Abstract
In the current model of the pathogenesis of polycythemia vera (PV), the JAK2V617F mutation arises in hematopoietic stem cells (HSCs) that maintain the disease, while erythroid precursor populations expand, resulting in excessive red blood cell production. We examined the role of these specific cell populations using a conditional Jak2V617F knockin murine model. We demonstrate that the most immature long-term (LT) HSCs are solely responsible for initiating and maintaining the disease in vivo and that Jak2V617F mutant LT-HSCs dominate hematopoiesis over time. When we induced Jak2V617F expression in erythropoietin receptor expressing precursor cells, the mice developed elevated hematocrit, expanded erythroid precursors, and suppressed erythropoietin levels. However, the disease phenotype was significantly attenuated compared with mice expressing Jak2V617F in LT-HSCs. In addition to developing a PV phenotype, all mice transplanted with Jak2V617F LT-HSCs underwent myelofibrotic transformation over time. These findings recapitulate the development of post-PV myelofibrosis in human myeloproliferative neoplasms. In aggregate, these results demonstrate the distinct roles of LT-HSCs and erythroid precursors in the pathogenesis of PV.
Introduction
Polycythemia vera (PV) is a clonal disorder that arises in the hematopoietic stem cell (HSC) compartment1-3 and is characterized by an expansion of myeloid progenitors and an overproduction of mature red blood cells (RBCs).4 In this study, we use a Jak2V617F murine model to delineate the specific contributions of the long-term (LT) HSC and erythroid precursor cell populations to disease pathogenesis.
Erythropoietin (EPO) receptor signaling plays a central role in the pathogenesis of PV. In 1974, Prchal et al reported that bone marrow (BM) cells from patients with PV could form erythroid colonies in vitro without the addition of EPO to the culture media, a pathognomonic feature of the disease termed endogenous erythroid colony (EEC) formation.5 James et al subsequently demonstrated that knocking down JAK2 expression in cells from PV patients markedly inhibited EEC, a finding that ultimately lead them, and others, to identify JAK2V617F, a somatic mutation present in virtually all PV patients.6-9 Although JAK2V617F is an activating mutation, it requires binding to a dimerized cytokine receptor to bring about this activation.10 This suggests that cytokine receptors that use JAK2 and are expressed in early hematopoietic differentiation are important in the establishment of the JAK2V617F mutant clone early in the course of the disease. Epo null and Epo receptor (Epo-R) null mice develop burst-forming unit erythroid (BFU-E) and colony-forming unit erythroid (CFU-E) precursors indicating that EPO signaling is not required for early erythroid differentiation.11
Using the murine Jak2V617F model, we previously showed that distinct cellular populations have unique roles in PV pathogenesis: the HSC-enriched LSK (LineagelowSca1+cKithigh) compartment is responsible for disease initiation while the erythroid progenitor compartment expands and confers a polycythemia phenotype.12 This work raises 2 linked questions regarding the role of specific cellular populations in the pathogenesis of JAK2V617F-mutant PV. First, because the LSK compartment is a heterogeneous population, the precise disease-initiating population has not been defined or functionally characterized. Second, given the effect of JAK2V617F on EPO-R signaling, the specific role of cells expressing EPO-R has not been determined. We addressed these questions by conditional expression of the Jak2V617F allele either in all hematopoietic cells or in Epo-R–expressing cells.
Methods
Experimental mice
We have previously described the conditional Jak2V617F knockin model in which after Cre recombination Jak2V617F is expressed from the endogenous murine Jak2 promoter resulting in physiologic expression of the mutant allele in vivo.12 EPO receptor GFP Cre (ErGFPcre) mice express a GFPcre fusion protein under the control of the endogenous EPO receptor (EpoR) promoter.13 E2Acre transgenic mice target expression of Cre recombinase to the early mouse embryo.14 All experiments were conducted using an institutional animal care and use committee–approved animal protocol at Brigham and Women's Hospital.
Flow cytometry
Flow cytometric examination of erythropoiesis was performed using CD71 (C2) and Ter119 (TER-119) Abs (BD Pharmingen). For hematopoietic stem and progenitor cell (HSPC) analysis, cells were first stained with a lineage cocktail of biotin-labeled anti–mouse Abs (Ter119, CD3, CD4, CD8, B220, Mac1, Gr1; BD Pharmingen) followed by a secondary stain with streptavidin-ApcCy7, cKit (2B8), Sca-1 (D7), and CD34 (RAM34) plus FcγRII/III(93) or CD150 (TC15-12F12.2) plus CD48 (HM48-1). For chimerism assessment 45.1 (A20) and 45.2(104) were used (Biolegend and BD Pharmingen, respectively). Flow cytometry was performed on a FACSCanto cytometer or LSR II (Becton Dickinson). For cell-sorting experiments, lineage-positive cells were depleted with Dynabeads (Invitrogen), stained with Streptavidin-ApcCy7, cKit (2B8), Sca-1 (D7), and sorted on a FACSAria (BD Biosciences).
BM transplantation
BM cells were resuspended in HBSS (Invitrogen) and injected into the lateral tail veins of lethally irradiated (2 × 5.5 Gy [550 rad]) wild-type (WT) recipient mice.
Purified BM subpopulation transplantations were performed using 3.5 × 103 Jak2+/VF LT-HSCs (CD150+CD48− LSKs) plus 2 × 105 supportive WT BM cells or a total of 2.8 × 104 short-term (ST) HSCs and multipotent progenitors (MPPs; CD150−CD48− LSKs and CD150−CD48+ LSKs, respectively) plus 2 × 105 supportive WT BM cells injected into lethally irradiated C57Bl/6 recipients (n = 5 in each recipient group). BM derived from Jak2+/VF mice expressed the CD45.2 Ag; recipient mice expressed both CD45.1 and CD45.2 and supportive WT BM cells expressed CD45.1 alone.
Competitive transplantation experiments were performed using a total of 1.35 × 104 LSK cells in the following Jak2+/VF to Jak2+/+ ratios: 75:25, 50:50, or 25:75, respectively, in combination with 2.5 × 105 supportive WT BM cells (that contained 450 WT LSK cells) injected into lethally irradiated C57Bl/6 recipients (n = 6 in each recipient group). BM derived from Jak2+/VF mice expressed the CD45.2 Ag; recipient mice expressed CD45.1 alone and supportive WT BM cells expressed both CD45.1 and CD45.2.
For unfractionated BM transplantations, a total of 2.5 × 106 whole BM cells from Jak2+/VFErGFPcre+ or Jak2+/VFE2Acre+ mice were transplanted into lethally irradiated C57Bl/6 recipients.
Histopathology
Tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, and stained with H&E or with reticulin, to assess for fibrosis. Images of histologic slides were obtained on a Nikon Eclipse E400 microscope equipped with a SPOT RT color digital camera model 2.1.1 (Diagnostic Instruments).
Jak2 allele-specific PCR
Primers for the Jak2 allele-specific PCR were designed using WASP software.15 RNA was isolated from purified LSK, megakaryocyte erythroid progenitor (MEP), and granulocyte macrophage progenitor (GMP) cells (RNeasy Mini Kit; QIAGEN). Complementary DNA was synthesized (TaqMan reagents), and PCR was performed using the following primer sequences: Jak2 WT forward primer 5′: TTTGAATTATGGTGTCTGCG; Jak2V617F mutant forward primer 5′: TTTGAATTATGGTGTCTGCT; common reverse primer 5′: CAGGTATGTATCCAGTGATCC.
Jak2 allele-specific quantitative RT-PCR
RNA was isolated from purified MEP and CD71+Ter119+ cells (RNeasy Mini Kit; QIAGEN). Complementary DNA was synthesized (TaqMan reagents), and relative transcript quantification was performed with SYBR Green reagents with the use of Applied Biosystems 7300 Real-Time PCR system and SDS software (Applied Biosystems). The primer sequences are listed in “Jak2 allele-specific PCR.”
EPO levels
Serum EPO levels were measured by ELISA (R&D Systems).
Statistical analysis
Prism Version 5.0a (Graphpad Software Inc) was used to analyze results and create graphs. All comparisons represent 2-tailed unpaired t test analysis (with Welch correction where appropriate) unless otherwise specified. Flow cytometry data were analyzed with FlowJo Version 8.8.7 software (TreeStar).
Results
Jak2V617F disease-initiating cells reside exclusively in the LT-HSC compartment
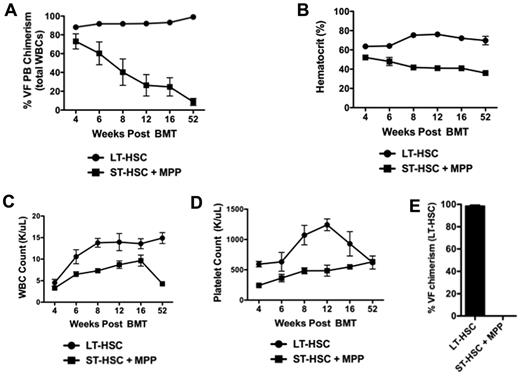
We have previously reported that the myeloproliferative neoplasm (MPN)–initiating population in the Jak2V617F model is contained within the LSK compartment that is enriched for HSCs.12 The LSK compartment is heterogeneous, comprising LT-HSCs, ST-HSCs, and MPPs. To define which subpopulation of the LSK compartment contains the disease-initiating cell, we purified Jak2V617F-expressing LT-HSCs (signaling lymphocyte activation molecule [SLAM] LSK: CD150+CD48− LSKs) or a combination of ST-HSCs (CD150−CD48− LSKs) and MPPs (CD150−CD48+ LSKs) from Jak2+/VFE2ACre+ mice and transplanted them into lethally irradiated congenic secondary recipients (LSKs from Jak2+/VF E2ACre+ mice express Jak2V617F12 ). To confirm that Jak2V617F is expressed in the ST-HSC + MPP cell population, we sequenced cDNA purified from these cells from Jak2+/VFE2ACre+ mice. The Jak2V617F allele was expressed in the ST-HSC + MPP cell population at heterozygous levels (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Donor chimerism and peripheral blood counts were followed for a period of 1 year in transplant recipients (Figure 1A-D). Only mice transplanted with LT-HSCs maintained LT donor chimerism (Figure 1A) and sustained elevation in hematocrit (Figure 1B). Consistent with this, at the time of sacrifice all mice in the LT-HSC recipient group had > 95% Jak2+/VF donor chimerism in the LT-HSC compartment while all mice in the ST-HSC + MPP group had 0% Jak2+/VF LT-HSC donor chimerism (Figure 1E). In aggregate, these results indicate that the LT-HSC compartment is solely responsible for initiating and maintaining the disease in vivo.
Jak2V617F disease-initiating cells are contained exclusively in the LT-HSC compartment. (A) The percentage of donor chimerism assessed in peripheral blood of lethally irradiated WT recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (B) Hematocrit of recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (C) WBC count of recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (D) Platelet count of recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (E) The percentage of Jak2+/VF(VF) donor chimerism assessed in CD150+CD48− LSK cells (LT-HSCs) from mice transplanted with Jak2+/VF LT-HSC cells or Jak2+/VF ST-HSC + MPP cells (mean ± SEM, n = 3-4 in each group).
Jak2V617F disease-initiating cells are contained exclusively in the LT-HSC compartment. (A) The percentage of donor chimerism assessed in peripheral blood of lethally irradiated WT recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (B) Hematocrit of recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (C) WBC count of recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (D) Platelet count of recipients of Jak2+/VFE2Acre+ LT-HSCs or a combination of Jak2+/VFE2Acre+ ST-HSCs and MPPs (mean ± SEM, n = 3-5 in each group). (E) The percentage of Jak2+/VF(VF) donor chimerism assessed in CD150+CD48− LSK cells (LT-HSCs) from mice transplanted with Jak2+/VF LT-HSC cells or Jak2+/VF ST-HSC + MPP cells (mean ± SEM, n = 3-4 in each group).
Jak2V617F disease-initiating cells gradually achieve clonal dominance over time
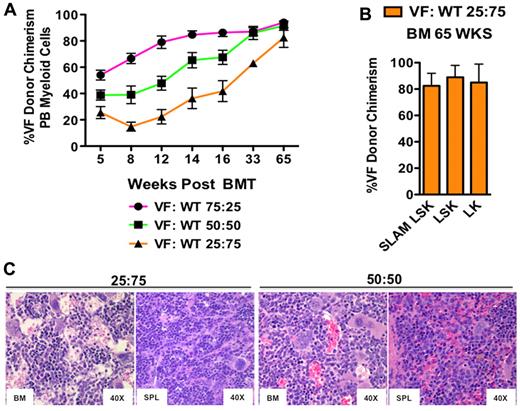
With standard LT repopulating assays (16-week), we previously observed only a minor competitive advantage for Jak2+/VF HSCs.12 We therefore sought to examine the selective advantage of Jak2V617F over a longer period of time that more closely corresponds to the gradual onset of human PV. We transplanted LSK cells into lethally irradiated congenic recipients using the following Jak2+/VF to Jak2+/+ ratios: 25:75, 50:50, and 75:25, respectively, in combination with 250 000 WT supportive BM cells. We have previously reported results at the 16-week time point12 and now present data for the remaining animals that were followed for an extended period of time (> 1 year). All recipient groups sustained increased hematocrit > 55% and white blood cell (WBC) count (> 10 000/μL) for > 6 months (supplemental Figure 1C). Interestingly, the degree of elevation in platelet count corresponded to the Jak2+/VF to Jak2+/+ input ratio, with mice that were transplanted with LSK cells in VF:WT ratio of 75:25 developing the highest platelet counts and those that received LSK cells in a VF:WT ratio of 25:75 having the lowest platelet counts (supplemental Figure 1C). Jak2+/VF chimerism in peripheral blood myeloid cells gradually increased over time, reaching 80% or greater in all groups 65 weeks after transplantation (Figure 2A). At 68 weeks after transplantation, all remaining animals in the 75:25 ratio group died and recipient mice in the 50:50 and 25:75 ratio groups were killed and analyzed. Jak2+/VF chimerism in the CD150+CD48− LSK compartment of recipients of LSKs in a VF:WT ratio of 25:75 was > 80% (Figure 2B). Jak2V617F expression was confirmed in Jak2+/VF LT-HSC cells purified from recipient mice at the time of sacrifice (68 weeks; supplemental Figure 1B). Recipient mice in the 50:50 and 25:75 groups all showed features of MPN similar to those observed in primary mice including erythroid and megakaryocytic hyperplasia (Figure 2C). Reticulin fibrosis was not seen. These results demonstrate that Jak2V617F mutant LT-HSCs gradually out-compete Jak2 WT LT-HSCs over time.
Jak2V617F MPN-initiating cells dominate hematopoiesis over time. (A) The percentage of Jak2+/VF (VF) donor myeloid chimerism assessed in peripheral blood (PB) from lethally irradiated secondary recipients of Jak2+/VFE2Acre+ and Jak2+/+E2Acre+ LSK cells in 75:25, 50:50, and 25:75 ratios, respectively, measured 5-65 weeks posttransplantation (mean ± SEM; n = 2-6 in each group). (B) Jak2+/VF (VF) to Jak2+/+ (WT) chimerism ratios assessed in BM SLAM LSK, LSK, and LK compartments from lethally irradiated WT recipients of Jak2+/VFE2Acre+ and Jak2+/+E2Acre+ LSK cells in a 25:75 ratio, measured 65 weeks after transplantation (mean ± SEM; n = 2 in each group). (C) Histopathologic (HE) sections of BM and spleen (SPL) from representative mice transplanted with Jak2+/VFE2Acre+ and Jak2+/+E2Acre+ LSK cells in 50:50 or 25:75 ratios, demonstrating erythroid and megakaryocytic hyperplasia in both groups.
Jak2V617F MPN-initiating cells dominate hematopoiesis over time. (A) The percentage of Jak2+/VF (VF) donor myeloid chimerism assessed in peripheral blood (PB) from lethally irradiated secondary recipients of Jak2+/VFE2Acre+ and Jak2+/+E2Acre+ LSK cells in 75:25, 50:50, and 25:75 ratios, respectively, measured 5-65 weeks posttransplantation (mean ± SEM; n = 2-6 in each group). (B) Jak2+/VF (VF) to Jak2+/+ (WT) chimerism ratios assessed in BM SLAM LSK, LSK, and LK compartments from lethally irradiated WT recipients of Jak2+/VFE2Acre+ and Jak2+/+E2Acre+ LSK cells in a 25:75 ratio, measured 65 weeks after transplantation (mean ± SEM; n = 2 in each group). (C) Histopathologic (HE) sections of BM and spleen (SPL) from representative mice transplanted with Jak2+/VFE2Acre+ and Jak2+/+E2Acre+ LSK cells in 50:50 or 25:75 ratios, demonstrating erythroid and megakaryocytic hyperplasia in both groups.
Erythroid lineage–restricted Jak2V617F expression results in an attenuated disease phenotype
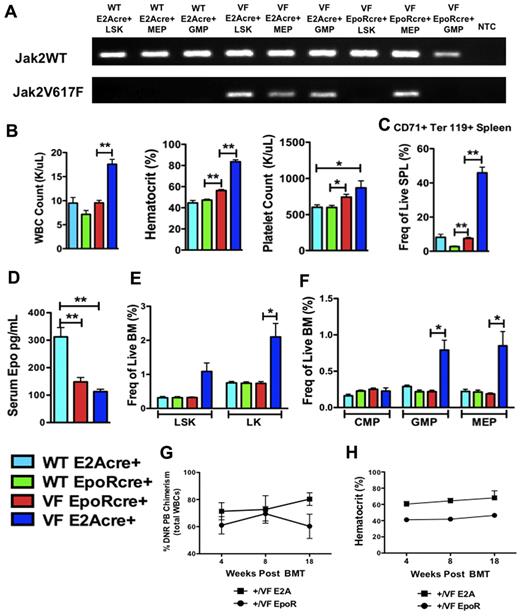
Having demonstrated that the MPN-initiating population is contained within the LT-HSC compartment and that Jak2V617F-expressing MPN stem cells have a competitive advantage, we next sought to delineate the effects of Jak2V617F expression in more differentiated cell populations. To evaluate the precise role of Jak2V617F in Epo-R–expressing cells, we expressed Jak2V617F from the endogenous Epo-R promoter using Epo-R GFP Cre (ErGFPcre) mice.13 Although ErGFPcre mice have only one functional Epo-R copy, Epo-R heterozygote mice have been shown to have no hematopoietic defects11 and using lineage tracking experiments ErGFPcre mice have recently been found to have no Epo-R expression in CD150+CD48− LSKs (LT-HCSs).16 Within the erythroid lineage, Epo-R expression is robust from the pre-CFU-E population onward. We found a similar pattern of expression for the EPO-R gene in human hematopoiesis17 (supplemental Figure 2). Using Jak2 allele-specific PCR, we found that Jak2V617F was not expressed in Jak2+/VFErGFPcre+ LSK or GMP cells but was expressed in MEP cells and committed erythroid precursors (Figure 3A; supplemental Figure 3A-B). In contrast, Jak2+/VFE2Acre+ mice expressed Jak2V617F in LSK, MEP, and GMP cells as previously described.12
Erythroid lineage–restricted Jak2V617F expression results in an attenuated MPN phenotype. (A) Jak2 allele-specific PCR performed on Jak2+/+ E2Acre+, Jak2+/VFE2Acre+, and Jak2+/VFEpoRCre+ LSK, MEP, or GMP cells purified from primary mice. Jak2WT and Jak2V617F refer to forward primers (a common reverse primer was used). NTC indicates no template control. Results demonstrate absent Jak2V617F expression in Jak2+/+ cells, Jak2V617F expression in all Jak2+/VFE2Acre+ cell populations and Jak2V617F expression in Jak2+/VFEpoRCre+ MEP cells only. (B) WBC count, hematocrit, and platelet counts of age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice aged 8-12 weeks (mean ± SEM; n = 4 in each group);*P < .05, **P < .0005. (C) Frequency of CD71+, Ter119+ erythroid precursors in spleen from age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 4 in each group); **P < .0005. (D) Serum EPO levels from age-matched Jak2+/+E2Acre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 8 in each group); **P < .001. (E) Frequency of LSK and LK cells in BM from age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 4 in each group); *P < .05. (F) Frequency of CMP, GMP, and MEP cells in BM from age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 4 in each group); *P < .05. (G) The percentage of donor chimerism assessed in peripheral blood of recipients of Jak2+/VFE2Acre+ or Jak2+/VFEpoRcre+ unfractionated BM (mean ± SEM, n = 4 in each group). (H) Hematocrit of recipients of Jak2+/VFE2Acre+ or Jak2+/VFEpoRcre+ unfractionated BM (mean ± SEM, n = 4 in each group).
Erythroid lineage–restricted Jak2V617F expression results in an attenuated MPN phenotype. (A) Jak2 allele-specific PCR performed on Jak2+/+ E2Acre+, Jak2+/VFE2Acre+, and Jak2+/VFEpoRCre+ LSK, MEP, or GMP cells purified from primary mice. Jak2WT and Jak2V617F refer to forward primers (a common reverse primer was used). NTC indicates no template control. Results demonstrate absent Jak2V617F expression in Jak2+/+ cells, Jak2V617F expression in all Jak2+/VFE2Acre+ cell populations and Jak2V617F expression in Jak2+/VFEpoRCre+ MEP cells only. (B) WBC count, hematocrit, and platelet counts of age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice aged 8-12 weeks (mean ± SEM; n = 4 in each group);*P < .05, **P < .0005. (C) Frequency of CD71+, Ter119+ erythroid precursors in spleen from age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 4 in each group); **P < .0005. (D) Serum EPO levels from age-matched Jak2+/+E2Acre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 8 in each group); **P < .001. (E) Frequency of LSK and LK cells in BM from age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 4 in each group); *P < .05. (F) Frequency of CMP, GMP, and MEP cells in BM from age-matched Jak2+/+E2Acre+, Jak2+/+EpoRcre+, Jak2+/VFEpoRcre+, and Jak2+/VFE2Acre+ mice (mean ± SEM; n = 4 in each group); *P < .05. (G) The percentage of donor chimerism assessed in peripheral blood of recipients of Jak2+/VFE2Acre+ or Jak2+/VFEpoRcre+ unfractionated BM (mean ± SEM, n = 4 in each group). (H) Hematocrit of recipients of Jak2+/VFE2Acre+ or Jak2+/VFEpoRcre+ unfractionated BM (mean ± SEM, n = 4 in each group).
Jak2+/VFErGFPcre+ mice developed a significant elevation in hematocrit and platelet count compared with Jak2+/+ErGFPcre+ mice (Figure 3B). Consistent with this, we found that CD71+Ter119+ committed erythroid precursors were significantly expanded in the spleens of Jak2+/VFErGFPcre+ mice compared with Jak2+/+ErGFPcre+ mice (Figure 3C) and that EPO levels were suppressed in Jak2+/VFErGFPcre+ animals (Figure 3D). In contrast, Jak2+/VFE2Acre+ mice demonstrated a marked elevation in white cells, hematocrit, and platelets and a massive expansion of CD71+Ter119+ cells in the spleen (Figure 3B-C) that resulted in profound splenomegaly (supplemental Figure 3C). In the BM, Jak2+/VFErGFPcre+ mice demonstrated mild erythroid and megakaryocytic dysplasia with clustering, nuclear atypia, hyperlobulation, and abnormal megakaryocyte localization. Myelofibrosis was not observed (supplemental Figure 3F). We found that myeloid progenitor cells (LK, LineagelowSca1−cKithigh) were not increased in the BM of Jak2+/VFErGFPcre+ mice (Figure 3E) and that none of the subpopulations of the myeloid progenitor compartment (including MEP cells) was expanded either (Figure 3F). LSK cells were not expanded in either the BM (Figure 3E) or spleen (supplemental Figure 3D) of Jak2+/VFErGFPcre+ mice. In contrast, Jak2+/VFE2Acre+ mice demonstrated significant expansion of myeloid progenitor (LK), GMP, and MEP cells in the BM, a trend toward significant LSK expansion in the BM (P < .056) and significant LSK expansion in the spleen (Figure 3E-F, supplemental Figure 3D). We have previously purified and transplanted MEP cells from Jak2+/VFE2Acre mice and demonstrated that they are incapable of propagating the MPN.12 Consistent with this, when we transplanted unfractionated BM from Jak2+/VFErGFPcre+ mice, recipients did not develop an MPN phenotype despite sustained donor chimerism (Figure 3G-H). In aggregate, these data indicate that when Jak2V617F expression is limited to Epo-R–expressing cells this results in attenuated disease and confirm our earlier findings that Jak2V617F does not transform erythroid progenitor cells.12
Mice transplanted with Jak2V617F mutant LT-HSCs develop MPN with myelofibrosis
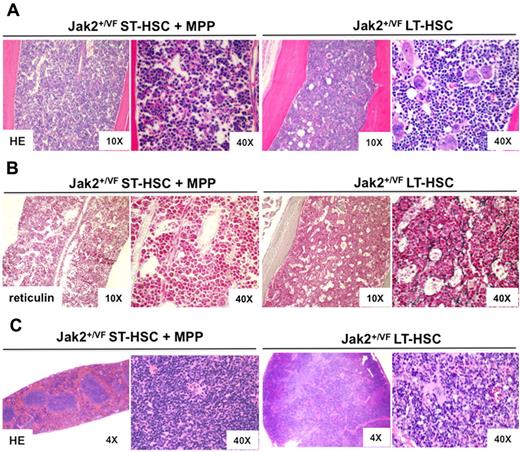
Myelofibrosis, a significant cause of morbidity and mortality in PV, develops as a late complication of the disease. We examined the BM pathology of mice 17 months after transplantation with LT-HSC or ST-HSC + MPP cell populations. Recipient mice that received Jak2+/VF LT-HSC demonstrated features of MPN similar to those observed in primary mice including megakaryocytic and erythroid hyperplasia in the BM (Figure 4A) and splenomegaly (supplemental Figure 3E) as result of extramedullary hematopoiesis (Figure 4C). Histopathology of the spleen demonstrated marked erythroid and mild megakaryocytic hyperplasia within the red pulp, with effacement of normal splenic architecture. Megakaryocytic abnormalities were particularly striking in both BM and spleen, with evidence of megakaryocyte clustering, atypical nuclear features such as hyperlobulation and prominent emperipolesis (Figure 4A,C). One mouse in the LT-HSC group developed evidence of accelerated disease as indicated by a hypercellular BM, left shifted myelopoiesis with immature forms, moderate myelofibrosis with neovascularization and marked hepatosplenomegaly with prominent myeloid infiltrates (supplemental Figure 4). Of note, all mice in the Jak2+/VF LT-HSC group developed reticulin-positive fibrosis, while none in the Jak2+/VF ST-HSC + MPP group did (Figure 4B). These results demonstrate that Jak2V617F mutant hematopoietic cells can induce changes in a WT BM niche that result in the development of reticulin fibrosis.
Mice transplanted with Jak2V617F mutant LT-HSCs develop MPN and myelofibrosis. (A) Histopathologic (HE) sections of BM from a representative mouse transplanted with Jak2+/VF ST-HSC + MPP cells (n = 4 in group) or Jak2+/VF LT-HSC cells (n = 3 in group), demonstrating diffuse erythroid hyperplasia and megakaryocyte hyperplasia in the Jak2+/VF LT-HSC recipient. The Jak2+/VF LT-HSC recipient also shows evidence of megakaryocytic clustering, enlarged forms with bizarre, hypernucleated nuclei, marked emperipolesis, and abnormal megakaryocyte localization in marrow sinuses or in association with trabecular bone. These abnormalities are not seen in the Jak2+/VF ST-HSC + MPP recipient. (B) Reticulin sections of BM from a representative mouse transplanted with Jak2+/VF ST-HSC + MPP cells (n = 4 in group) or Jak2+/VF LT-HSC cells (n = 3 in group), demonstrating a fine reticulin fiber network with crossing fibers deposition consistent with grade 1-2 reticulin fibrosis in the Jak2+/VF LT-HSC recipient. These abnormalities are not seen in the Jak2+/VF ST-HSC + MPP recipient. (C) Histopathologic (HE) sections of spleen from a representative mouse transplanted with Jak2+/VF ST-HSC + MPP cells (n = 4 in group) or Jak2+/VF LT-HSC cells (n = 3 in group), demonstrating erythroid hyperplasia and megakaryocyte hyperplasia with clustering and atypical nuclear features in the Jak2+/VF LT-HSC recipient. These abnormalities are not seen in the Jak2+/VF ST-HSC + MPP recipient.
Mice transplanted with Jak2V617F mutant LT-HSCs develop MPN and myelofibrosis. (A) Histopathologic (HE) sections of BM from a representative mouse transplanted with Jak2+/VF ST-HSC + MPP cells (n = 4 in group) or Jak2+/VF LT-HSC cells (n = 3 in group), demonstrating diffuse erythroid hyperplasia and megakaryocyte hyperplasia in the Jak2+/VF LT-HSC recipient. The Jak2+/VF LT-HSC recipient also shows evidence of megakaryocytic clustering, enlarged forms with bizarre, hypernucleated nuclei, marked emperipolesis, and abnormal megakaryocyte localization in marrow sinuses or in association with trabecular bone. These abnormalities are not seen in the Jak2+/VF ST-HSC + MPP recipient. (B) Reticulin sections of BM from a representative mouse transplanted with Jak2+/VF ST-HSC + MPP cells (n = 4 in group) or Jak2+/VF LT-HSC cells (n = 3 in group), demonstrating a fine reticulin fiber network with crossing fibers deposition consistent with grade 1-2 reticulin fibrosis in the Jak2+/VF LT-HSC recipient. These abnormalities are not seen in the Jak2+/VF ST-HSC + MPP recipient. (C) Histopathologic (HE) sections of spleen from a representative mouse transplanted with Jak2+/VF ST-HSC + MPP cells (n = 4 in group) or Jak2+/VF LT-HSC cells (n = 3 in group), demonstrating erythroid hyperplasia and megakaryocyte hyperplasia with clustering and atypical nuclear features in the Jak2+/VF LT-HSC recipient. These abnormalities are not seen in the Jak2+/VF ST-HSC + MPP recipient.
Discussion
In this study, we use a Jak2V617F conditional knockin model, the phenotype of which closely recapitulates the clinical features of human PV, to investigate the hierarchical organization of hematopoiesis in MPN. We refine the characterization of MPN disease-propagating cells and demonstrate that these cells reside exclusively in the LT-HSC compartment. We elucidate the contribution of Epo-R–expressing cells to PV pathogenesis and demonstrate that Jak2V617F expression in hematopoietic stem and progenitor cell populations is required for a full PV phenotype to develop. We show that sustained in vivo Jak2V617F expression in hematopoietic cells can induce reticulin fibrotic transformation within the BM niche.
The finding that Jak2V617F MPN disease-propagating stem cells are contained exclusively in the LT-HSC compartment is similar to that recently reported in a murine model of chronic myelogenous leukemia (CML),18 indicating that this appears to be a general feature of MPN-inducing oncogenic kinases. Unlike more potent oncogenes such as MLL-AF9,19 BCR-ABL has been shown to be incapable of fully transforming progenitor cell populations.20,21 This study and our earlier work indicate that this is also the case for JAK2V617F.12 In our initial study evaluating the impact of the Jak2V617F mutation on HSC self-renewal, we found that that Jak2+/VF LSK cells had a minor competitive advantage over Jak2+/+ LSK cells at 16 weeks.12 In this study in which we followed competitive repopulation in recipient mice for > 1 year, we find that Jak2V617F mutant LT-HSCs gradually out-compete WT LT-HSCs to dominate hematopoiesis. Similar findings were recently reported in abstract form in another Jak2V617F knock-in model22 and in a JAK2V617F transgenic model,23 although the competitive repopulating advantage observed in both of these studies was greater than what we have found. In another knock-in model, JAK2V617F was actually found to be detrimental in the HSC compartment, in that JAK2V617F mutant HSCs were out-competed by WT cells in transplant recipients as early as 5 weeks posttransplantation, such that the MPN was not transplanted.24 It remains unclear whether this finding is related to expressing human JAK2V617F from the endogenous murine Jak2 promoter or to another cause of increased replicative stress in the model or to immunologic rejection. In aggregate, the results from murine models to date appear to indicate that the Jak2V617F mutation confers a clonal advantage to HSCs.
Human PV is a disease characterized by a chronic stable clinical course.25 Clonal expansion occurs later in myeloid differentiation26 and consistent with this the JAK2V617F CD34+ allele burden in PV is lower than the JAK2V617F neutrophil allele burden.27 In essential thrombocythemia (ET) patients where the JAK2V617F mutant clone is heterozygous, the clone can remain stable without expansion over many years.28 Human in vitro studies evaluating the effects of the JAK2V617F mutation on the HSC compartment in PV have shown contrasting results, with one finding expansion of the HSC population1 and another finding no alteration in HSC compartment size.29 In vivo functional studies of JAK2V617F mutant HSCs have proved challenging as a result of poor engraftment of JAK2V617F mutant CD34+ cells from PV patients in immunocompromised mice.30,31 A particular concern in using xenotransplantation models for in vivo functional studies of JAK2V617F, an allele that activates cytokine receptor signaling, is that human cells are evaluated in a murine microenvironment and differences between murine and human cytokines may be important in this context. For these reasons, Jak2V617F knock-in models, such as the one we use in this study, are attractive for assessing the functional effects of the Jak2V617F mutation, within the HSC compartment, in isolation, in vivo. In interpreting the findings from these in vivo studies, however, it is important to bear in mind a few caveats of the competitive repopulation assay. These include the fact that BM transplantation experiments require that recipient mice be irradiated resulting in perturbation of the BM niche and also that hematopoietic reconstitution in competitive repopulating transplants is likely polyclonal. Additional studies addressing how JAK2V617F mutant LT-HSCs emerge and persist to establish clonal hematopoiesis will be critical to understanding the mechanisms of clonal dominance in MPN.
EPO hypersensitivity is regarded as the sine qua non of PV. To evaluate the precise contribution of Epo-R signaling to the pathogenesis of Jak2V617F-mediated MPN, we expressed Jak2V617F in vivo only in cells that express the Epo-R and found that the PV phenotype that arose was markedly attenuated compared with the MPN that occurs when Jak2V617F is expressed in HSCs and in all hematopoietic lineages. These findings indicate that cell populations that are “upstream” of the EPO-R in terms of hematopoietic differentiation mediate significant aspects of the PV phenotype. These cells include the LT-HSC population, which is critical for establishing and maintaining the JAK2V617F mutant clone in vivo, and other myeloid progenitor cell populations that are expanded in MPN. The effects of JAK2V617F expression early in hematopoietic differentiation may be mediated via other cytokine receptors that signal through JAK2. These effects could include altered HSC quiescence (via the thrombopoietin receptor, MPL) and/or enhanced myeloid progenitor cell proliferation (via G-CSFR and IL3R). Epigenetic effects of the JAK2V617F mutation may also alter the function of JAK2V617F mutant hematopoietic stem and progenitor cells.32 Therapeutic targeting of JAK2V617F in cells at an early stage of hematopoietic differentiation has the potential to not only ameliorate the clinical manifestations of PV but also to eradicate the disease-propagating HSC population for curative PV therapy.
In this study, we find that mice that were transplanted with Jak2+/VF LT-HSCs and followed for an extended period (> 1 year) develop myelofibrosis. The megakaryocytic lineage is thought to play a central role in the development of myelofibrosis33 and is it notable in our study that Jak2+/VF LT-HSC recipient mice with fibrotic transformation all demonstrated marked megakaryocytic abnormalities. As we have previously reported, we did not see evidence of myelofibrosis in primary Jak2V617F germline-expressing mice,12 possibly as these mice die prematurely from probable thrombotic events by age 6 months. There has been some heterogeneity in the myelofibrosis phenotype between the various Jak2V617F knock-in models reported.12,24,34,35 The most prominent fibrosis was seen in the homozygote model of Akada et al suggesting that the level of JAK2V617F expression is important in the development of a fibrosis phenotype,34 and consistent with the finding in MPN patients that higher JAK2V617F allele burden is associated with transformation to myelofibrosis.36 Fibrotic transformation occurs with long latency in our study, similar to the development of post-PV myelofibrosis in MPN patients.37 The Jak2V617F knock-in models will be valuable for the further study of myelofibrosis.
In conclusion, we report the specific roles of the LT-HSC and erythroid precursor cell populations in the pathogenesis of PV using a conditional Jak2V617F knock-in murine model. We find that Jak2V617F MPN disease-propagating stem cells are contained exclusively in the LT-HSC compartment and that these cells have a competitive advantage over WT LT-HSCs. We show that erythroid lineage-restricted Jak2V617F expression results in an attenuated MPN phenotype that is not transplantable. We use LT transplantation assays to demonstrate that a pathologic interaction between Jak2V617F mutant hematopoietic cells and the BM niche can induce fibrotic transformation over time.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Ursula Klingmuller for ErGFPcre mice, and Dr Dirk Heckl for critically reviewing the manuscript.
This work was supported by the National Institutes of Health (P01 CA108631 [B.L.E.] and K08 HL109734 [A.M.]) and the MPN Foundation (B.L.E.). A.M. has received support from the Jeanne D. Housman Fund for Research on Myeloproliferative Disorders and is an ASH Scholar recipient. S.W.L. is supported by the Leukemia Foundation of Australia and the National Health and Medical Research Council.
National Institutes of Health
Authorship
Contribution: A.M., L.P., S.W.L., and B.L.E. designed experiments and interpreted data; A.M., L.P., and S.W.L. performed experiments; R.K.S. reviewed and interpreted all histopathology; F.A.-S. analyzed gene expression profiling data; A.M. and B.L.E. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benjamin L. Ebert, Division of Hematology, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, 1 Blackfan Circle, Karp Building 5.210, Boston, MA 02115; e-mail: bebert@partners.org.