 (21%) |
| 3 | 5 (36%) | 6 (32%) |
| > 3 | 8 (57%) | 9 (47%) |
| Prior autologous transplantation | ||
| Yes | 6 (12%) | 11 (22%) |
dUCB indicates double umbilical cord blood; and haplo-marrow, HLA-haploidentical related donor bone marrow.
dUCB and Haplo-marrow graft characteristics
| CTN 0604 dUCB . | . |
|---|---|
| Number of patients | 50 |
| Combined pre-cryopreservation total nucleated cell count, ×107/kg | |
| Median | 5.0 |
| Range | 2.4-9.3 |
| Combined post-thaw total nucleated cell count, ×107/kg | |
| Median | 4.2 |
| Range | 2.3-13.6 |
| HLA matching | |
| 4 of 6 + 4 of 6 | 21 (42%) |
| 4 of 6 + 5 of 6 | 11 (22%) |
| 4 of 6 + 6 of 6 | 1 (2%) |
| 5 of 6 + 5 of 6 | 13 (26%) |
| 5 of 6 + 6 of 6 | 1 (2%) |
| 6 of 6 + 6 of 6 | 3 (6%) |
| HLA typing match score (1st cord to 2nd cord) | |
| 4 of 6 | 35 (70%) |
| 5 of 6 | 11 (22%) |
| 6 of 6 | 4 (8%) |
| CTN 0603 Haplo-marrow | |
| Number of patients | 50 |
| Median donor age, y (range) | 41 (19-79) |
| Donor relationship to patient | |
| Parent | 15 (30%) |
| Child | 18 (36%) |
| Sibling | 17 (34%) |
| HLA Typing match score (GVH direction) | |
| 5 of 10 | 28 (56%) |
| 6 of 10 | 12 (24%) |
| 7 of 10 | 9 (18%) |
| 8 of 10 | 1 (2%) |
| HLA typing match score (HVG direction) | |
| 5 of 10 | 22 (44%) |
| 6 of 10 | 22 (44%) |
| 7 of 10 | 3 (6%) |
| 8 of 10 | 1 (2%) |
| 9 of 10 | 1 (2%) |
| 10 of 10 | 1 (2%) |
| CTN 0604 dUCB . | . |
|---|---|
| Number of patients | 50 |
| Combined pre-cryopreservation total nucleated cell count, ×107/kg | |
| Median | 5.0 |
| Range | 2.4-9.3 |
| Combined post-thaw total nucleated cell count, ×107/kg | |
| Median | 4.2 |
| Range | 2.3-13.6 |
| HLA matching | |
| 4 of 6 + 4 of 6 | 21 (42%) |
| 4 of 6 + 5 of 6 | 11 (22%) |
| 4 of 6 + 6 of 6 | 1 (2%) |
| 5 of 6 + 5 of 6 | 13 (26%) |
| 5 of 6 + 6 of 6 | 1 (2%) |
| 6 of 6 + 6 of 6 | 3 (6%) |
| HLA typing match score (1st cord to 2nd cord) | |
| 4 of 6 | 35 (70%) |
| 5 of 6 | 11 (22%) |
| 6 of 6 | 4 (8%) |
| CTN 0603 Haplo-marrow | |
| Number of patients | 50 |
| Median donor age, y (range) | 41 (19-79) |
| Donor relationship to patient | |
| Parent | 15 (30%) |
| Child | 18 (36%) |
| Sibling | 17 (34%) |
| HLA Typing match score (GVH direction) | |
| 5 of 10 | 28 (56%) |
| 6 of 10 | 12 (24%) |
| 7 of 10 | 9 (18%) |
| 8 of 10 | 1 (2%) |
| HLA typing match score (HVG direction) | |
| 5 of 10 | 22 (44%) |
| 6 of 10 | 22 (44%) |
| 7 of 10 | 3 (6%) |
| 8 of 10 | 1 (2%) |
| 9 of 10 | 1 (2%) |
| 10 of 10 | 1 (2%) |
dUCB indicates double umbilical cord blood; and haplo-marrow, HLA-haploidentical related donor bone marrow.
Fifty-five patients were registered in the Haplo-marrow trial. Five were not treated according to the protocol and were excluded from the analysis: 1 withdrew consent, 2 were found to have disease relapsed/progression, and 2 were not eligible for the protocol. Accrual occurred between December 2008 and May 2010. The median age of Haplo-marrow recipients was 48 (range 7-70). Three patients were < 18 years of age at transplantation. Approximately 75% of patients had a Karnofsky performance status ≥ 90%. Thirty-one patients had a diagnosis of acute leukemia and 19 patients had a diagnosis of lymphoma. Seventeen donors were siblings of the recipient, 15 were parents, and 18 were children. More than 75% of the HLA-haploidentical related donors were mismatched for 4 or more HLA alleles (HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1) in both the GVH and HVG directions (Table 2).
Hematopoietic recovery and chimerism
After dUCB transplantation, the cumulative incidence of neutrophil recovery > 500/μL at day +56 was 94% (95% confidence interval [95% CI], 87%-100%) with a median time to recovery of 15 days (range, 4-47; Figure 2A). The cumulative incidence of platelet recovery ≥ 20 000/μL at day +100 was 82% (95% CI, 71%-93%), with a median time to recovery of 38 days (range, 3-87). The corresponding probability for platelets ≥ 50 000/μL was 59% (95% CI, 44%-73%), with a median time to recovery of 43 days (range, 29-323; Figure 2B). There were 5 cases of pri-mary graft failure and 1 secondary graft failure; 3 graft failure patients died at days +23, +28, and +193 (after the second dUCB transplantation) and 2 patients had autologous reconstitution and died of relapse at day +99 and +117. The patient with secondary graft failure was determined to have lost chimerism at day +183, had leukemia relapse at day +330, and died at day +347. Median donor chimerism in marrow or peripheral blood was 92% (range, 0%-100%) on day +28 and 100% (range, 25%-100%) on day +56 after transplantation. The 2 patients with autologous reconstitution who relapsed before day +56 were excluded for chimerism reporting at day +56. Six patients had mixed chimerism at day +56; 4 of these patients were 100% donor at day +180. The remaining 2 patients died, one from recurrent disease and the other from acute GVHD before the day +180 chimerism assay.
Hematopoietic recovery. Neutrophil (A,C) and platelet recovery (B,D) after RIC and dUCB (A-B) or Haplo-marrow (C-D) transplantation.
Hematopoietic recovery. Neutrophil (A,C) and platelet recovery (B,D) after RIC and dUCB (A-B) or Haplo-marrow (C-D) transplantation.
After Haplo-marrow transplantation, the cumulative incidence of neutrophil recovery > 500/μL at day +56 was 96% (95% CI, 90%-100%), with a median time to recovery of 16 days (range, 12-83; Figure 2D). The cumulative incidence of platelet recovery ≥ 20 000/μL at day +100 was 98% (95% CI, 93%-100%), with a median time to recovery of 24 days (range, 1-92). The corresponding probability for platelets ≥ 50 000/μL was 76% (95% CI, 64%-88%), with a median time to recovery of 26 days (range, 1-126; Figure 2E). There was 1 case of primary graft failure; this patient did not receive a second transplantation and died at day +67. Median donor chimerism in the marrow or peripheral blood was 100% (range 72%-100%) on day +28 and 100% (range 0%-100%) on day +56 after transplantation. All patients had 100% donor chimerism at day +56.
Toxicities
Targeted grade 3-5 toxicities were reported on 56% and 30% of patients after dUCB and Haplo-marrow transplantation, respectively. The frequency of targeted toxicities observed by day +180 after transplantation by organ system and grade attribution and the number of patients experiencing toxicities are summarized in Table 3; there were no grade 5 toxicities reported in either trial between days 0 and +180. In the dUCB recipients, grade 3-5 infusion-related toxicities included hypertension (n = 5), dyspnea/hypoxia (n = 3), allergic/chills (n = 2), nausea/vomiting (n = 2), arrhythmia (n = 1), and hypotension (n = 1). No grade 3-5 toxicities were reported within 24 hours of Haplo-marrow infusion.
Protocol targeted grade 3-4 toxicity day 0-180*
| Organ/system . | CTN 0604 dUCB no. of events (no. of patients) . | CTN 0603 Haplo-marrow no. of events (no. of patients) . | ||
|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | |
| Hypertension | 8 (8) | 0 (0) | 7 (5) | 0 (0) |
| Hypotension | 7 (6) | 0 (0) | 1 (1) | 0 (0) |
| Cardiac arrhythmia | 4 (3) | 1 (1) | 1 (1) | 0 (0) |
| Left ventricular systolic dysfunction | 3 (3) | 2 (2) | 0 (0) | 0 (0) |
| Hepatic† | 9 (6) | 1 (1) | 3 (3) | 1 (1) |
| Pulmonary† | 9 (6) | 9 (6) | 4 (4) | 5 (2) |
| Hemorrhagic cystitis | 5 (3) | 0 (0) | 3 (2) | 0 (0) |
| Hemorrhage | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Mucositis stomatitis | 2 (2) | 0 (0) | 2 (2) | 0 (0) |
| Somnolence | 7 (5) | 0 (0) | 0 (0) | 0 (0) |
| Seizure | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Organ/system . | CTN 0604 dUCB no. of events (no. of patients) . | CTN 0603 Haplo-marrow no. of events (no. of patients) . | ||
|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | |
| Hypertension | 8 (8) | 0 (0) | 7 (5) | 0 (0) |
| Hypotension | 7 (6) | 0 (0) | 1 (1) | 0 (0) |
| Cardiac arrhythmia | 4 (3) | 1 (1) | 1 (1) | 0 (0) |
| Left ventricular systolic dysfunction | 3 (3) | 2 (2) | 0 (0) | 0 (0) |
| Hepatic† | 9 (6) | 1 (1) | 3 (3) | 1 (1) |
| Pulmonary† | 9 (6) | 9 (6) | 4 (4) | 5 (2) |
| Hemorrhagic cystitis | 5 (3) | 0 (0) | 3 (2) | 0 (0) |
| Hemorrhage | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Mucositis stomatitis | 2 (2) | 0 (0) | 2 (2) | 0 (0) |
| Somnolence | 7 (5) | 0 (0) | 0 (0) | 0 (0) |
| Seizure | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
dUCB indicates double umbilical cord blood; and haplo-marrow, HLA-haploidentical related donor bone marrow.
Excludes dUCB infusional toxicity.
Hepatic indicates alanine aminotransferase and/or alkaline phosphatase; pulmonary indicates hypoxia and/or dyspnea.
GVHD
After dUCB transplantation, the cumulative incidences of grade II-IV and III-IV acute GVHD at day +100 were 40% (95% CI, 26%-54%) and 21% (95% CI, 6%-37%), respectively (Figure 3A). The cumulative incidence of chronic GVHD at 1 year was 25% (95% CI, 12%-39%; Figure 3B).
GVHD. Cumulative incidences of acute (A,C) and chronic (B,D) GVHC after RIC with either dUCB transplantation (A-B) or Haplo-marrow transplantation (C-D).
GVHD. Cumulative incidences of acute (A,C) and chronic (B,D) GVHC after RIC with either dUCB transplantation (A-B) or Haplo-marrow transplantation (C-D).
NRM, relapse, and survival
After dUCB transplantation, the median follow-up of surviving patients was 365 days (range, 56-411 days). The 1-year cumulative incidence of NRM was 24% (95% CI, 11%-36%) and relapse/progression, 31% (95% CI, 17%-44%; Figure 4A). The most frequent cause of death was relapse (Table 4). Six-month survival, which was the primary end point, was 74% (95% CI, 59%-84%). The 1-year probability of PFS was 46% (95% CI, 31%-60%) and OS 54% (95% CI, 38%-67%; Figure 4B).
Long-term outcomes. Relapse and NRM (A,C), OS, and event-free survival (B,D) after RIC and either dUCB (A-B) or Haplo-marrow transplantation (C-D).
Long-term outcomes. Relapse and NRM (A,C), OS, and event-free survival (B,D) after RIC and either dUCB (A-B) or Haplo-marrow transplantation (C-D).
Causes of death
| . | CTN 0604 dUCB . | CTN 0603 haplo-marrow . |
|---|---|---|
| Relapse | 10 | 13 |
| Graft failure | 3 | 1 |
| Acute GVHD | 3 | 0 |
| Chronic GVHD | 1 | 0 |
| Infection | 1 | 2 |
| Organ failure | 1 | 0 |
| Hemorrhage | 1 | 0 |
| Other | 1 | 0 |
| Total | 21 of 50 | 16 of 50 |
| . | CTN 0604 dUCB . | CTN 0603 haplo-marrow . |
|---|---|---|
| Relapse | 10 | 13 |
| Graft failure | 3 | 1 |
| Acute GVHD | 3 | 0 |
| Chronic GVHD | 1 | 0 |
| Infection | 1 | 2 |
| Organ failure | 1 | 0 |
| Hemorrhage | 1 | 0 |
| Other | 1 | 0 |
| Total | 21 of 50 | 16 of 50 |
dUCB indicates double umbilical cord blood; and haplo-marrow, HLA-haploidentical related donor bone marrow.
After Haplo-marrow transplantation, the median follow-up of surviving patients was 357 days (range 103-441). The 1-year cumulative incidence of NRM was 7% (95% CI, 0%-15%) and relapse/progression was 45% (95% CI, 30%-61%; Figure 4C). The most frequent cause of death was also relapse (Table 4). Six-month survival, which was the primary end point, was 84% (95% CI, 70%-92%). The 1-year probability of PFS was 48% (95% CI, 32%-62%) and OS 62% (95% CI, 44%-76%; Figure 4D).
Discussion
Our approach of running parallel multicenter phase 2 trials with identical objectives, eligibility criteria, and clinical end points achieved the important strategic objective of reproducing the encouraging single-center results with dUCB and Haplo-marrow transplantation reported previously.8,10 Centers were not required to participate in both trials and patients were not randomly assigned to one trial or the other, so the outcomes were not and should not be compared directly. However, our data suggest that survival rates after RIC and either dUCB or Haplo-marrow transplantation are comparable to survival rates in patients with high-risk hematologic malignancies who were transplanted with blood or marrow from matched unrelated donors after RIC in a registry-based study.1
RIC increases the number of potential candidates for allogeneic BMT by increasing the number of patients expected to tolerate the procedure. For the many patients without available related donors, most transplantation centers have established algorithms for utilization of alternative donor sources, with the choice of alternative donor largely influenced by institutional expertise and research priorities. Whereas several transplantation centers search the unrelated donor registries simultaneously for adult donors and UCB, others favor related HLA-haploidentical donors.
The prospective trials described here sought to determine whether the promising 1- or 2-center outcomes8,10 could be reproduced in multicenter, cooperative group settings. Although accrual of 50 patients in each trial was estimated to require 3 years, both trials met their accrual goals in approximately half that time. The rapid patient accrual can be attributed to the great interest in better understanding the effectiveness of these alternative donor sources to close the gap in donor availability.
The dUCB strategy has been widely used to overcome the cell-dose limitation of a single UCB unit for adults and larger adolescents. The outcomes observed in this trial were consistent with those reported from a single center and registry data showing promising outcomes after RIC dUCB transplantation.7,8,13 Whereas data on the outcomes of patients undergoing a RIC UCB comparing 1 with 2 UCB units grafted are limited,8,13 single UCB transplantation is feasible if the total nucleated cell dose of the unit is deemed acceptable.14-17 However, large numbers of adults cannot find a single unit8 with the required minimum cryopreserved cell dose of 2.5-3.0 × 107/kg.18 Therefore, to keep the treatment homogeneous, we chose to use dUCB grafts for all patients.
The primary limitation to Haplo-marrow transplantation has been intense bidirectional alloreactivity resulting in high incidences of graft failure and severe GVHD. High-dose posttransplantation cyclophosphamide was initially developed in animal models as a method for inducing tolerance to histocompatibility antigens, and was found to mitigate both graft rejection and GVHD after major histocompatibility complex–mismatched BMT.19 An initial concern was that high-dose posttransplantation cyclophosphamide might be toxic to donor stem cells, significantly delaying hematopoietic recovery. However, lympho-hematopoietic stem cells are relatively quiescent and express high levels of aldehyde dehydrogenase, which likely confers cellular resistance to cyclophosphamide.20,21 Indeed, the kinetics of donor neutrophil and platelet recovery were acceptable in the initial phase 1/2 trials.9,10
The limited numbers of patients enrolled per center prevented us from looking for a transplantation center effect on survival. Nevertheless, these parallel multicenter phase 2 trials achieved 2 important goals: (1) reproducing results reported at single institutions in the multicenter setting and (2) providing preliminary data suggesting that outcomes achieved with dUCB and Haplo-marrow transplantation with RIC regimens are comparable to those reported after matched unrelated donor transplantation.1 These results set the stage for a multicenter, randomized phase 3 trial to evaluate the relative risks and benefits of dUCB versus Haplo-marrow transplantation with RIC regimens.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Richard Jones (Haplo-marrow) and Dr John Wagner (dUCB) for their work developing the platforms that were tested in these phase 2 trials and for their encouragement and support that made these trials possible; Jennierose D'Elia and Jason Thompson from the EMMES Corporation for coordinating these clinical trials; and the research nurses and coordinators at the transplantation centers.
This work was supported in part by grant UO1-HL069294 from the National Heart, Lung, and Blood Institute (National Institutes of Health, Bethesda, MD) and the National Cancer Institute (NIH).
National Institutes of Health
Authorship
Contribution: C.G.B., E.J.F., M.E., and P.V.O. designed the trial, reviewed data, interpreted results, and jointly drafted the manuscript; S.L.C. designed the trial, analyzed data, interpreted results, and critically reviewed the manuscript; J.W. analyzed and interpreted data and critically reviewed the manuscript; and C.K., L.J.C., S.M.D., J.R.W., O.S.A., C.S.C., M.H.J., and K.K.B. reviewed data, interpreted results, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio G. Brunstein, MD, PhD, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN, 55403; e-mail: bruns072@umn.edu (regarding cord blood transplantations) or Ephraim J. Fuchs, MD, MBA, 288 Cancer Research Bldg, 1650 Orleans St, Baltimore, MD 21287; e-mail: fuchsep@jhmi.edu (regarding HLA-haploidentical BMTs).
References
Author notes
C.G.B. and E.J.F. share first authorship.
M.E. and P.V.O. share senior authorship.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal