Abstract
Survivin, a member of the inhibitors of apoptosis protein family, plays important roles in cell proliferation and survival and is highly expressed in various malignancies, including leukemias. To better understand its role in acute myeloid leukemia (AML), we profiled survivin expression in samples obtained from 511 newly diagnosed AML patients and in CD34+38− AML stem/progenitor cells using a validated reverse-phase protein array; we correlated its levels with clinical outcomes and with levels of other proteins in the same sample set. We found that survivin levels were higher in bone marrow than in paired peripheral blood leukemic cells (n = 140, P = .0001) and that higher survivin levels significantly predicted shorter overall (P = .016) and event-free (P = .023) survival in multivariate Cox model analysis. Importantly, survivin levels were significantly higher in CD34+38− AML stem/progenitor cells than in bulk blasts and total CD34+ AML cells (P < .05). Survivin expression correlated with the expressions of multiple proteins involved with cell proliferation and survival. Particularly, its expression strongly correlated with HIF1α in the stem/progenitor cell compartment. These results suggest that survivin is a prognostic biomarker in AML and that survivin, which is overexpressed in AML stem/progenitor cells, remains a potentially important target for leukemia therapy.
Introduction
Resistance to chemotherapy, which is the primary treatment for acute myeloid leukemia (AML), is a major obstacle in the cure of AML patients, and is often attributed to the deregulation of apoptosis in AML cells, particularly in AML stem cells. Although cytogenetic analysis at the time of diagnosis provides important prognostic information, molecular markers have also been used to provide further prognostic information and direct patients to targeted treatment options, especially for patients with normal cytogenetics.1-3 Thus, identifying deregulated apoptosis regulators that may be prognostic markers and understanding their roles in cell death and chemoresistance may facilitate the selection of treatment options and benefit patients.
Survivin, a member of the inhibitors of apoptosis (IAP) protein family, is one of the most frequently up-regulated transcripts in cancer but is expressed at low or undetectable levels in many normal adult tissues.4 Survivin in malignant cells is up-regulated by multiple signaling pathways and by tumor microenvironments including PI3K, MAPK, STAT3, Wnt/β-catenin, hypoxia, angiogenesis, and NF-κB signaling pathways.5-11 Survivin is part of the Aurora B-survivin-INCENP-Borealin/Dasra B complex, the chromosomal passenger essential for cell-cycle progression and cytokinesis.12,13 Its roles in regulating cell proliferation and cell death and its differential expression in many cancers make survivin a promising therapeutic target and a potential prognostic marker.14-17 Overexpression of survivin has been identified in several hematologic malignancies.18 We found that survivin is highly expressed in AML blasts and its expression is regulated by hematopoietic cytokines through MAPK and PI3K signaling.5 In addition, we found that targeting survivin by antisense oligonucleotide (ASO) induces cell proliferation defects and subsequent cell death in AML cells.19 Recently, survivin expression has also been found to be stimulated by the AML1/ETO fusion protein in AMLs carrying the t(8;21)(q22;q22) chromosome translocation.20 We have also reported that survivin is regulated through Bcr-Abl/MAPK signaling in chronic myeloid leukemia (CML) cells, and that targeting survivin overcomes imatinib resistance, decreases colony formation in samples from CML patients in blast crisis, and increases imatinib sensitivity in imatinib-responsive CML cells.21 Furthermore, the role of survivin in promoting leukemogenesis was supported by a recent study showing that overexpression of survivin initiates hematologic malignancies in transgenic mice.22 High levels of survivin have been reported to predict unfavorable prognoses in several hematologic malignancies.23-26 Although survivin was reported to be highly expressed in AML, its prognostic impact is not clearly defined. Some found that survivin predicts poor clinical outcomes, others did not.26-28 This lack of a definitive answer is largely related to small sample sizes and different ways of measuring survivin levels such as protein versus RNA.
Reverse-phase protein array (RPPA) is a robust and reproducible high-throughput proteomics system that can quantitatively determine protein expression levels in large sample sets and requires only small amounts of protein. Our group has established RPPA and demonstrated that it is a valuable tool for the functional profiling of protein expression in AML.29-31 To better understand the roles of survivin in AML, we took advantage of this state-of-the-art novel technology,29,30 determined expression levels of survivin and of 206 additional proteins of interest in samples obtained from 511 patients newly diagnosed with AML, and analyzed the correlation of survivin levels with clinical outcomes and with the levels of other proteins. AML stem cells, which give rise to leukemic blasts, are known to be more resistant to therapy and responsible for disease relapse. We therefore also measured survivin levels in CD34+38− AML stem/progenitor cells taking advantage of the minimal sample size requirement of RPPA and correlated survivin with an additional 120 other proteins probed in the same dataset. We found that survivin is a prognostic marker in AML and that its expression is higher in the AML stem/progenitor cell compartment than in blasts and total CD34+ AML cells, and correlated with multiple proteins involved in cell proliferation and survival.
Methods
Patient population
The patient population was the same as previously described.31 Briefly, peripheral blood (PB) and bone marrow (BM) specimens were collected from 511 patients with newly diagnosed AML evaluated at The University of Texas MD Anderson Cancer Center from September 1999 to March 2007. A total of 387 BM and 283 PB samples were studied; for 140 patients, both BM and PB samples were available. Survivin levels in BM samples were used in the main analysis if both BM and PB were available. A paired relapse sample was available for 47 of the AML patients. All but one of the relapse samples were from BM. The demographics and clinical characteristics of the newly diagnosed patients are described in Table 1. This patient population is typical of the MD Anderson referral pattern: a high percentage of patients with unfavorable cytogenetics (49%) and a very high percentage with an antecedent hematologic disorder (AHD; 40%). The samples were classified by cytogenetic risk groups using the Cancer and Leukemia Group B system, as described previously.32
Demographics and clinical characteristics of newly diagnosed AML patients in the study
| . | . | All cases . | Treated cases . |
|---|---|---|---|
| N | 511 | 415 | |
| Male:Female | 291:220 | 218:197 | |
| Age, y | Min | 15.8 | 15.8 |
| Max | 87.23 | 87.4 | |
| Median | 65.7 | 64.3 | |
| Cyto, % | Favorable | 6.7 | 8.0 |
| Intermediate | 44.0 | 47.0 | |
| Unfavorable | 49.3 | 45.1 | |
| FLT3, % | ITD | 14.9 | 17.8 |
| D835 | 3.1 | 5.8 | |
| Both | 1.6 | 1.9 | |
| Zubrod PS, % | 3 or 4 | 3.3 | 3.1 |
| AHD, % | ≥ 2 | 39.9 | 37.1 |
| Infection, % | Yes | 19.8 | 22.2 |
| WBC | Median | 8.8 | 9.9 |
| Platelet | Median | 56 | 55.5 |
| Hemoglobin | Median | 9.6 | 9.6 |
| BM blast, % | Median | 46 | 50 |
| PB blast, % | Median | 18 | 20.5 |
| Response, % | CR | 57.1 | |
| Resistant | 32.8 | ||
| Fail | 10.1 | ||
| Relapse | 61.6% | ||
| Alive | 25.8% | ||
| Median overall survival, wk | 49.14 | ||
| Median remission duration, wk | 45.86 |
| . | . | All cases . | Treated cases . |
|---|---|---|---|
| N | 511 | 415 | |
| Male:Female | 291:220 | 218:197 | |
| Age, y | Min | 15.8 | 15.8 |
| Max | 87.23 | 87.4 | |
| Median | 65.7 | 64.3 | |
| Cyto, % | Favorable | 6.7 | 8.0 |
| Intermediate | 44.0 | 47.0 | |
| Unfavorable | 49.3 | 45.1 | |
| FLT3, % | ITD | 14.9 | 17.8 |
| D835 | 3.1 | 5.8 | |
| Both | 1.6 | 1.9 | |
| Zubrod PS, % | 3 or 4 | 3.3 | 3.1 |
| AHD, % | ≥ 2 | 39.9 | 37.1 |
| Infection, % | Yes | 19.8 | 22.2 |
| WBC | Median | 8.8 | 9.9 |
| Platelet | Median | 56 | 55.5 |
| Hemoglobin | Median | 9.6 | 9.6 |
| BM blast, % | Median | 46 | 50 |
| PB blast, % | Median | 18 | 20.5 |
| Response, % | CR | 57.1 | |
| Resistant | 32.8 | ||
| Fail | 10.1 | ||
| Relapse | 61.6% | ||
| Alive | 25.8% | ||
| Median overall survival, wk | 49.14 | ||
| Median remission duration, wk | 45.86 |
AML indicates acute myeloid leukemia; Cyto, cytogenetic; FLT3, FMS-like tyrosine kinase 3; PS, performance status; AHD, antecedent hematologic disorder; WBC, white blood cell; and PB, peripheral blood.
Of the 511 AML patients, 415 were treated at MD Anderson and were evaluable for outcome. Among the treated patients, 277 received regimens that contained high-dose Ara-C, 35 received standard-dose Ara-C and 8 received low-dose Ara-C. Most of the Ara-C–treated patients also received other treatments. A variety of regimens were given to the other 95 patients.
Sample collection, sample preparation, and RPPA
Proteomic profiling was performed on samples from patients with AML as previously described.31 Samples had been acquired during routine diagnostic assessments and were analyzed in accordance with the regulations and protocols approved by the MD Anderson Institutional Review Board. Informed consent had been obtained in accordance with the Declaration of Helsinki. Samples were enriched for leukemic cells by performing Ficoll-Hypaque (Sigma-Aldrich) density-gradient separation to yield a mononuclear fraction, followed by CD3/CD19 depletion to remove contaminating T and B cells if they were calculated to be > 5% on the basis of the differential measurement. After this procedure, the blast purity reached ∼ 98%, as determined in ∼ 20 samples by flow cytometry. Proteomic profiling of AML stem/progenitor cells was performed in the CD34+38− stem cell fraction sorted by flow cytometry as previously described.30 Briefly, CD34+ cells were purified from the leukemia-enriched fraction (ficolled and CD3/CD14 depleted) by MACS (Miltenyi Biotec) and then separated into CD34+38− and CD34+38+ fractions by flow sorting after incubation with anti-CD34, anti-CD38 Abs, and IgG controls (BD Biosciences). The samples were normalized to a concentration of 1 × 104 cells/μL, and whole-cell lysates were prepared. RPPA was carried out after the methods and validation procedures described fully in previous publications.29,30 Briefly, patient samples were printed onto the slides in 5 serial dilutions along with controls for normalization and expression. The slides were probed with strictly validated primary Abs against survivin (Cell Signaling Technology) and 200 additional proteins (published elsewhere), a secondary Ab was used to amplify the signal, and a stable dye33 was precipitated. The expression levels were quantified from the stained slides using MicroVigene software (Version 3.4; Vigene Tech).
Statistical analysis
Supercurve algorithms were used to generate a single value from the 5 serial dilutions.34 The loading control35 and topographical normalization procedures accounted for variations in protein concentrations and background staining. Normalization procedures were performed using the R software program (Version 2.8.0, 2008-10-20). For correlation of survivin expression with the expression of other proteins, we accounted for multiple testing using a Bonferroni correction and thus accepted any proteins with a Pearson correlation −0.2 ≥ R ≥ 0.2 (P < .0001) in bulk AML cells and −0.4 > R > 0.4 (P ≤ .01) in CD34+38− AML stem/progenitor cells. Comparison of survivin levels between paired samples was done using the paired t test and Pearson product-moment correlation analysis and between different cell compartments using ANOVA and the Tukey multiple comparison test. Unbiased clustering, perturbation bootstrap clustering, and principal component analyses were performed as described previously.29,30 Associations between survivin expression levels and categorical clinical variables were assessed in R using standard t tests, linear regression, or mixed-effects linear models. Associations between the protein level and continuous variables were assessed using Pearson and Spearman correlation and linear regression analysis. Bonferroni corrections were performed to account for the multiple statistical parameters used for calculating statistical significance. A Cox proportional hazards regression model (1972) was used to evaluate the ability of patient prognostic variables and survivin to predict overall survival (OS) and event-free survival (EFS). Patients with missing data were excluded from the analysis. A Kaplan-Meier plot was generated to evaluate differences in OS and EFS between the 2 groups separated by the median value of survivin expression. Stepwise model selection using the Akaike information criterion was used to finalize the multivariate Cox model of the prognostic variables. All demographic and clinical variables (but not survivin or other biomarkers) were included in the stepwise variable selection to create a base model. Then, survivin was added to the base model to evaluate its effects. Outcome analyses and multivariate analyses were carried out using the software R (Version 2.12.1).36
Results
Survivin is differentially expressed in BM and PB samples obtained from newly diagnosed AML patients
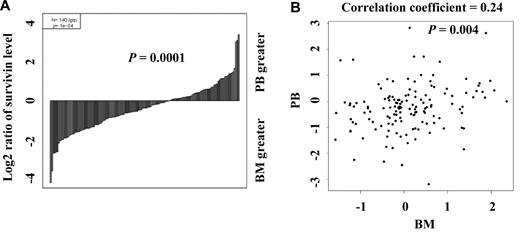
We determined survivin expression by RPPA in the samples obtained from 511 newly diagnosed AML patients (Table 1) and found that it was variably expressed. Paired BM and PB were available from 140 patients. To assess whether survivin levels differ depending on the source of the samples, we compared the levels of survivin in the 140 paired BM and PB samples using the paired t test and Pearson product-moment correlation analysis and found that survivin levels were significantly higher in samples from BM than from PB (Figure 1) as demonstrated by both waterfall plot (P = .0001, Figure 1A) and scatter plot (correlation coefficient = 0.24, P = .004, Figure 1B) consistent with its function in cell proliferation and its induction by growth factors and hematopoietic cytokines.
Comparison of survivin levels in paired PB and BM samples. (A) Survivin levels in 140 paired PB and BM samples were determined by PRRA and compared by paired t test (P = .0001) and (B) Pearson product-moment correlation analysis (correlation coefficient = 0.24, P = .004).
Comparison of survivin levels in paired PB and BM samples. (A) Survivin levels in 140 paired PB and BM samples were determined by PRRA and compared by paired t test (P = .0001) and (B) Pearson product-moment correlation analysis (correlation coefficient = 0.24, P = .004).
Correlation of survivin expression with clinical characteristics of patients
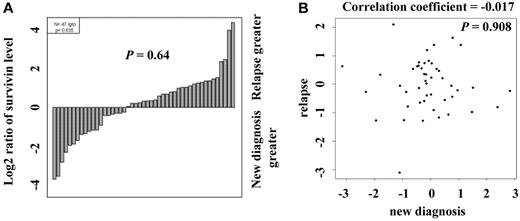
Survivin levels were determined from samples taken at diagnosis and relapse, and the levels were compared using the paired t test and Pearson product-moment correlation analysis. Among the 47 cases with paired diagnosis and relapse samples, there was no consistent pattern of change in survivin levels (Figure 2) as demonstrated by both waterfall plot (P = .64, Figure 2A) and scatter plot (correlation coefficient = -0.017, P = .908, Figure 2B). In most cases, the expression levels in the paired samples were within 2-fold of each other. However, more pairs were found to have higher survivin levels at relapse than at diagnosis (28 pairs vs 19 pairs; Figure 2A). Survivin expression did not correlate with any demographics of patients and clinical characteristics shown in Table 1: survivin levels were not associated with cytogenetic groups (P = .22), nucleophosmin mutation status (P = .20), or FLT3 mutation status (neither with FMS-like tyrosine kinase-3 internal tandem duplication [FLT3-ITD] mutation, P = .76 nor with D835 mutations, P = .76). No correlation between survivin levels and FLT3 mutation status was observed either when the analysis was done within intermediate cytogenetic group. Furthermore, survivin levels were not associated with treatment response (P = .81) or relapse status (P = .93). In addition, we found no significant relationship between survivin levels and any of the following variables: sex, age, French-American-British (FAB) subtype, BM or PB blast counts, patient performance status, AHD status, infection status, white blood count (WBC), platelet count, or hemoglobin levels. Survivin levels also did not correlate with whether the patients had a history of prior malignancy, chemotherapy, or radiation therapy. By univariate analysis, although high survivin levels had a trend to be associated with worse OS or EFS, these associations were not statistically significant, using either Kaplan-Meier plots (P = .14 and P = .23, respectively, with high and low survivin expression level groups defined by the median value; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) or a Cox regression model (hazard ratio [HR] = 1.08, P = .18 and HR = 1.10, P = .12, respectively, for 1 unit increase in the continuous survivin expression level; supplemental Table 1).
Comparison of survivin levels in paired samples from patients at diagnosis and relapse. (A) Survivin levels in 47 paired new diagnosis and relapse samples were determined by PRRA and compared by paired t test (P = .64) and (B) Pearson product-moment correlation analysis (correlation coefficient = -0.017, P = .908).
Comparison of survivin levels in paired samples from patients at diagnosis and relapse. (A) Survivin levels in 47 paired new diagnosis and relapse samples were determined by PRRA and compared by paired t test (P = .64) and (B) Pearson product-moment correlation analysis (correlation coefficient = -0.017, P = .908).
Survivin expression is an independent predictor of OS and EFS in multivariate analysis
To determine the prognostic impact of survivin levels in AML in multivariate analysis, Cox proportional hazards modeling was performed based on the stepwise model selection method using survivin levels as a continuous variable. Results showed that survivin was a significant factor for both OS (P = .016) and EFS (P = .023). As demonstrated in Table 2 for OS and for EFS, for a one unit increase in the expression level of survivin, the hazard ratio was increased by a factor of 1.17 and 1.15 for OS and EFS, respectively. Similar results were obtained when FLT3-ITD and FLT3-D835 were included in the model and survivin was a significant predictor for both OS (P = .026; supplemental Table 2.1) and EFS (P = .035; supplemental Table 2.2). We then distinguished the intensively treated patients from others, and reanalyzed survivin levels and outcomes by multivariate analyses. Patients were classified into 3 groups: patients who received less-intensive treatments and patients not treated at MD Anderson Cancer Center (No Rx) were compared with patients who received intensive treatments. Accounting for these treatment differences and other important factors, survivin was prognostic for both OS (P = .012) and EFS (P = .017; supplemental Table 3). The intensively treated group was patients who received the high-dose Ara-C (HDAC)–based regimens including HDAC, HDAC plus fludarabine, HDAC plus anthracycline, and HDAC plus nonanthracycline and standard Ara-C plus anthracycline. The less intensively treated group was patients who received low-dose Ara-C, demethylating agents, histone deacetylating agents, and various targeted therapies. Next, we analyzed survivin levels and clinical outcomes considering stem cell transplantation as a censoring event. Sixty-six patients underwent stem cell transplantation: syngeneic, 1; related donors, 39; and unrelated donors, 26. Again, survivin was highly prognostic for both OS (P = .007) and EFS (P = .008; supplemental Table 4). Because the survivin levels in BM and PB were different, we also analyzed the datasets generated from BM (n = 372) and PB (n = 270) samples separately. Multivariate analysis using the BM survivin level as a continuous variable indicated that survivin level in BM was a statistically significant independent predictor of OS (P = .011) in AML (Table 3 for OS). Although not statistically significant, patients with lower BM survivin tended to have longer EFS (P = .082; Table 3 for EFS). Multivariate analysis using the PB survivin level as a continuous variable also showed that the level of survivin in PB was a statistically significant independent predictor of both OS (P = .033) and EFS (P = .007) in AML (Table 4 for OS and for EFS).
Multivariate Cox model by survivin level
| Variable . | Estimate . | Hazard ratio . | 95% CI for hazard ratio . | P . |
|---|---|---|---|---|
| For OS | ||||
| Age | 0.04 | 1.04 | (1.04, 1.05) | < .001 |
| Sex, male | −0.20 | 0.82 | (0.66, 1.01) | .063 |
| Log2 (WBC) | 0.12 | 1.12 | (1.06, 1.19) | < .001 |
| BM blast | 0.005 | 1.005 | (1.00, 1.01) | .021 |
| Cyto (intermediate) | 0.85 | 2.34 | (1.18,4.65) | .015 |
| Cyto (unfavorable) | 1.38 | 3.97 | (2.01,7.84) | < .001 |
| Survivin | 0.16 | 1.17 | (1.03,1.33) | .016 |
| For EFS | ||||
| Age | 0.03 | 1.03 | (1.03, 1.04) | < .001 |
| Sex, male | −0.24 | 0.78 | (0.64, 0.96) | .021 |
| Log2 (WBC) | 0.09 | 1.10 | (1.04, 1.16) | .001 |
| BM blast | 0.01 | 1.01 | (1.00, 1.01) | .001 |
| Cyto (intermediate) | 0.70 | 2.02 | (1.13,3.59) | .017 |
| Cyto (unfavorable) | 1.19 | 3.30 | (1.86, 5.86) | < .001 |
| Survivin | 0.14 | 1.15 | (1.02,1.30) | .023 |
| Variable . | Estimate . | Hazard ratio . | 95% CI for hazard ratio . | P . |
|---|---|---|---|---|
| For OS | ||||
| Age | 0.04 | 1.04 | (1.04, 1.05) | < .001 |
| Sex, male | −0.20 | 0.82 | (0.66, 1.01) | .063 |
| Log2 (WBC) | 0.12 | 1.12 | (1.06, 1.19) | < .001 |
| BM blast | 0.005 | 1.005 | (1.00, 1.01) | .021 |
| Cyto (intermediate) | 0.85 | 2.34 | (1.18,4.65) | .015 |
| Cyto (unfavorable) | 1.38 | 3.97 | (2.01,7.84) | < .001 |
| Survivin | 0.16 | 1.17 | (1.03,1.33) | .016 |
| For EFS | ||||
| Age | 0.03 | 1.03 | (1.03, 1.04) | < .001 |
| Sex, male | −0.24 | 0.78 | (0.64, 0.96) | .021 |
| Log2 (WBC) | 0.09 | 1.10 | (1.04, 1.16) | .001 |
| BM blast | 0.01 | 1.01 | (1.00, 1.01) | .001 |
| Cyto (intermediate) | 0.70 | 2.02 | (1.13,3.59) | .017 |
| Cyto (unfavorable) | 1.19 | 3.30 | (1.86, 5.86) | < .001 |
| Survivin | 0.14 | 1.15 | (1.02,1.30) | .023 |
CI indicates confidence interval; OS, overall survival; and EFS, event-free survival.
Multivariate Cox model by survivin level (BM samples)
| Variable . | Estimate . | Hazard ratio . | 95% CI for hazard ratio . | P . |
|---|---|---|---|---|
| For OS | ||||
| Age | 0.05 | 1.05 | (1.04, 1.06) | < .001 |
| Sex, male | −0.31 | 0.73 | (0.57, 0.95) | .019 |
| Log2 (WBC) | 0.12 | 1.13 | (1.06, 1.21) | < .001 |
| BM blast | 0.01 | 1.01 | (1.00, 1.01) | .008 |
| Cyto (intermediate) | 0.73 | 2.08 | (0.95, 4.55) | .068 |
| Cyto (unfavorable) | 1.22 | 3.38 | (1.56, 7.35) | .002 |
| Survivin | 0.20 | 1.23 | (1.05, 1.43) | .011 |
| For EFS | ||||
| Age | 0.04 | 1.04 | (1.03, 1.05) | < .001 |
| Sex, male | −0.32 | 0.73 | (0.57, 0.93) | .012 |
| Log2 (WBC) | 0.10 | 1.11 | (1.04, 1.18) | .001 |
| BM blast | 0.01 | 1.01 | (1.00, 1.01) | < .001 |
| Cyto (intermediate) | 0.63 | 1.87 | (0.96, 3.64) | .064 |
| Cyto (unfavorable) | 1.09 | 2.97 | (1.54, 5.74) | .001 |
| Survivin | 0.13 | 1.14 | (0.98, 1.32) | .082 |
| Variable . | Estimate . | Hazard ratio . | 95% CI for hazard ratio . | P . |
|---|---|---|---|---|
| For OS | ||||
| Age | 0.05 | 1.05 | (1.04, 1.06) | < .001 |
| Sex, male | −0.31 | 0.73 | (0.57, 0.95) | .019 |
| Log2 (WBC) | 0.12 | 1.13 | (1.06, 1.21) | < .001 |
| BM blast | 0.01 | 1.01 | (1.00, 1.01) | .008 |
| Cyto (intermediate) | 0.73 | 2.08 | (0.95, 4.55) | .068 |
| Cyto (unfavorable) | 1.22 | 3.38 | (1.56, 7.35) | .002 |
| Survivin | 0.20 | 1.23 | (1.05, 1.43) | .011 |
| For EFS | ||||
| Age | 0.04 | 1.04 | (1.03, 1.05) | < .001 |
| Sex, male | −0.32 | 0.73 | (0.57, 0.93) | .012 |
| Log2 (WBC) | 0.10 | 1.11 | (1.04, 1.18) | .001 |
| BM blast | 0.01 | 1.01 | (1.00, 1.01) | < .001 |
| Cyto (intermediate) | 0.63 | 1.87 | (0.96, 3.64) | .064 |
| Cyto (unfavorable) | 1.09 | 2.97 | (1.54, 5.74) | .001 |
| Survivin | 0.13 | 1.14 | (0.98, 1.32) | .082 |
Multivariate Cox model by survivin level (PB samples)
| Variable . | Estimate . | Hazard ratio . | 95% CI for hazard ratio . | P . |
|---|---|---|---|---|
| For OS | ||||
| Age | 0.04 | 1.04 | (1.03, 1.05) | < .001 |
| Log2 (WBC) | 0.11 | 1.12 | (1.03, 1.20) | .005 |
| Cyto (intermediate) | 1.13 | 3.10 | (0.97, 9.90) | .056 |
| Cyto (unfavorable) | 1.66 | 5.27 | (1.66, 16.79) | .005 |
| Survivin | 0.18 | 1.20 | (1.02, 1.42) | .033 |
| For EFS | ||||
| Age | 0.03 | 1.03 | (1.02, 1.04) | < .001 |
| Log2 (WBC) | 0.10 | 1.10 | (1.02, 1.18) | .009 |
| Cyto (intermediate) | 0.89 | 2.44 | (0.98, 6.05) | .054 |
| Cyto (unfavorable) | 1.38 | 3.97 | (1.61, 9.84) | .003 |
| Survivin | 0.22 | 1.25 | (1.06, 1.46) | .007 |
| Variable . | Estimate . | Hazard ratio . | 95% CI for hazard ratio . | P . |
|---|---|---|---|---|
| For OS | ||||
| Age | 0.04 | 1.04 | (1.03, 1.05) | < .001 |
| Log2 (WBC) | 0.11 | 1.12 | (1.03, 1.20) | .005 |
| Cyto (intermediate) | 1.13 | 3.10 | (0.97, 9.90) | .056 |
| Cyto (unfavorable) | 1.66 | 5.27 | (1.66, 16.79) | .005 |
| Survivin | 0.18 | 1.20 | (1.02, 1.42) | .033 |
| For EFS | ||||
| Age | 0.03 | 1.03 | (1.02, 1.04) | < .001 |
| Log2 (WBC) | 0.10 | 1.10 | (1.02, 1.18) | .009 |
| Cyto (intermediate) | 0.89 | 2.44 | (0.98, 6.05) | .054 |
| Cyto (unfavorable) | 1.38 | 3.97 | (1.61, 9.84) | .003 |
| Survivin | 0.22 | 1.25 | (1.06, 1.46) | .007 |
Survivin expression is higher in CD34+38− AML stem/progenitor cells than in bulk blasts or total CD34+ leukemic cells
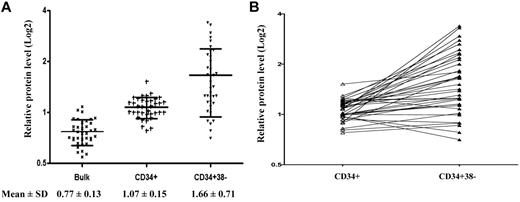
AML stem cells are known to be more resistant to various therapies and contribute to disease relapse, and survivin has been shown to play a role in hematopoietic stem cell proliferation.37 To assess whether survivin is overexpressed in AML stem/progenitor cells, we measured survivin expression in CD34+38− cells isolated from AML samples (n = 37) by RPPA and compared the levels with those in bulk blasts and CD34+ AML cells. We found that survivin was differentially expressed in various cell compartments (P < .001; Figure 3): the more primitive the cells, the higher the survivin level. Survivin expression was significantly higher in CD34+ cells than in bulk blasts (P < .05; 1.39-fold) and CD34+38− cells had the highest survivin levels (1.55-fold of CD34+ cells and 2.16-fold of total blasts, respectively, P < .05). The relative levels of survivin expressed as mean ± SD in bulk blasts, CD34+ cells, and CD34+38− AML stem/progenitor cells were 0.77 ± 0.13, 1.07 ± 0.15, and 1.66 ± 0.71, respectively (Figure 3A). To determine whether high survivin expression in CD34+38− AML stem/progenitor cells is a general phenomenon or occurs only in separate groups, we used a line graph to directly compare survivin levels in paired CD34+ and CD34+38− cells. As shown in Figure 3B, survivin levels are higher in CD34+38− cells than CD34+ cells in most pairs.
Comparison of survivin levels in bulk blasts, CD34+ AML cells, and CD34+38− AML cells. (A) CD34+ and CD34+38− cells were isolated from blasts of 37 AML patient samples. Survivin levels in bulk, CD34+ and CD34+38− cells were determined by RPPA and compared. (B) For direct comparison of survivin levels in CD34+ cells and CD34+38− cells of paired samples, a line graph is shown.
Comparison of survivin levels in bulk blasts, CD34+ AML cells, and CD34+38− AML cells. (A) CD34+ and CD34+38− cells were isolated from blasts of 37 AML patient samples. Survivin levels in bulk, CD34+ and CD34+38− cells were determined by RPPA and compared. (B) For direct comparison of survivin levels in CD34+ cells and CD34+38− cells of paired samples, a line graph is shown.
Correlation of survivin expression with the expression of other proteins
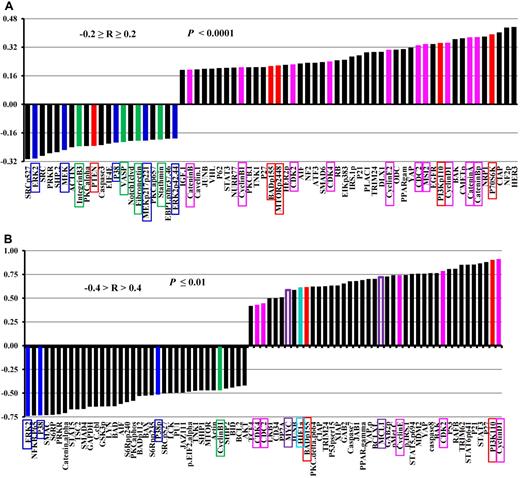
In addition to survivin, the same set of samples was also probed for 206 (supplemental Table 5) other proteins for samples from 511 newly diagnosed AML patients and 120 (supplemental Table 6) proteins for CD34+38− cells isolated from blasts of 37 AML patients, enabling us to correlate survivin expression levels with the levels of various proteins. As shown in Figure 4, survivin expression correlated with the expressions of multiple proteins. Figure 4A shows the results in samples from 511 newly diagnosed AML patients. Survivin levels positively correlated with levels of various cell proliferation–related proteins (pink, Figure 4A), such as cyclins, cyclin-dependent kinases, MSI2, and catenins consistent with its function in cell proliferation. Survivin levels also significantly correlated with the levels of proteins in the PI3K signaling pathway (red, Figure 4A): positively with PI3Kp110, phosphorylated mTOR, p70S6K, and BADp155, and negatively with PTEN in agreement with the induction of survivin by PI3K signaling as described by us previously.5 Surprisingly, survivin levels correlated negatively with levels of several proteins in the MAPK signaling pathway (blue, Figure 4A) and proteins involved with cell migration/adhesion such as integrin B3, VASP, fibronectin, and stathmin (green, Figure 4A) and FAK (R = 0.19 and P < .0001, not shown).
Correlation of survivin expression levels with the levels of other proteins in samples from 511 newly diagnosed AML patients and in CD34+38− cells isolated from blasts of AML patients. (A) Levels of survivin and 206 other proteins were determined by RPPA in the same sample set of 511 newly diagnosed AML samples. The expression level of survivin was correlated with the expression of 206 proteins and those with Pearson correlation −0.2 ≥ R ≥ 0.2 (P < .0001) are shown. (B) Levels of survivin and 120 other proteins were determined by RPPA in CD34+38− cells isolated from blasts of 37 AML patients. The expression level of survivin was correlated with the expression of 120 other proteins and those with Pearson correlation −0.4 > R > 0.4 (P ≤ .01) are shown.
Correlation of survivin expression levels with the levels of other proteins in samples from 511 newly diagnosed AML patients and in CD34+38− cells isolated from blasts of AML patients. (A) Levels of survivin and 206 other proteins were determined by RPPA in the same sample set of 511 newly diagnosed AML samples. The expression level of survivin was correlated with the expression of 206 proteins and those with Pearson correlation −0.2 ≥ R ≥ 0.2 (P < .0001) are shown. (B) Levels of survivin and 120 other proteins were determined by RPPA in CD34+38− cells isolated from blasts of 37 AML patients. The expression level of survivin was correlated with the expression of 120 other proteins and those with Pearson correlation −0.4 > R > 0.4 (P ≤ .01) are shown.
Figure 4B shows proteins whose expression levels significantly correlated with survivin levels in CD34+38− AML stem/progenitor cells. Survivin strongly correlated with PI3K and its downstream target BADp155 (red, Figure 4B). It positively correlated with multiple cyclin-dependent kinases, cyclins (pink, Figure 4B), but negatively correlated with cyclin B1 (green, Figure 4B). It also correlated negatively with members of MAPK signaling proteins such as ERK, p38, and phospho-p38 (blue, Figure 4B). Specifically, levels of survivin strongly correlated with HIF1α levels (cyan, Figure 4B), in agreement with survivin regulation by the hypoxia microenvironment and the highly hypoxic stem cell niche.38 Survivin also strongly correlated with several proteins important for stem cell functions, such as Myc and Mcl-1 (purple open boxes, Figure 4B). Note that survivin expression was correlated with ∼ 200 proteins on samples from 511 newly diagnosed AML patients and with 120 proteins for CD34+38− cells isolated from blasts of 37 AML patients. Therefore, some proteins correlated with survivin in the former but not in the latter samples, because they were not included in the stem cell sample set, or the sample size for the CD34+38− group was too small.
Discussion
In this study, we found that survivin levels were significantly higher in CD34+38− AML stem/progenitor cells than in bulk blasts and CD34+ AML cells, and that survivin expression correlated with the expressions of multiple proteins involved with cell proliferation and survival. In the stem/progenitor cell compartment, its expression strongly correlated with HIF1α. We also found that survivin levels were higher in BM than in paired PB leukemic cells and that higher survivin levels significantly predicted shorter OS and EFS. These findings support the roles of survivin in cell proliferation and survival, and suggest that survivin expression predicts poor clinical outcome in AML and that survivin plays important roles in AML stem cells.
Survivin levels were found to be higher in BM than in PB in 140 paired samples from newly diagnosed AML patients. Blasts in the BM and PB reside in very different microenvironments, with BM blasts in direct contact with BM stromal cells. BM-derived stromal cells secret multiple growth factors and hematopoietic cytokines that are essential for hematopoiesis and also play important roles in promoting the growth and survival of leukemic cells. We previously reported that survivin expression in AML cells is induced by various hematopoietic cytokines.5 Thus, these cytokines likely induce the expression of survivin in BM.
A recent study in rectal cancer showed that higher survivin expression correlated with advanced disease, and failure to down-regulate survivin after neoadjuvant radiochemotherapy was associated with distant metastases and shorter survival.39 Among the 47 cases with paired diagnosis and relapse samples in the current study, we did not observe a consistent pattern of change in survivin levels (P = .64) and saw both increases and decreases in survivin levels in the relapsed samples. However, we did observe that survivin levels were more likely to be increased (n = 28) than decreased (n = 19) at relapse. Thus, more paired samples are needed to determine whether survivin levels are different between diagnosis and relapse samples in AML.
It was reported that survivin predicts poor clinical outcome in different hematologic malignancies including AML.23-28 We previously determined survivin expression by Western blot in 116 AML patient samples.27 Although log-rank tests and Cox regression analysis showed that the risk of mortality increased as the survivin level increased, it did not reach statistical significance. Using Martingale residual analysis, we identified a small group of patients with very high survivin levels who were at high risk for death, but this group was too small for the results to be statistically significant.27 In addition, not all 116 samples were obtained from newly diagnosed patients. In the current study, we took advantage of the robust RPPA method to profile survivin expression in 511 samples, all from newly diagnosed AML patients. Multivariate analysis showed that its expression level is of clinical significance for both OS and EFS.
Correlative studies showed that survivin levels correlated with multiple proteins related to survivin regulation and function. Correlation of survivin expression with PI3K signaling supports the regulation of survivin by PI3K signaling and its prosurvival role. Correlation of survivin with cyclin-dependent kinases and cyclins supports cell cycle–dependent regulation of survivin, and its role in cell proliferation as survivin is known to be essential for cell-cycle progression and cytokinesis. Correlation of survivin with catenins supports the regulation of survivin by the wnt/β-catenin pathway.10,11 Correlation of survivin with HIF1α in AML stem/progenitor cells is consistent with regulation of survivin by hypoxia.9 It is interesting that survivin positively correlated with MSI2, a protein expressed in HSC and essential for HSC self-renewal and long-term hematopoietic engraftment.40 A recent study demonstrated that MSI2 protein expression predicts unfavorable outcome in AML.41 It was surprising that survivin levels negatively correlated with MAPK signaling, and multiple proteins related to cell migration/adhesion, since we and others have shown that survivin expression is induced by MAPK/ERK signaling5 and that survivin was reported to activate NF-κB in cooperation with XIAP (leading to fibronectin gene expression) and to regulate metastasis in epithelial tumors.42 We believe that the correlation results are valid as survivin was negatively correlated with not just one but multiple proteins in the MAPK signaling pathway and various proteins related to cell migration/adhesion. The negative correlation of survivin with MAPK signaling and members of cell adhesion/migration proteins does not imply that survivin is not regulated by MAPK signaling or that survivin does not affect cell adhesion and migration. In addition, although survivin was reported to be up-regulated by FLT3-ITD and regulate the expansion of FLT3-ITD–transformed hematopoietic progenitor cells with self-renewal capacity and development of FLT3-ITD acute leukemia in mice,43 we did not find survivin levels correlated with FLT3-ITD mutation status. Clearly, survivin's relation with these proteins is not simply 1-directional, but it may involve cross talk, feedback, and/or other unknown mechanisms. Survivin was also found to correlate positively with p21, p27, and p53. However, the phosphorylation status of p21 and p27 is unknown and mutation status of p53 is not completely determined in these patient samples. Although the correlation study will help us to understand the regulation of survivin and provide a basis for optimal combinations to target various survival pathways for therapies, more mechanistic studies are needed to better understand the regulation of survivin and interactions of survivin with other proteins and signaling pathways in AML cells.
AML is a stem cell disease, and the ineffectiveness of chemotherapy in eradicating leukemic stem cells contributes at least in part to the inevitable relapse of AML. In this regard, our finding that survivin levels are higher in the CD34+38− compartment of AML is of particular interest. Higher levels of survivin in stem cells may suggest that survivin confers leukemia stem cells a survival and self-renewal advantage and contributes to disease relapse. It may also suggest that survivin plays role in the initiation of AML. This notion is supported by a recent study demonstrating that overexpression of survivin is sufficient to initiate hematologic malignancies in an in vivo mouse model, and that hematopoietic cells engineered to overexpress survivin are less susceptible to apoptosis.22 Thus, strategies selectively targeting survivin may not only eliminate bulk AML blasts but also efficiently eradicate AML stem cells, preventing the recurrence of the disease. To date, however, this strategy has not been successfully translated to clinic. Although a proof-of-principle study using a survivin ASO (LY2183108) in a dose-escalation study in solid tumor in humans reportedly showed down-regulation of survivin both in RNA and protein,44 its effectiveness for treating AML and cancer in general has not been demonstrated. YM155,45 developed as a small-molecule suppressor of survivin, has entered phase 2 clinical trials in various cancers and showed limited single-agent activity; combination trials are ongoing.46-49 However, the effect of YM155 and its combination with chemotherapies on AML cells and AML stem/progenitor cells has not been investigated. Interestingly, survivin levels were shown to correlate with various catenins. Survivin is a known downstream target of the Wnt/β-catenin pathway10,11 and the Wnt/β-catenin pathway is required for self-renewal of leukemia stem cells.50 Inhibitors of the Wnt/β-catenin pathway are under clinical development and targeting this pathway may hold promise for inhibition of survivin and also for elimination of AML stem cells.
In conclusion, we demonstrated that survivin is an adverse predictor of survival in AML. Survivin is overexpressed in AML stem/progenitor cells and its expression correlates with the expression of multiple proteins participating in cell growth and survival. Given survivin's role in cell proliferation and apoptosis suppression, survivin remains a potentially important target for leukemia therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Deanna Alexander for helping with the manuscript preparation.
This work was supported in part by grants from the Leukemia & Lymphoma Society (S.M.K.), and National Institutes of Health grants (P01 CA55164 and P01 CA16672) and the Paul and Mary Haas Chair in Genetics (M.A.).
National Institutes of Health
Authorship
Contribution: B.Z.C. conceptualized the study and wrote the manuscript; Y.Q. and D.H.M. performed experiments; X.H., L.D., N.Z., and K.R.C. analyzed the data; M.K., J.C., and H.M.K. helped with patient data and patient samples; G.B.M. supported RPPA and edited the manuscript; M.A. supported the study, helped in analysis of the data, and edited the manuscript; and S.M.K. performed RPPA, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bing Z. Carter, PhD, or Steven M. Kornblau, MD, Section of Molecular Hematology and Therapy, Department of Leukemia, Unit 448, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: bicarter@mdanderson.org or skornbla@mdanderson.org.