Abstract
We studied the impact of risk stratification–directed interventions for minimal residual disease (MRD) on relapse and disease-free survival (DFS) prospectively in 814 subjects with standard-risk acute leukemia receiving allotransplantation in first or second complete remission. A total of 709 subjects were MRD− after transplantation (Group A); 105 subjects were MRD+, 49 received low-dose IL-2 (Group B), and 56 received modified donor lymphocyte infusion (DLI) with or without low-dose IL-2 (Group C). Posttransplantation immune suppression for GVHD was also modified based on MRD state. The cumulative risk of relapse was significantly less and DFS was significantly better in subjects in Group C than in subjects in Group B (P = .001 and P = .002, respectively), but was not different from subjects in Group A (P = .269 and P = .688, respectively). Multivariate analyses confirmed that MRD state and modified DLI were significantly correlated with relapse (P = .000, odds ratio [OR] = 0.255 and P = .000, OR = 0.269) and DFS (P = .001, OR = 0.511 and P = .006, OR = 0.436, respectively). These data suggest that risk stratification–directed interventions with modified DLI in patients with standard-risk acute leukemia who are MRD+ after transplantation may improve transplantation outcomes.
Introduction
Allogeneic hematopoietic cell transplantation (HSCT) is an effective therapy for acute leukemia, but relapse remains an important problem.1,2 Therapy options for relapse include stopping immune suppression, reinduction of chemotherapy, donor lymphocyte infusion (DLI), and another transplantation used alone or in combination. However, the efficacy of these interventions is limited. One approach to the relapse problem in persons with standard-risk acute leukemia is to intervene before hematologic or pathologic relapse occurs based on posttransplantation analysis or minimal residual disease (MRD) using immune or molecular techniques.
Recent studies have indicated that leukemia-associated aberrant immune phenotypes (LAIPs) identified by multiparameter flow cytometry (FCM) and specific fusion genes detected by real-time quantitative PCR (RQ-PCR) can be used to detect and monitor MRD.3,4 Other studies have also confirmed the feasibility of using MRD state as determined by FCM and RQ-PCR as prognostic variables in acute leukemia treated with chemotherapy.5,6 The use of leukemia-specific fusion genes is also effective for monitoring MRD, but is only useful in approximately 25% of cases. Wilms tumor gene-1 (WT1) is overexpressed in approximately 90% of cases of acute leukemia and is therefore useful in monitoring MRD in most persons with standard-risk acute leukemia. Several studies have suggested that quantification of WT1 by RQ-PCR can be used to monitor MRD after chemotherapy and can predict relapse.7,8 Recently, we reported a strong correlation between LAIPs and WT1 and leukemia relapse, disease-free survival (DFS), and survival in patients with acute leukemia receiving allotransplantation.9,10
DLI is an effective posttransplantation therapy for leukemia relapse,11,12 but is associated with a substantial risk of GVHD. Recently, we modified the usual DLI procedure by treating patients with G-CSF–mobilized peripheral blood cells, followed by short-term immune suppression after DLI. Our data suggest that modified DLI is associated with less GVHD and that post-DLI immune suppression does not reduce the GVL effects.13,14 Our recent data also indicate that low-dose IL-2 given after transplantation reduces relapse risk in persons with high-risk acute lymphoblastic leukemia (ALL) receiving allotransplantation.15 Based on these observations, we performed a prospective study to investigate the impact of risk stratification–directed interventions on transplantation outcomes in persons with standard-risk acute leukemia receiving allotransplantations based on MRD state.
Methods
Eligibility criteria
Consecutive subjects receiving non-T cell–depleted allotransplantations at Peking University Institute of Hematology from January 1, 2006 to November 30, 2010 were eligible if they met one of these criteria: (1) had standard-risk acute leukemia defined as first or second complete remission without t(9;22), t(15;17), inv(16), t(16;16), or t(8;21) mutations; or (2) had myelodysplastic syndrome–refractory anemia with excess blasts. The study was approved by the ethics committee of Peking University People's Hospital and written informed consent was obtained from all subjects before study entry in accordance with the Declaration of Helsinki.
Transplantations
Transplantations were performed as described previously.16,17 Recipients of HLA-identical related transplantations received busulfan (0.8 mg/kg IV every 6 hours for 12 doses) and cyclophosphamide (1.8 g/m2/d for 2 days) or total body irradiation (7.7 Gy) given as 1 fraction followed by cyclophosphamide. Recipients of HLA-haploidentical related transplantation and recipients of HLA-matched transplantation from unrelated donors were conditioned with busulfan, cyclophosphamide, and human antithymocyte globulin 2.5 mg/kg/d IV for 4 days (Sangstat) or total body irradiation, cyclophosphamide, and antithymocyte globulin. All subjects received G-CSF–mobilized BM and blood cells. After transplantation, they received cyclosporine (CSA), mycophenolate mofetil, and short-term methotrexate (MTX).
MRD monitoring and definitions
Data were available on LAIPs at diagnosis in 91.8% (747 of 814) of subjects and on WT1 overexpression in 63.1% (514 of 814) of subjects; 91.8% of subjects had at least 1 measurement, 63.1% of subjects had both. All subjects were monitored for MRD after transplantation using LAIPs and WT1. LAIPs were detected by 4-color FCM. Different Ab combinations were used in B-ALL, T-ALL, and acute myeloid leukemia as described previously.10,18 A total of 1 000 000 events were collected for analysis routinely. When cell numbers were limited, a minimal 750 000 events were collected. FCM+ was defined as > 0.001% of cells with a LAIP phenotype in ≥ 1 BM samples after transplantation. Sensitivity was 79% and specificity was 85% for persons with ALL.10 WT1 expression was evaluated by TaqMan-based RQ-PCR technology, as described previously.9 WT1+ was defined as a transcript level > 0.60%. Sensitivity was 96% and specificity was 79% for persons with acute leukemia.9 Samples were analyzed at 1, 2, 3, 4.5, 6, 9, and 12 months after transplantation and at 6-month intervals thereafter. When FCM− or WT1+ was detected, the test was repeated 2 weeks later. Subjects were scored as MRD+ if they had 2 consecutive positive results using FCM or WT1 or were both FCM+ and WT1+ in a single sample within 1 year after transplantation.
Therapy of MRD+ subjects after transplantation
Discontinuing immune suppression.
Posttransplantation immune suppression was immediately tapered and then discontinued in subjects who were MRD+ ≤ 100 days after transplantation. Subjects who were MRD+ > 100 days after transplantation had immune suppression immediately discontinued.
IL-2 therapy.
Modified DLI.
For modified DLI,13,16 G-CSF–mobilized peripheral blood stem cells were given instead of the more common unstimulated donor blood lymphocytes. The median doses of mononuclear cells, CD3+ cells, CD4+ lymphocytes, and CD8+ lymphocytes were 1 (range, 0.75-2.00) × 108/kg, 0.38 (range, 0.15-0.93) × 108/kg, 0.19 (range, 0.10-0.54) × 108/kg, and 0.14 (range, 0.06-0.43) × 108/kg, respectively. After DLI, subjects received immunosuppressive drugs such as CSA or MTX to prevent GVHD. Subjects receiving DLI from an HLA-identical related donor received GVHD prophylaxis for 2-4 weeks; subjects receiving DLI from an HLA-matched unrelated or HLA-haploidentical donor received GVHD prophylaxis for 4-6 weeks at the discretion of the attending physicians (and usually depending on the patient's GVHD status after DLI). The starting dose of CSA was 2.5 mg/kg/d and the dose was adjusted to maintain a plasma concentration > 100 ng/mL. MTX was given at 10 mg IV on days 1, 4, 8, and weekly thereafter for 2-6 weeks.
Intervention strategy based on MRD state
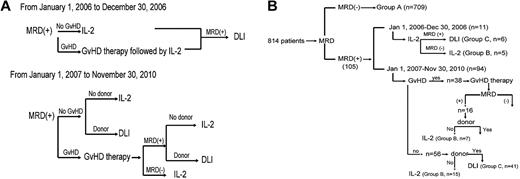
From January 1, 2006 to December 30, 2006, subjects who were MRD+ and without GVHD received low-dose IL-2, whereas subjects with GVHD received GVHD therapy followed by low-dose IL-2. DLI was given if the subject remained MRD+ after IL-2 therapy (the intervention strategy is shown in Figure 1A).
Intervention strategy. Diagram of intervention strategy based on MRD state (A) and subgroup (B).
Intervention strategy. Diagram of intervention strategy based on MRD state (A) and subgroup (B).
From January 1, 2007 to November 30, 2010, DLI or IL-2 was given to MRD+ subjects based on whether a DLI donor was available. However, subjects with GVHD first received GVHD therapy, after which time MRD testing was repeated. Those subjects who then became MRD− received low-dose IL-2, whereas those subjects who remained MRD+ received DLI or IL-2 based on donor availability. Subjects receiving DLI also received antileukemia chemotherapy 48-72 hours before DLI if they agreed. Antileukemia chemotherapy consisted of aclacinomycin 20 mg/d for 7 days and cytarabine 150 mg/m2 for 7 days in subjects with acute myeloid leukemia or myelodysplastic syndrome and MTX 1 g/m2 for 1 day in subjects with ALL. MRD state was monitored at 1, 2, 3, 4.5, 6, 9, and 12 months after the interventions and at 6-month intervals thereafter.
Definitions and evaluation
Leukemia relapse was scored as BM, extramedullary, or both by common morphological criteria. FCM and molecular data were not used to define relapse. GVHD was scored as acute or chronic based on published criteria.20 Chimerism analyses were done by DNA fingerprinting of short tandem-repeat on blood samples and/or chromosome FISH of BM samples. Evaluations were done at 1, 2, 3, 4.5, 6, 9, and 12 months after transplantation and at 6-month intervals thereafter.
Statistical analyses
Cumulative incidences of relapse, transplant-related mortality (TRM), and GVHD were calculated by accounting for competing risks.21 DFS was plotted using the Kaplan-Meier method and was compared using the log-rank test.22 Univariate analyses were performed using the χ2 test for categorical variables and the Mann-Whitney test for continuous variables. Multivariate analyses were performed using a Cox proportional hazards model. The end point of follow-up for all surviving subjects was May 31, 2011. Unless otherwise specified, all P values are 2-sided and P < .05 was considered significant. The SPSS 13.0 and R 2.6.1 software packages were used for data analyses.
Results
Subject groups
A total of 709 of 814 consecutive subjects were MRD− at all of the time points tested after transplantation (Group A); 105 subjects were MRD+ and in 12 of these, this was based on FCM and WT1 data in 1 sample. Ten subjects had 2 FCM+ results and 83 had 2 WT1+ results; 49 of these 105 MRD+ subjects only received low-dose IL-2 (Group B) and another 56 received DLI with or without low-dose IL-2 (Group C).
From January 1, 2006 to December 30, 2006, 11 subjects received IL-2, 6 of whom finally received DLI because of persistent MRD+. From January 1, 2007 to November 30, 2010, 94 subjects received interventions: 41 of 56 subjects without GVHD at the time of MRD+ received DLI; the remaining 15 received IL-2 because a DLI donor was unavailable. Thirty-eight additional subjects had GVHD at the time of MRD+ diagnosis, 16 of whom remained MRD+ after GVHD therapy. Nine of these subjects then received DLI and 7 received IL-2 because of DLI-donor unavailability. The 22 remaining subjects became MRD− after control of GVHD and received IL-2 only (Figure 1B).
Relapse
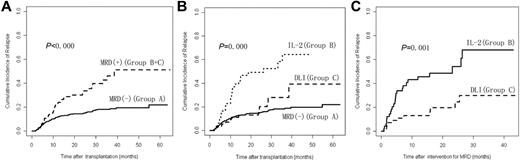
Cumulative incidence of leukemia relapse at 3 years after transplantation was 22.0% (95% confidence interval [95% CI], 18.4%-25.6%) in the 814 subjects; 18.1% (95% CI, 14.6%-21.6%) in Group A, 64.4% (95% CI, 44.8%-84.0%) in Group B, and 27.8% (95% CI, 12.1%-43.5%) in Group C. Cumulative incidence of relapse in Group A was significantly lower than in Groups B and C combined (P < .000) or Group B alone (P < .000). Cumulative incidence of relapse in Group C was significantly lower than Group B (P = .001) but not significantly different from Group A (P = .269). In addition, there was no significant difference in incidence of relapse after transplantation between the subjects receiving chemotherapy before DLI and subjects not receiving chemotherapy in Group C (P = .822; Figure 2 and Table 1).
Relapse after transplantation. (A) Cumulative incidence of relapse after transplantation between Group A and Groups B and C (n = 814). (B) Cumulative incidence of relapse after transplantation comparing Groups A, B, and C (n = 814). (C) Cumulative incidence of relapse after intervention for MRD between Groups B and C (n = 105). A total of 709 subjects were MRD− after transplantation (Group A); 105 subjects were MRD+ after transplantation (Groups B and C); 49 subjects received IL-2 (Group B) and 56 subjects received DLI (Group C).
Relapse after transplantation. (A) Cumulative incidence of relapse after transplantation between Group A and Groups B and C (n = 814). (B) Cumulative incidence of relapse after transplantation comparing Groups A, B, and C (n = 814). (C) Cumulative incidence of relapse after intervention for MRD between Groups B and C (n = 105). A total of 709 subjects were MRD− after transplantation (Group A); 105 subjects were MRD+ after transplantation (Groups B and C); 49 subjects received IL-2 (Group B) and 56 subjects received DLI (Group C).
Subject characteristics and outcomes (N = 814)
| Variable . | MRD− . | MRD+ . | P† . | MRD+ . | P‡ . | DLI . | P§ . | ||
|---|---|---|---|---|---|---|---|---|---|
| IL-2 . | DLI . | Chemo-therapy . | No chemo-therapy . | ||||||
| No. of subjects | 709 | 105 | 49 | 56 | 32 | 24 | |||
| Subject age, y (range) | 29 (2-59) | 23 (4-59) | .012 | 25 (4-59) | 22 (6-55) | .882 | 20 (6-54) | 24.5 (12-55) | .379 |
| Subject sex, n (%) | .556 | .181 | .938 | ||||||
| Male | 438 (61.8) | 68 (64.8) | 35 (71.4) | 33 (58.9) | 19 (59.4) | 14 (58.3) | |||
| Female | 271 (38.2) | 37 (35.2) | 14 (28.6) | 23 (41.1) | 13 (40.6) | 10 (41.7) | |||
| Disease type, n (%) | .170 | .950 | .940 | ||||||
| AML | 378 (53.3) | 61 (58.1) | 29 (59.2) | 32 (57.1) | 18 (56.3) | 14 (58.3) | |||
| ALL | 247 (34.8) | 38 (36.2) | 17 (34.7) | 21 (37.5) | 12 (37.5) | 9 (37.5) | |||
| MDS-RAEB | 84 (11.8) | 6 (5.7) | 3 (6.1) | 3 (5.4) | 2 (6.3) | 1 (4.2) | |||
| Remission state, n (%) | .103 | .037 | .679 | ||||||
| CR1 | 559 (88.6) | 85 (81.0) | 42 (85.7) | 43 (76.8) | 22 (73.3) | 18 (78.3) | |||
| CR2 | 72 (11.4) | 20 (19.0) | 7 (14.3) | 13 (23.2) | 8 (26.7) | 5 (21.7) | |||
| Cytogenetic subGroup, n (%)* | .103 | .615 | .626 | ||||||
| Intermediate | 466 (65.7) | 62 (59.0) | 30 (61.2) | 32 (57.1) | 22 (68.8) | 16 (66.7) | |||
| Poor | 63 (8.9) | 14 (13.3) | 5 (10.2) | 9 (16.1) | 4 (12.5) | 5 (20.8) | |||
| Not available | 180 (25.4) | 29 (27.6) | 14 (28.6) | 15 (26.8) | 8 (18.8) | 3 (12.5) | |||
| No. of induction chemotherapies, n (%) | .095 | .989 | .892 | ||||||
| 1 | 433 (68.6) | 66 (62.9) | 31 (63.3) | 35 (62.5) | 20 (66.7) | 14 (60.9) | |||
| 2 | 149 (23.6) | 22 (20.9) | 10 (20.4) | 12 (21.4) | 6 (20.0) | 5 (21.7) | |||
| > 2 | 49 (7.8) | 17 (16.2) | 8 (16.3) | 9 (16.1) | 4 (13.3) | 4 (17.4) | |||
| Type of donor, n (%) | .080 | .002 | .214 | ||||||
| HLA-identical related | 215 (30.3) | 40 (38.1) | 14 (28.6) | 26 (46.4) | 13 (40.6) | 13 (54.2) | |||
| HLA-haploidentical related | 438 (61.8) | 53 (50.5) | 24 (49.0) | 29 (51.8) | 19 (59.4) | 10 (41.7) | |||
| Unrelated | 56 (7.9) | 12 (11.4) | 11 (22.4) | 1 (1.8) | 0 (0.0) | 1 (4.2) | |||
| Conditioning regimen, n (%) | .003 | .376 | .797 | ||||||
| TBI-based | 2 (0.3) | 4 (3.8) | 1 (2.0) | 1 (1.8) | 1 (3.1) | 2 (8.3) | |||
| BU-based | 707 (99.7) | 101 (96.2) | 48 (98.0) | 55 (98.2) | 31 (96.9) | 22 (91.7) | |||
| Acute GvHD pre-MRD, n (%) | 15 (30.6) | 18 (32.1) | .762 | 11 (34.3) | 7 (29.1) | .370 | |||
| ≥ Grade 2 | 8 (16.3) | 8 (14.3) | .708 | 6 (18.8) | 2 (8.3) | .269 | |||
| ≥ Grade 3 | 0 (0.0) | 0 (0.0) | .989 | 0 (0.0) | 0 (0.0) | 1.000 | |||
| Chronic GvHD pre-MRD, n (%) | 13 (26.5) | 12 (21.4) | .326 | 7 (21.9) | 5 (20.8) | .788 | |||
| Extensive GvHD pre-MRD | 4 (8.2) | 5 (8.9) | .768 | 3 (9.3) | 2 (8.3) | .990 | |||
| Chimerism status pre-MRD, n (%) | .856 | .986 | |||||||
| Full donor | 46 (93.9) | 54 (96.4) | 31 (96.9) | 23 (95.8) | |||||
| Mixed | 3 (6.1) | 2 (3.6) | 1 (3.1) | 1 (4.2) | |||||
| Full recipient | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Interval from HSCT to MRD, d (range) | 140 (30-365) | 135 (30-435) | .455 | 132 (30-435) | 135 (30-365) | .980 | |||
| Chemotherapy before intervention, n (%) | 0 (0.0) | 32 (57.1) | .001 | ||||||
| Discontinuing immunosuppressions before intervention, n (%) | 26 (53.1) | 28 (50.0) | .754 | 16 (50.0) | 12 (50.0) | .989 | |||
| Acute GvHD after intervention, n (%) | 10.2 | 30.8 | .017 | 33.3 | 29.0 | .655 | |||
| ≥ Grade 2 | 8.2 | 27.9 | .017 | 30.3 | 26.2 | .701 | |||
| ≥ Grade 3 | 4.1 | 8.4 | .471 | 9.8 | 7.3 | .752 | |||
| Chronic GvHD after intervention, n (%) | 37.3 | 42.9 | .982 | 45.5 | 39.4 | .934 | |||
| Extensive chronic GvHD | 30.5 | 34.2 | .858 | 39.4 | 30.1 | .283 | |||
| Incidence of TRM at 3 y after HSCT, n (%) | 19.7 | 12.7 | .080 | 11.4 | 14.4 | .897 | 22.4 | 8.5 | .055 |
| Actual relapse rate, n (%) | 103 (14.5) | 37 (35.2) | .000 | 25 (51.0) | 12 (21.4) | .002 | 5 (15.6) | 7 (29.2) | .222 |
| Incidence of relapse at 3 y after HSCT, n (%) | 18.1 | 46.0 | .000 | 64.4 | 27.8 | .001 | 26.4 | 32.7 | .822 |
| OS at 3 y after HSCT, n (%) | 66.0 | 44.0 | .005 | 28.1 | 58.3 | .022 | 60.5 | 57.8 | .496 |
| Median OS after HSCT, mo (95% CI) | 44.39 (33.87-54.90) | 33.17 (25.23-41.11) | .005 | 18.97 (3.13-34.80) | 38.53 (33.78-50.86) | .022 | 40.32 (33.78-50.86) | 38.53 (33.32-54.25) | .496 |
| DFS at 3 y after HSCT, n | 61.6 | 40.3 | .041 | 24.1 | 55.6 | .002 | 51.2 | 57.8 | .327 |
| Median DFS after HSCT, mo (95% CI) | 42.40 (33.85-54.90) | 28.00 (19.12-36.88) | .041 | 13.33 (8.78-17.88) | 38.53 (32.00-59.92) | .002 | 40.52 (32.0-49.04) | 38.53 (32.40-54.05) | .327 |
| Variable . | MRD− . | MRD+ . | P† . | MRD+ . | P‡ . | DLI . | P§ . | ||
|---|---|---|---|---|---|---|---|---|---|
| IL-2 . | DLI . | Chemo-therapy . | No chemo-therapy . | ||||||
| No. of subjects | 709 | 105 | 49 | 56 | 32 | 24 | |||
| Subject age, y (range) | 29 (2-59) | 23 (4-59) | .012 | 25 (4-59) | 22 (6-55) | .882 | 20 (6-54) | 24.5 (12-55) | .379 |
| Subject sex, n (%) | .556 | .181 | .938 | ||||||
| Male | 438 (61.8) | 68 (64.8) | 35 (71.4) | 33 (58.9) | 19 (59.4) | 14 (58.3) | |||
| Female | 271 (38.2) | 37 (35.2) | 14 (28.6) | 23 (41.1) | 13 (40.6) | 10 (41.7) | |||
| Disease type, n (%) | .170 | .950 | .940 | ||||||
| AML | 378 (53.3) | 61 (58.1) | 29 (59.2) | 32 (57.1) | 18 (56.3) | 14 (58.3) | |||
| ALL | 247 (34.8) | 38 (36.2) | 17 (34.7) | 21 (37.5) | 12 (37.5) | 9 (37.5) | |||
| MDS-RAEB | 84 (11.8) | 6 (5.7) | 3 (6.1) | 3 (5.4) | 2 (6.3) | 1 (4.2) | |||
| Remission state, n (%) | .103 | .037 | .679 | ||||||
| CR1 | 559 (88.6) | 85 (81.0) | 42 (85.7) | 43 (76.8) | 22 (73.3) | 18 (78.3) | |||
| CR2 | 72 (11.4) | 20 (19.0) | 7 (14.3) | 13 (23.2) | 8 (26.7) | 5 (21.7) | |||
| Cytogenetic subGroup, n (%)* | .103 | .615 | .626 | ||||||
| Intermediate | 466 (65.7) | 62 (59.0) | 30 (61.2) | 32 (57.1) | 22 (68.8) | 16 (66.7) | |||
| Poor | 63 (8.9) | 14 (13.3) | 5 (10.2) | 9 (16.1) | 4 (12.5) | 5 (20.8) | |||
| Not available | 180 (25.4) | 29 (27.6) | 14 (28.6) | 15 (26.8) | 8 (18.8) | 3 (12.5) | |||
| No. of induction chemotherapies, n (%) | .095 | .989 | .892 | ||||||
| 1 | 433 (68.6) | 66 (62.9) | 31 (63.3) | 35 (62.5) | 20 (66.7) | 14 (60.9) | |||
| 2 | 149 (23.6) | 22 (20.9) | 10 (20.4) | 12 (21.4) | 6 (20.0) | 5 (21.7) | |||
| > 2 | 49 (7.8) | 17 (16.2) | 8 (16.3) | 9 (16.1) | 4 (13.3) | 4 (17.4) | |||
| Type of donor, n (%) | .080 | .002 | .214 | ||||||
| HLA-identical related | 215 (30.3) | 40 (38.1) | 14 (28.6) | 26 (46.4) | 13 (40.6) | 13 (54.2) | |||
| HLA-haploidentical related | 438 (61.8) | 53 (50.5) | 24 (49.0) | 29 (51.8) | 19 (59.4) | 10 (41.7) | |||
| Unrelated | 56 (7.9) | 12 (11.4) | 11 (22.4) | 1 (1.8) | 0 (0.0) | 1 (4.2) | |||
| Conditioning regimen, n (%) | .003 | .376 | .797 | ||||||
| TBI-based | 2 (0.3) | 4 (3.8) | 1 (2.0) | 1 (1.8) | 1 (3.1) | 2 (8.3) | |||
| BU-based | 707 (99.7) | 101 (96.2) | 48 (98.0) | 55 (98.2) | 31 (96.9) | 22 (91.7) | |||
| Acute GvHD pre-MRD, n (%) | 15 (30.6) | 18 (32.1) | .762 | 11 (34.3) | 7 (29.1) | .370 | |||
| ≥ Grade 2 | 8 (16.3) | 8 (14.3) | .708 | 6 (18.8) | 2 (8.3) | .269 | |||
| ≥ Grade 3 | 0 (0.0) | 0 (0.0) | .989 | 0 (0.0) | 0 (0.0) | 1.000 | |||
| Chronic GvHD pre-MRD, n (%) | 13 (26.5) | 12 (21.4) | .326 | 7 (21.9) | 5 (20.8) | .788 | |||
| Extensive GvHD pre-MRD | 4 (8.2) | 5 (8.9) | .768 | 3 (9.3) | 2 (8.3) | .990 | |||
| Chimerism status pre-MRD, n (%) | .856 | .986 | |||||||
| Full donor | 46 (93.9) | 54 (96.4) | 31 (96.9) | 23 (95.8) | |||||
| Mixed | 3 (6.1) | 2 (3.6) | 1 (3.1) | 1 (4.2) | |||||
| Full recipient | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Interval from HSCT to MRD, d (range) | 140 (30-365) | 135 (30-435) | .455 | 132 (30-435) | 135 (30-365) | .980 | |||
| Chemotherapy before intervention, n (%) | 0 (0.0) | 32 (57.1) | .001 | ||||||
| Discontinuing immunosuppressions before intervention, n (%) | 26 (53.1) | 28 (50.0) | .754 | 16 (50.0) | 12 (50.0) | .989 | |||
| Acute GvHD after intervention, n (%) | 10.2 | 30.8 | .017 | 33.3 | 29.0 | .655 | |||
| ≥ Grade 2 | 8.2 | 27.9 | .017 | 30.3 | 26.2 | .701 | |||
| ≥ Grade 3 | 4.1 | 8.4 | .471 | 9.8 | 7.3 | .752 | |||
| Chronic GvHD after intervention, n (%) | 37.3 | 42.9 | .982 | 45.5 | 39.4 | .934 | |||
| Extensive chronic GvHD | 30.5 | 34.2 | .858 | 39.4 | 30.1 | .283 | |||
| Incidence of TRM at 3 y after HSCT, n (%) | 19.7 | 12.7 | .080 | 11.4 | 14.4 | .897 | 22.4 | 8.5 | .055 |
| Actual relapse rate, n (%) | 103 (14.5) | 37 (35.2) | .000 | 25 (51.0) | 12 (21.4) | .002 | 5 (15.6) | 7 (29.2) | .222 |
| Incidence of relapse at 3 y after HSCT, n (%) | 18.1 | 46.0 | .000 | 64.4 | 27.8 | .001 | 26.4 | 32.7 | .822 |
| OS at 3 y after HSCT, n (%) | 66.0 | 44.0 | .005 | 28.1 | 58.3 | .022 | 60.5 | 57.8 | .496 |
| Median OS after HSCT, mo (95% CI) | 44.39 (33.87-54.90) | 33.17 (25.23-41.11) | .005 | 18.97 (3.13-34.80) | 38.53 (33.78-50.86) | .022 | 40.32 (33.78-50.86) | 38.53 (33.32-54.25) | .496 |
| DFS at 3 y after HSCT, n | 61.6 | 40.3 | .041 | 24.1 | 55.6 | .002 | 51.2 | 57.8 | .327 |
| Median DFS after HSCT, mo (95% CI) | 42.40 (33.85-54.90) | 28.00 (19.12-36.88) | .041 | 13.33 (8.78-17.88) | 38.53 (32.00-59.92) | .002 | 40.52 (32.0-49.04) | 38.53 (32.40-54.05) | .327 |
AML indicates acute myelogenous leukemia; MDS-RAEB, myelodysplastic syndrome–refractory anemia with excess blasts; CR, complete remission; BU, busulfan; and TBI, total body irradiation.
Cytogenetic subGroups were according to the published data.23-25
Comparison was performed between Group A and Groups B and C.
Comparison was performed between Groups B and C.
Comparison was performed between the subjects receiving chemotherapy prior to DLI and subjects not receiving chemotherapy in Group C.
Disease type (P = .016), remission state (P = .001), MRD state after transplantation (P = .001) and intervention for MRD (P = .001) were significantly correlated with cumulative risk of relapse in univariate analyses. No other variable tested significantly correlated with relapse risk in univariate analysis, including age, patient's sex, cytogenetic subgroup, number of induction chemotherapies, donor type, conditioning regimen, acute and chronic GVHD before MRD, interval from HSCT to MRD, chimerism status and acute and chronic GVHD after intervention of MRD. In multivariate analysis MRD-negativity after transplantation (P = .000, OR = 0.255) and receiving DLI (P = .000, OR = 0.269) were significantly correlated with a lower cumulative risk of leukemia relapse.
TRM
There were no significant differences in the incidence of TRM between Group A as well as Groups B and C combined (P = .080), between Groups B and C (P = .897), between Groups A and C (P = .115) or between Groups A and B (P = .112). In addition, there was a trend that the incidence of TRM after transplantation in the subjects receiving chemotherapy before DLI was higher than that in subjects not receiving chemotherapy in Group C (22.4% vs 8.5%, P = .055; Table 1).
GVHD
Cumulative incidences of acute GVHD of any grade, ≥ grade 2 and ≥ grade 3 after transplantation were 30.2%, 15.2% and 4.9% in Group A, respectively. Cumulative incidences of chronic and extensive chronic GVHD after transplantation were 38.8% and 32.9% in Group A.
Cumulative incidence of acute GVHD of any grade after interventions was significantly higher in Group C than in Group B (P = .017). There were no significant differences in incidences of ≥ grade 3 acute GVHD (P = .471), total chronic GVHD (P = .982) and extensive chronic GVHD (P = .858) after interventions between the Groups (Table 1). In addition, between the subjects receiving chemotherapy before DLI and subjects not receiving chemotherapy in Group C, there were no significant difference in incidence of acute GVHD of any grade (P = .655), ≥ grade 3 acute GVHD (P = .752), total chronic GVHD (P = .934) and extensive chronic GVHD (P = .283).
OS
The median overall survival (OS) was 44 months (95% CI, 42-46 months). OS in Group A was significantly longer than that in Groups B and C combined (P = .005) or Group B alone (P = .027). OS in Group C was significantly longer than in Group B (P = .022) and was not significantly different from Group A (P = .695). In addition, there was no significant difference in OS between the subjects receiving chemotherapy before DLI and subjects not receiving chemotherapy in Group C (P = .496; Figure 3 and Table 1).
OS after transplantation. (A) OS after transplantation between Group A and Groups B and C (n = 814). (B) OS after transplantation comparing Groups A, B, and C (n = 814). (C) OS after intervention for MRD between Groups B and C (n = 105). A total of 709 subjects were MRD− after transplantation (Group A); 105 subjects were MRD+ after transplantation (Groups B and C); 49 subjects received IL-2 (Group B) and 56 subjects received DLI (Group C).
OS after transplantation. (A) OS after transplantation between Group A and Groups B and C (n = 814). (B) OS after transplantation comparing Groups A, B, and C (n = 814). (C) OS after intervention for MRD between Groups B and C (n = 105). A total of 709 subjects were MRD− after transplantation (Group A); 105 subjects were MRD+ after transplantation (Groups B and C); 49 subjects received IL-2 (Group B) and 56 subjects received DLI (Group C).
Disease type (P = .025), remission state (P = .002), MRD state after transplantation (P = .003), and intervention for MRD (P = .001) were significantly correlated with OS in univariate analyses. No other variable tested was correlated significantly with OS in univariate analyses, including age, sex, cytogenetic subgroup, number of induction chemotherapies, donor type, conditioning regimen, acute and chronic GVHD pre-MRD, interval from HSCT to MRD, chimerism status, and acute and chronic GVHD after treatment of MRD. In multivariate analyses, MRD-negativity after transplantation (P = .007, OR = 0.644) and receiving DLI (P = .002, OR = 0.553) were correlated significantly with longer OS.
DFS
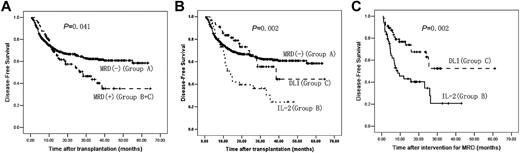
The median DFS was 42 months (95% CI, 40-44 months). DFS in Group A was significantly longer than that in Groups B and C combined (P = .041) or Group B alone (P = .001). DFS in Group C was significantly longer than in Group B (P = .002) and was not significantly different from Group A (P = .688). In addition, there was no significant difference in DFS between the subjects receiving chemotherapy before DLI and subjects not receiving chemotherapy in Group C (P = .327; Figure 4 and Table 1).
DFS after transplantation. (A) DFS after transplantation between Group A and Groups B and C (n = 814). (B) DFS after transplantation comparing Groups A, B, and C (n = 814). (C) DFS after intervention for MRD between Groups B and C (n = 105). A total of 709 subjects were MRD− after transplantation (Group A); 105 subjects were MRD+ after transplantation (Groups B and C); 49 subjects received IL-2 (Group B) and 56 subjects received DLI (Group C).
DFS after transplantation. (A) DFS after transplantation between Group A and Groups B and C (n = 814). (B) DFS after transplantation comparing Groups A, B, and C (n = 814). (C) DFS after intervention for MRD between Groups B and C (n = 105). A total of 709 subjects were MRD− after transplantation (Group A); 105 subjects were MRD+ after transplantation (Groups B and C); 49 subjects received IL-2 (Group B) and 56 subjects received DLI (Group C).
Disease type (P = .017), remission state (P = .002), MRD state after transplantation (P = .003), and intervention for MRD (P = .005) were correlated significantly with DFS in univariate analyses. In multivariate analyses, MRD-negativity after transplantation (P = .001, OR = 0.511) and receiving DLI (P = .006, OR = 0.436) were correlated significantly with longer DFS.
Discussion
The incidence of relapse in subjects who were MRD− after transplantation was lower than in subjects who were MRD+ after transplantation (Groups B and C combined) and was lower than in the subset of patients who received only low-dose IL-2 (Group B). However, subjects in Group C who were MRD+ after transplantation and received modified DLI with or without IL-2 had a leukemia relapse rate similar to subjects in Group A. These data suggest that the intervention of DLI with or without IL-2 in MRD+ subjects after transplantation reduced relapse risk.
Similar results were seen with a DFS end point: comparable results with Groups A and C, both of which were significantly better than Group B. These data suggest that giving DLI with or without IL-2 to subjects who are MRD+ after transplantation results in no decrement in survival from causes other than relapse. Clearly, modified DLI with or without IL-2 produced better results than IL-2 only. Subjects receiving DLI with or without IL-2 had lower relapse rates (29.8% vs 68.2%, P = .001) and better DFS (52.5% vs 20.8%, P = .002).
In the present study, the incidence of relapse was 22.0% in the total population and 18.1% in subjects who were MRD− after transplantation. Recent studies, however, have reported the cumulative relapse risk to be approximately 30%-40%.26-31 This may be partly because the application of risk stratification–directed interventions for persons who were MRD+ reduced relapse after transplantation. These results also confirmed that risk stratification by MRD state after transplantation is reliable and is the foundation of risk stratification–directed interventions. However, it is notable that data from Group A subjects indicate that MRD negativity after transplantation does not guarantee continued complete remission. The imperfect sensitivity was possibly because the MRD-detection platform itself was imperfect. Although the sensitivities for FCM+ in ALL subjects10 and WT1+ for acute leukemia subjects9 were more than 75% in previous studies, our definition of MRD+ in this study was stricter than in most other reports to avoid the excessive TRM related to the interventions for MRD+ state after transplantation. This certainly resulted in an imperfect sensitivity of MRD testing but increasing specificity. In the future, MRD markers used in risk stratification should be analyzed further to increase the sensitivity but to not reduce specificity. Nevertheless, in the present study, persons with standard-risk acute leukemia still benefited from risk stratification and risk stratification–directed interventions.
Our study was not randomized, a factor that mandates a replication of our results, ideally in a randomized clinical trial. We tried to compensate for the lack of randomization by considering variables know to be correlated with transplantation outcomes in this setting. Most were comparable between the cohort except for remission state before transplantation (P = .037), donor types (P = .002), and antileukemia chemotherapy pre-intervention (P = .001). In subjects receiving DLI with or without IL-2, more were in second complete remission before transplantation than those receiving only IL-2 (23.2% vs 14.3%, P = .037). However, the impact of this imbalance should be to increase rather than to decrease relapse risk. In addition, more subjects in the DLI cohort received antileukemia chemotherapy pre-intervention than in the IL-2–only cohort (57.1% vs none, P = .001). Although this may have potentially biased the results in favor of the patients receiving DLI, our data showed that there were no significant differences in the incidence of relapse, OS, or DFS in the subjects receiving chemotherapy before DLI and subjects not receiving chemotherapy in Group C (P > .05). In this study, the subjects in Group C received chemotherapy based on their choice and were not randomized. Furthermore, because the interval from chemotherapy to DLI was only 48-72 hours, it is difficult to evaluate whether the efficacy observed in subjects in Group C was due to chemotherapy or to DLI. However, in the present study, chemotherapy with DLI or DLI alone were effective methods of preventing relapse and improving survival in persons with standard-risk acute leukemia. In the future, a randomized study should be designed to compare the effect of chemotherapy plus DLI or DLI or chemotherapy alone on relapse and survival. In the present study, it is difficult to separate the role of discontinuation of immunosuppression from interventions (IL-2/DLI), because approximately 50% of subjects receiving interventions had already discontinued immunosuppression.
Although results in subjects in Group B were inferior to those in Group C, our data do not rule out a benefit for intervention with IL-2 only in subjects MRD+ after transplantation. Our previous study suggested a 75% relapse rate in subjects who were MRD+ after transplantation but not receiving any interventions.9 These results suggest that the GVL effects of IL-2 were probably inferior to DLI, but that treatment with IL-2 before MRD+ may be useful in patients with standard-risk acute leukemia.
GVHD is an important risk of DLI. However, we found no significant differences in subjects receiving IL-2 or DLI (P = .471) in the incidence of ≥ grade 3 acute GVHD or TRM (P = .897). Our previous study suggested that modified DLI has the same antileukemia efficacy as conventional DLI but less acute GVHD and better survival. These observations may result from the different composition of the infused cells and short-term use of immunosuppressive drugs.13,14 Data from a randomized trial are needed to confirm our observation. Huang et al reported that the use of G-CSF may decrease T-cell responses indirectly through selective increase of plasmacytoid dendritic cell and monocytes and down-regulation of the costimulatory signal of CD28/B7.32-34 Morris et al confirmed that the use of G-CSF during blood-cell mobilization augments natural killer/T-cell–dependent CD8+ cytotoxicity, purportedly separating GVHD and GVL.34
In conclusion, our study is the first to suggest that risk stratification–directed modified DLI may reduce relapse and improve survival of subjects with standard-risk acute leukemia after HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Robert Peter Gale (Imperial College, London, United Kingdom, and Celgene Corporation, Summit, NJ), for revising the manuscript.
This study was supported by National Natural Science Foundation of China (grant number 30971292), the HI-Tech Research Development Program of China 863 (2010-2014), and the Leading Program of Clinical Faculty accredited by the Ministry of Health of China (2010-2012).
Authorship
Contribution: C.-H.Y. and D.-H.L. analyzed the data and wrote the manuscript; Y.-R.L. and Y.-Z.Q. detected WT1 and LAIPs and analyzed the data; X.-J.H. designed the research; and all authors provided patient data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Jun Huang, Peking University People's Hospital, Peking University Institute of Hematology, Beijing 100044, China; e-mail: xjhrm@medmail.com.cn.
References
Author notes
C.-H.Y. and D.-H.L. contributed equally to this work.