Abstract
BH3 mimetics are a new class of proapo-ptotic anticancer agents that have shown considerable promise in preclinical animal models and early-stage human trials. These agents act by inhibiting the pro-survival function of one or more Bcl-2–related proteins. Agents that inhibit Bcl-xL induce rapid platelet death that leads to thrombocytopenia; however, their impact on the function of residual circulating platelets remains unclear. In this study, we demonstrate that the BH3 mimetics, ABT-737 or ABT-263, induce a time- and dose-dependent decrease in platelet adhesive function that correlates with ectodomain shedding of the major platelet adhesion receptors, glycoprotein Ibα and glycoprotein VI, and functional down-regulation of integrin αIIbβ3. Analysis of platelets from mice treated with higher doses of BH3 mimetics revealed the presence of a subpopulation of circulating platelets undergoing cell death that have impaired activation responses to soluble agonists. Functional analysis of platelets by intravital microscopy revealed a time-dependent defect in platelet aggregation at sites of vascular injury that correlated with an increase in tail bleeding time. Overall, these studies demonstrate that Bcl-xL–inhibitory BH3 mimetics not only induce thrombocytopenia but also a transient thrombocytopathy that can undermine the hemostatic function of platelets.
Introduction
Apoptosis is an evolutionarily conserved process important for mammalian development, tissue homeostasis, and immune tolerance. Apoptosis also acts as an important barrier against malignant transformation. Resistance to apoptosis is a hallmark feature of cancer, and several pathways regulating apoptosis are commonly altered in human malignancies.1,2 For example, > 50% of all human cancers contain mutations in the p53 tumor suppressor gene, with nearly all of these mutations preventing p53 from triggering apoptosis.3 Furthermore, the overexpression or enhanced function of certain prosurvival proteins, such as Bcl-2,2 Bcl-xL,2 PI3K/Akt/mTOR,4 and nuclear factor-κB,5 is found in many types of human cancers. As a consequence, strategies targeting specific prosurvival pathways have gained prominence as novel anticancer therapies.
Progress unraveling the complex pathways underlying apoptosis and cell survival, and elucidation of their roles in human cancers, has led to the development of several potential anticancer drugs, such as inhibitors of the Bcl-2 protein family,6 inhibitors of apoptosis (IAPs),7 MDM2,8 PI3K/Akt/mTOR,9 as well as TNF-related apoptosis-inducing ligand.10 A particularly promising approach is the ability to inhibit tumor cell survival through the use of agents that mimic the proapoptotic Bcl-2 homology 3 (BH3) domain proteins. BH3 proteins play an important role in inducing apoptosis by antagonizing the function of prosurvival Bcl-2 family proteins. Preclinical studies with the BH3 mimetic ABT-737, which selectively antagonizes Bcl-2, Bcl-xL, and Bcl-w, have demonstrated potent cytotoxic activity against a broad range of tumor cell lines in vitro and tumor models in vivo.11-18
A consistent feature of BH3 mimetics that inhibit Bcl-xL, including ABT-737 and ABT-263, is their capacity to induce rapid thrombocytopenia in preclinical animal models and humans.19-23 The reduction in platelet count is dose-dependent and can lead to severe thrombocytopenia at high concentrations.21,23 Platelets possess many of the conventional apoptotic regulators, including members of the Bcl-2 family such as prosurvival Bcl-xL and the proapoptotic members Bak and Bax. In addition, platelets possess other key components of programmed cell death, including Apaf-1, caspase-3, and caspase-9. Deficiency of Bcl-xL leads to a marked reduction in platelet life span and severe thrombocytopenia, whereas loss of Bak and Bax is associated with prolonged platelet survival and persistent thrombocytosis.21 In addition to its central role in regulating platelet life span, numerous reports have indicated that the levels of Bcl-xL decrease during platelet storage ex vivo,24 coinciding with increased phosphatidylserine (PS) exposure and decreased platelet function, raising the possibility that Bcl-xL may regulate platelet viability during prolonged storage.
The central importance of apoptosis in regulating platelet life span is now well established21,23 ; however, its importance in regulating platelet function remains unclear.25,26 Agents that perturb the normal function of mitochondria induce “apoptotic-like” changes in platelets, leading to PS exposure and a procoagulant phenotype.27,28 Consistent with this, direct induction of apoptosis in platelets by BH3 mimetics induces platelet procoagulant function in vitro in a caspase-, Bak-, and Bax-dependent manner,26 raising the possibility that BH3 mimetics may promote blood coagulation and thrombin generation in vivo. However, to date, there have been no data from preclinical animal studies or clinical trials indicating that BH3 mimetics induce a prothrombotic state. Preliminary reports from early-phase clinical trials have confirmed predictable dose-related falls in platelet counts in patients treated with ABT-26319,29 but have not identified an increased incidence of thromboembolic events.19,20 Whether BH3 mimetics perturb hemostasis beyond their effects on platelet number is unknown. Furthermore, it is currently unclear whether dying platelets expressing PS actually circulate in the bloodstream and whether they retain their adhesive function to arrest bleeding (hemostasis) at sites of vascular injury.
In this study, we have examined the effect of Bcl-xL–inhibitory BH3 mimetics on platelet adhesive function and investigated the potential consequence(s) of triggering platelet cell death pathways on physiologic hemostatic responses. Using ABT-737 or ABT-263, we demonstrate a time- and dose-dependent defect in platelet adhesive function that correlates with ectodomain shedding of the major platelet adhesion receptors, glycoprotein Ibα (GPIbα) and GPVI, and the functional down-regulation of integrin αIIbβ3. Analysis of platelets obtained from the circulation of ABT-263– or ABT-737–treated mice confirmed that the residual circulating platelets shed GPIbα and GPVI and have impaired integrin αIIbβ3 adhesive function. Significantly, these platelets also expressed surface PS, although they do not promote thrombin generation in vivo. Analysis of the platelet adhesive response to vascular injury, using intravital microscopy and a mouse tail bleeding time, revealed that ABT-737 and ABT-263 induced a down-regulation in platelet adhesive function that exacerbates the hemostatic defect associated with thrombocytopenia.
Methods
Materials and antibodies
A detailed description of specific materials and antibodies used in these studies is presented in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Animal studies
All procedures involving the use of C57BL/6 mice were approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee (Melbourne, Australia), under project numbers E/0569/2007/M and E/0997/2010/M.
In vivo administration of ABT compounds.
Mice were administered vehicle or ABT-737 (75 mg/kg) via intraperitoneal injection, according to previous methods.21 ABT-263 (5-25 mg/kg) or corresponding vehicle (10% ethanol, 30% PEG400, 60% Phosal 50 PG)13,22 was administered via oral gavage. Blood sampling at the indicated times was achieved via submandibular bleed, with whole blood collected into hirudin containing the caspase inhibitor QVD-OPh (100μM).
Platelet count.
Platelet count was quantified using a Sysmex (Roche Diagnostics) automated blood analyzer.
Tail bleeding studies.
Posttransfusion recovery of ABT-737-treated mouse platelets.
Washed platelets were labeled with 5-chloromethylfluorescein diacetate followed by pretreatment with vehicle or ABT-737 (0.1-1.0μM) for 90 minutes. Before transfusion, platelet PS exposure was measured by annexin V binding as described previously.26 Recipient mice were intravenously injected with 108 5-chloromethylfluorescein diacetate-labeled platelets and peripheral blood sampled via tail vein bleed at the time points indicated. Posttransfusion recovery was determined by flow cytometric measurement of the proportion of 5-chloromethylfluorescein diacetate–labeled platelets in the peripheral blood at each time point after injection.
Intravital microscopy.
Intravital studies were performed according to a modified method of Denis et al.32 After ABT-263 or vehicle treatment, mice (∼ 18-20 g) were anesthetized (60 mg/kg sodium pentobarbitone) and the mesentery exteriorized via abdominal incision. Mesenteric venules (100-200 μm) were identified and vessels injured by micropuncture using a microinjector needle with a standardized tip diameter of 2 to 4 μm. Microinjector needles (glass capillaries, GD-1, 1 × 50 mm, Narishige) were prepared using a Narishige needle puller (PC-10 capillary puller, Narishige). Axial control of the microinjection needle was obtained using an Injectman micromanipulator (Eppendorf). The tip of the microinjector needle was placed in the center of the vessel, 20 μm above the vessel bottom. Platelet aggregate formation was allowed to develop on the tip of the microinjector needle for a period of 15 minutes. Needle punctures were performed between 1.5 and 3 hours after ABT-263 or vehicle treatment. Platelet interactions were visualized using differential interference contrast microscopy (Olympus IX81 inverted microscope; objective: 40× PlanSAPO, NA 1.35) and captured for off-line analysis using a digital EMCCD camera (QuantEM, photometrics), in combination with Metamorph, Version 7.5 (Biostrategy). Platelet aggregate size was analyzed at 50-second intervals by 2-dimensional surface area analysis performed using ImageJ Version 1.44 software.
Measurement of integrin αIIbβ3 activation, glycoprotein ectodomain shedding, and PS exposure
Integrin αIIbβ3 activation, glycoprotein ectodomain shedding, and surface exposure of PS were quantified using flow cytometry and SDS-PAGE /Western blot analysis. A detailed description of these methods is presented in supplemental Methods.
Analysis of platelet morphology and function
A detailed description of the methods used to characterize platelet morphology and function in vitro, including scanning electron microscopy, platelet aggregation, and in vitro flow based assays, is presented in supplemental Methods.
Statistical analysis
Statistical significance between multiple treatment groups was analyzed using a 1-way ANOVA with Dunnett multiple comparison test. Statistical significance between multiple treatment groups over time was performed using 2-way ANOVA, with Bonferroni post-tests. Statistical significance between 2 treatment groups was analyzed using an unpaired Student t test with 2-tailed P values (Prism software, GraphPad Software for Science). Data are presented as means ± either SEM or SD (where indicated), where n = the number of independent experiments performed.
Results
Morphologic changes in apoptotic platelets
The BH3 mimetics ABT-737 and ABT-263 antagonize the prosurvival function of Bcl-XL in platelets, unleashing the proapoptotic executioners Bak/Bax to induce mitochondrial outer membrane permeability, cytochrome C release, and caspase activation. ABT-737 treatment of washed platelets induces surface exposure of PS, independent of platelet activation26 ; however, its impact on the morphology of platelets has yet to be defined. Temporal analysis of platelet morphology using high magnification differential interference contrast microscopy and scanning electron microscopy revealed that the earliest morphologic changes observed in ABT-737–treated washed platelets (1μM) was the conversion of discoid platelets to spherical forms expressing numerous filopodia (Figure 1A; supplemental Figure 1A). These morphologic changes typically occurred slowly, with initial changes apparent after 15 minutes and maximal shape change occurring after 45 minutes of ABT-737 treatment. Later time points were characterized by extensive membrane blebbing (Figure 1A, 180 minutes; supplemental Figure 1A) and microvesiculation, leading to the clumping of a subset of platelets (data not shown). Previous reports have demonstrated that ABT-737 can induce leakage of serotonin from platelet dense granules,23 raising the possibility that platelet shape change may be induced by dense granule-derived serotonin or from the release of nucleotides, such as adenosine diphosphate (ADP). However, pretreating platelets with the serotonin receptor antagonist ketanserin, the ADPase apyrase, and the purinergic receptor antagonists MRS187 and 2-MeSAMP (supplemental Figure 1C; and data not shown) had no inhibitory effect on ABT-737–induced platelet shape change. Moreover, the potent platelet activation inhibitors PGE1 and theophylline had no effect on ABT-737–induced shape change (supplemental Figure 1C), suggesting that these morphologic changes occurred independent of platelet activation and the release of endogenous platelet agonists. Consistent with this, time course studies of serotonin release from ABT-737–treated platelets revealed minimal loss of serotonin within the first hour of incubation, well after platelet shape change had occurred (data not shown).
Morphologic changes associated with platelet apoptosis. Washed human platelets (3.0 × 108/mL) were resuspended in Tyrode buffer in the presence (A-B) or absence (C) of bovine serum albumin (BSA; 5 mg/mL), then incubated with vehicle (dimethyl sulfoxide [DMSO]) or ABT-737 (1μM) for the indicated times. In some experiments, platelets were preincubated with Q-VD-Oph (50μM) or Y27632 (30μM) before treatment with ABT-737. At the indicated time point, platelets were either (A-B) fixed in suspension with paraformaldehyde (2% final) and processed for scanning electron microscopy, or (C) lysed for Western blot analysis using an anti-ROCK1 polyclonal antibody, as described in supplemental Methods. Images are representative of 3 independent experiments. When the experiments described in panel A were performed in the absence of BSA, the ABT-737–induced morphologic change depicted here at 45 minutes was observed after 15-minute treatment, a difference attributed to binding of ABT-737 to BSA.
Morphologic changes associated with platelet apoptosis. Washed human platelets (3.0 × 108/mL) were resuspended in Tyrode buffer in the presence (A-B) or absence (C) of bovine serum albumin (BSA; 5 mg/mL), then incubated with vehicle (dimethyl sulfoxide [DMSO]) or ABT-737 (1μM) for the indicated times. In some experiments, platelets were preincubated with Q-VD-Oph (50μM) or Y27632 (30μM) before treatment with ABT-737. At the indicated time point, platelets were either (A-B) fixed in suspension with paraformaldehyde (2% final) and processed for scanning electron microscopy, or (C) lysed for Western blot analysis using an anti-ROCK1 polyclonal antibody, as described in supplemental Methods. Images are representative of 3 independent experiments. When the experiments described in panel A were performed in the absence of BSA, the ABT-737–induced morphologic change depicted here at 45 minutes was observed after 15-minute treatment, a difference attributed to binding of ABT-737 to BSA.
Platelet shape change is regulated by cytosolic calcium flux and activation of Rho kinase-1 (ROCK-1).33,34 Analysis of cytosolic calcium levels in ABT-737– and ABT-263–treated platelets revealed that platelet shape change was not associated with significant changes in cytosolic calcium flux (data not shown). Furthermore, chelators of extracellular (ethyleneglycoltetraacetic acid) or intracellular calcium (ethyleneglycoltetraacetic acid-AM), had no inhibitory effect on platelet shape change (data not shown). In contrast, pretreating platelets with pharmacologic inhibitors against ROCK (Figure 1B; ABT-737 + Y27632; supplemental Figure 1B) abolished ABT-737–induced platelet shape and delayed the onset of membrane blebbing and microvesiculation. All morphologic changes associated with apoptosis were inhibited by caspase inhibition (Figure 1B, ABT-737 + QVD-Oph; supplemental Figure 1B) and immunoblot analysis revealed the caspase-dependent cleavage of ROCK1 over time (Figure 1C), previously reported to produce a constitutively active form of the kinase.35
Apoptosis down-regulates platelet adhesive function
Apoptosis of nucleated cells is associated with marked alterations in the adhesive function of surface receptors, particularly integrins.36 To investigate the impact of apoptosis on the adhesive function of platelets, ABT-737–treated platelets were evaluated for their ability to adhere to thrombogenic surfaces under physiologic blood flow conditions. Vehicle-treated control platelets (3 × 108/mL), in the presence of red blood cells, adhered rapidly to a type I fibrillar collagen substrate under conditions of high shear (1800 s−1), forming discrete platelet thrombi on large collagen fibrils (Figure 2A). In contrast, ABT-737 treatment of platelets (3 × 108/mL) resulted in a time-dependent defect in platelet adhesion and thrombus growth, with complete elimination of platelet adhesion after treatment with ABT-737 for 180 minutes (Figure 2A-B). A similar defect in platelet adhesion was observed on a purified von Willebrand factor (VWF) matrix (Figure 2C). Both platelet adhesion and thrombus formation on the collagen substrate were rescued by preincubating platelets with Q-VD-Oph (Figure 2A-B), confirming a major role for caspases in this process.
Apoptotic platelets demonstrate reduced adhesion to collagen and VWF. Washed human platelets (3.0 × 108/mL) resuspended in Tyrode buffer (containing BSA [5 mg/mL]) were incubated with vehicle (DMSO) or ABT-737 (1μM), in the presence or absence of Q-VD-Oph (Q-VD: 50μM) for up to 180 minutes. Treated platelets reconstituted with red blood cells were perfused through (A-B) collagen (2.0 mg/mL)- or (C) VWF (100 μg/mL)-coated microcapillary tubes at 1800 s−1. Platelet adhesion was recorded in real time, for off-line analysis as described in supplemental Methods. (A) Snapshots of adherent platelets after 6-minute perfusion across collagen are representative of 3 independent experiments. (B) Quantification of the number of adherent platelets per field after 30-second perfusion across collagen (mean ± SEM, n = 3). (C) Quantification of the number of adherent platelets per field, after 1-minute perfusion across VWF (mean ± SEM, n = 3). At 45 minutes, the rate of translocation of ABT-737–treated platelets was 2-fold faster than that of vehicle-treated platelets. ns indicates not significant. **P < .01. ***P < .001.
Apoptotic platelets demonstrate reduced adhesion to collagen and VWF. Washed human platelets (3.0 × 108/mL) resuspended in Tyrode buffer (containing BSA [5 mg/mL]) were incubated with vehicle (DMSO) or ABT-737 (1μM), in the presence or absence of Q-VD-Oph (Q-VD: 50μM) for up to 180 minutes. Treated platelets reconstituted with red blood cells were perfused through (A-B) collagen (2.0 mg/mL)- or (C) VWF (100 μg/mL)-coated microcapillary tubes at 1800 s−1. Platelet adhesion was recorded in real time, for off-line analysis as described in supplemental Methods. (A) Snapshots of adherent platelets after 6-minute perfusion across collagen are representative of 3 independent experiments. (B) Quantification of the number of adherent platelets per field after 30-second perfusion across collagen (mean ± SEM, n = 3). (C) Quantification of the number of adherent platelets per field, after 1-minute perfusion across VWF (mean ± SEM, n = 3). At 45 minutes, the rate of translocation of ABT-737–treated platelets was 2-fold faster than that of vehicle-treated platelets. ns indicates not significant. **P < .01. ***P < .001.
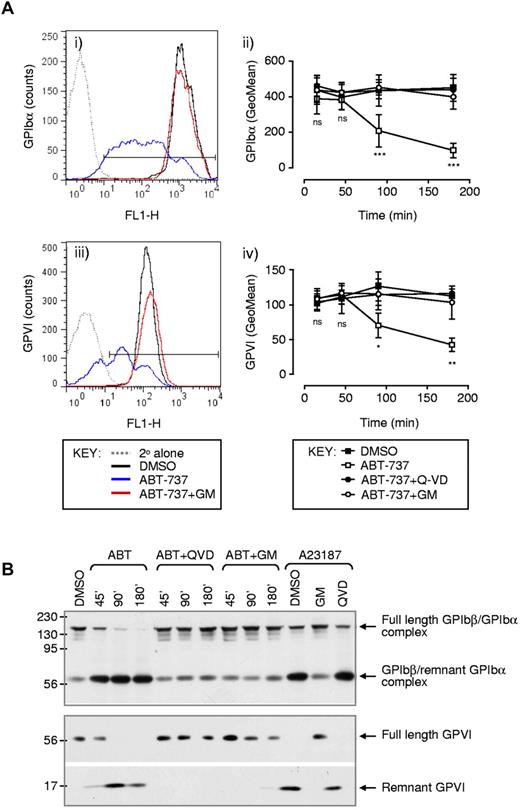
Platelet adhesion to a collagen matrix under high shear is dependent on the adhesive interaction between the platelet GPIb/V/IX complex and VWF adsorbed onto the collagen substrate. To examine whether alterations in the surface levels of intact GPIb/V/IX complex were responsible for defects in platelet adhesion in ABT-737–treated platelets, the level of membrane-expressed GPIbα (the major VWF binding component of the GPIb/V/IX complex) was quantified by flow cytometry. As demonstrated in Figure 3Ai-ii, there was a time-dependent reduction in surface-expressed GPIbα that paralleled the defect in platelet adhesion. In contrast, surface expression of the other major components of the receptor complex, GPIbβ and GPIX (data not shown) remained intact, suggesting that loss of the entire receptor complex by microvesiculation was an unlikely explanation for the loss of GPIbα receptor expression. Ectodomain shedding of GPIbα primarily by the metalloproteinase A disintegrin and metalloproteinase-17 has been shown to result in the loss of up to 80% of the receptor in response to platelet activation by physiologic agonists.37,38 Metalloproteolytic cleavage of GPIbα occurs within a membrane-proximal protease-sensitive region and causes the release of an approximately 95-kDa soluble GPIbα ectodomain fragment and membrane retention of an approximately 16-kDa remnant receptor (composed of a predicted residual 25–amino acid extracellular sequence, a single transmembrane spanning domain, and a 96–amino acid cytosolic tail). This remnant GPIbα receptor is disulfide-linked to GPIbβ and under native conditions migrates on SDS-PAGE gel electrophoresis gel as an approximately 60-kDa complex. Western blot analysis of platelet lysates using a polyclonal antibody against the cytoplasmic domain of GPIbα37,39 demonstrated a time-dependent loss of full-length GPIbα after ABT-737 treatment of washed platelets (Figure 3B). This decrease in full-length GPIbα was associated with the appearance of an approximately 60-kDa fragment, which was identical in size to the remnant GPIbα/GPIbβ receptor complex generated in ionophore A23187-stimulated platelets. Two additional lines of evidence suggested that ectodomain shedding was the dominant mechanism of GPIbα down-regulation in apoptosing platelets. First, analysis of shed proteins from the surface of platelets, by initially labeling surface proteins with biotin before treatment with ABT-737, revealed that > 90% of the shed protein consisted of GPIbα (data not shown). Second, pretreating platelets with the metalloproteinase inhibitor GM6001 prevented ABT-737–induced loss of GPIbα from the surface of platelets and the generation of GPIbα proteolytic fragments (Figure 3; data not shown).
Ectodomain shedding of GPIbα and GPVI from the surface of apoptotic platelets. Washed human platelets were preincubated with vehicle (DMSO), Q-VD-Oph (Q-VD: 50μM), or GM6001 (GM: 100μM) before treatment with vehicle (DMSO) or ABT-737 (1μM) for the indicated times or 180 minutes (Ai,iii). (A) Platelets were fixed and the surface expression of GPIbα (i-ii), and GPVI (iii-iv) was examined by flow cytometry, as described in supplemental Methods. In some experiments (B), platelets were lysed under nonreducing conditions and GPIbα and GPVI were immunoblotted, as described in supplemental Methods. Images and flow cytometry profiles (Ai,iii,B) are taken from 1 experiment representative of 3 independent experiments. (Aii,iv) Date are the mean ± SEM (n = 3). ns indicates not significant. *P < .05. **P < .01. ***P < .001.
Ectodomain shedding of GPIbα and GPVI from the surface of apoptotic platelets. Washed human platelets were preincubated with vehicle (DMSO), Q-VD-Oph (Q-VD: 50μM), or GM6001 (GM: 100μM) before treatment with vehicle (DMSO) or ABT-737 (1μM) for the indicated times or 180 minutes (Ai,iii). (A) Platelets were fixed and the surface expression of GPIbα (i-ii), and GPVI (iii-iv) was examined by flow cytometry, as described in supplemental Methods. In some experiments (B), platelets were lysed under nonreducing conditions and GPIbα and GPVI were immunoblotted, as described in supplemental Methods. Images and flow cytometry profiles (Ai,iii,B) are taken from 1 experiment representative of 3 independent experiments. (Aii,iv) Date are the mean ± SEM (n = 3). ns indicates not significant. *P < .05. **P < .01. ***P < .001.
The other major platelet adhesion receptor, which is proteolytically shed from the surface of platelets in response to physiologic agonists, is the major collagen receptor GPVI.40 Data shown in Figure 3 demonstrate that GPVI was also proteolytically shed from the surface of ABT-737–treated platelets (Figure 3Aiii-iv,B). Similar to GPIbα, the loss of GPVI ectodomain was time-dependent (Figure 3Aiv) and resulted in the appearance of the approximately 10-kDa proteolytic remnant of GPVI detected in Western blots using an anti-GPVI cytoplasmic tail antibody (Figure 3B).37 GPVI proteolysis was also blocked by caspase and metalloproteinase inhibitors (Figure 3A-B). Taken together, these studies demonstrate that platelet apoptosis is associated with the ectodomain shedding of GPIbα and GPVI, resulting in a major defect in the ability of platelets to adhere to thrombogenic matrices.
Apoptosis down-regulates integrin αIIbβ3 adhesive function
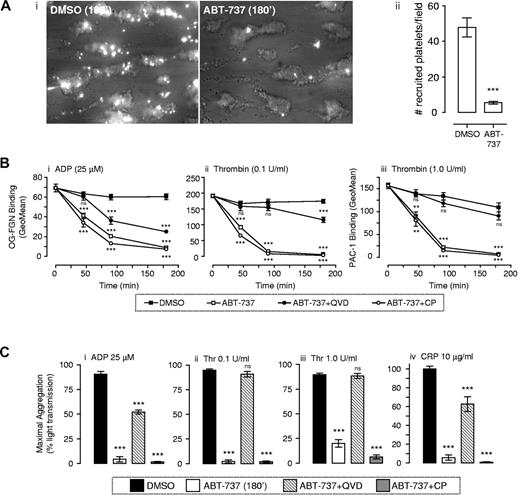
Platelet adhesion to thrombogenic surfaces is critically dependent on GPIb/V/IX and GPVI, whereas the formation of stable platelet aggregates is dependent on the adhesive function of integrin αIIbβ3. Previous reports have demonstrated that ABT-737 treatment of platelets does not induce integrin αIIbβ3 activation23,26 or promote aggregation of platelets,23 consistent with the inability of BH3 mimetics to induce platelet activation.23,26 To investigate the impact of apoptosis on platelet aggregation, fluorescently labeled ABT-737–treated platelets were perfused over the surface of preformed thrombi and the ability of flowing platelets to tether and form stable adhesion contacts with the thrombus surface quantified. As demonstrated in Figure 4Ai, vehicle-treated platelets were efficiently recruited to the surface of preformed thrombi, with a high proportion of tethered platelets forming stable adhesion contacts (Figure 4Aii). In contrast, there was an 88% reduction in the number of ABT-737–treated platelets tethering and forming stable adhesion contacts with the thrombus surface (Figure 4Ai-ii). This defect in platelet aggregation was unlikely to be simply explained by defective platelet tethering because of GPIbα shedding, as a similar defect in platelet recruitment to the thrombus surface was observed at low shear rates (data not shown). To examine more directly the impact of ABT-737 treatment on integrin αIIbβ3 adhesive function, ligand binding studies using Oregon green–labeled fibrinogen or the activation-specific anti-integrin αIIbβ3 antibody PAC-1 were performed. We and others have previously demonstrated that incubation of platelets with ABT-737 did not induce fibrinogen or PAC-1 binding to platelets.23,26 Agonist stimulation of platelets (ADP 25μM; thrombin 0.1-1.0 U/mL) in the presence of ABT-737 did not alter integrin αIIbβ3 activation (Figure 4B; t = 0). However, preincubation of platelets with ABT-737 before agonist stimulation of platelets resulted in a time-dependent reduction in integrin αIIbβ3 adhesive function, such that, after complete conversion of platelets to an apoptotic (PS-positive) state,26 platelets were unable to bind fibrinogen or PAC-1, even in response to potent stimuli, such as 1.0 U/mL thrombin (Figure 4B). The time course of functional decline in integrin αIIbβ3 adhesive function correlated well with the time course of ABT-induced PS exposure26 and was mirrored by defective platelet aggregation in response to ADP (25μM), collagen-related peptide (CRP; 10 μg/mL), or thrombin (0.1-1.0 U/mL; Figure 4C). Notably, this defect in integrin αIIbβ3 adhesive function was not attributable to proteolytic loss of the receptor from the platelet surface, as normal levels of integrin αIIbβ3 were maintained on the surface of apoptotic platelets (data not shown). Nonetheless, proteolytic events were essential for integrin αIIbβ3 down-regulation as the ligand binding and aggregation defect in ABT-737–treated platelets could be largely rescued by caspase inhibitors, whereas calpain inhibitors were without effect (Figure 4B-C). Overall, these studies indicate that apoptotic conversion of platelets leads to a profound defect in platelet adhesive function, partially through a metalloproteinase-dependent shedding of the ligand binding regions of GPIbα and GPVI and a caspase-dependent down-regulation of integrin αIIbβ3 adhesive function.
Apoptotic platelets demonstrate reduced integrin αIIbβ3 activation, aggregation, and recruitment to forming thrombi under flow conditions. (A) Human whole blood was “spiked” with fluorescently labeled washed platelets treated with either vehicle (DMSO) or ABT-737 (1μM). The recruitment of vehicle or ABT-737–incubated platelets to forming thrombi was quantified as described in supplemental Methods. (i) Images are taken from 1 experiment representative of 3 independent experiments, with the mean ± SEM (n = 3) depicted in subpanel ii. (B-C) Effect of ABT-737 on integrin αIIbβ3 activation and platelet aggregation. Washed platelets resuspended in Tyrode buffer in the presence of BSA (5 mg/mL) were pretreated with vehicle (DMSO) or ABT-737 (1μM) for the indicated time periods, or 180 minutes. In some experiments, platelets were pretreated with Q-VD-Oph (Q-VD: 50μM) or calpeptin (CP: 100 μg/mL). (B) Integrin αIIbβ3 activation was measured after stimulation with ADP (25μM, 30 seconds), thrombin (0.1 or 1.0 U/mL, 5 minutes), or CRP (10 μg/mL, 5 minutes) in the presence of Oregon green–labeled fibrinogen (OG-FGN; 20 μg/mL) or FITC-PAC1, and quantified by flow cytometry, as indicated in supplemental Methods. Line graphs represent the mean ± SEM (n = 3). (C) Aggregation in response to the indicated concentration of agonist was monitored as described in supplemental Methods. Histograms represent maximal aggregation (mean ± SEM, n = 4). ns indicates not significant. **P < .01. ***P < .001.
Apoptotic platelets demonstrate reduced integrin αIIbβ3 activation, aggregation, and recruitment to forming thrombi under flow conditions. (A) Human whole blood was “spiked” with fluorescently labeled washed platelets treated with either vehicle (DMSO) or ABT-737 (1μM). The recruitment of vehicle or ABT-737–incubated platelets to forming thrombi was quantified as described in supplemental Methods. (i) Images are taken from 1 experiment representative of 3 independent experiments, with the mean ± SEM (n = 3) depicted in subpanel ii. (B-C) Effect of ABT-737 on integrin αIIbβ3 activation and platelet aggregation. Washed platelets resuspended in Tyrode buffer in the presence of BSA (5 mg/mL) were pretreated with vehicle (DMSO) or ABT-737 (1μM) for the indicated time periods, or 180 minutes. In some experiments, platelets were pretreated with Q-VD-Oph (Q-VD: 50μM) or calpeptin (CP: 100 μg/mL). (B) Integrin αIIbβ3 activation was measured after stimulation with ADP (25μM, 30 seconds), thrombin (0.1 or 1.0 U/mL, 5 minutes), or CRP (10 μg/mL, 5 minutes) in the presence of Oregon green–labeled fibrinogen (OG-FGN; 20 μg/mL) or FITC-PAC1, and quantified by flow cytometry, as indicated in supplemental Methods. Line graphs represent the mean ± SEM (n = 3). (C) Aggregation in response to the indicated concentration of agonist was monitored as described in supplemental Methods. Histograms represent maximal aggregation (mean ± SEM, n = 4). ns indicates not significant. **P < .01. ***P < .001.
Identification of circulating platelets undergoing apoptosis in mice treated with BH3 mimetics
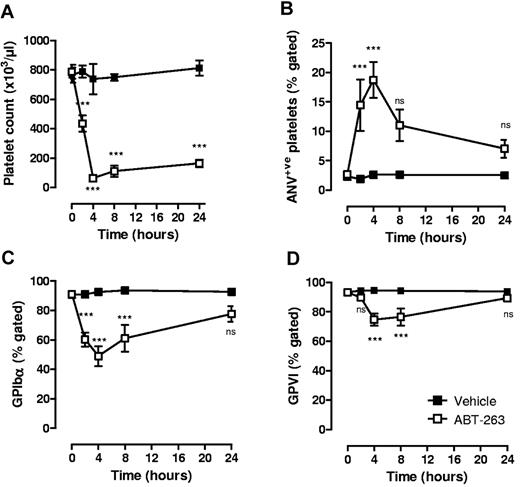
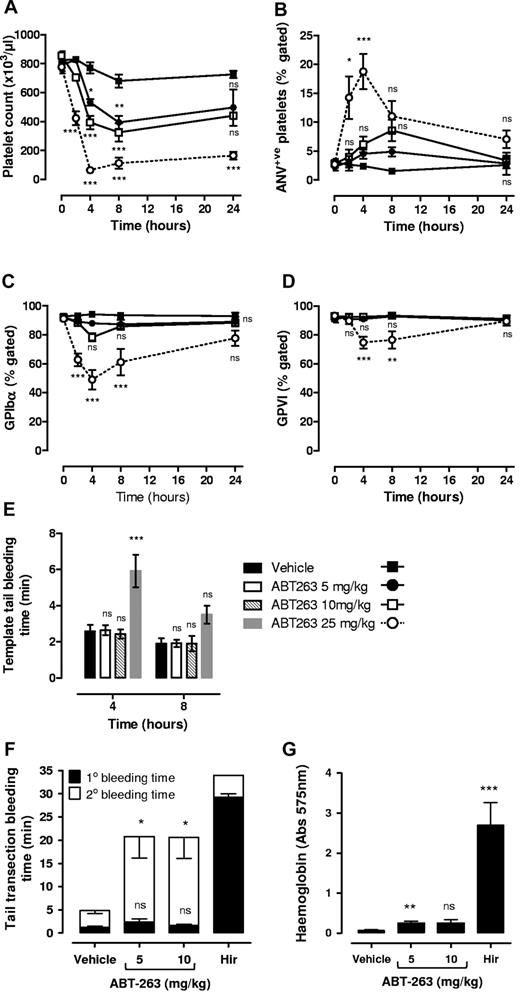
Previous studies have demonstrated that the single administration of ABT-737 or ABT-263 in mice and dogs13,21-23 results in rapid and reversible thrombocytopenia. Whether the residual circulating platelets are resistant to the proapoptotic effects of these molecules or exhibit features of apoptosis remains unclear. Consistent with previous reports,21,22 a single administration of either ABT-737 (75 mg/kg intraperitoneally; supplemental Figure 2A) or ABT-263 (25 mg/kg orally; Figure 5A) to mice resulted in a > 90% reduction in platelet count within 4 hours of administration. At the nadir of thrombocytopenia, residual circulating platelets displayed signs of apoptosis, including reduced surface expression of GPIbα (Figure 5C; supplemental Figure 2C) and GPVI (Figure 5D; supplemental Figure 2D) and increased surface exposure of PS (Figure 5B; supplemental Figure 2B). To investigate the relationship between changes in platelet count with alterations in GPIbα/GPVI receptor levels and PS expression, time course experiments were performed on ABT-263–treated mice. ABT-263 was chosen over ABT-737 for these studies because of its improved solubility and more reliable and consistent absorption in vivo.22 PS exposure and reduced GPIbα/GPVI expression were detected as early as 2 hours after drug administration and persisted for up to 8 hours (Figure 5). At 24 hours after drug administration, despite persistent thrombocytopenia (80% reduction in platelet count, Figure 5A), GPIbα/GPVI receptor expression and PS exposure on residual circulating platelets were equivalent to vehicle-treated controls (Figure 5B-D). Notably, the level of PS surface expression and loss of GPIbα/GPVI receptors on residual circulating platelets at 2 and 4 hours were considerably less than that observed from ABT-737– or ABT-263–treated platelets in vitro (Figure 5; supplemental Figure 3, 19% PS-positive; mean fluorescence intensity = 8.5, 4 hours after ABT-737 in vivo vs 86.5% PS-positive; mean fluorescence intensity = 120, 3 hours after ABT-737 in vitro). This indicates that the majority of platelets undergoing major alterations in their surface membrane are probably rapidly cleared in vivo. Consistent with this, ABT-737–treated platelets expressing high levels of surface PS in vitro were rapidly cleared after their infusion in vivo (supplemental Figure 2F).
Effect of ABT-263 on platelet count and residual circulating platelets in vivo. C57BL6 mice were administered ABT-263 (25 mg/kg) or an equal volume of vehicle by oral gavage, and whole blood sampled over a 24-hour time period, as described in “In vivo administration of ABT compounds.” The effect of ABT-263 administration on (A) platelet count, (B) exposure of PS, and expression of (C) GPIbα and (D) GPVI on the surface of circulating platelets was assessed as described in supplemental Methods. Graphs represent the data collected from 6 to 8 independent experiments (mean ± SEM). ns indicates not significant. ***P < .001.
Effect of ABT-263 on platelet count and residual circulating platelets in vivo. C57BL6 mice were administered ABT-263 (25 mg/kg) or an equal volume of vehicle by oral gavage, and whole blood sampled over a 24-hour time period, as described in “In vivo administration of ABT compounds.” The effect of ABT-263 administration on (A) platelet count, (B) exposure of PS, and expression of (C) GPIbα and (D) GPVI on the surface of circulating platelets was assessed as described in supplemental Methods. Graphs represent the data collected from 6 to 8 independent experiments (mean ± SEM). ns indicates not significant. ***P < .001.
Impact of apoptosis on platelet functional responses
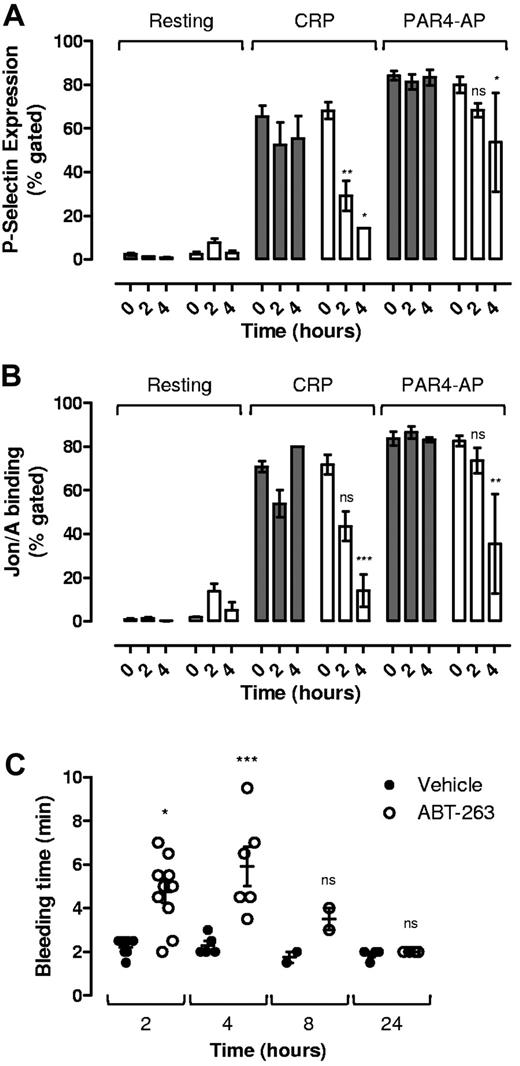
To investigate whether the ABT-263–induced changes in circulating platelets was sufficient to alter platelet functional responses, we examined the ability of soluble agonists to induce platelet α-granule release and integrin αIIbβ3 activation 2 and 4 hours after administration of ABT-263. As demonstrated in Figure 6, at both 2 and 4 hours there was a marked reduction in CRP-induced P-selectin expression (Figure 6A) and integrin αIIbβ3 activation (Figure 6B). Platelet stimulation by the PAR4 agonist was less affected, although still significantly reduced at the 4-hour time point (Figure 6A-B). These findings suggest that ABT-263 not only induces thrombocytopenia but also a thrombocytopathy that undermines key platelet functional responses. To investigate the impact of these changes on the hemostatic function of platelets, tail bleeding time assays were performed. For these studies, we chose the template bleeding time assay, as this assay was less sensitive to changes in platelet count than tail transection assays (data not shown). As demonstrated in Figure 6, an approximately 3-fold increase in bleeding time was evident 2 and 4 hours after ABT-263 administration in mice (Figure 6C). A similar prolongation of bleeding was observed 4 hours after administration of ABT-737 (supplemental Figure 2E). An increase in bleeding time was still evident 8 hours after drug; however, by 24 hours tail bleeding times had normalized (Figure 6C). Given that the circulating platelet counts at 24 hours (164 ± 25 × 103/μL) were considerably lower than the counts at 2 hours after drug (422 ± 47 × 103/μL; Figure 5A), these findings indicate that the decrease in platelet functional responses noted at 2 and 4 hours probably cause a significant defect in the ability of platelets to form an effective hemostatic plug. Notably, although residual circulating platelets also demonstrated elevated levels of surface-exposed PS (Figure 5B; supplemental Figure 2B), no elevation in thrombin-antithrombin levels were detected in these animals (supplemental Figure 4), suggesting that apoptotic platelets do not promote thrombin generation in vivo.
Effect of ABT-263 on platelet glycoprotein receptor expression and platelet activation potential in residual circulating platelets in vivo. C57BL6 mice were administered ABT-263 (25 mg/kg) or an equal volume of vehicle by oral gavage, and whole blood sampled over a 24-hour time period as described in “In vivo administration of ABT compounds.” Whole blood was analyzed for (A) P-selectin expression and (B) integrin αIIbβ3 activation in response to CRP (10 μg/mL) or PAR4-AP (300μM; 10 minutes). Histograms represent the data collected from 6 to 8 independent experiments (mean ± SEM). (C) Template tail bleeding time was quantified as described in “Tail bleeding studies.” Each point represents data from 1 mouse, with the mean ± SEM indicated. ns indicates not significant. *P < .05. **P < .01. ***P < .001.
Effect of ABT-263 on platelet glycoprotein receptor expression and platelet activation potential in residual circulating platelets in vivo. C57BL6 mice were administered ABT-263 (25 mg/kg) or an equal volume of vehicle by oral gavage, and whole blood sampled over a 24-hour time period as described in “In vivo administration of ABT compounds.” Whole blood was analyzed for (A) P-selectin expression and (B) integrin αIIbβ3 activation in response to CRP (10 μg/mL) or PAR4-AP (300μM; 10 minutes). Histograms represent the data collected from 6 to 8 independent experiments (mean ± SEM). (C) Template tail bleeding time was quantified as described in “Tail bleeding studies.” Each point represents data from 1 mouse, with the mean ± SEM indicated. ns indicates not significant. *P < .05. **P < .01. ***P < .001.
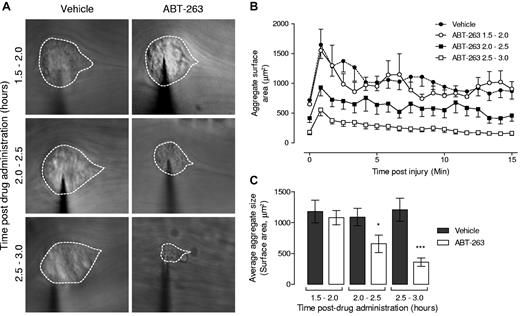
To demonstrate directly that ABT-263 was undermining the efficiency of platelet recruitment to sites of vascular injury, we performed intravital microscopy on mesenteric venules after localized vascular injury with a microinjector needle. As demonstrated in Figure 7, platelet recruitment to sites of vessel injury occurred in a dynamic manner (Figure 7B), with maximal aggregate size typically observed in the first 60 seconds after vascular injury, plateauing to approximately 60% of the peak aggregate size 5 minutes after injury (Figure 7A,C). ABT-263 had no significant inhibitory effect on platelet recruitment within the first 90 minutes after administration of the drug; however, between 2 and 3 hours after drug, there was a progressive reduction in the efficiency of platelet recruitment to sites of vascular injury (Figure 7). These results, in combination with the tail bleeding time data, indicate that ABT-263 administration is associated with a time-dependent reduction in the adhesive function of residual circulating platelets.
ABT-263 treatment inhibits platelet thrombus formation in vivo. Platelet aggregate formation following ABT-263 (25 mg/kg orally) treatment in vivo was examined in mouse mesenteric venules after needle puncture injury, performed between 1.5 and 3 hours after ABT-263 treatment, and data grouped accordingly in half-hour intervals after treatment. Subsequent platelet accrual over a 15-minute period was monitored by intravital microscopy. (A) Representative images of average aggregate surface area from vehicle- or ABT-263–treated mice during the indicated time period. For clarity, platelet aggregates are demarcated. (B) The cumulative size of forming aggregates (μm2) measured over 50-second intervals for a maximum of 900 frames (15 minutes; 1 frame = 1 second). (C) Quantification of the average aggregate surface area (μm2) measured over 50-second intervals for a maximum of 900 frames (15 minutes). (B-C) Data represent the mean ± SEM where: Ctl 1.5 to 2.0 hours, n = 10; ABT-263 1.5 to 2.0 hours, n = 10; Ctl 2.0 to 2.5 hours, n = 20; ABT-263 2.0 to 2.5 hours, n = 23; Ctl 2.5 to 3.0 hours, n = 15; and ABT-263 2.5 to 3.0 hours, n = 22. *P < .05, ***P < .001.
ABT-263 treatment inhibits platelet thrombus formation in vivo. Platelet aggregate formation following ABT-263 (25 mg/kg orally) treatment in vivo was examined in mouse mesenteric venules after needle puncture injury, performed between 1.5 and 3 hours after ABT-263 treatment, and data grouped accordingly in half-hour intervals after treatment. Subsequent platelet accrual over a 15-minute period was monitored by intravital microscopy. (A) Representative images of average aggregate surface area from vehicle- or ABT-263–treated mice during the indicated time period. For clarity, platelet aggregates are demarcated. (B) The cumulative size of forming aggregates (μm2) measured over 50-second intervals for a maximum of 900 frames (15 minutes; 1 frame = 1 second). (C) Quantification of the average aggregate surface area (μm2) measured over 50-second intervals for a maximum of 900 frames (15 minutes). (B-C) Data represent the mean ± SEM where: Ctl 1.5 to 2.0 hours, n = 10; ABT-263 1.5 to 2.0 hours, n = 10; Ctl 2.0 to 2.5 hours, n = 20; ABT-263 2.0 to 2.5 hours, n = 23; Ctl 2.5 to 3.0 hours, n = 15; and ABT-263 2.5 to 3.0 hours, n = 22. *P < .05, ***P < .001.
Dose-dependent effects of ABT-263 on receptor ectodomain shedding, PS exposure, and platelet hemostatic function
The rate and extent of thrombocytopenia induced by Bcl-xL–inhibitory BH3 mimetics are dose related.21-23 At high concentrations, ABT-263 and ABT-737 induce a rapid, severe reduction in platelet count, whereas at lower doses the rate and extent of thrombocytopenia are progressively less. In clinical studies, ABT-263 is administered at doses that cause a gradual reduction in platelet count over several days and result in a maximal reduction in platelet count of between 50% and 70%.22,29 Investigation of the dose-dependent effects of ABT-263 (5 and 10 mg/kg) on platelet count, GPIbα/GPVI receptor levels, and PS surface exposure revealed a slower reduction in platelet count at these lower drug concentrations with a nadir typically at approximately 40% of vehicle-treated control (Figure 8). Notably, the proportion of circulating platelets exhibiting features of apoptosis was dose-dependent with < 10% platelets PS-positive 4 hours after drug at 10 mg/kg versus > 20% PS-positive 4 hours after drug at 25 mg/kg (Figure 8A-D). Consistent with this, template tail bleeding time assays were normal in mice receiving lower doses of ABT-263 (5 and 10 mg/kg; Figure 8E); however, using a more sensitive tail transection method, a slight increase in blood loss was observed (Figure 8G). This subtle hemostatic defect was primarily the result of a tendency for secondary rebleeding (Figure 8F). Taken together, these findings indicate that the extent of the thrombocytopathy induced by BH3 mimetics is dose-related.
Effect of ABT-263 on platelet receptor expression, PS exposure, and platelet function is dose-dependent. C57BL6 mice were administered ABT-263 (5 or 10 mg/kg) or an equal volume of vehicle by oral gavage, and whole blood sampled at the indicated times as described in “In vivo administration of ABT compounds.” Whole blood was analyzed for (A) platelet count as well as (B) PS surface expression (ANV+ve), (C) GPIbα, and (D) GPVI surface expression. (E) Template tail bleeding time was quantified as described in “Tail bleeding studies.” Histograms represent the data collected from 5 independent experiments (mean ± SEM). Data obtained from mice treated with 25 mg/kg ABT-263 have been included here for comparison purposes. These data have been taken from Figures 5 and 6 and are therefore represented here as broken lines/gray bars. (F-G) Bleeding time (F) and blood loss (hemoglobin levels) (G) were quantified following 3-mm tail transection as described in supplemental Methods. Histograms represent the data collected from 5 independent experiments (mean ± SEM), where closed bars represent the initial cessation of bleeding (1° bleeding time), and open bars represent rebleeding (2° bleeding time). Identical experiments performed on mice administered 1 mg/kg hirudin (Hir) have been provided for comparison. ns indicates not significant. *P < .05. **P < .01. ***P < .001.
Effect of ABT-263 on platelet receptor expression, PS exposure, and platelet function is dose-dependent. C57BL6 mice were administered ABT-263 (5 or 10 mg/kg) or an equal volume of vehicle by oral gavage, and whole blood sampled at the indicated times as described in “In vivo administration of ABT compounds.” Whole blood was analyzed for (A) platelet count as well as (B) PS surface expression (ANV+ve), (C) GPIbα, and (D) GPVI surface expression. (E) Template tail bleeding time was quantified as described in “Tail bleeding studies.” Histograms represent the data collected from 5 independent experiments (mean ± SEM). Data obtained from mice treated with 25 mg/kg ABT-263 have been included here for comparison purposes. These data have been taken from Figures 5 and 6 and are therefore represented here as broken lines/gray bars. (F-G) Bleeding time (F) and blood loss (hemoglobin levels) (G) were quantified following 3-mm tail transection as described in supplemental Methods. Histograms represent the data collected from 5 independent experiments (mean ± SEM), where closed bars represent the initial cessation of bleeding (1° bleeding time), and open bars represent rebleeding (2° bleeding time). Identical experiments performed on mice administered 1 mg/kg hirudin (Hir) have been provided for comparison. ns indicates not significant. *P < .05. **P < .01. ***P < .001.
Discussion
BH3 mimetics are a promising new class of anticancer agents that are highly effective at inducing apoptosis in malignant cells. In particular, the BH3 mimetics, ABT-737 and ABT-263, which target Bcl-xL, Bcl-2, and Bcl-w, have demonstrated considerable efficacy in preclinical tumor models11 and for ABT-263 in human clinical trials.19,20 However, the major side effect of these agents is thrombocytopenia, a feature that is dose limiting and has the potential to undermine their full antitumor benefit. In addition to inducing rapid platelet clearance from the circulation, BH3 mimetics also have the potential to induce functional changes in the remaining circulating platelets, in particular the expression of PS on the cell surface, which may result in increased thrombin generation. However, to date, there have been no data from preclinical animal models or clinical studies indicating that BH3 mimetics increase bleeding or thrombotic complications in patients receiving these drugs. Bleeding is a potentially important issue given that BH3 mimetics that target Bcl-xL are being evaluated in patients with advanced lymphoproliferative disorders and preclinically show efficacy in other hematologic malignancies associated with thrombocytopenia, such as relapsed acute lymphoblastic leukemia.17
The studies presented here provide evidence that BH3 mimetics targeting Bcl-xL can lead to changes in residual circulating platelets that can undermine their hemostatic function. We have demonstrated that ABT-737 or ABT-263 induces time- and dose-dependent defects in platelet adhesive function that correlates with ectodomain shedding of the major platelet adhesion receptors, GPIbα and GPVI, and the functional down-regulation of integrin αIIbβ3. Consistent with these findings, analysis of platelet function in vivo has revealed that BH3 mimetics induce a transient down-regulation in platelet adhesive function that exacerbates the hemostatic defect associated with thrombocytopenia. The exact mechanism(s) by which BH3 mimetics promote ectodomain shedding is not clear but may involve the activation of p38 MAPK, as this serine/threonine kinase has recently been demonstrated to promote A disintegrin and metalloproteinase-17–mediated shedding of GPIbα during platelet storage.41 In addition, shedding of GPIbα and GPVI can be induced by calmodulin inhibitors or thiol-modifying reagents that may directly activate sheddases.37,42 Whether either of these mechanisms is relevant to platelets treated with BH-3 mimetics remains to be determined.
The negative impact of loading doses of ABT-263 and ABT-737 on the hemostatic function of platelets is best exemplified by our tail bleeding time studies. For example, despite only a 40% to 50% drop in platelet count 2 hours after administration of 25 mg/kg ABT-263, there was typically a 3-fold increase in bleeding time. This increase in tail bleeding was almost certainly attributable to defective platelet function, as a similar time-dependent defect in platelet thrombus formation was observed in our intravital studies. Although rapid platelet clearance would exacerbate the bleeding risk, thrombocytopenia per se is unlikely to be the dominant cause of the hemostatic defect, as similar reductions in platelet counts using lower doses of ABT-263 (5 or 10 mg/kg) were not associated with substantial increases in bleeding time. Similarly, no increase in template bleeding time was observed 24 hours after a single administration of 25 mg/kg ABT-263, despite an approximately 80% decrease in platelet count, further highlighting the functional significance of the thrombocytopathy within the first few hours of drug administration.
Our bleeding time and intravital studies, in combination with our studies examining the ex vivo function of platelets, reveal that relatively subtle changes in adhesion receptor levels and PS exposure in dying platelets can be associated with marked defects in platelet function. For example, 2 hours after administration of ABT-263, we observed marked defects in CRP-induced platelet P-selectin expression and integrin αIIββ3 activation, despite subtle changes in GPVI levels. Although the degree of CRP stimulation of platelets is sensitive to modest reductions in GPVI levels,43,44 it is probable that other caspase-dependent proteolytic events inside the cell play a major role in undermining platelet stimulation through GPVI. One of these events is probably proteolysis of membrane skeletal proteins, including filamain A, gelsolin, and spectrin, which play important roles in regulating the submembranous cytoskeleton and the formation of cytoskeletal signaling complexes.45 Such changes may also contribute to the adhesion and aggregation defects observed in ABT-737– and ABT-263–treated platelets.
Based on our previous findings that ABT-737–treated platelets can support efficient blood coagulation and thrombin generation in vitro,26 combined with our findings that circulating dying platelets express low levels of PS in vivo, it may have been expected that increased thrombin-antithrombin complexes would have been detected in mouse blood samples. Thrombin-antithrombin is a very sensitive marker of blood coagulation and α-thrombin generation, and we isolated blood samples during phases where platelet PS expression was maximal; therefore, it is unlikely that we would have missed a small transient peak of thrombin generation in our studies. Recent experimental studies have demonstrated that thrombin generation and fibrin accumulation during thrombus formation in the mouse microvasculature predominantly occurs within the confines of a developing thrombus.46 In this situation, formation of the tenase and prothrombinase complexes on the surface of platelets in the base of thrombi would be partially protected from the washout effects of flowing blood. It is well known that blood coagulation reactions are most efficient under conditions of low blood flow, and it is possible that dying platelets in free flow are unable to support the efficient assembly of coagulation complexes and therefore do not promote significant thrombin generation. Future studies will be required to address this issue.
Our studies have revealed that the presence of circulating PS-positive platelets with reduced adhesive function is a transient phenomenon after a single administration of BH3 mimetics. The relatively rapid recovery in platelet hemostatic function 24 hours after drug is likely to reflect a number of factors, including the rapid clearance of functionally effete dying platelets from the circulation; their replacement by new platelets that are more resistant to the effects of ABT-263; as well as the relatively short half-life of ABT-263. Pharmacokinetic profiling of ABT-263 in mice has demonstrated a plasma half-life of 5.7 hours.22 Thus, over a 24-hour time period, new platelets would be exposed to rapidly declining blood levels of the drug. Furthermore, younger, reticulated platelets are more resistant to the effects of BH3 mimetics.21 With an expected 20% turnover of platelet numbers over a 24-hour time period, it is probable that the newly released platelets would account for the return of normal hemostatic function.
Our mouse studies also demonstrate that the deleterious effects of ABT-263 on platelet function are greatest at high doses and therefore suggest that many of the negative effects may be minimized clinically by avoiding a loading dose of the drug that rapidly induces platelet death. Indeed, a strategy of commencing treatment with low lead-in dosing for 1 week before dose escalation has proven safe and effective in early-stage clinical trials of ABT-263 monotherapy.19,20 Nonetheless, our studies suggest that caution should be exercised on the timing of invasive procedures after administration of these drugs in humans. Although, in mice, the first 4 hours after administration of the drug has the greatest impact on platelet function, future studies will be required to determine the optimal safety window in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Joan Clark for assistance with scanning electron microscopy, and Michelle Meilak, Sharelle Sturgeon, Jing Jing, and Naomi Sprigg for technical assistance.
This work was supported by the National Health and Medical Research Council of Australia (project grants 516725 and 545848; program grants 461221, 461219, and IRIISS 361646; and fellowships, S.P.J., B.T.K., R.K.A., D.C.S.H., and A.W.R), the Victorian Cancer Agency (fellowships, K.D.M. and A.W.R.), the Victorian State Government (OIS grant), the Cancer Council of Victoria, the Sylvia and Charles Viertel Charitable Foundation (fellowship, B.T.K.), the Leukemia & Lymphoma Society, White Plains, NY (fellowship, E.C.J.), and the China Scholarship Council (scholarship, J.Q.).
Authorship
Contribution: S.M.S. designed and performed research, analyzed and interpreted data, and wrote the paper; K.E.J. designed and performed research and analyzed and interpreted data; E.E.G. designed research and analyzed and interpreted data; M.J.W. and E.C.J. performed research and analyzed and interpreted data; M.H., J.Q., I.A., A.O., and A.W. performed research; R.K.A., K.D.M., H.H.S., and D.C.S.H. provided intellectual input; B.T.K. and A.W.R. designed research, analyzed and interpreted data, and provided intellectual input; and S.P.J. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The Walter and Eliza Hall Institute of Medical Research has an ongoing research collaboration agreement with Genentech in the field of apoptosis, specifically Bcl-2 family proteins. A.W.R. is an investigator on 3 clinical trials funded by Abbott and Genentech and receives funding for laboratory research that is part of those trials. The work described in this manuscript was not funded by either Abbott or Genentech. The remaining authors declare no competing financial interests.
Correspondence: Shaun P. Jackson, Australian Centre for Blood Diseases, 6th Level Burnet Building, 89 Commercial Road, Melbourne, Victoria, Australia 3004; e-mail: Shaun.Jackson@med.monash.edu.au.
References
Author notes
S.M.S. and K.E.J. contributed equally to this study.

![Figure 1. Morphologic changes associated with platelet apoptosis. Washed human platelets (3.0 × 108/mL) were resuspended in Tyrode buffer in the presence (A-B) or absence (C) of bovine serum albumin (BSA; 5 mg/mL), then incubated with vehicle (dimethyl sulfoxide [DMSO]) or ABT-737 (1μM) for the indicated times. In some experiments, platelets were preincubated with Q-VD-Oph (50μM) or Y27632 (30μM) before treatment with ABT-737. At the indicated time point, platelets were either (A-B) fixed in suspension with paraformaldehyde (2% final) and processed for scanning electron microscopy, or (C) lysed for Western blot analysis using an anti-ROCK1 polyclonal antibody, as described in supplemental Methods. Images are representative of 3 independent experiments. When the experiments described in panel A were performed in the absence of BSA, the ABT-737–induced morphologic change depicted here at 45 minutes was observed after 15-minute treatment, a difference attributed to binding of ABT-737 to BSA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/6/10.1182_blood-2011-04-347849/4/m_zh89991176000001.jpeg?Expires=1769092661&Signature=fHmCad0q6tEhY1ao6UCWjfuj0mw0T2NFomKDBgV9nfQ6lxDR0vkzP5psVBZTOgi3n6htaJnEXOrcsbwdQk3LJ31iH3hsLngF7pgzbLkn~5uRMmJ0cGJySV3aEw1z9afWPA58jnGLAjvr7J20jFLiowplWXTyH07b9yP5QgKXV6j1ucXCVv6NDyzKzlV7pllXTELUksid5A3rrzqeKXetkBInMSYCm7WYX0htr0O2LODKcrl4I3tD8SPM8hvK-riwgAs36Cpvf~5MoNnOXCb-ijrtQQzvFZWGIEKuyg0azwpslsDbzxjIrSV4DR8y2Pd3E4-aAXrai0mT3PvoFez-gA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Apoptotic platelets demonstrate reduced adhesion to collagen and VWF. Washed human platelets (3.0 × 108/mL) resuspended in Tyrode buffer (containing BSA [5 mg/mL]) were incubated with vehicle (DMSO) or ABT-737 (1μM), in the presence or absence of Q-VD-Oph (Q-VD: 50μM) for up to 180 minutes. Treated platelets reconstituted with red blood cells were perfused through (A-B) collagen (2.0 mg/mL)- or (C) VWF (100 μg/mL)-coated microcapillary tubes at 1800 s−1. Platelet adhesion was recorded in real time, for off-line analysis as described in supplemental Methods. (A) Snapshots of adherent platelets after 6-minute perfusion across collagen are representative of 3 independent experiments. (B) Quantification of the number of adherent platelets per field after 30-second perfusion across collagen (mean ± SEM, n = 3). (C) Quantification of the number of adherent platelets per field, after 1-minute perfusion across VWF (mean ± SEM, n = 3). At 45 minutes, the rate of translocation of ABT-737–treated platelets was 2-fold faster than that of vehicle-treated platelets. ns indicates not significant. **P < .01. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/6/10.1182_blood-2011-04-347849/4/m_zh89991176000002.jpeg?Expires=1769092661&Signature=l9AVpHqX6PnEHSp6kl2Vlk8NDk1gjYtxbhTslfpqpXaY2wi9UoVGulGDT5gKuHsU3kAqtKFLMDp0mn7qJ8bsOaCdMD4YTqvULzBxnwqkygLMBlPXU4vTXEv1JqliYOk7UUtmENRST0v8KH4jVQ4yamDYbL9xiYGya5MYw22KcQ1fpKzORGEhqUWo34ZOi~JPRT5IB-iGizJa3O9nf87dQXFc-izaL2G4s3EbHJq0N3CsAUYMJVPgqCMvoy2f6K-8pzICc5EPTcfuwzk20ace~SOwhcgRv8ryz3pzM613PNbPG5DgSK2O2QSguKH43i1i9bCTKYrYRQgBfi~SafwzpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)