Abstract
Fanconi anemia, complementation group C (FANCC)–deficient hematopoietic stem and progenitor cells are hypersensitive to a variety of inhibitory cytokines, one of which, TNFα, can induce BM failure and clonal evolution in Fancc-deficient mice. FANCC-deficient macrophages are also hypersensitive to TLR activation and produce TNFα in an unrestrained fashion. Reasoning that suppression of inhibitory cytokine production might enhance hematopoiesis, we screened small molecules using TLR agonist–stimulated FANCC- and Fanconi anemia, complementation group A (FANCA)–deficient macrophages containing an NF-κB/AP-1–responsive reporter gene (SEAP). Of the 75 small molecules screened, the p38 MAPK inhibitor BIRB 796 and dasatinib potently suppressed TLR8-dependent expression of the reporter gene. Fanconi anemia (FA) macrophages were hypersensitive to the TLR7/8 activator R848, overproducing SEAP and TNFα in response to all doses of the agonist. Low doses (50nM) of both agents inhibited p38 MAPK–dependent activation of MAPKAPK2 (MK2) and suppressed MK2-dependent TNFα production without substantially influencing TNFα gene transcription. Overproduction of TNFα by primary FA cells was likewise suppressed by these agents and involved inhibition of MK2 activation. Because MK2 is also known to influence production and/or sensitivity to 2 other suppressive factors (MIP-1α and IFNγ) to which FA hematopoietic progenitor cells are uniquely vulnerable, targeting of p38 MAPK in FA hematopoietic cells is a rational objective for preclinical evaluation.
Introduction
BM failure is a nearly universal complication of Fanconi anemia (FA), an inherited disease caused by biallelic inactivating mutations of any one of 15 genes.1–4 FA gene products collectively facilitate responses to DNA damage,1 and therefore it is often presumed (notwithstanding a lack of direct evidence supporting the idea) that hematopoietic defects simply reflect attrition of hematopoietic stem cells (HSCs) that have specifically suffered excessive DNA damage. An alternative explanation is that the FA proteins are multifunctional and play a direct role in stem cell maintenance, and therefore, DNA damage in FA HSCs is not necessarily required to suppress their function.5–9 In normal cells, for example, Fanconi anemia, complementation group C (FANCC) modulates the hematopoietic inhibitory effects of TNFα, IFNγ, and MIP-1α, each of which normally function to suppress hematopoiesis.6,10–12 FANCC influences TNFα responsiveness at least in part by modulating the activation state of the IFN-inducible double-stranded RNA activated protein kinase.13 FANCC also suppresses the activation potential of certain TLR pathways in normal mononuclear phagocytes.14 Therefore, in hematopoietic tissues, FANCC deficiency results in a TLR-dependent overproduction of TNFα, one of the cytokines to which the stem-cell pool is uniquely intolerant.10,15–19 These abnormalities are important elements in the pathogenesis of BM failure.6,20 There is also experimental evidence that this TNFα–inhibitory loop is a selective pressure that enhances the ultimate emergence of TNF-resistant leukemic and preleukemic clones.21–23 Therefore, interdiction of TNFα-induced BM failure, particularly in ways that might have an additional favorable influence on IFNγ- and MIP-1α–activated signaling pathways, may improve BM function and reduce the likelihood of clonal evolution by improving the fitness landscape and altering the coefficient of selection.21
Seeking small molecules with these attributes, in the present study we exploited the TLR-hypersensitive phenotype as a screening tool to identify therapeutic agents that might suppress that pathway in FA cells. Using a TLR8-hypersensitive, FANCC-deficient mononuclear phagocyte cell line that we described previously,14 we screened 75 small molecules, approximately 50 of which were kinase inhibitors. We identified 2 inhibitors, BIRB 796 and dasatinib, that functioned to suppress the TLR-dependent overproduction of TNFα in FANCC- and Fanconi anemia, complementation group A (FANCA)–deficient macrophages. We also determined that both agents functioned to suppress TNFα gene expression posttranscriptionally by inhibiting p38 MAPK activation of MAPKAPK2 (MK2). Others have demonstrated that short-term p38 MAPK inhibition enhances the repopulating capacity of Fancc-deficient HSCs,24 that p38 MAPK modulates responses to IFNγ in some cell types,25 and that MK2 controls expression of not only TNFα, but also IFNγ and MIP-1α production.26,27 Therefore, preclinical evaluation of p38 MAPK inhibitors is clearly warranted using murine models of FA.
Methods
Abs and reagents
Anti–acetyl-NF-κB p65 (Lys310), anti–phospho-NF-κB p65 (Ser536), anti-MAPKAPK2 (MK2), anti–phospho-MAPKAPK2 (Thr334), anti-p38 MAPK, anti–phospho-p38 MAPK(Thr 180/Tyr182), and anti–phospho-c-Jun (Ser73) rabbit mAbs were all purchased from Cell Signaling Technology. Anti–c-Jun (H-79) and anti–NF-κB p65 rabbit polyclonal Abs and anti-FANCD2 mouse mAb were purchased from Santa Cruz Biotechnology. BIRB 796 (doramapimod) was obtained from Axon Medchem. Dasatinib was obtained from LC Laboratories. Other inhibitors (Table 1) were obtained from LC Laboratories, Axon Medchem, and Sigma-Aldrich. MMP V Inhibitor was obtained from Santa Cruz Biotechnology. MMP Inhibitor II, PP-2, GM-6001, and the negative control for GM-6001 were obtained from EMD Chemicals. The pNiFty plasmid, QUANTI-Blue, and lipopolysaccharide (LPS) were obtained from InvivoGen. R848 was obtained from Enzo Life Sciences. Actinomycin D and mitomycin C were obtained from Sigma-Aldrich.
Small molecules used in screening
| 17-AAG | CHIR258 | JAK1i | Nilotinib | SB-43152 |
| 8 Azaguanine | CHIR265 | JAK3i | P38i | SB203580 |
| ABT-864 | CI-1033 | JNK2i | Pan-JAKi | Sorafenib |
| Adiphenine | CP-690550 | JNKIIi | PD153035 | SRCi |
| AG490 | CYC-202 | KN92 | PD325901 | Staurosporine |
| AKTIV | Dasatinib | KN93 | PD98059 | STO609 |
| AKTX | EGFRi | Lapatinib | PI-103 | Sunitinib |
| AMPK | Erlotinib | LSD1 | Piperine | SYKi |
| Anisomycin | Estradiol | LY294002 | PKC412 | Thapsigargin |
| AZD-1152 | Gefitinib | LY333531 | PP2 | Trichostatin A |
| Azithromycin | Go6976 | MEK1/2 | Proguanil | Tunicamycin |
| BAY-11 | GW-786034 | Menadione | PTK787 | Vorinostat |
| BIRB-796 | H-89 | MLN-8054 | R1881 | VX-680 |
| Boldine | Harmane | MLN518 | Resveratrol | Zardaverine |
| cAMP | Imatinib | QNZ | SB-202190 | ZD-6474 |
| 17-AAG | CHIR258 | JAK1i | Nilotinib | SB-43152 |
| 8 Azaguanine | CHIR265 | JAK3i | P38i | SB203580 |
| ABT-864 | CI-1033 | JNK2i | Pan-JAKi | Sorafenib |
| Adiphenine | CP-690550 | JNKIIi | PD153035 | SRCi |
| AG490 | CYC-202 | KN92 | PD325901 | Staurosporine |
| AKTIV | Dasatinib | KN93 | PD98059 | STO609 |
| AKTX | EGFRi | Lapatinib | PI-103 | Sunitinib |
| AMPK | Erlotinib | LSD1 | Piperine | SYKi |
| Anisomycin | Estradiol | LY294002 | PKC412 | Thapsigargin |
| AZD-1152 | Gefitinib | LY333531 | PP2 | Trichostatin A |
| Azithromycin | Go6976 | MEK1/2 | Proguanil | Tunicamycin |
| BAY-11 | GW-786034 | Menadione | PTK787 | Vorinostat |
| BIRB-796 | H-89 | MLN-8054 | R1881 | VX-680 |
| Boldine | Harmane | MLN518 | Resveratrol | Zardaverine |
| cAMP | Imatinib | QNZ | SB-202190 | ZD-6474 |
For each molecule, at least 3 doses were tested. Cell viability was quantified using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay.
Cell lines and primary cells
THP-1 cells were obtained from the American Type Culture Collection and THP1-Blue cells were obtained from InvivoGen. The development and culture conditions of the cell lines T-shNT and T-shFC were described previously.14 The EBV-transformed patient-derived lymphoblast cell line HSC536N (FANCC-deficient) and isogenic cells complemented with normal human FANCC cDNA (HSC536N/FANCC) were used as described previously.28 BM-derived macrophages were prepared from low-density (Ficoll-Paque PLUS; GE Healthcare) BM mononuclear cells obtained from 2-month-old wild-type or Fancc-deficient mice using published methods.29–31 Specifically, mononuclear cells were cultured for 8 days in IMDM (Life Technologies), 20% FBS (Hyclone), and 40 ng/mL of recombinant murine GM-CSF (R&D Systems), followed by a 2-day treatment in serum-free medium. Macrophages were replated into 96-well dishes (10 000 cells/well) and treated with 1 μg/mL of LPS for 24 hours. Conditioned media were then harvested for TNFα quantification using the Quantikine ELISA Kit (R&D Systems). All murine studies were approved by the Portland Veterans Administration Institutional Animal Care and Use Committee. Primary CD14+ cells were prepared from peripheral blood mononuclear leukocytes (isolated using Ficoll-Paque Plus) using magnetic microbeads (EasySep; StemCell Technologies). Cells were then cultured at a concentration of 10 000cells/200 μL for 24 hours in RPMI medium and 10% FBS in the presence of multiple doses of R848. Conditioned media were harvested for TNFα quantification using the Quantikine ELISA Kit (R&D Systems). Cells from healthy volunteers and from patients were obtained after having obtained informed consent from either the subject or the subject's parents on a clinical study approved by the Institutional Review Board of G. Gaslini Children's Institute.
Stable suppression of FANCC and FANCA with lentiviral shRNA
THP-1 Blue cells were transduced with lentiviral particles expressing shRNA targeting FANCC, as we described previously.14 A similar strategy was used to suppress FANCA. Specifically, 5 lentiviral shFANCA MISSION vectors (TRCN0000118982, TRCN0000118983, TRCN0000118984, TRCN0000118985, and TRCN0000118986; Sigma-Aldrich) were tested. shRNA-expressing cells were selected with 0.6 μg/mL of puromycin for 2-3 weeks, after which time the cells were maintained in medium containing 0.3 μg/mL of puromycin. Knockdown was quantified using immunoblots for FANCA and FANCD2. TRCN0000118982 (a coding region target sequence of GCCGACCTCAAGGTTTCTATA) provided the greatest knockdown of FANCA and FANCD2L (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and was therefore used in all subsequent experiments.
Drug-screening assay
Recently, we reported the development of an shRNA-transduced, FANCC-deficient THP1-Blue cell line (T-shFC) in which the TLR7/8 agonist R848 induced increased TNFα production compared with the control cell line (T-shNT, transduced with a nontargeted shRNA).14 Likewise, treatment with R848 induced the expression of the NF-κB– and AP-1–dependent reporter gene and secreted embryonic alkaline phosphatase (SEAP), the activity of which was quantified using the QUANTI-Blue reagent, as described previously.14 In the present study, we used T-shFC cells in a functional screening assay to identify molecules that suppressed or induced SEAP. We tested 75 inhibitors (Table 1). For each of the inhibitors tested, at least 3 doses were analyzed and cell viability was assessed with the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay. The requirements for considering any screening result to be positive were: (1) ≥ 70% suppression of SEAP expression in R848-stimulated cells; and (2) greater than 80% viability of the cell population at the end of the 24-hour culture period. Studies using the SEAP reporter driven only by 5 NF-κB–binding sites (and lacking the AP-1 sites) were accomplished by transfecting the pNiFty plasmid into THP-1 cells using Nucleofector II with Program X-001 (Amaxa). The cells were selected in vitro with Zeocin (200 μg/mL; Life Technologies).
TNFα and MMP7 assays
A total of 100 000 cells/200 μL were cultured in 96-well plates and either maintained in culture medium (HSC536N and HSC536N/FANCC) or treated with 30μM R848 for 24 hours (T-shNT and T-shFAC), after which time culture supernatants were assayed for TNFα or MMP7 using Quantikine ELISA Kits (R&D Systems). In some experiments with T-shNT and T-shFAC cells, cultures were pretreated with MMP inhibitors for 6 hours before the addition of 30μM R848 for 24 hours and subsequent medium collection.
Immunoblotting
Real-time qRT-PCR
For real-time quantitative RT-PCR (qRT-PCR), total RNA was prepared from 1-5 × 106 cells using the RNeasy Mini kit (QIAGEN). Complementary DNA synthesis and real-time PCR were performed as described previously.14 Primer and probe sets for TNFα (Hs00174128_m1) and MMP7 (Hs01042795_m1) were purchased as TaqMan Gene Expression Assays (Life Technologies). Primers and TaqMan probes for SEAP detection were designed using Primer Express Version 3.0 software (Life Technologies) and were as follows: probe 6-FAM-ACACGCGGCAACG-MGB; 5′ primer CCGCTTTAACCAGTGCAACA; 3′ primer CCCGATTCATCACGGAGATG. Primers were purchased from IDT and probes from Life Technologies.
Quantifying mRNA decay
Actinomycin D (5 μg/mL) was added to cells pretreated for 24 hours with 30μM R848. Total RNA was prepared at regular time intervals thereafter. TNFα mRNA was quantified using real-time qRT-PCR.
siRNA
SMARTpool siRNA targeting MK2 and control siRNA were purchased from Thermo Scientific Dharmacon. The pool consisted of 4 distinct sequences: GAACCACCCUUGGAUCAUG, GAAUGACCAUCACCGAGUU, CGAAUGGGCCAGUAUGAAU, and UGAUUGUCAUGGAAUGUUU. Cells were transfected with siRNA using Nucleofector II (Amaxa), as described previously.14 After transfection, the cells were cultured for 72 hours, after which time MK2 expression was maximally suppressed.
Statistical analysis
All statistical analyses were performed using a 2-tailed Student t test. Unless otherwise stated, data are presented as mean values ± SD of 3 or more independent experiments. P < .05 was considered statistically significant.
Results
Agents that inhibit TNFα gene expression in FANCC- and FANCA-deficient cells
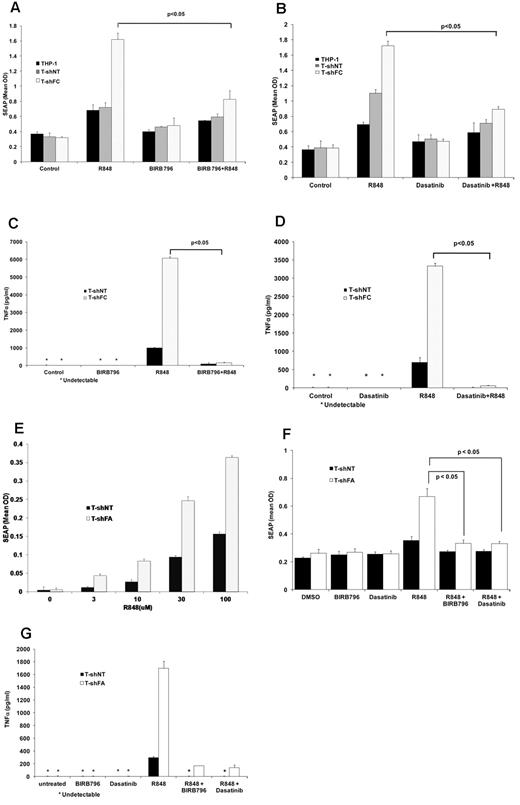
THP1-Blue cells report TLR-induced NF-κB/AP-1 activation by expressing SEAP. Using our inhibitor-screening assay, we tested small molecules for their capacity to suppress the TLR-dependent expression of SEAP. Two of the 75 small-molecule inhibitors initially screened (Table 1), BIRB 796, a specific p38 MAPK inhibitor, and dasatinib, a dual Abl/Src kinase inhibitor, were among the most potent inhibitors of SEAP production. BIRB 796 and dasatinib effectively suppressed R848-induced SEAP production in both T-shFC and T-shNT cells to the same level as that observed in controls (Figure 1A-B). Both agents also inhibited TNFα production (as quantified by ELISA) by R848-stimulated T-shNT and T-shFC cells (Figure 1C-D). Compared with T-shNT cells, FANCA-deficient THP-1 cells (T-shFA) were also hypersensitive to R848. Specifically, T-shFA cells produced more SEAP at all 4 doses of R848 tested (Figure 1E), and the overproduction of both SEAP (Figure 1F) and TNFα (Figure 1G) by these cells was suppressed by both BIRB 796 and dasatinib.
BIRB 796 and dasatinib inhibit NF-κB/AP-1–dependent SEAP production in T-shFC cells. Mean values ± SD are shown. All P values were calculated using the Student t test. (A) BIRB 796 inhibits R848-induced SEAP gene expression in FANCC-deficient cells. THP1-Blue cells (THP1) or THP1-Blue cells stably expressing either control (nontarget) shRNA (T-shNT) or shRNA targeting FANCC (T-shFC) were cultured in medium alone (control) or medium with the TLR7/8 agonist R848 (30μM) alone, BIRB 796 (500nM) alone, or R848 (30μM) alone after a 6-hour pretreatment with BIRB 796 (500nM). Conditioned media were collected after 24 hours of R848 exposure and SEAP was quantified colorimetrically using QUANTI-Blue reagent. (B) Dasatinib inhibits R848-induced SEAP expression in FANCC-deficient cells. The experimental design was identical to that of 1A except that dasatinib (500nM) was used as the inhibitor. (C) BIRB 796 inhibits TNFα secretion in THP-1 cells. T-shNT and T-shFC were treated as in panel A with BIRB 796 (500nM) and/or R848 (30μM). Conditioned media were collected after 24 hours of incubation with R848 and TNFα was quantified by ELISA. Untreated cells or cells treated only with BIRB 796 did not produce detectable TNFα (indicated with an asterisk). (D) Dasatinib inhibits TNFα secretion in THP-1 cells. T-shNT and T-shFC cells were treated as in panel B with R848 (30μM) and dasatinib (500nM). Untreated cells or cells treated with dasatinib alone did not produce detectable TNFα (indicated with an asterisk). (E) FANCA-deficient THP-1 cells are hypersensitive to R848. THP1-Blue cells stably expressing shRNA targeting FANCA (T-shFA) and T-shNT cells were exposed to R848 (0-100μM) for 24 hours. Supernatant media were collected and SEAP was quantified colorimetrically using QUANTI-Blue reagent. At each dose of R848, T-shFA cells produced more SEAP than did T-shNT cells (P < .05). (F) BIRB 796 and dasatinib suppress the production of SEAP in both T-shFA and T-shNT cells. The design of these studies on T-shFA cells was identical to that of studies on T-shFC cells (A-B). (G) BIRB 796 and dasatinib suppress TNFα production in R848-stimulated T-shFA and T-shNT cells. The design of these studies on T-shFA cells was identical to that used with T-shFC cells (C-D).
BIRB 796 and dasatinib inhibit NF-κB/AP-1–dependent SEAP production in T-shFC cells. Mean values ± SD are shown. All P values were calculated using the Student t test. (A) BIRB 796 inhibits R848-induced SEAP gene expression in FANCC-deficient cells. THP1-Blue cells (THP1) or THP1-Blue cells stably expressing either control (nontarget) shRNA (T-shNT) or shRNA targeting FANCC (T-shFC) were cultured in medium alone (control) or medium with the TLR7/8 agonist R848 (30μM) alone, BIRB 796 (500nM) alone, or R848 (30μM) alone after a 6-hour pretreatment with BIRB 796 (500nM). Conditioned media were collected after 24 hours of R848 exposure and SEAP was quantified colorimetrically using QUANTI-Blue reagent. (B) Dasatinib inhibits R848-induced SEAP expression in FANCC-deficient cells. The experimental design was identical to that of 1A except that dasatinib (500nM) was used as the inhibitor. (C) BIRB 796 inhibits TNFα secretion in THP-1 cells. T-shNT and T-shFC were treated as in panel A with BIRB 796 (500nM) and/or R848 (30μM). Conditioned media were collected after 24 hours of incubation with R848 and TNFα was quantified by ELISA. Untreated cells or cells treated only with BIRB 796 did not produce detectable TNFα (indicated with an asterisk). (D) Dasatinib inhibits TNFα secretion in THP-1 cells. T-shNT and T-shFC cells were treated as in panel B with R848 (30μM) and dasatinib (500nM). Untreated cells or cells treated with dasatinib alone did not produce detectable TNFα (indicated with an asterisk). (E) FANCA-deficient THP-1 cells are hypersensitive to R848. THP1-Blue cells stably expressing shRNA targeting FANCA (T-shFA) and T-shNT cells were exposed to R848 (0-100μM) for 24 hours. Supernatant media were collected and SEAP was quantified colorimetrically using QUANTI-Blue reagent. At each dose of R848, T-shFA cells produced more SEAP than did T-shNT cells (P < .05). (F) BIRB 796 and dasatinib suppress the production of SEAP in both T-shFA and T-shNT cells. The design of these studies on T-shFA cells was identical to that of studies on T-shFC cells (A-B). (G) BIRB 796 and dasatinib suppress TNFα production in R848-stimulated T-shFA and T-shNT cells. The design of these studies on T-shFA cells was identical to that used with T-shFC cells (C-D).
BIRB 796 and dasatinib inhibit TNFα overproduction in a patient-derived cell line and in primary Fancc- and FANCA-deficient macrophages
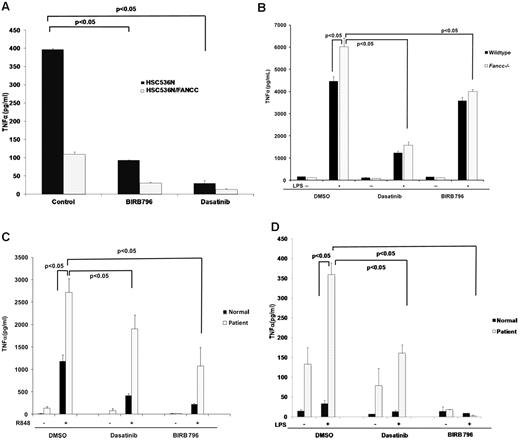
The B-cell line HSC536N, derived from a FANCC-deficient patient, produces TNFα constitutively, and TNFα production in these cells is completely suppressed by complementation with wild-type FANCC cDNA.14 Both BIRB 796 and dasatinib fully suppressed TNFα production in HSC536N cells, matching TNFα levels produced by the complemented isogenic cell line (HSC536N/FANCC; Figure 2A). Because LPS induces TNFα overproduction and TNFα-dependent BM failure in Fancc−/− mice,18 we tested the effectiveness of BIRB 796 and dasatinib as inhibitors of TNFα production by LPS-treated BM-derived murine macrophages from wild-type and Fancc−/− mice. Both BIRB 796 and dasatinib suppressed TNFα production effectively (Figure 2B). As was the case with T-shFA cells (Figure 1G), primary peripheral blood mononuclear phagocytes from a Fanconi group A patient (FANCA-deficient) were hypersensitive to both R848 and LPS compared with an age-matched control sample studied in parallel. Moreover, both small molecules suppressed TNFα gene expression induced by both TLR agonists (Figure 2C-D).
BIRB 796 and dasatinib suppress TLR-activated TNFα gene expression in primary and patient-derived FA cells. Means ± SD are shown. All P values were calculated using the Student t test. (A) BIRB 796 and dasatinib inhibit constitutive TNFα secretion in the patient-derived FANCC−/− lymphoblast cell line HSC536N. HSC536N and HSC536N/FANCC (complemented with normal FANCC cDNA) were cultured in medium alone (control) or medium treated with BIRB 796 (500nM) or dasatinib (500nM) for 6 hours. After 6 hours, supernatant media were collected and TNFα measured by ELISA. (B) BIRB 796 and dasatinib inhibit LPS-induced TNFα production in Fancc-deficient murine macrophages. Primary murine BM-derived macrophages obtained from Fancc−/− and wild-type mice were maintained in control medium (plus DMSO) or treated for 6 hours with BIRB 796 (500nM) or dasatinib (500nM), followed by stimulation with LPS (1 μg/mL) for 24 hours. TNFα in conditioned media was measured by ELISA. (C-D) BIRB 796 and dasatinib inhibit R848- and LPS-induced TNFα production in FANCA-deficient human macrophages. Primary human CD14+ macrophages obtained from a normal age-matched volunteer and a FANCA-deficient patient were cultured for 6 hours in control medium (plus DMSO), BIRB 796 (500nM), or dasatinib (500nM), before stimulation for 24 hours with R848 (1μM; C) or LPS (0.1 ng/mL; D). TNFα in conditioned media was measured by ELISA.
BIRB 796 and dasatinib suppress TLR-activated TNFα gene expression in primary and patient-derived FA cells. Means ± SD are shown. All P values were calculated using the Student t test. (A) BIRB 796 and dasatinib inhibit constitutive TNFα secretion in the patient-derived FANCC−/− lymphoblast cell line HSC536N. HSC536N and HSC536N/FANCC (complemented with normal FANCC cDNA) were cultured in medium alone (control) or medium treated with BIRB 796 (500nM) or dasatinib (500nM) for 6 hours. After 6 hours, supernatant media were collected and TNFα measured by ELISA. (B) BIRB 796 and dasatinib inhibit LPS-induced TNFα production in Fancc-deficient murine macrophages. Primary murine BM-derived macrophages obtained from Fancc−/− and wild-type mice were maintained in control medium (plus DMSO) or treated for 6 hours with BIRB 796 (500nM) or dasatinib (500nM), followed by stimulation with LPS (1 μg/mL) for 24 hours. TNFα in conditioned media was measured by ELISA. (C-D) BIRB 796 and dasatinib inhibit R848- and LPS-induced TNFα production in FANCA-deficient human macrophages. Primary human CD14+ macrophages obtained from a normal age-matched volunteer and a FANCA-deficient patient were cultured for 6 hours in control medium (plus DMSO), BIRB 796 (500nM), or dasatinib (500nM), before stimulation for 24 hours with R848 (1μM; C) or LPS (0.1 ng/mL; D). TNFα in conditioned media was measured by ELISA.
High doses of BIRB 796 and dasatinib inhibit TNFα gene transcription
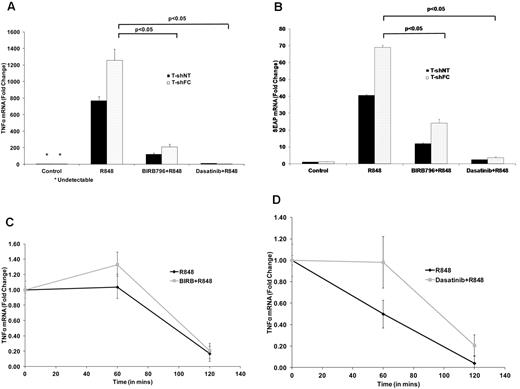
We quantified TNFα mRNA in R848-stimulated and unstimulated T-shFC and T-shNT cells with and without exposure to each drug. High doses of BIRB 796 and dasatinib (500nM) suppressed TNFα mRNA, although suppression by dasatinib was more complete (Figure 3A). Both drugs likewise inhibited SEAP mRNA (Figure 3B). Because neither BIRB 796 nor dasatinib reduced the TNFα mRNA half-life (Figure 3C-D), inhibition of both SEAP mRNA and TNFα mRNA reflects transcriptional suppression by these 2 agents at 500nM doses.
BIRB 796 and dasatinib suppress the production of TNFαand SEAP transcripts. T-shNT and T-shFC were maintained in medium alone (control), or medium treated with R848 (30μM) for 24 hours after 6 hours of pretreatment with either BIRB 796 (500nM) or dasatinib (500nM). Total RNA was prepared and TNFα (A) and SEAP (B) mRNAs quantified using real-time qRT-PCR. Data are expressed as the mean ± SD -fold change relative to transcripts in unexposed (control) cells and based on 3 technical replicates normalized to 18S rRNA. Dasatinib suppressed TNFα mRNA levels to a greater degree than did BIRB 796. (C-D) Neither BIRB 796 nor dasatinib enhanced TNFα mRNA degradation. T-shFC cells were either cultured in medium alone or treated with BIRB 796 (500nM; C) or dasatinib (500nM; D) for 6 hours, after which time all cells were stimulated with R848 (30μM) for 24 hours. After adding actinomycin D (5 μg/mL), total RNA was collected at 0, 60, and 120 minutes and TNFα mRNA was measured by real-time qRT-PCR. For each drug, 1 representative of 2 experiments with identical results is shown.
BIRB 796 and dasatinib suppress the production of TNFαand SEAP transcripts. T-shNT and T-shFC were maintained in medium alone (control), or medium treated with R848 (30μM) for 24 hours after 6 hours of pretreatment with either BIRB 796 (500nM) or dasatinib (500nM). Total RNA was prepared and TNFα (A) and SEAP (B) mRNAs quantified using real-time qRT-PCR. Data are expressed as the mean ± SD -fold change relative to transcripts in unexposed (control) cells and based on 3 technical replicates normalized to 18S rRNA. Dasatinib suppressed TNFα mRNA levels to a greater degree than did BIRB 796. (C-D) Neither BIRB 796 nor dasatinib enhanced TNFα mRNA degradation. T-shFC cells were either cultured in medium alone or treated with BIRB 796 (500nM; C) or dasatinib (500nM; D) for 6 hours, after which time all cells were stimulated with R848 (30μM) for 24 hours. After adding actinomycin D (5 μg/mL), total RNA was collected at 0, 60, and 120 minutes and TNFα mRNA was measured by real-time qRT-PCR. For each drug, 1 representative of 2 experiments with identical results is shown.
BIRB 796 and dasatinib differentially influence c-Jun activation
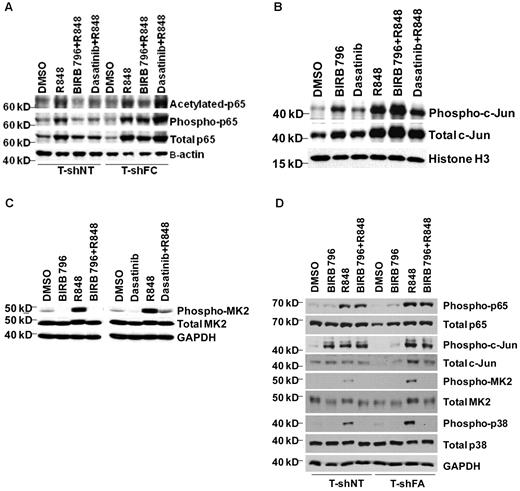
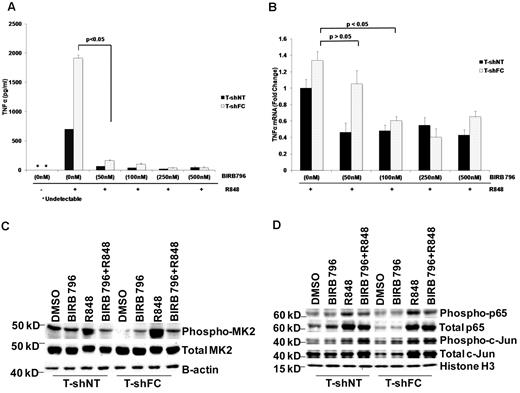
The reporter we used in our screens was responsive to a promoter containing both NFκB and AP-1 sites. To determine whether the AP-1 site was required for SEAP and TNFα gene expression in THP1-Blue cells, we transfected THP-1 cells with the pNiFty plasmid featuring only 5 NF-κB sites (and no AP-1 site)32 that governs the expression of SEAP. pNiFty-transfected cells did not produce SEAP, but did produce TNFα in response to R848 (supplemental Figure 2A-B), indicating that additional elements in the native TNFα promoter are required and that NF-κB activation alone is insufficient to account for the R848-induced TNFα gene expression. Indeed, results of studies on the states of activation of NF-κB p65 and c-Jun supported the requirement for both factors in FANCC-deficient and wild-type cells. Transcription of TNFα relies both on phosphorylation (Ser536)24 and acetylation (Lys310)25 of p65. Both posttranslational modifications are overrepresented in R848-exposed T-shFC cells, but only BIRB 796 suppressed p65 acetylation and phosphorylation (Figure 4A) and only dasatinib suppressed c-Jun activation (Figure 4B). Moreover, the degree to which the activation of these proteins was suppressed by either agent was minimal and was not observed at low (50nM) doses of these agents (Figure 5D).
Dasatinib and BIRB 796 do not inhibit R848-induced NF-κB p65 or c-Jun levels in FANCC- or FANCA-deficient mononuclear phagocytes, but do inhibit MK2 phosphorylation. T-shNT cells and T-shFC cells were treated with BIRB 796 (500nM) or dasatinib (500nM) for 6 hours and then stimulated with R848 (30μM) for 24 hours. (A) Western blot analyses of nuclear extracts were performed using Abs for total p65, phospho-p65(Ser536), and acetylated p65(Lys310). Whereas both BIRB 796 and dasatinib suppressed p65 phosphorylation and acetylation in T-shNT cells, neither agent suppressed p65 phosphorylation in T-shFC cells, and only BIRB 796 inhibited p65 acetylation. One representative example of 3 identical experiments is shown. (B) Western blot analyses of nuclear extracts were performed as above, but Abs to total c-Jun and phosphorylated (Ser73) c-Jun were used. BIRB 796 did not suppress c-Jun phosphorylation and the suppressive effect of dasatinib was minimal (lane 6 vs lane 4). One representative example of 3 identical experiments is shown. (C) Immunoblot analysis of whole-cell lysates of shFC cells exposed to R848 with and without either dasatinib or BIRB 796 demonstrated marked suppression of MK2 phosphorylation by both small molecules (lanes 4 and 8 vs lanes 3 and 7). One representative example of 3 identical experiments is shown. (D) T-shFA and T-shNT cells were exposed to R848 alone for 24 hours or to BIRB 796 for 6 hours, followed by R848 for 24 hours. Whole-cell lysates were used in immunoblot assays for total and phosphorylated p65, c-jun, p38, and MK2. BIRB 796 had no influence on p65 or c-jun phosphorylation, but profoundly suppressed p38 and MK2 phosphorylation. One representative experiment of 2 is shown.
Dasatinib and BIRB 796 do not inhibit R848-induced NF-κB p65 or c-Jun levels in FANCC- or FANCA-deficient mononuclear phagocytes, but do inhibit MK2 phosphorylation. T-shNT cells and T-shFC cells were treated with BIRB 796 (500nM) or dasatinib (500nM) for 6 hours and then stimulated with R848 (30μM) for 24 hours. (A) Western blot analyses of nuclear extracts were performed using Abs for total p65, phospho-p65(Ser536), and acetylated p65(Lys310). Whereas both BIRB 796 and dasatinib suppressed p65 phosphorylation and acetylation in T-shNT cells, neither agent suppressed p65 phosphorylation in T-shFC cells, and only BIRB 796 inhibited p65 acetylation. One representative example of 3 identical experiments is shown. (B) Western blot analyses of nuclear extracts were performed as above, but Abs to total c-Jun and phosphorylated (Ser73) c-Jun were used. BIRB 796 did not suppress c-Jun phosphorylation and the suppressive effect of dasatinib was minimal (lane 6 vs lane 4). One representative example of 3 identical experiments is shown. (C) Immunoblot analysis of whole-cell lysates of shFC cells exposed to R848 with and without either dasatinib or BIRB 796 demonstrated marked suppression of MK2 phosphorylation by both small molecules (lanes 4 and 8 vs lanes 3 and 7). One representative example of 3 identical experiments is shown. (D) T-shFA and T-shNT cells were exposed to R848 alone for 24 hours or to BIRB 796 for 6 hours, followed by R848 for 24 hours. Whole-cell lysates were used in immunoblot assays for total and phosphorylated p65, c-jun, p38, and MK2. BIRB 796 had no influence on p65 or c-jun phosphorylation, but profoundly suppressed p38 and MK2 phosphorylation. One representative experiment of 2 is shown.
BIRB 796 and dasatinib influence TNFα gene expression posttranscriptionally. Means ± SD are shown. All P values were calculated using the Student t test. (A) BIRB 796 effectively inhibited secreted TNFα in monocytes. T-shNT and T-shFC cells were treated with BIRB 796 (0-500nM) for 6 hours, followed by treatment with R848 (30μM) for 24 hours. Supernatant media were then collected and TNFα was quantified by ELISA. Low doses of BIRB 796 (50nM) suppressed secreted TNFα protein by 20-fold. (B) T-shNT and T-shFC cells were treated with BIRB 796 (0-500nM) for 6 hours, followed by treatment with R848 (30μM) for 24 hours. mRNA was then prepared and TNFα transcripts measured by real-time qRT-PCR. Data are expressed as the fold change normalized to 18S rRNA and are relative to mRNA levels in unexposed (control) T-shNT cells. TNFα mRNA is reduced modestly in the presence of BIRB 796 and minimally at the 50nM dose that was fully suppressive of TNFα protein production (A). One of 2 identical experiments is shown. (C) Low doses of BIRB 796 (50nM) suppress R848-induced MK2 phosphorylation in whole-cell lysates from T-shFC and T-shNT cells. One of 2 identical experiments is shown. (D) Immunoblot analysis for p65 and c-Jun phosphorylation in nuclear extracts of T-shFC and T-shNT cells revealed that low doses of BIRB 796 had no effect on p65 or c-Jun phosphorylation in R848-induced T-shFC and T-shNT cells. One representative immunoblot of 3 identical experiments is shown.
BIRB 796 and dasatinib influence TNFα gene expression posttranscriptionally. Means ± SD are shown. All P values were calculated using the Student t test. (A) BIRB 796 effectively inhibited secreted TNFα in monocytes. T-shNT and T-shFC cells were treated with BIRB 796 (0-500nM) for 6 hours, followed by treatment with R848 (30μM) for 24 hours. Supernatant media were then collected and TNFα was quantified by ELISA. Low doses of BIRB 796 (50nM) suppressed secreted TNFα protein by 20-fold. (B) T-shNT and T-shFC cells were treated with BIRB 796 (0-500nM) for 6 hours, followed by treatment with R848 (30μM) for 24 hours. mRNA was then prepared and TNFα transcripts measured by real-time qRT-PCR. Data are expressed as the fold change normalized to 18S rRNA and are relative to mRNA levels in unexposed (control) T-shNT cells. TNFα mRNA is reduced modestly in the presence of BIRB 796 and minimally at the 50nM dose that was fully suppressive of TNFα protein production (A). One of 2 identical experiments is shown. (C) Low doses of BIRB 796 (50nM) suppress R848-induced MK2 phosphorylation in whole-cell lysates from T-shFC and T-shNT cells. One of 2 identical experiments is shown. (D) Immunoblot analysis for p65 and c-Jun phosphorylation in nuclear extracts of T-shFC and T-shNT cells revealed that low doses of BIRB 796 had no effect on p65 or c-Jun phosphorylation in R848-induced T-shFC and T-shNT cells. One representative immunoblot of 3 identical experiments is shown.
BIRB 796 and dasatinib suppress TNFα expression posttranscriptionally
Because both agents were equally potent in suppressing TNFα production, we reasoned that they might function more effectively to suppress posttranscriptional steps in TNFα gene expression. Therefore, in light of the known suppressive effects of BIRB 796 on p38 MAPK function, we tested the capacity of both agents to inhibit MK2, a major effector of TNFα mRNA translation and canonical p38 MAPK substrate.33,34 Specifically, when phosphorylated on Thr334 in some cell types, MK2 enhances TNFα production by inhibiting TNFα mRNA degradation35 and enhancing TNFα mRNA translation.34,36 Indeed, exposure of FANCC-deficient THP-1 cells to R848 resulted in robust phosphorylation of MK2, and both BIRB 796 and dasatinib inhibited MK2 phosphorylation profoundly (Figure 4C). We also quantified activation of p65, c-Jun, p38, and MK2 in FANCA-deficient THP-1 cells and determined that: (1) only MK2 and p38 were overactivated in FANCA-deficient cells; and (2) BIRB 796 profoundly suppressed the phosphorylation of both (Figure 4D).
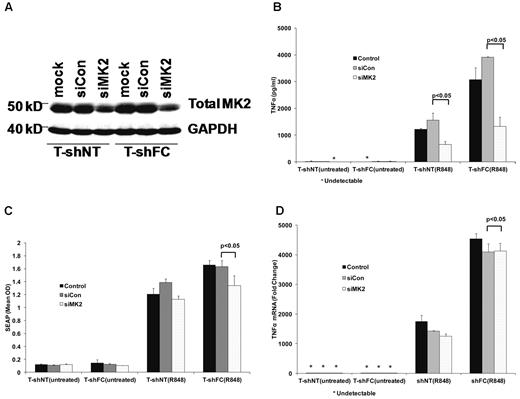
To confirm that BIRB 796 functions most potently to suppress TNFα gene expression largely at a posttranscriptional control point, we took advantage of the fact that this agent inhibits TNFα release completely at doses as low as 50nM (Figure 5A). We first determined that TNFα mRNA was minimally suppressed in R848-stimulated shFC cells by this dose of BIRB 796 (Figure 5B) and then that this dose effectively suppressed MK2 phosphorylation without inhibiting either p65 or c-Jun activation (Figure 5C-D). This indicates that the primary effect of this agent in FANCC- and FANCA-deficient cells is to suppress translation of TNFα mRNA. Likewise, 50nM dasatinib suppressed SEAP expression and TNFα mRNA only marginally (supplemental Figure 3A-B), but inhibited TNFα production by more than 3-fold (supplemental Figure 3C). To confirm that MK2 hyperactivation plays a role in the FA phenotype, we suppressed MK2 gene expression in T-shFC and T-shNT cells using MK2 RNAi. We confirmed that the siRNA we used suppressed MK2 gene expression by approximately 50% (Figure 6A) and that TNFα protein production was suppressed in both T-shNT and T-shFC cells exposed to R848 by approximately 50% (Figure 6B). MK2 RNAi did not have an equivalent effect on either SEAP expression (Figure 6C) or TNFα mRNA (Figure 6D). Therefore, TNFα overproduction in FANCC-deficient THP-1 cells is dependent on MK2 phosphorylation by p38 MAPK. Moreover, because neither BIRB 796 nor dasatinib suppresses TNFα mRNA half-life (Figure 3), and taking into account the role of MK2 in facilitating TNFα mRNA translation,34,36 the control point for this agent is likely suppression of TNFα mRNA translation.
MK2 regulates TNFα production posttranscriptionally. T-shFC and T-shNT cells were transfected with nontargeted siRNAs (siCon) or siRNA targeting MK2 (siMK2) by nucleofection. Cells were also subjected to nucleofection without siRNA present (mock). At the time of maximal MK2 knockdown (72 hours), the cells were stimulated with R848 (30μM) for 24 hours or were left untreated. (A) MK2 suppression (lanes 3 and 6) was confirmed by Western blot of whole-cell extracts 72 hours after nucleofection. (B) TNFα protein in supernatant media was quantified by ELISA 24 hours after R848 stimulation. siMK2 suppressed TNFα levels produced by both T-shFC and T-shNT cells and the degree of suppression matched the degree of MK2 knockdown with RNAi (6A). (C) SEAP expression, as quantified by QUANTI-Blue, was minimally suppressed by siMK2. (D) TNFα mRNA measured 24 hours after R848 stimulation by real-time qRT-PCR revealed no suppression by treatment of cells with siMK2. Therefore, MK2 suppression inhibited TNFα production and/or release, but not SEAP or TNFα gene transcription in T-shFC cells.
MK2 regulates TNFα production posttranscriptionally. T-shFC and T-shNT cells were transfected with nontargeted siRNAs (siCon) or siRNA targeting MK2 (siMK2) by nucleofection. Cells were also subjected to nucleofection without siRNA present (mock). At the time of maximal MK2 knockdown (72 hours), the cells were stimulated with R848 (30μM) for 24 hours or were left untreated. (A) MK2 suppression (lanes 3 and 6) was confirmed by Western blot of whole-cell extracts 72 hours after nucleofection. (B) TNFα protein in supernatant media was quantified by ELISA 24 hours after R848 stimulation. siMK2 suppressed TNFα levels produced by both T-shFC and T-shNT cells and the degree of suppression matched the degree of MK2 knockdown with RNAi (6A). (C) SEAP expression, as quantified by QUANTI-Blue, was minimally suppressed by siMK2. (D) TNFα mRNA measured 24 hours after R848 stimulation by real-time qRT-PCR revealed no suppression by treatment of cells with siMK2. Therefore, MK2 suppression inhibited TNFα production and/or release, but not SEAP or TNFα gene transcription in T-shFC cells.
Dasatinib's effect reflects its Src-kinase inhibitory activity
Dasatinib is a well-characterized Src kinase inhibitor but also targets other non-Src-family kinases involved in innate immune signaling.37 Therefore, to determine whether the Src kinase inhibitory activity suppressed p38 activation, we used pyrazolopyrimidine (PP2), a highly specific Src kinase inhibitor known to inhibit TNFα gene expression in endotoxin-stimulated human macrophages.38,39 PP2 not only inhibited TNFα in R848-induced macrophages in our studies (supplemental Figure 4A), but also suppressed the activation of both p38 MAPK and its substrate, MK2 (supplemental Figure 4B). Therefore, it is likely that the effectiveness of dasatinib in posttranscriptionally suppressing p38-dependent TNFα gene expression depends on its activity as an inhibitor of Src family kinases.
MMPs are not involved in regulating TNFα gene expression in THP-1 cells
Others have reported alternative p38 MAPK-dependent control points for TNFα gene expression in FANCC-deficient lymphoblasts (a few of which produce TNFα constitutively). p38-activated MMP7 was found to enhance the secretion of TNFα from these cell lines.40 Although we did detect a significant amount of MMP7 mRNA and secreted matrix metalloproteinase 7 (MMP7) in FANCC-deficient lymphoblasts (supplemental Figure 5A-B), we detected no secreted MMP7 protein or mRNA in THP-1 cells (not shown). We were also surprised to find that an MMP7 inhibitor had no impact on TNFα production by FANCC-deficient lymphoblasts (supplemental Figure 5C). We therefore tested broader-spectrum MMP inhibitors, including GM-6001 (an inhibitor of MMPs 1, 2, 3, 8, and 9) and MMP inhibitor V (which inhibits MMPs 2, 3, 8, 9, 12, and 13) and also found no inhibition of TNFα secretion at multiple doses in either FANCC-deficient lymphoblasts (not shown) or R848-stimulated, FANCC-deficient THP-1 cells (supplemental Figure 5D-E). This result indicates that MMPs do not play a role in controlling TNFα secretion in FANCC-deficient monocytes.
Discussion
Seeking small molecules that might suppress aberrant activation of the TLR pathway in FA cells, we used FANCC- and FANCA-deficient cell lines (T-shFC and T-shFA, respectively) and a control cell line (T-shNT), each of which has a stably integrated TLR pathway–responsive reporter gene under the control of NF-κB and AP-1 response elements.14 Using these cell lines to screen 75 small molecules (Table 1), we identified 2, BIRB 796 and dasatinib, that potently suppressed expression of the reporter gene (SEAP). We then confirmed that these agents also suppressed the TLR-dependent expression of TNFα in the T-shFC and T-shFA cells (Figure 1C-D) in a Fanconi group C patient–derived cell line (Figure 2A), in primary murine Fancc-deficient mononuclear phagocytes (Figure 2B), and in primary human FANCA-deficient mononuclear phagocytes (Figure 2C-D).
Whereas both agents are known to suppress TLR-induced expression of TNFα,41,42 they are in different functional classes. Therefore, we sought pathways influenced by both of them using TNFα gene expression. Interestingly, although we identified these agents using a screening tool that relied on NF-κB– and c-Jun–dependent transcription of a reporter gene, our mechanistic studies indicate unambiguously that both agents have a greater effect on TNFα gene expression at a posttranscriptional control point, likely by suppressing TNFα mRNA translation. To confirm that BIRB 796 functions most potently to suppress TNFα gene expression largely at a posttranscriptional control point, we took advantage of the fact that this agent inhibits TNFα release completely at doses as low as 50nM (Figure 5A). We first determined that TNFα mRNA was minimally suppressed in R848-stimulated shFC cells by this dose of BIRB 796 (Figure 5B), and then that this dose effectively suppressed MK2 phosphorylation without inhibiting either p65 or c-Jun activation (Figure 5C-D). This result indicates that the primary effect of this agent in FANCC- and FANCA-deficient cells is to suppress translation of TNFα mRNA or secretion of the protein. Likewise, 50nM dasatinib suppressed SEAP expression and TNFα mRNA only marginally (supplemental Figure 3A-B), but inhibited TNFα production by more than 3-fold (supplemental Figure 3C). To confirm that MK2 hyperactivation plays a role in the FA phenotype, we suppressed MK2 gene expression in T-shFC and T-shNT cells using MK2 RNAi. We confirmed that the siRNA we used suppressed MK2 gene expression by approximately 50% (Figure 6A), and that TNFα protein production was suppressed in both T-shNT and T-shFC cells exposed to R848 by approximately 50% (Figure 6B). MK2 RNAi did not have an equivalent effect on either SEAP expression (Figure 6C) or TNFα mRNA (Figure 6D). Therefore, TNFα overproduction in FANCC-deficient THP-1 cells is dependent on MK2 phosphorylation by p38 MAPK. Moreover, because neither BIRB 796 nor dasatinib suppresses TNFα mRNA half-life (Figure 3), and taking into account the role of MK2 in facilitating TNFα mRNA translation,34,36 the control point for this agent is likely suppression of TNFα mRNA translation.
The effect of BIRB 796, an inhibitor of all 4 isoforms of p38, is not unexpected,43 but the influence of dasatinib on p38 activation was less anticipated, in large part because wholesale deficiency of the 3 most highly expressed Src kinases in murine macrophages (Hck, Lyn, and Fgr) impairs neither p38 and NF-KB activation nor TNFα production.44 Nonetheless, it has been reported that this agent does suppress both LPS-induced TNFα gene expression in mice,41,45 and Tlr4- and Tlr9-dependent TNFα expression in rodent macrophages.45 Our finding that a highly specific Src kinase inhibitor suppressed R848-induced TNFα gene expression and suppressed p38 MAPK, MK2, and c-Jun activation but not p65 phosphorylation (supplemental Figure 4A-B), a profile identical to that which we describe for dasatinib, is compatible with the notion that dasatinib's effect in our studies reflects its Src kinase–inhibitory activity.
Both BIRB 796 and dasatinib inhibit p38 MAPK activity, inhibit phosphorylation of its canonical substrate MK2, and suppress TNFα protein production. The importance of MK2 suppression by these agents as a key mechanism is emphasized by studies showing that LPS does not induce expression of TNFα in Mk2-deficient mice,33 and that MK2 plays a role in modulating the production26,27 of 2 other key cytokines to which FA hematopoietic progenitors and stem cells are hypersensitive,10,11,28,46 namely IFNγ and MIP-1α. Whereas p38 MAPK-activated MK2 is known to enhance the half-life and translation of TNFα mRNA in some cell types,34 in our experiments BIRB 796 and dasatinib suppressed TNFα production without reducing TNFα mRNA half-life, (Figure 3C-D), suggesting that the effects of MK2 activation were exclusively translational. Others have reported that an important p38 MAPK-dependent control point for TNFα gene expression in FA cells is the expression of MMP7, which functions to enhance TNFα secretion.40 However, we detected no secreted MMP7 protein or mRNA in THP-1 cells (not shown) and found that neither MMP7 specific nor more general MMP inhibitors influenced R848-induced TNFα gene expression in either FANCC-deficient or -proficient cells (supplemental Figure 4C-E).
The results of the present study show that both FANCA- and FANCC-deficient mononuclear phagocytes exhibit the TLR-hypersensitive phenotype, that the phenotype is effected by hyperactivation of p38 MAPK and its substrate MK2, and that 2 effective inhibitors of the phenotype act by suppressing the activation states of these 2 kinases. At higher doses, these agents also function to slightly suppress activation of either NF-κB or c-Jun; otherwise we would not have been able to ascertain them in our screening assay. This has persuaded us that the screening method we have used may give rise to false negative results in future screening experiments and that quantification of cytokine production would likely be a more reliable approach.
The precise biochemical mechanisms by which FANCA and FANCC influence TLR pathways are not yet clearly defined. Nor have we yet determined that other FA genes influence precisely the same pathways. However, the discovery that inhibition of p38 MAPK is a key mechanism underlying the effects of these 2 agents helps to better focus future mechanistic studies of FANCA and FANCC on pathways that modulate p38 and MK2 activation. For example, of the 2 well-known p38 activators, MEKK1 and ASK1,47,48 ASK1 is of substantial interest. Because its activity is inhibited by proteins49–51 known to interact with6,8 or to be stabilized by52,53 FA proteins, it has been implicated in aberrant TNFα responses in Fancc-deficient murine embryonic fibroblasts.20,54
FANCA and FANCC mutations account for at least 65% of patients with FA worldwide. Therefore, regardless of the mechanisms by which these 2 FA proteins modulate TLR-dependent p38 MAPK activation and whether these mechanisms are shared with other FA complementation groups, the control of p38 MAPK and MK2 with small molecules has substantial appeal. In light of the capacity of p38 inhibition to suppress IFNγ and TNFα responsiveness,20,25,54 and because inhibition of MK2 suppresses inducible production of MIP-1α, TNFα, and IFNγ,26,27 p38 MAPK and MK2 play a central role in the FA hematologic phenotype, at least in 2 of the most common FA complementation groups, A and C. Preclinical studies of new-generation p38 MAPK inhibitors are clearly warranted and should be designed in a way that tests their capacity in murine models of FA to safely correct the aberrant hematopoietic phenotype (both cytokine overproduction and hypersensitivity) without enhancing the evolution of neoplastic clones.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute (HL048546 to G.B.), the National Cancer Institute (NCI; CA138237 to G.B.), and the Department of Veterans Affairs (to G.B.). J.W.T. is supported by the William Lawrence and Blanche Hughes Fund, The Leukemia & Lymphoma Society, the NCI (grant K99 CA151457-01), and the Oregon Clinical and Translational Research Institute (grant UL1 RR024140 from the National Center for Research Resources, a component of the National Institutes of Health, and the National Institutes of Health Roadmap for Medical Research).
National Institutes of Health
Authorship
Contribution: P.A. and G.C.B. designed the project, performed the experiments, analyzed all of the primary data, developed the new assays, and wrote the manuscript; J.E.Y. conducted RT-PCR and immunoblot experiments and proofread and revised the final manuscript and figures; M.R.G. performed ELISA and immunoblot experiments and helped with manuscript revisions; S.V helped to design and perform the inhibitor screening and conducted the T-shFA experiments; W.K. maintained all cell lines; K.R. and L.E.H. developed the murine BM macrophage and murine splenocyte experiments; J.W.T. helped to design, perform, and analyze the inhibitor screening; J.S., E.C., and C.D. designed and performed all experiments using human samples and helped with manuscript revisions; and J.S. performed helpful studies on Fancc-deficient mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Grover C. Bagby Jr, MD, Knight Cancer Institute at OHSU, CR145, HRC 14D40, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; e-mail: grover@ohsu.edu.