Abstract
We explored adeno-associated viral vector (AAV)–mediated gene transfer in the perinatal period in animal models of severe congenital factor VII (FVII) deficiency, a disease associated with early postnatal life-threatening hemorrhage. In young adult mice with plasma FVII < 1% of normal, a single tail vein administration of AAV (1 × 1013 vector genomes [vg]/kg) resulted in expression of murine FVII at 266% ± 34% of normal for ≥ 67 days, which mediated protection against fatal hemorrhage and significantly improved survival. Codon optimization of human FVII (hFVIIcoop) improved AAV transgene expression by 37-fold compared with the wild-type hFVII cDNA. In adult macaques, a single peripheral vein injection of 2 × 1011 vg/kg of the hFVIIcoop AAV vector resulted in therapeutic levels of hFVII expression that were equivalent in males (10.7% ± 3.1%) and females (12.3% ± 0.8%). In utero delivery of this vector in the third trimester to fetal monkeys conferred expression of hFVII at birth of 20.4% ± 3.7%, with a gradual decline to > 1% by 7 weeks. Re-administration of an alternative serotype at 12 months postnatal age increased hFVII levels to 165% ± 6.2% of normal, which remained at therapeutic levels for a further 28 weeks without toxicity. Thus, perinatal AAV-mediated gene transfer shows promise for disorders with onset of pathology early after birth.
Introduction
Congenital FVII deficiency (CFVIID) is the most common autosomal recessive bleeding disorder, with an estimated prevalence of 1:500 000 in the West, but 4 times higher in parts of the world where consanguinity is common.1 CFVIID is caused by mutations in the coagulation factor VII (FVII) gene (13q34) resulting in low or undetectable plasma FVII levels. Factor VII is a vitamin K–dependent serine protease that is synthesized in the liver. The interaction of FVII with tissue factor (TF) generates the serine protease FVIIa, which is pivotal for activation of coagulation at the site of vascular injury. Deficiency of FVII results in a bleeding diathesis that is heterogeneous but severe or lethal in at least 20% of patients with a homozygous or compound heterozygous genotype. Infants with severe factor VII deficiency (factor VII 0%) invariably develop fatal intracranial hemorrhage within hours or days of birth. If undiagnosed and untreated this is fatal. Subjects with slightly less severe reduction of factor VII level to 1% or higher may escape this complication and present later with joint bleeding, epistaxis, or some other hemorrhagic manifestation.2,3
Currently, recombinant-activated FVII (rFVIIa) is the treatment of choice in the West. However, the very short half-life (∼ 2 hours) necessitates frequent IV infusions, more than is required for hemophilia A or B or any other coagulation factor deficiency. The annual cost of treating bleeding episodes with rFVIIa is ∼ 1 million pounds/child in the United Kingdom, which makes prophylaxis untenable on economic grounds for all but the wealthiest countries. To reduce the constant risk of life-threatening hemorrhage, many children with CFVIID undergo liver transplantation, despite the significant morbidity and mortality of this procedure.4 The situation is worse in the developing world, where treatment of bleeding episodes is limited to plasma-derived products, which are in scarce supply or contaminated with blood-borne pathogens.2,3,5
CFVIID is a good model for perinatal gene transfer because its clinical manifestations are attributable to the lack of a single gene product (FVII) that circulates in minute amounts in the plasma (500 ng/mL). Unlike other congenital liver disorders, the therapeutic goal for CFVIID is modest as an increase in the circulating levels of FVII to > 5% of normal will be sufficient to ameliorate the bleeding diathesis.6 Response to factor replacement therapy in CFVIID is not influenced by additional factors such as substrate flux and metabolic state, a substantial advantage for a proof-of-concept study over other liver gene therapy targets. Notably, FVII is structurally and functionally related to human FIX (hFIX), a molecule that is a focus of successful gene replacement strategies.7-12
In contrast to hemophilia A and B, gene therapy for CFVIID remains relatively unexplored. This is in part because effective gene therapy for CFVIID would require intervention during the perinatal period to prevent fatal neonatal hemorrhage which raises several unique biologic and ethical issues. Recombinant adeno-associated viral vectors (rAAV) are currently the vectors of choice for disorders affecting postmitotic tissues such as the liver. They are the focus of several phase 1/2 clinical trials involving a variety of different genetic disorders including hemophilia B, lysosomal storage disorders, inherited retinal degeneration, α-1 antitrypsin, and lipoprotein lipase deficiency.13-18 In addition to their excellent safety profile, the most attractive attribute of these vectors is their ability to mediate persistent therapeutic transgene expression after a single administration of vector in a variety of animal models.7,8,19-22 Emerging results from clinical trials suggest that long-term persistent expression of a transgene is indeed achievable in humans.17,23,24 We and others have recently shown that the potency of rAAV-mediated gene transfer can be further improved by the use of self-complementary vectors (scAAV) in animals and humans, which further enhances the safety profile of AAV vectors as well as easing the burden of vector production.9,25-27 While significant progress has been made with the use of these vectors in adults, their evaluation in the preclinical setting for gene transfer in the perinatal period to moderate a disease requires further investigation.
In this study, we used CFVIID as a model to establish the potential for perinatal AAV gene transfer in preventing death and long-term disease pathology. Our results show that gene transfer in early life using an AAV vector in mice was protective from disease-associated mortality. We have also shown that prenatal gene transfer using established techniques and scAAV vectors confers a therapeutic level of transgene expression without toxicity in young rhesus monkeys.
Methods
Vector design and production
The murine and human FVII cDNAs were cloned into our previously described expression cassette downstream of the LP1 liver-specific promoter and upstream of the SV40 poly-A signal (Figures 1A and 2A).25 The coding region of scAAV-LP1-hFVII-wt is based on human FVII cDNA (NM_019616.2) with its 5′ and 3′ untranslated regions removed to meet the packaging constraints of a self-complementary AAV vector. A codon-optimized version of this shorter form of hFVII cDNA was synthesised using codons most frequently found in highly expressed eukaryotic genes and inserted into a scAAV-LP1 backbone to form scAAV-LP1-hFVII-coop.
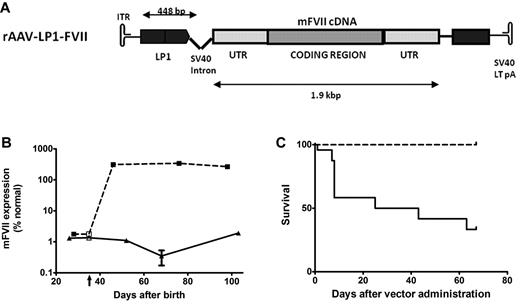
FVII-deficient neonatal mice treated with an AAV8 vector-encoding murine FVII. (A) A schematic of the rAAV-LP1-mFVII construct consisting of the murine FVII cDNA under the control of a liver-specific LP1 promoter/enhancer element, followed by the SV40 virus large T Ag poly-A signal. (B) FVII activity as assessed by a chromogenic assay in plasma of low FVII mice after treatment with 2 × 1013 vg/kg AAV8-LP1-mFVII (broken line, N = 9) compared with control untreated low FVII animals (solid line, N = 3). Vertical arrow indicates virus administration on day 35. (C) Kaplan-Meier survival curve showing that low FVII mice treated with a single tail vein injection of AAV8-LP1-mFVII survived for at least 65 days after administration (100 days postnatal; broken line, N = 19), a significant (P < .0001) improvement over untreated mice (solid line, N = 23).
FVII-deficient neonatal mice treated with an AAV8 vector-encoding murine FVII. (A) A schematic of the rAAV-LP1-mFVII construct consisting of the murine FVII cDNA under the control of a liver-specific LP1 promoter/enhancer element, followed by the SV40 virus large T Ag poly-A signal. (B) FVII activity as assessed by a chromogenic assay in plasma of low FVII mice after treatment with 2 × 1013 vg/kg AAV8-LP1-mFVII (broken line, N = 9) compared with control untreated low FVII animals (solid line, N = 3). Vertical arrow indicates virus administration on day 35. (C) Kaplan-Meier survival curve showing that low FVII mice treated with a single tail vein injection of AAV8-LP1-mFVII survived for at least 65 days after administration (100 days postnatal; broken line, N = 19), a significant (P < .0001) improvement over untreated mice (solid line, N = 23).
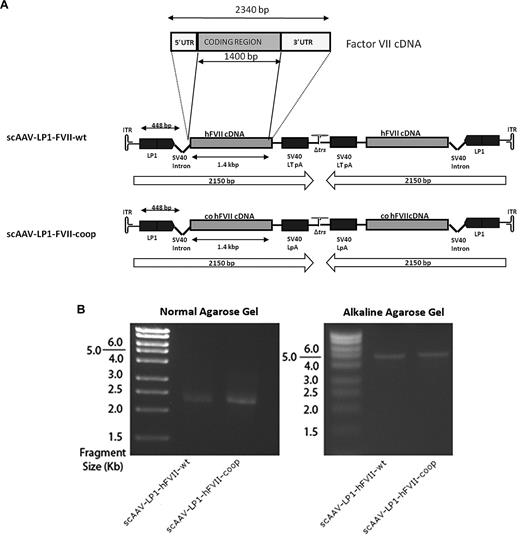
Construction and evaluation of human FVII AAV vectors. (A) Schematic of the human FVII constructs. In scAAV-LP1-FVII-wt, the coding region of the human FVII cDNA without the 3′ and 5′ UTRs was inserted into our scAAV backbone downstream of the LP1 and followed by the SV40 virus large T Ag poly-A signal. scAAV-LP1-FVII-coop is identical to scAAV-LP1-FVII-wt except that the wild-type human FVII cDNA has been replaced with a codon-optimized cognate. (B) Genomic configuration of the scAAV-LP1-FVII-wt and scAAV-LP1-FVII-coop provirus is shown under native (left) or alkaline (right) conditions.
Construction and evaluation of human FVII AAV vectors. (A) Schematic of the human FVII constructs. In scAAV-LP1-FVII-wt, the coding region of the human FVII cDNA without the 3′ and 5′ UTRs was inserted into our scAAV backbone downstream of the LP1 and followed by the SV40 virus large T Ag poly-A signal. scAAV-LP1-FVII-coop is identical to scAAV-LP1-FVII-wt except that the wild-type human FVII cDNA has been replaced with a codon-optimized cognate. (B) Genomic configuration of the scAAV-LP1-FVII-wt and scAAV-LP1-FVII-coop provirus is shown under native (left) or alkaline (right) conditions.
scAAV stocks were produced as described previously.28 Briefly, calcium phosphate precipitation was used to transfect 293 cells with an adenoviral helper plasmid, an AAV5 or 8 capsid packaging plasmid, and the AAV-FVII vectors which are based on the AAV2 genome. The harvested crude lysate was purified using an AVB-sepharose chromatography column (AAV8) or a mucin column (AAV5). Virus yield was quantified by quantitative PCR (qPCR) and capsid proteins visualized by Coomassie staining of virus aliquots run on a denaturing acrylamide gel.
Alkaline gel electrophoresis
Purified virus was exposed to DNAse I before genomic DNA extraction with SDS and proteinase K. Proviral DNA was isolated, then electrophoresed in nondenaturing or alkaline 1% agarose gel in TBE buffer and visualized using ethidium bromide.
Molecular analysis
DNA from monkey liver samples was digested using EcoRI or BsrDI (single and double cutters of the viral genome, respectively). The resulting fragments were separated by electrophoresis and transferred to a nitrocellulose membrane. The membrane was then probed with a 408-bp fragment of plasmid scAAV-LP1-hFVII-coop obtained by digestion with PfoI. A standard curve was made by serial dilution of scAAV-LP1-hFVIIcoop, similarly cut.
Transduction of HepG2 cells in vitro
HepG2 (human hepatoma, ATCC HB-8065) cells were transduced with 1 × 105 vg/cell/d over 4 days. On the fifth day, medium was replaced with X-Vivo 10 (BioWhittaker) without supplemental vitamin K. Conditioned media was then harvested after 20 hours and concentrated ∼ 20-fold using YM-30 centricon filtration columns (Millipore).
Evaluation of hFVII transgene expression
Biologic activity of codon-optimized and wild-type FVII expressed into cell media was assayed using the COASET Factor VII kit (Chromogenix). Plasma concentration of FVII was measured by ELISA, using a goat polyclonal anti-FVII Ab (R&D Systems). The secondary Ab was a sheep anti–human FVII polyclonal Ab (Affinity Biologicals). Binding was visualized using OPD substrate, reading absorbance at 490 nm. Standards were made by serially diluting recombinant hFVII (R&D Systems) into pooled normal mouse or monkey plasma, as appropriate (100% = 0.5 μg/mL). Using these reagents, an immunocapture assay was developed that could detect as little as 3 ng of human FVII in 1 mL of mouse plasma. The lower limit of detection of human FVII in rhesus plasma was 15 ng/mL because of cross-reactivity with rhesus FVII.
Comparing expression from wild-type and codon-optimized vectors, results were vulnerable to distortion by differences in transduction efficiency of the 2 virus stocks. To overcome this, expression levels were normalized against vector genome copy number. qPCR targeting the LP1 promoter (forward [Fw]: TGGTGGTGCCTGAAGCTGAG, [Rv]: GGAGACGAGCAGAGGTTGTC) was used to quantify the viral genomes and host genome copies in DNA extracted from liver tissue samples. To determine the number of viral genome copies per liver cell on day 45, FVII expression data as measured by ELISA were normalized by dividing measured FVII by these vector genome copy numbers, providing the FVII expression/vector genome/cell.
Western blotting of FVII was based on dilution of plasma 1/10 in RIPA cell lysis buffer before denaturing acrylamide electrophoresis and subsequent transfer to a PVDF membrane, which was then blocked using 1% BSA in TBS-Tween for 1 hour at room temperature. Abs and standards were used as described for the ELISA.
Evaluation of vectors in vivo
All animal procedures were performed in accordance with institutional guidelines under protocols approved by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at St Jude Children's Research Hospital and at the University of California, Davis. All animal work carried out in the United Kingdom was performed under the authority of the United Kingdom Home Office Project and Personal Licenses regulations and was compliant with the guidelines of the University College London ethical review committee. Viral vectors were administered to FVII-deficient mice at 35 days postnatal age, and to healthy mice at 40-50 days postnatal age, via tail vein injection.
In utero, IP delivery of vector to fetal monkeys was performed as described previously in the Center for Fetal Monkey Gene Transfer for Heart, Lung and Blood Diseases.29,30 Procedures conformed to the requirements of the Animal Welfare Act, and protocols were approved before implementation by the Institutional Animal Care and Use Committee at the University of California–Davis. Normally cycling, adult female rhesus monkeys (Macaca mulatta; N = 3) with a history of prior pregnancy were bred and identified as pregnant, using established methods.31 Fetuses were monitored sonographically during gestation to ensure normal growth and development and sex confirmed (female fetuses selected). Pregnant animals were screened for the presence of anti-AAV5 Abs to ensure that in utero gene transfer was only performed in dams that were anti-AAV5 Ab negative. In the late third trimester (135 days gestation; term 165 ± 10 days) AAV vector was administered under ultrasound guidance by the IP route using established protocols in telazol-sedated dams.31 Newborns were delivered by Cesarean section at term (160 ± 2 days gestation), umbilical cord blood samples collected (∼ 15 mL), simian Apgar scores assessed, then infants were placed in incubators postdelivery and nursery-reared.31 Infant health, food intake, and body weights were recorded daily or weekly (dependent on age) in the nursery according to established protocols. At ∼ 3 months postnatal age, animals were placed in juvenile housing and weighed regularly. Blood samples were collected from a peripheral vessel at 1 week then monthly postnatal age for complete blood counts (CBCs), serum chemistry panels, and coagulation profiles (∼ 3-5 mL depending on age). At 1-year postnatal age, animals were sedated with ketamine (10 mg/kg IM) and injected intravenously with scAAV-LP1-hFVII-coop vector, pseudotyped with serotype 8-capsid (scAAV8-LP1-hFVII-coop; 4 × 1011 vg/kg). Blood samples were collected before injection then at monthly intervals postinjection until tissue harvest.
Animals were euthanized by an overdose of pentobarbital and tissue harvests performed according to established protocols at ∼ 1.5 years of age.31 Blood samples were obtained (CBCs, chemistry panels, serum, plasma, clotting profiles), then the following tissues were collected: thymus, spleen, liver (all lobes: right and left lateral, caudate, quadrate), lymph nodes (right and left axillary and inguinal, mesenteric), pancreas, right and left adrenals, right and left kidneys, reproductive tract including gonads, gastrointestinal (GI) tract (stomach, duodenum, jejunum, ileum, colon), brain (cerebrum, cerebellum), spinal cord, heart (right and left ventricles), diaphragm, all lung lobes (right and left cranial, right and left middle, right and left caudal, accessory), skin, muscle, and BM. Select tissues were weighed (thymus, spleen, liver, adrenals, kidneys, gonads, brain) and multiple blocks of tissues were collected as follows: fixed in formalin for 24 hours for routine histopathology and snap frozen after embedding in OCT compound. Sections of each tissue collected were also quick frozen over liquid nitrogen for qPCR analysis. Representative sections of all tissues were embedded and sectioned at 5-6 μm then stained with H&E for routine histopathology.
Adult rhesus monkeys received vector at the St Jude Children's Research Hospital nonhuman primate facility. Three females, ages 9-10 years (average weight = 6.8 kg) and 3 males, 3.5-9 years (average weight = 6.5kg) were used. Vector was administered as a bolus infusion via a saphenous vein as previously described.11 Blood samples were subsequently collected monthly. At the time of euthanasia, liver and gonadal tissues were collected.
Abs against AAV5, AAV8, and hFVII-coop were assayed by ELISA
Immunocapture plates were coated with the appropriate Ag (AAV8, AAV5, or recombinant hFVII) before incubation with plasma samples. Bound Ab was detected using HRP-conjugated anti–rhesus IgG Ab, visualized using the OPD substrate, reading absorbance at 490 nm. The titer of the Ab is defined arbitrarily as the reciprocal of the dilution whose absorbance is equal to 3 times the mean background absorbance level, as described previously.32 Murine FVII activity levels were analyzed using a chromogenic assay (Hyphen BioMed) according to the kit instructions.
Results
rAAV-LP1-mFVII mediated gene transfer in a murine model of FVII deficiency
To demonstrate the potential of AAV-mediated FVII expression, a vector encoding murine FVII pseudotyped with AAV8 capsid (Figure 1A) was administered as a bolus injection (2 × 1013 vg/kg) at day 35 of life into low-expressing FVII mice described previously by Castellino et al.33 These mice express FVII at 1.96% ± 0.46 of wild-type levels as measured by a chromogenic assay, sufficient for them to survive birth and for several weeks after, but not high enough to prevent a range of pathologies including spontaneous hemorrhage.33
In AAV FVII-treated mice, the plasma mFVII level reached 312% ± 40% at 11 days after gene transfer and was maintained at this level for the duration of the study (2 months) following gene transfer, as assessed by a chromogenic assay (N = 9). In contrast, untransduced low-expressing FVII littermates exhibited stable FVII activity of 1.18% ± 0.61% of normal levels (Figure 1B). This difference was highly significant (P < .0001).
Kaplan-Meier survival curves were plotted for the AAV FVII-treated low-expressing FVII mice and compared using the log-rank test with survival in control animals. As shown in Figure 1C, transduced mice had a significant survival advantage compared with control mice. After 67 days postadministration (100 days postnatal), 57% of untransduced low-FVII mice had died (N = 13/23). Postmortem inspection showed that the deceased untreated low-FVII mice invariably had evidence of intracranial hemorrhage but no bleeding elsewhere including the peritoneal and thoracic cavities. This suggested that CNS bleeding was likely a significant contributing factor to death. Early death or postmortem evidence of hemorrhage were not observed in FVII-low mice treated with rAAV8-LP1-mFVII vector, with survival of all (N = 19/19) mice in this cohort at 67 days after administration (100 days postnatal). This survival advantage was highly significant (P < .0001). The stable mFVII expression and significant survival advantage observed in mice following a single injection of AAV vector justified further exploration of AAV-mediated perinatal gene transfer using the CFVIID model.
In vitro evaluation of scAAV-LP1-hFVII constructs
In preparation for evaluation of this strategy in a context relevant to humans, we constructed a self-complementary AAV (scAAV) vector encoding wild-type human FVII (hFVII, Figure 2A), as murine FVII cannot be activated by primate tissue factor. scAAV vectors are more potent than single-stranded AAV but have a limited packaging capacity of ∼ 2.3 kb. To meet the packaging constraints of scAAV, the 5′ and 3′ untranslated regions of the hFVII cDNA were deleted (scAAV-LP1-hFVII-wt). In a second vector, the shortened wild-type hFVII cDNA was replaced with a codon-optimized form of the gene (scAAV-LP1-hFVII-coop) in which codon usage had been altered to better reflect the tRNA availability in mammalian cells in a manner described before for human FIX.9 The 1336-bp wild-type and codon-optimized sequences of FVII cDNA were 84% identical at the nucleotide level and showed 100% identity at the peptide level. Full sequences of each cDNA and an analysis of their codon usages are outlined in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The 2 scAAV vectors were pseudotyped with AAV serotype 5 and 8 capsid protein. Packaging of self-complementing viral genome was confirmed by agarose gel electrophoresis (Figure 2B). Under nondenaturing conditions, the viral DNA migrated at a size of ∼ 2.3 kb, while under alkaline conditions its apparent size was ∼ 4.6 kb.
Next, the HepG2 human liver hepatoma cell line was transduced with scAAV-LP1-hFVII-coop, and secreted proteins collected in serum-replacement media X-Vivo 10. Media harvested from transduced cells had 2.4-fold higher levels of hFVII than media harvested from untransduced HepG2 cells (Figure 3A), which as expected naturally secrete low levels of endogenous hFVII.
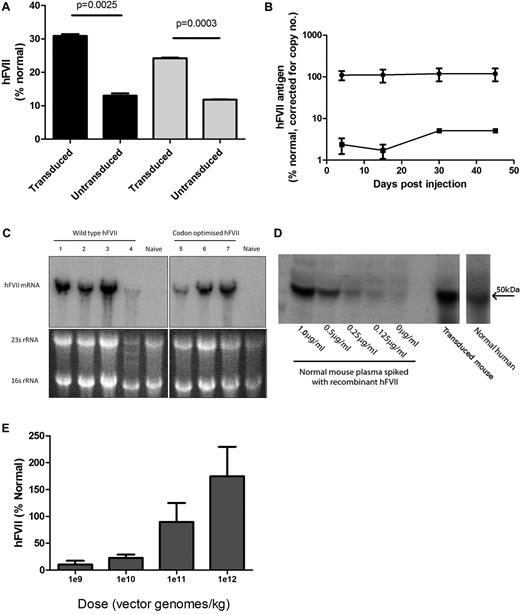
Evaluation of AAV-mediated FVII expression. (A) FVII Ag (left side, ■) and activity (right side, ▩) in supernatant harvested from HepG2 cells 72 hours after the final transduction with scAAV8-LP1-hFVII-coop at an moi of 1 × 105 vg/cell/d over 4 days. (B) Human FVII levels in plasma of male C57Bl/6 mice following a single tail vein administration of 2.8 × 1012 vg/kg scAAV-encoding human FVII (circles = hFVII-coop, squares = hFVII-wt). (C) Top panels: Northern blot showing total human FVII mRNA levels in the liver of mice transduced with 2.8 × 1012 vg/kg scAAV-LP1-FVII-wt (left) and scAAV-LP1-FVII-coop (right) compared with control untransduced animals (naive). Bottom image: Ethidium-stained agarose gel showing equal loading of liver RNA before transfer onto the nitrocellulose membrane. (D) Representative Western blot showing that human FVII-coop expressed following scAAV-mediated gene transfer (transduced mouse) has the same molecular weight as recombinant FVII and FVII detected in human pooled plasma. Serial dilution of recombinant FVII in naive mouse plasma suggests that the amount of human FVII in the experimental animal is > 1 μg/mL. (E) Human FVII levels in mice at 6 weeks after a single tail vein injection of between 1 × 109-1 × 1012 vg/kg scAAV-LP1-FVII-coop. Results are shown as means ± SEM.
Evaluation of AAV-mediated FVII expression. (A) FVII Ag (left side, ■) and activity (right side, ▩) in supernatant harvested from HepG2 cells 72 hours after the final transduction with scAAV8-LP1-hFVII-coop at an moi of 1 × 105 vg/cell/d over 4 days. (B) Human FVII levels in plasma of male C57Bl/6 mice following a single tail vein administration of 2.8 × 1012 vg/kg scAAV-encoding human FVII (circles = hFVII-coop, squares = hFVII-wt). (C) Top panels: Northern blot showing total human FVII mRNA levels in the liver of mice transduced with 2.8 × 1012 vg/kg scAAV-LP1-FVII-wt (left) and scAAV-LP1-FVII-coop (right) compared with control untransduced animals (naive). Bottom image: Ethidium-stained agarose gel showing equal loading of liver RNA before transfer onto the nitrocellulose membrane. (D) Representative Western blot showing that human FVII-coop expressed following scAAV-mediated gene transfer (transduced mouse) has the same molecular weight as recombinant FVII and FVII detected in human pooled plasma. Serial dilution of recombinant FVII in naive mouse plasma suggests that the amount of human FVII in the experimental animal is > 1 μg/mL. (E) Human FVII levels in mice at 6 weeks after a single tail vein injection of between 1 × 109-1 × 1012 vg/kg scAAV-LP1-FVII-coop. Results are shown as means ± SEM.
The biologic activity of hFVII secreted from these cells was confirmed using a chromogenic assay. The ratio of FVII protein (as detected by ELISA) to FVII activity was similar for untransduced (0.92) and transduced (0.78) cells. The lower biologic activity is probably explained by the lack of supplemental vitamin K in the culture media. Nevertheless, these data confirm that the codon-optimized form of hFVII generated biologically active FVII at levels comparable with those of the wild-type coding sequence (P = .11).
Evaluation of hFVII constructs in mice
Before evaluation in nonhuman primates the scAAV-LP1-hFVII constructs were evaluated in wild-type mice. A dose of 2.8 × 1012 vg/kg scAAV8-LP1-hFVII-coop or scAAV8-LP1-hFVII-wt pseudotyped with AAV serotype 8 capsid was injected into the tail vein of 2 groups of 4 male mice. As shown in Figure 3B, after correction for viral copies/liver cell, mice transduced with scAAV8-LP1-hFVII-coop expressed FVII (118.5% ± 40.79%) at a level that was 37-fold higher than those transduced with scAAV8-LP1-hFVII-wt (5.1% ± 0.11%) on day 45 after gene transfer.
Northern blot analysis showed similar levels of hFVII mRNA in the liver of the scAAV8-LP1-hFVII-wt and scAAV8-LP1-hFVII-coop cohorts (Figure 3C). This finding supports the hypothesis that the observed increased efficiency of FVII expression from the codon-optimized gene is because of improved posttranscriptional processing.
Western blot analysis of plasma derived from mice transduced with scAAV8-LP1-hFVII-coop showed a band at 50 kDa, the expected size of hFVII, thus confirming that full-length hFVII was being expressed in mice (Figure 4).
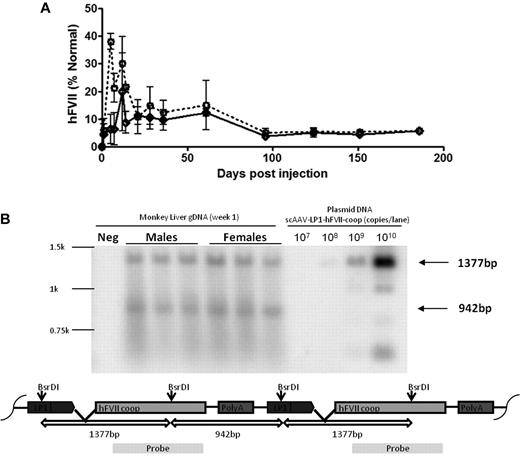
AAV-mediated expression of codon-optimizedhFVII in monkeys. (A) Human FVII levels over time in male (N = 3, dotted line) and female (N = 3, solid line) adult monkeys following a single peripheral vein administration of 4 × 1011 vg/kg scAAV5-LP1-hFVII-coop. (B) Southern blot of DNA extracted from male and female rhesus monkey liver at 4 weeks after administration of scAAV5-LP1-hFVII-coop following digestion with BsRDI (double cutter) and probed with a hFVII-coop probe. As shown in the schematic, the probe is designed to detect the 1377- and 942-bp fragments expected from digest of head-to-tail concatemers.
AAV-mediated expression of codon-optimizedhFVII in monkeys. (A) Human FVII levels over time in male (N = 3, dotted line) and female (N = 3, solid line) adult monkeys following a single peripheral vein administration of 4 × 1011 vg/kg scAAV5-LP1-hFVII-coop. (B) Southern blot of DNA extracted from male and female rhesus monkey liver at 4 weeks after administration of scAAV5-LP1-hFVII-coop following digestion with BsRDI (double cutter) and probed with a hFVII-coop probe. As shown in the schematic, the probe is designed to detect the 1377- and 942-bp fragments expected from digest of head-to-tail concatemers.
Next, varying doses of scAAV8-LP1-hFVII-coop vector (range: 1 × 109 to 1 × 1012 vg/kg) were injected into the tail vein of 6- to 8-week-old male C57Bl/6 mice (N = 5-8 animals/cohort). Levels of hFVII circulating in mouse plasma showed a dose-dependent response to the vector dose, as quantified by ELISA (Figure 3E). Doses of scAAV8-LP1-hFVII-coop as low as 1 × 109 vg/kg were sufficient to mediate hFVII at levels > 5% of normal, which are likely to be therapeutic. Administration of 1 × 1012 vg/kg resulted in expression of hFVII at 174.7% ± 54.96% of normal without toxicity.
Codon-optimized hFVII is expressed at equivalent levels by male and female adult rhesus monkeys
Previous work with AAV vectors in mice showed that liver transduction in females was 5- to 13-fold less efficient than in males.34 Although this relative inefficiency in females can be partially corrected through the use of self-complementing vectors or pretreatment with bortezomib and testosterone,34,35 this is a critical concern for CFVIID, an autosomal-recessive condition.
To investigate sex-dependent differences in transgene expression in nonhuman primates, 3 male-female pairs of adult rhesus monkeys were injected with scAAV5-LP1-hFVII-coop. Plasma hFVII levels were measured at varying time points using an immune capture assay that can detect as little as 15 ng/mL hFVII in normal rhesus monkey plasma (Figure 4A). Transgene expression reached peak levels of 15.0% ± 5.1% and 12.28% ± 0.68% in male and female monkeys, respectively, before declining to steady-state level of ∼ 7.2%. This pattern of AAV-mediated transgene expression has been described before for AAV vectors encoding human FIX.25 Transgene expression was maintained at this level for at least 180 days (duration of the study) after gene transfer with no significant difference in average plasma FVII levels between the male and female cohorts.
Viral genome copy numbers were assessed in liver samples by Southern blot (Figure 4B). This assay confirmed that the virus transduced male and female livers with equal efficiency. These results suggest that the sex-specific difference in transgene expression seen previously in mice is species-specific.
hFVII is expressed at therapeutic levels in neonatal monkeys after fetal gene transfer
Three newborn monkeys were delivered uneventfully at term following in utero gene transfer of scAAV5-LP1-hFVII-coop. They remained healthy during the study period with no evidence of adverse findings. All CBCs and clinical chemistry panels were within normal limits for animals in this age group compared with concurrent and historical controls. All coagulation panels were also found to be within normal limits. Body weights at birth and throughout the study period paralleled the normal historical control range.
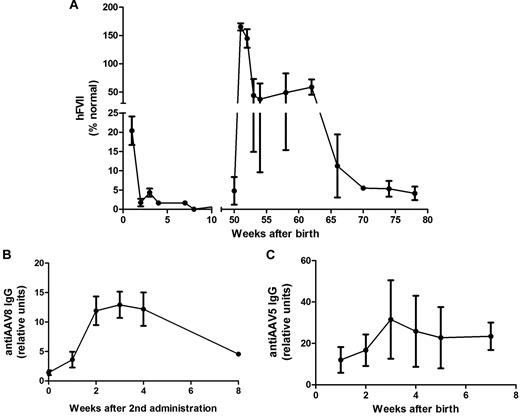
Levels of circulating hFVII were assessed from frequent blood samples and measured by ELISA. As shown in Figure 5A, hFVII was detected at birth in all 3 newborns at an average of 20.4% ± 3.7%. Plasma samples from a fourth, untreated newborn and from several adult rhesus monkeys were consistently measured at 0% hFVII. Transgene expression was observed to decline over time to levels below the threshold of detection (3 months postnatal) possibly because of loss of episomally maintained proviral DNA as the animals grew and matured. This decline was not associated with an increase in serum transaminases or with the development of inhibitory anti-FVII Abs. IgG Abs against the AAV5 capsid were detected in plasma samples at birth (Figure 5B), consistent with the relatively mature state of the primate immune system during the last trimester.
Expression of hFVII in young rhesus monkeys following fetal administration of vector supernatant andreadministration postnatally. (A) Plasma human FVII levels in young rhesus monkeys following administration of 4 × 1011 vg/kg scAAV5-LP1-hFVII-coop prenatally and scAAV8-LP1-hFVII-coop at 1 year postnatal age. (B) Anti-AAV5 IgG levels in plasma after fetal administration of AAV5 LP1 hFVII coop. (C) Anti-AAV8 IgG levels following administration of scAAV8-LP1-hFVII-coop (second administration of vector) in monkeys at 1 year postnatal age.
Expression of hFVII in young rhesus monkeys following fetal administration of vector supernatant andreadministration postnatally. (A) Plasma human FVII levels in young rhesus monkeys following administration of 4 × 1011 vg/kg scAAV5-LP1-hFVII-coop prenatally and scAAV8-LP1-hFVII-coop at 1 year postnatal age. (B) Anti-AAV5 IgG levels in plasma after fetal administration of AAV5 LP1 hFVII coop. (C) Anti-AAV8 IgG levels following administration of scAAV8-LP1-hFVII-coop (second administration of vector) in monkeys at 1 year postnatal age.
Approximately 1 year after fetal gene transfer, the monkeys were rechallenged with scAAV-LP1-hFVII-coop vector, pseudotyped with serotype 8 capsid (scAAV8-LP1-hFVII-coop), to evade the humoral immune response against AAV5 during the initial treatment. The vector was administered in ketamine-sedated monkeys as a slow bolus infusion into a peripheral vein at a dose of 4 × 1011 vg/kg, and was well tolerated.
After IV injection of scAAV8-LP1-hFVII-coop plasma hFVII levels increased, with a mean peak of 165% ± 10.8% at 1 month postinjection (Figure 5A). After this initial peak, expression stabilized at ∼ 47.3% ± 27.7% until 3 months postinjection and was still detectable at therapeutic levels until tissue harvest. As before, the decline in expression was not associated with an increase in serum transaminase levels or with the development of inhibitory Abs against FVII, as assessed by ELISA and a modified Bethesda assay. As expected, IgG Abs against the AAV8 capsid were detected in plasma samples within 4 weeks of administration of scAAV8-LP1-hFVII-coop (Figure 5C).
Analysis of tissues collected for biodistribution studies using a qPCR assay confirmed that the highest concentration of viral genomes was in the liver. Approximately 10-fold lower levels of the FVII provirus were detected in other tissues (see Table 1). This biodistribution profile is consistent with prior reports.11 It is important to note that other studies have shown that a positive PCR finding in gonadal sections does not imply that vector sequences are present in the germ cells.29 Laser capture microdissection and subsequent qPCR would be required to confirm this finding.
Vector biodistribution in selected rhesus monkey tissues following in utero and postnatal readministration of scAAV-LP1-FVII-coop
| Tissue . | Viral genome copies per cell . |
|---|---|
| BM | 0.38 |
| Cerebellum | 0.02 |
| Jejunum | 0.03 |
| Left ventricle | 2.65 |
| Liver caudate | 35.89 |
| Liver left lateral | 9.35 |
| Liver right lateral | 41.86 |
| Lung right middle | 1.94 |
| Right adrenal gland | 1.05 |
| Right axial lymph node | 0.03 |
| Right ovary | 4.53 |
| Spinal cord | 0.045 |
| Spleen | 0.40 |
| Naive liver | 0.00 |
| Water | 0.00 |
| Tissue . | Viral genome copies per cell . |
|---|---|
| BM | 0.38 |
| Cerebellum | 0.02 |
| Jejunum | 0.03 |
| Left ventricle | 2.65 |
| Liver caudate | 35.89 |
| Liver left lateral | 9.35 |
| Liver right lateral | 41.86 |
| Lung right middle | 1.94 |
| Right adrenal gland | 1.05 |
| Right axial lymph node | 0.03 |
| Right ovary | 4.53 |
| Spinal cord | 0.045 |
| Spleen | 0.40 |
| Naive liver | 0.00 |
| Water | 0.00 |
Rhesus monkeys were injected in utero with 4 × 1011 vg/kg scAAV5-LP1-hFVII-coop near term, and administered a second dose of 4 × 1011 vg scAAV8-LP1-hFVII-coop IV 1 year after birth. Tissue samples were collected at ∼ 1.5 years of age and viral genome copy number per cell was quantified by quantitative PCR.
Discussion
Gene therapy is beginning to show promise for the treatment of a variety of monogenic diseases in adults. However, many genetic diseases present with early onset of irreversible organ damage or mortality. To treat these disorders successfully, safe and efficacious gene therapy vectors and treatment protocols are needed.30,31 In this study, we assessed the safety and efficacy of perinatal gene transfer for CFVIID because of early-onset presentation with intracranial hemorrhage. A single tail-vein administration of AAV-encoding murine FVII was found to be sufficient to provide young adult mice with supraphysiologic levels of FVII expression for at least 67 days postgene transfer. All treated mice were protected from disease-associated hemorrhage and consequent mortality for this time period with no associated toxicity. Our results are consistent with reports of perinatal gene transfer with AAV vectors in murine models of Leber congenital amaurosis,36 Pompe disease,37 and hemophilia B,38 as well as studies focused on safety and gene transfer efficiency in rhesus monkeys.30,39
Codon optimization has been shown to improve the efficiency of expression of certain genes but the algorithms underlying the codon substitutions are not yet perfect, leading to inconsistent results.40,41 For instance, codon optimization of the murine FVII cDNA resulted in complete loss of transgene expression following AAV-mediated gene transfer in mice (data not shown). In contrast, codon optimization of the human FVII gene resulted in substantial improvement of FVII protein expression, apparently because of improved posttranscriptional processing. This is consistent with our experience with the human FIX and FVIII genes.42 The reason for the variability in the success of codon optimization is currently unclear but may relate to moderation of translation which could impact protein folding.
We, and others, have previously shown that AAV vectors are more efficient at mediating gene transfer in males compared with females in rodents and canine models.9,34,35,43-46 However, in nonhuman primates, gene transfer efficiency in males and females has consistently been shown to be equivalent.30 While the reason for this species-specific difference remains unknown, it is consistent with other variations in the biologic processing of AAV vectors in primates compared with other animal models.25 This observation clearly has significant implications for CFVIID as well as other disorders where females are affected by disease in significant numbers. Our data in nonhuman primates suggests that there is no need to develop different treatment protocols for female patients involving the use of androgens or proteosome inhibitors to achieve the same level of gene transfer as in male patients,25,35 and emphasizes the importance of choice of species for preclinical studies.
In utero gene transfer using viral vectors remains a controversial area and is prohibited in humans by a variety of regulatory bodies around the world, in part because of a poor understanding of the toxicity associated with this procedure. Our data suggest that ultrasound-guided in utero administration of AAV vector in the late third trimester in nonhuman primates is feasible and safe for the fetus and the dam. This is consistent with other reports from our group and others in large animal models.11,29-31,47 In utero gene transfer of human FVII in late gestation conferred expression at the time of birth and shortly thereafter within the range of values that could offer protection to children with severe CFVIID in the early postnatal period, which is the critical time associated with perinatal death. However, as in other large animal models, rAAV-mediated transgene expression declined over time. This decline in the postnatal period is most likely because of loss of episomally retained transgene as shown previously in juvenile mice48,49 and by our group in monkeys and sheep.11,47 Nevertheless, readministration of the vector pseudotyped with an antigenically distinct capsid was successful in restoring expression in young rhesus monkeys, which was then maintained at therapeutic levels until the end of the study.
The formation of inhibitory Abs against therapeutic factors is a major concern in the treatment of the hemophilias, and must be considered in the development of gene therapy vectors. Throughout our study, no inhibitory Abs against hFVII were detected, corresponding to the low incidence of inhibitor formation in patients receiving FVII infusions,50 and to our previous data suggesting that early expression of transgenes from the liver can help to confer immunologic tolerance.11,47 While these data are very encouraging, the potential for species-specific differences in the immune response means that this must be interpreted with caution when beginning to consider the exploration of these techniques in human patients.
In summary, perinatal gene transfer with the AAV vectors used in these studies was found to be safe and efficient, with transgene expression at therapeutic levels in mice and rhesus monkeys. These findings further support careful evaluation of this strategy for the treatment of congenital FVII deficiency as well as other disorders with early onset of pathology and neonatal mortality such as Crigler Najjar type I disease, lysosomal storage, and urea cycle disorders, where current treatment options are nonexistent or suboptimal.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by The Wellcome Trust, United Kingdom; Department of Health's National Institute for Health Research (NIHR) Biomedical Research Centres funding award to UCLH/UCL; NIHR Program Grant A for Molecular and Tissue Engineering, United Kingdom; The Katharine Dormandy Trust; The ASSISI Foundation of Memphis; the American Lebanese Syrian Associated Charities (ALSAC); the National Heart, Lung, and Blood Institute (NHLBI) Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases (grant HL085794); the California National Primate Research Center base operating grant (RR00169); and the National Cancer Institute Cancer Center Support grant CA027165.
National Institutes of Health
Wellcome Trust
Authorship
Contribution: C.B. performed research and wrote the manuscript; J.M., M.D.P., S.M.K.B., Y.S., and C.L.M. performed research; E.G.D.T., J.H.M., and A.J.T. designed research; S.N.W., J.T.G., and F.J.C. designed/performed research; A.F.T. designed/performed research and wrote the manuscript; and A.M.D. and A.C.N. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amit C. Nathwani, MD, PhD, UCL Cancer Institute, Paul O'Gorman Building, University College London, 72 Huntley Street, London WC1E 6BT, United Kingdom; e-mail: a.nathwani@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal