Abstract
Abstract 2606
For the last 40 years, cytosine arabinoside (ara-C) combined with anthracycline (at different dose schedules) has been considered standard therapy for AML. Multiple novel agents have been added to that combination in an attempt to improve AML outcomes. We evaluated the survival of patients with AML treated at our institution over the last 5 decades.
A total of 3,631 patients with newly-diagnosed non-APL AML, 294 CBF-AML, and 237 APL registered in the MDACC Leukemia Database (1965–2010) were evaluated. Patients were divided by decade of referral. Outcomes in specific AML subtypes were evaluated in patients enrolled in 80 different chemotherapeutic protocols used since 1985 when cytogenetic information was consistently available. Analyses comparing different regimens included only protocols that accrued a minimum of 30 patients.
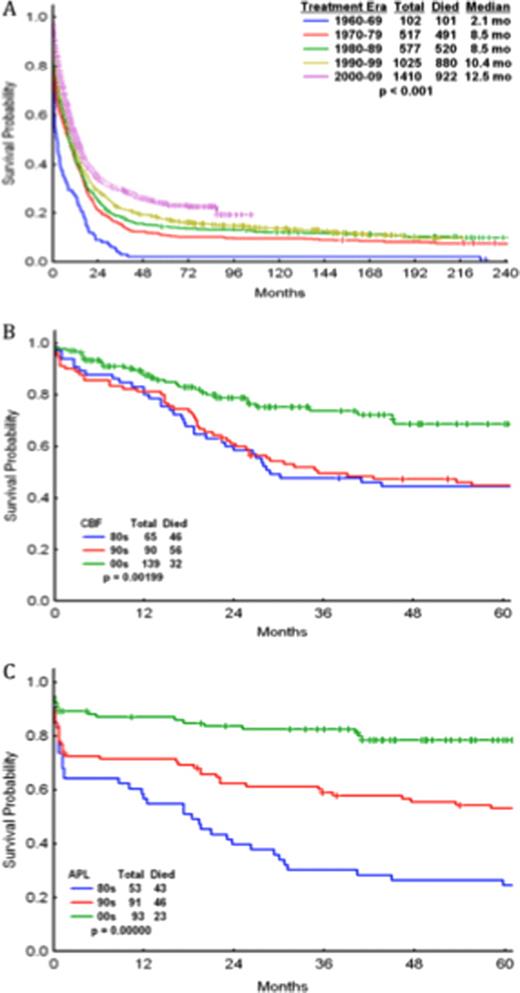
Median overall survival (OS) per decade (1960 to 2010) was 2.1, 8.5, 8.5, 10.4, and 12.5 mos. 1-yr OS was 26%, 41%, 41%, 45%, 51% (Fig 1A). While the prognosis remains poor, incremental improvements in median OS were observed both in patients <60yr (3.7, 11.8, 13.8, 15.7, 19.4 mos; p<0.001) and ≥60yr (0.7, 1.6, 3.7, 5.8, 7.6 mos; p<0.001). As expected, cytogenetics predicted response to chemotherapy, with complete response (CR) rates of 90%, 66%, 58%, 38% for patients with core-binding factor (CBF) AML, cytogenetically-normal (CN) AML, complex cytogenetics, or -5/-7 abnormalities, respectively. The achievement of CR during induction remained a critical prognostic factor across decades; regimens that produced CR rates ≥60% rendered improved OS rates over those inducing CR rates in <60% of patients (53 vs 28wks; p=0.0001). Patients treated with regimens containing ≥1g/m2 versus <1g/m2 of ara-C per course rendered higher CR (65% vs 58%; p=0.0001), CR duration (67 vs 48wks; p=0.002), and median OS (55 vs 48wks; p=0.06) rates, with similar low early mortality (first 8wks) rates among patients <60yr (12% vs 13%) but higher early mortality among patients ≥60yr (30% vs 21%). When comparing idarubicin (12mg/m2x3) vs daunorubicin (45mg to 60m/m2×3) combined with equal ara-C doses, idarubicin-containing regimens induced higher CR (66% vs 52%; p=0.005) and OS (61 vs 46; p=0.06). Based on these data, we developed the AI regimen (ara-C: 1.5g/m2×3d and idarubicin: 12mg/m2×3d) in 1990's for the treatment of newly diagnosed AML (n=486; CR, CR duration, and OS rates: 61%, 63wks, and 48wks). Four agents (sorafenib, SAHA, G-CSF and tipifarnib) improved CR, CR duration, and OS rates when added to the AI backbone (compared to AI alone) amongst patients with non-CBF, non-APL AML: 84%/58wks/NR, 74%/42wks/68wks, 76%/82wks/83wks, 61%/72wks/54wks, respectively. The addition of G-CSF to AI (AIG; n=137) appeared to improve CR, CR duration, and OS rates (77%/87wks/86wks) over the AI regimens, even after removing CBF-AMLs (n=22; 76%/82wks/83wks) but not when fludarabine was substituted for idarubicin (AFG; n=413; 57%/42wks/35wks). Fludarabine-based regimens designed in the 1990's such as FA (n=290; 72%/NR/85wks) but not FAI (n=265; 54%/42wks/32wks) improved upon AI. However, when CBF-AMLs were removed, the outcomes with FA (n=159; 55%/47wks/34wks) and FAI (n=260; 54%/41wks/32wks) were both inferior to AI-based combinations (n=860; 65%/64wks/55wks). In the 2000's, the FLAG-gemtuzumab regimen improved CR, CR duration, and OS (92%/NR/NR) compared with outcomes in the 80's (89%/21mo/28mo) and 90's (90%/22mo/35mo) (Fig 1B). Similarly, the use of ATRA+ATO+/−gemtuzumab improved outcomes in APL in the 2000's (88%/NR/NR) compared with the 80's (64%/18mo/18mo) and 90's (71%/NR/126mo) (Fig 1C).
Albeit modestly, AML outcomes have improved every decade since 1960 due to improvements in supportive care, the use of higher doses of ara-C and idarubicin (over daunorubicin). The addition of certain targeted agents to chemotherapy backbones (e.g. sorafenib, SAHA, G-CSF, tipifarnib) may further improve outcomes in non-APL, non-CBF AML. CBF-AML outcomes have improved remarkably with FLAG-gentuzumab and APL outcomes have done so with ATRA+arsenic+/−gemtuzumab.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal