Abstract
A main complication of treatment of patients with hemophilia A is the development of anti–factor VIII (fVIII) antibodies. The immunogenicity of fVIII potentially is a function of its procoagulant activity, which may result in danger signals that drive the immune response. Alternatively, intrinsic structural elements in fVIII may be particularly immunogenic. Finally, VWF, the carrier protein for fVIII in plasma, may play a role in immune recognition. We compared the immunogenicity of wild-type (wt) B domain–deleted fVIII and 2 inactive fVIII molecules, R372A/R1689A fVIII and V634M fVIII in fVIII−/− and fVIII−/−/VWF−/− mice. R372A/R1689A fVIII lacks proteolytic recognition sites and is not released from VWF. In contrast, V634M fVIII undergoes proteolytic cleavage and dissociation from VWF. No significant difference was observed in the immunogenicity of wt fVIII and V634M fVIII. R372A/R1689A fVIII was slightly less immunogenic in a subset of immunization regimens tested. High doses of wt fVIII were required to produce an immune response in fVIII−/−/VWF−/− mice. Our results indicate that a main component of the immune response to fVIII is independent of its procoagulant function, is both positively and negatively affected by its association with VWF, and may involve intrinsic elements of fVIII structure.
Introduction
Most patients with severe hemophilia A have undetectable circulating factor VIII (fVIII). The recognition of fVIII as “self” and the development of immunologic tolerance during neonatal development and early life presumably do not develop in most of these patients. On exposure to exogenous fVIII, which usually occurs during the first year, ∼ 30% of patients with severe hemophilia A develop inhibitory anti-fVIII antibodies (inhibitors).1 The immune response to fVIII currently is the most significant complication in the management of patients with hemophilia A. In addition, antibodies to fVIII can develop in persons without hemophilia, producing an autoimmune condition called acquired hemophilia A, which frequently results in life- or limb-threatening bleeding.
FVIII inhibitor formation in patients with hemophilia A and in mice with hemophilia A (fVIII−/−) is a MHC class II T cell–dependent process.2 During T cell–dependent antibody formation, T-cell receptors on naive T cells recognize antigen bound to MHC class II molecules on the surface of APCs, including dendritic cells (DCs), macrophages, and B cells, that are present in secondary lymphoid organs (eg, lymph nodes and the spleen). Antigen presentation when combined with appropriate costimulatory signals results in T-cell activation and proliferation and differentiation into T-helper cells.
Because fVIII is an immunologically foreign protein or an altered self-protein in patients with severe hemophilia A and fVIII−/− mice, it may not seem surprising that it produces an antibody response. However, it usually is difficult to raise antibodies to a foreign protein without using adjuvants,3 especially when the protein is delivered by intravenous administration. In a direct comparison of the immunogenicity of equal doses of adjuvant-free ovalbumin and human fVIII in fVIII−/− mice, anti-fVIII antibody titers were ∼ 100-fold higher than antiovalbumin titers.4 Model monomeric protein immunogens such as ovalbumin or lysozyme typically are given with adjuvants with a dose > 50-fold higher than the adjuvant-free doses of ∼ 10 μg/kg or less that are required to produce fVIII inhibitors in patients with hemophilia A and fVIII−/− mice. In addition, although the concentration of fVIII in plasma is lower than all the other coagulation factors, it is the most commonly targeted coagulation factor in autoimmunity. Thus, fVIII evidently is an unusually immunogenic protein. FVIII circulates noncovalently bound to VWF, which must be considered as a possible factor in the immunogenicity of fVIII.
The requirement for adjuvants to produce an immune response has been termed the “immunologist's dirty little secret.”5 Adjuvants typically include microbial products, such as the inactivated Mycobactrium tuberculosis bacilli in complete Freund adjuvant, which activate APCs. Host products of infection and inflammation serve as natural adjuvants, generating “danger” signals that provide specificity to the immune response and prevent autoimmunity.6
Blood coagulation and thrombin production generate a proinflammatory milieu that may provide danger signals to stimulate an immune response.7 Thus, the immunogenicity of fVIII may be driven by the hemostatic and inflammatory process it helps produce and not the structure of fVIII or the fVIII/VWF complex per se. To test this hypothesis, Skupsky et al studied the immunogenicity of heat-inactivated, denatured fVIII in fVIII−/− mice and found that it was less immunogenic than native fVIII.4 The heat-inactivated fVIII preparation lacked many of the B-cell epitopes present in native fVIII but retained fVIII-specific T-cell epitopes. In addition, Skupsky et al observed a decrease in antibody formation and T-cell responses when fVIII was given to fVIII−/− mice that received anticoagulation agents such as warfarin or the specific thrombin inhibitor, hirudin.4 On the basis of these findings, they concluded that the immunogenicity of fVIII is primarily linked to its function as a procoagulant. However, the disruption of B-cell epitopes and possible loss of VWF interactive sites in heat-inactivated fVIII leaves open the possibility that fVIII structure may contribute significantly to its immunogenicity.
To address the immunogenicity of fVIII independent of its procoagulant and potentially proinflammatory function and the role of VWF in the process, we compared the immune response of 2 inactive, conformationally intact recombinant B domain-deleted (BDD) fVIII molecules to wild-type (wt) in fVIII−/− and fVIII−/−/VWF−/− mice. R372A/R1689A fVIII lacks cleavage sites that are recognized by thrombin and factor Xa. Cleavage at R372 between the A1 and A2 domains of fVIII is necessary for production of factor IXa cofactor activity that is the basis of the procoagulant function of fVIII. Cleavage at R1689 in the light chain of fVIII leads to the dissociation of activated fVIII from VWF, binding to activated platelets and assembly of the intrinsic pathway factor X-activating complex. Because R372A/R1689A fVIII is not released from VWF, it should not localize to procoagulant sites or promote thrombin formation. V634M fVIII contains a single substitution in the A2 domain that leads to a profound loss of procoagulant activity.8 It has normal proteolytic recognition sites and dissociates from VWF on exposure to thrombin. Thus, it should not contribute to thrombin production, but, unlike R372A/R1689A fVIII, it should localize to procoagulant sites.
Methods
Materials used in this study are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Isolation and characterization of recombinant wt BDD fVIII, R372A/R1689A fVIII, and V634M fVIII
cDNAs encoding “wild-type” human BDD wt fVIII and V634M fVIII, both containing a 14-amino acid segment, SFSQNPPVLKRHQR, in place of the B domain9 in a mammalian expression plasmid designated ReNeo have been described previously.10,11 Wt fVIII/ReNeo was used as a template to produce cDNAs encoding R372A/R1689A fVIII by splicing-by-overlap extension mutagenesis as described in supplemental Methods. Stable expression, purification, and characterization of R372A/R1689A fVIII and V634M fVIII from baby hamster kidney-derived cells was performed as described previously for BDD wt fVIII with minor modifications,12 as described in supplemental Methods.
Binding of fVIII to VWF
The binding of wt fVIII and R372A/R1689A fVIII to VWF after thrombin exposure was measured by ELISA as described previously,13 with the following modifications. Dilutions of wt fVIII, R372A/R1689A fVIII, and V634M fVIII were made with 0.15M NaCl/0.02M HEPES/5mM CaCl2/0.05% Tween-80, pH 7.4 and added in duplicate to wells coated with VWF. In thrombin-treated wells, fVIII was activated with 40nM thrombin for 30 seconds, immediately removed, and replaced with 120nM hirudin for 1 minute. The binding of fVIII to VWF in the presence or absence of thrombin was quantitated with biotinylated anti-fVIII A2 domain 2-76, alkaline phosphatase–conjugated streptavidin, and p-nitrophenylphosphate.
FVIII bioassays
FVIII coagulant activity was measured with the activated partial thromboplastin reagent-based 1-stage coagulation assay as previously described,14 except that preincubation of fVIII-deficient plasma and activated partial thromboplastin time reagent was conducted for 4 minutes at 37°C. Pooled normal human plasma referenced against a World Health Organization standard was used as the standard. In addition, fVIII was measured with a chromogenic substrate assay that was based on factor X activation by an enzymatic complex consisting of factor IXa, phosphatidylcholine-phosphatidylserine (PCPS), calcium, and limiting amounts of fVIIIa as described previously.15 Factor Xa formation was measured with the chromogenic substrate Spectrazyme Xa as described previously,16 using a VersaMax kinetic plate reader (Molecular Devices).
FVIII-dependent thrombin generation in plasma at 37°C after initiation of coagulation with tissue factor and phospholipid was measured with the fluorometric thrombin substrate Z-Gly-Gly-Arg-AMC. Wt fVIII, R372A/R1689A fVIII, and V634M fVIII constructs were diluted to 2 μg/mL into fVIII-deficient plasma. In a 96-well MicroFluor 2B plate (ThermoScientific), 20 μL of reconstituted plasma was combined with 25 μL of TGA substrate (1mM Z-Gly-Gly-Arg-AMC with 15mM CaCl2) and 5 μL TGA RC low. Fluorescence was read 460 nm after excitation at 360 nm in a BioTek SynergyMX fluorescence plate reader. Relative fluorescence units were compared with a calibration curve that was based on a thrombin calibrator obtained from Technoclone (TGA CAL; DiaPharma). Data were analyzed with an Excel (Microsoft) spreadsheet derived from Technoclone's evaluation software. Parameters analyzed included the peak thrombin concentration and endogenous thrombin potential.
FVIII−/− and fVIII−/−/VWF−/− mice
FVIII exon 16–disrupted hemophilia A male (fVIII−/y) and female (fVIII−/−) mice (both designated fVIII−/−) in a mixed S129/C57BL/6 background17,18 were obtained from Dr Leon Hoyer and backcrossed further to ∼ 75% C57BL/6 background. VWF−/− mice in a C57BL/6 background19 were a generous gift from Dr Denisa Wagner (Harvard Medical School). FVIII−/−/VWF−/−were produced by cross-breeding the 2 strains. Offspring of male VWF−/− × female FVIII−/− and female VWF−/− × male FVIII−/− breeding pairs were genotyped by PCR to select FVIII−/−/VWF−/− pups. FVIII−/−/VWF−/− offspring were bred for 4 generations with continued genotyping before experiments were begun. Genotyping was performed with DNA isolated from ear punches with the use of the Qiagen QIAamp DNA Micro Kit according to the manufacturer's protocol for isolation and concentration of DNA from laser-microdissected tissues as described in supplemental Methods.
FVIII immunization regimens
Eight- to 12-week-old fVIII−/− or fVIII−/−/VWF−/− mice were warmed on a heating pad for 5 minutes to dilate tail veins. Wt fVIII, R372A/R1689A fVIII, and V634M fVIII samples were diluted in 100 μL of sterile saline and immediately injected by tail vein. Silver nitrate was used to cauterize bleeding after injection. Blood was collected by terminal cardiac puncture 7 days after the final injection. Samples were held on ice before centrifugation at 2000g for 15 minutes at 4°C to collect plasma for analysis by ELISA and Bethesda assay.
Three regimens were used, designated fVIII−/− low dose, fVIII−/− increasing dose, and fVIII−/−/VWF−/− immunizations. For the low-dose regimen, fVIII−/− mice were divided into wt fVIII and R372A/R1689A fVIII groups (n = 25 in each group) and received 6 weekly injections of 0.2 μg, followed by 2 injections of 0.5 μg of protein. For the increasing-dose regimen fVIII−/− mice were divided into wt fVIII, R372A/R1689A fVIII, and V634M fVIII groups (n = 37 in each group). Each of the groups were further divided into equal subgroups to receive 4 weekly injections of fVIII at doses of 0.5, 1.0, 1.5, and 2.0 μg, followed by an additional injection at twice the initial dose. In the fVIII−/−/VWF−/− regimen, mice were divided into wt fVIII, R372A/R1689A fVIII, and V634M fVIII groups (n = 16 in each group). Mice received 6 weekly injections of 0.6 μg, followed by 2 weekly injections of 1.5 μg.
Anti-fVIII ELISAs
Anti-fVIII IgG in plasma from immunized mice was measured by direct ELISA with the use of a modification of previously described procedures,13,20 as described in supplemental Methods. Murine anti-fVIII antibodies specific for the classic C2 domain epitope, which binds VWF and phospholipid,13,20 were measured with a competition ELISA. ELISA plates were coated with fVIII and initially preincubated for 1 hour with 3 μg/mL of a nonbiotinylated murine nonclassic anti–human C2 “blocking” mAb 2-77 to prevent binding of nonclassic anti-C2 domain antibodies. Test plasma or control, murine hemophilia A noninhibitor plasma was diluted 1/24 in blocking buffer and added to a separate 96-well polypropylene plate. The biotinylated classic anti–human C2 mAb ESH-4 was serially diluted into the plasma, and then samples were transferred to the ELISA plate. Bound biotinylated mAb was measured as a function of test plasma dilution with the use of alkaline phosphatase–conjugated streptavidin and p-nitrophenylphosphate and fitted to the 4-parameter logistic equation. The mAb concentration required to produce an A405 of 0.5 (EC0.5) was calculated by interpolation on the fitted curve. The corresponding mAb titer is defined as EC0.5−1. The normal range of EC0.5 values for the binding of biotinylated mAbs was estimated by performing 8 replicate mAb titrations of the control plasma EC0.5 values (means and SDs) for ESH-4. The corresponding normal range of control plasma ELISA titers was defined with EC0.5 values within 2 SDs from the mean.
FVIII inhibitor assay
FVIII inhibitor titers were measured with the Bethesda assay,21 using the modifications previously described.22 Pooled citrated normal human plasma was used as the source of fVIII activity. One Bethesda unit (BU) per milliliter is defined as the dilution of inhibitor that produces 50% inhibition of fVIII activity. At least 2 dilutions that resulted in 40%-60% residual fVIII activity were used to calculate the inhibitor titers.
Clearance of wt fVIII, R372A/R1689A fVIII, and V634M fVIII in fVIII−/− mice
Wt fVIII, R372A/R1689A fVIII, and V634M fVIII were prepared at a dose of 1.0 μg and injected as described in “FVIII immunization regimens.” Blood was collected by terminal cardiac puncture 0.25, 0.5, 1, 2, and 4 hours after the final injection with 3 mice at each time point. FVIII antigen levels were measured with an ELISA with 2-116 as the capture mAb and biotinylated 1B5 as the detection antibody (see supplemental Methods for full details). Mouse plasma samples to be tested were incubated for 30 minutes at room temperature with 100mM β-mercaptoethanol and then serially diluted to 1/32, 1/64, 1/128, and 1/256. The dilutions were used as the antigens in the ELISA, and the amount of fVIII antigen was measured off a standard curve from the same fVIII construct.
Statistical analysis
Comparisons between groups were made with the Mann-Whitney U test and SigmaStat Version 3.5 (Systat). A P value < .05 was considered statistically significant.
Results
Characterization of inactive fVIII constructs R372A/R1689A fVIII and V634M fVIII
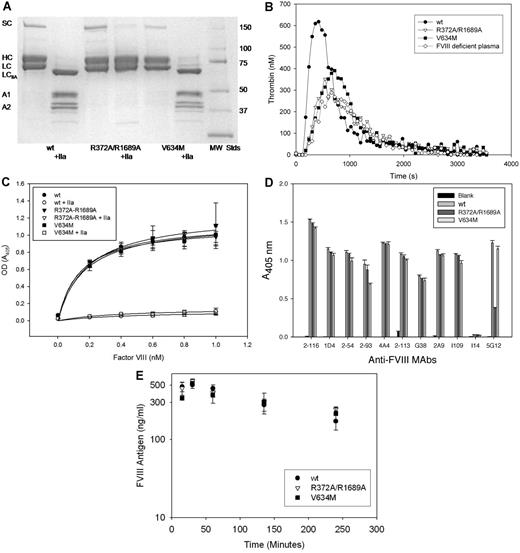
Two inactive BDD fVIII molecules were constructed to investigate the roles of fVIII activation, localization of fVIII at sites of hemostasis and inflammation, and fVIII release from VWF on the immunogenicity of fVIII in murine model systems. R372A/R1689A fVIII lacked thrombin and factor Xa cleavage sites at R372 and R1689. As a result, it did not undergo heavy chain or light cleavage by thrombin (Figure 1A). The specific procoagulant activity of R372A/R1689A fVIII was < 1% of wt fVIII, and it lacked detectable cofactor activity in a purified intrinsic factor Xase assay (Table 1). In addition, it did not correct the defect in endogenous thrombin potential or peak thrombin generation of fVIII-deficient plasma in a thrombin generation assay (Figure 1B; Table 1). Cleavage at R1689 in the light chain of fVIII was necessary for its dissociation from VWF.23 R372A/R1689A fVIII bound normally to VWF, but it did not dissociate from VWF on exposure to thrombin (Figure 1C). Therefore, it was unlikely that R372A/R1689A fVIII would localize to the phospholipid membrane at a hemostatic site.
Characterization of inactive fVIII constructs. (A) SDS-PAGE of wt fVIII, R372A/R1689A fVIII, and V634M fVIII with and without exposure to thrombin (factor IIa). MW STDS indicates molecular weight standards; SC, single chain fVIII; HC, fVIII heavy chain (A1-A2 domains); LC, fVIII light chain; LCIIa, thrombin-cleaved light chain; A1, A1 domain; and A2, A2 domain. (B) Thrombin generation in fVIII-deficient plasma reconstituted with 2 μg/mL wt fVIII, R372A/R1689A fVIII, or V634M fVIII. (C) Binding of wt fVIII, R372A/R1689A fVIII, or V634M fVIII to immobilized VWF in the presence or absence of exposure to thrombin detected by ELISA as described in “Binding of fVIII to VWF.” (D) ELISA of binding of wt fVIII, R372A/R1689A fVIII, or V634M fVIII to immobilized domain-specific anti-fVIII capture mAbs 2-116 (A1), 1D4 (A2), 2-54 (A2), 2-93 (A2), 4A4 (A2), 2-113 (A3), G38 (A3), 5G12, 2A9 (C1), I-109 (C2), and I14 (C2, negative control). Biotinylated I14 was used as the detection antibody. (E) Clearance of 1 μg of wt fVIII, R372A/R1689A fVIII, or V634M fVIII after tail-vein injection in FVIII−/− mice. Errors represent sample SDs.
Characterization of inactive fVIII constructs. (A) SDS-PAGE of wt fVIII, R372A/R1689A fVIII, and V634M fVIII with and without exposure to thrombin (factor IIa). MW STDS indicates molecular weight standards; SC, single chain fVIII; HC, fVIII heavy chain (A1-A2 domains); LC, fVIII light chain; LCIIa, thrombin-cleaved light chain; A1, A1 domain; and A2, A2 domain. (B) Thrombin generation in fVIII-deficient plasma reconstituted with 2 μg/mL wt fVIII, R372A/R1689A fVIII, or V634M fVIII. (C) Binding of wt fVIII, R372A/R1689A fVIII, or V634M fVIII to immobilized VWF in the presence or absence of exposure to thrombin detected by ELISA as described in “Binding of fVIII to VWF.” (D) ELISA of binding of wt fVIII, R372A/R1689A fVIII, or V634M fVIII to immobilized domain-specific anti-fVIII capture mAbs 2-116 (A1), 1D4 (A2), 2-54 (A2), 2-93 (A2), 4A4 (A2), 2-113 (A3), G38 (A3), 5G12, 2A9 (C1), I-109 (C2), and I14 (C2, negative control). Biotinylated I14 was used as the detection antibody. (E) Clearance of 1 μg of wt fVIII, R372A/R1689A fVIII, or V634M fVIII after tail-vein injection in FVIII−/− mice. Errors represent sample SDs.
Bioassay of purified fVIII constructs with comparison to fVIII-deficient plasma
| FVIII construct . | Specific activity, U/mg* . | Intrinsic factor Xase assay† . | Endogenous thrombin potential‡ . | Peak thrombin generation‡ . |
|---|---|---|---|---|
| wt fVIII | 8075 | 100 | 6880 ± 760 | 780 ± 140 |
| R372A/R1689A fVIII | 36 | < 1 | 5330 ± 740 | 260 ± 37 |
| V634M fVIII | 57 | < 1 | 5640 ± 610 | 420 ± 87 |
| fVIII-deficient plasma | 5120 ± 1220 | 280 ± 95 |
| FVIII construct . | Specific activity, U/mg* . | Intrinsic factor Xase assay† . | Endogenous thrombin potential‡ . | Peak thrombin generation‡ . |
|---|---|---|---|---|
| wt fVIII | 8075 | 100 | 6880 ± 760 | 780 ± 140 |
| R372A/R1689A fVIII | 36 | < 1 | 5330 ± 740 | 260 ± 37 |
| V634M fVIII | 57 | < 1 | 5640 ± 610 | 420 ± 87 |
| fVIII-deficient plasma | 5120 ± 1220 | 280 ± 95 |
FVIII activity was determined by 1-stage coagulation assay as described in “FVIII bioassays.”
FVIII-dependent intrinsic factor Xase activity was measured as described in “FVIII bioassays” and expressed relative to wt fVIII.
Thrombin generation assays were performed as described in “FVIII bioassays.” Errors represent sample SDs derived from triplicates from each of 2 independent experiments.
V634M fVIII is a severe hemophilia A mutation associated with normal fVIII antigen levels but < 1% coagulant activity.8 Unlike R372A/R1689A fVIII, V634M was cleaved normally by thrombin (Figure 1A) and dissociated from VWF after exposure to thrombin (Figure 1C) However, like R372A/R1689A fVIII, it had < 1% activity of wt fVIII by 1-stage coagulation assay or by purified intrinsic Xase assay (Table 1) and did not correct the defect in thrombin generation of fVIII-deficient plasma (Figure 1B; Table 1). Thus, V634M fVIII should localize to sites of hemostasis but not promote fibrin formation.
During purification, the chromatographic behavior of R372A/R1689A fVIII and V634M fVIII was indistinguishable from wt fVIII (supplemental Table 1), indicating that they maintain structural integrity. To examine their structural integrity further, the ability of the constructs to bind to a panel of 11 nonoverlapping mAbs that collectively recognize all the domains of BDD fVIII was investigated by ELISA. R372A/R1689A fVIII and V634M fVIII bound all mAbs similarly to wt fVIII, except for 5G12. 5G12, an anti-A3 mAb, bound to R372A/R1689A but with lower absorbance than wt fVIII and V634M. These results indicated that R372A/R1689A and V634M are structurally intact (Figure 1D). The clearance of R372A/R1689A, V634M, and wt fVIII was similar in fVIII−/− mice (Figure 1E), indicating that the potential differential immunogenicity of these constructs was not because of alternative clearance mechanisms.
Immunogenicity of low-dose wt fVIII and R372A/R1689A fVIII in fVIII−/− mice
The immunogenicity of wt fVIII and R372A/R1689A fVIII was compared in fVIII−/− mice with the use of doses similar to the dose of fVIII used in humans on the basis of body weight. In this low-dose model, mice received 6 weekly tail vein injections of 0.2 μg, followed by 2 injections of 0.5 μg. For mice injected with wt fVIII, 68% had positive ELISA titers with a mean titer of 1490 and a median titer of 400 (Figure 2A; Table 2). In contrast, R372A/R1689A fVIII produced positive ELISA titers in 40% of mice with a mean titer of 470 and a median titer of 0 (Figure 2A). The difference in ELISA titers between the 2 groups was significant (P = .03, Mann-Whitney test). FVIII inhibitor titers in mice injected with wt fVIII displayed a mean of 44 BU/mL and a median of 10 BU/mL compared with a mean of 4.3 BU/mL and a median of 0 in the R372A/R1689A fVIII group (Figure 2B). The difference in fVIII inhibitor titers between the 2 groups also was significant (P = .02, Mann-Whitney test). Although R372A/R1689A fVIII, which is not released from VWF after thrombin exposure, was less immunogenic than wt fVIII in this model, it retained significant immunogenicity with 40% of mice showing evidence of an immune response.
The antibody response to low-dose wt fVIII and R372A/R1689A fVIII in fVIII−/− mice. FVIII−/− mice were injected with 6 weekly doses of wt fVIII (n = 25) or R372A/R1689A fVIII (n = 25) of 0.2 μg, followed by 2 additional doses of 0.5 μg. One week after the last dose, plasma was collected for measurement of (A) total anti-fVIII IgG by ELISA and (B) fVIII inhibitor titers by the Bethesda assay. The difference in ELISA titers and inhibitor titers between the 2 groups was significant (P = .03 and 0.02, Mann-Whitney test). (C) The binding of biotinylated ESH4, a mAb that recognizes the classic C2 domain epitope overlapping a VWF binding site, was measured in the absence (filled circles) and presence (open triangles) of a 1/24 dilution of a high-titer plasma from a mouse immunized with R372A/R1689A fVIII. *P < .05.
The antibody response to low-dose wt fVIII and R372A/R1689A fVIII in fVIII−/− mice. FVIII−/− mice were injected with 6 weekly doses of wt fVIII (n = 25) or R372A/R1689A fVIII (n = 25) of 0.2 μg, followed by 2 additional doses of 0.5 μg. One week after the last dose, plasma was collected for measurement of (A) total anti-fVIII IgG by ELISA and (B) fVIII inhibitor titers by the Bethesda assay. The difference in ELISA titers and inhibitor titers between the 2 groups was significant (P = .03 and 0.02, Mann-Whitney test). (C) The binding of biotinylated ESH4, a mAb that recognizes the classic C2 domain epitope overlapping a VWF binding site, was measured in the absence (filled circles) and presence (open triangles) of a 1/24 dilution of a high-titer plasma from a mouse immunized with R372A/R1689A fVIII. *P < .05.
Comparative immunogenicity of wt fVIII, R372A/R1689A fVIII, and R372A/R1689A fVIII in fVIII−/− mice
| fVIII construct . | Dose, μg . | No. of ELISA titer positive . | Mean/median ELISA titer . | No. of inhibitor titer positive . | Mean/median inhibitor titer, BU/mL . |
|---|---|---|---|---|---|
| wt fVIII | 0.2* | 17/25 | 1490/400 | 13/25 | 44/10 |
| 0.5 | 2/9 | 9/0 | 1/9 | 1/0 | |
| 1.0 | 5/9 | 100/11 | 3/9 | 14/0 | |
| 1.5 | 9/9 | 460/426 | 9/9 | 100/73 | |
| 2.0 | 10/10 | 1540/1760 | 10/10 | 310/310 | |
| R372A/R1689A fVIII | 0.2* | 10/25 | 470/0 | 6/25 | 4/0 |
| 0.5 | 1/9 | 120/0 | 1/9 | 8/0 | |
| 1.0 | 5/9 | 170/16 | 3/9 | 29/0 | |
| 1.5 | 6/9 | 1170/74 | 6/9 | 160/8 | |
| 2.0 | 10/10 | 730/450 | 10/10 | 160/103 | |
| V634M fVIII | 0.5 | 3/9 | 190/0 | 1/9 | 11/0 |
| 1.0 | 5/9 | 140/10 | 4/9 | 16/0 | |
| 1.5 | 6/9 | 630/560 | 6/9 | 130/88 | |
| 2.0 | 9/10 | 2810/1480 | 9/10 | 450/290 |
| fVIII construct . | Dose, μg . | No. of ELISA titer positive . | Mean/median ELISA titer . | No. of inhibitor titer positive . | Mean/median inhibitor titer, BU/mL . |
|---|---|---|---|---|---|
| wt fVIII | 0.2* | 17/25 | 1490/400 | 13/25 | 44/10 |
| 0.5 | 2/9 | 9/0 | 1/9 | 1/0 | |
| 1.0 | 5/9 | 100/11 | 3/9 | 14/0 | |
| 1.5 | 9/9 | 460/426 | 9/9 | 100/73 | |
| 2.0 | 10/10 | 1540/1760 | 10/10 | 310/310 | |
| R372A/R1689A fVIII | 0.2* | 10/25 | 470/0 | 6/25 | 4/0 |
| 0.5 | 1/9 | 120/0 | 1/9 | 8/0 | |
| 1.0 | 5/9 | 170/16 | 3/9 | 29/0 | |
| 1.5 | 6/9 | 1170/74 | 6/9 | 160/8 | |
| 2.0 | 10/10 | 730/450 | 10/10 | 160/103 | |
| V634M fVIII | 0.5 | 3/9 | 190/0 | 1/9 | 11/0 |
| 1.0 | 5/9 | 140/10 | 4/9 | 16/0 | |
| 1.5 | 6/9 | 630/560 | 6/9 | 130/88 | |
| 2.0 | 9/10 | 2810/1480 | 9/10 | 450/290 |
P < .05 when compared with wt fVIII.
Epitope mapping with the use of domain-specific anti-fVIII antibodies found that both wt fVIII and R372A/R1689A fVIII produced polyclonal responses to both heavy and light chain epitopes (data not shown). Because R372A/R1689A fVIII was not released from VWF after exposure to thrombin, and because VWF bound to the fVIII C2 domain at a site that overlapped the binding site for classic anti-C2 antibodies,13 we determined whether VWF shielded the classic C2 epitope from antibody development. FVIII was immobilized on microtiter wells, and the binding of the biotinylated classic anti-C2 mAb, ESH4, was measured in the presence or absence of plasma from immunized fVIII−/− mice by ELISA. In Figure 2C, the rightward shift of the biotinylated ESH4 binding curve for 1 of the 4 high-titer fVIII inhibitor plasmas shown in Figure 2B indicated the presence of antibodies directed against classic C2 domain epitopes. Two of the 4 high-titer R372A/R1689A fVIII had anti-classic C2 domain antibodies that were detected with this method. Thus, the inability of fVIII to dissociate from VWF after thrombin cleavage did not protect against formation of classic anti-C2 antibodies.
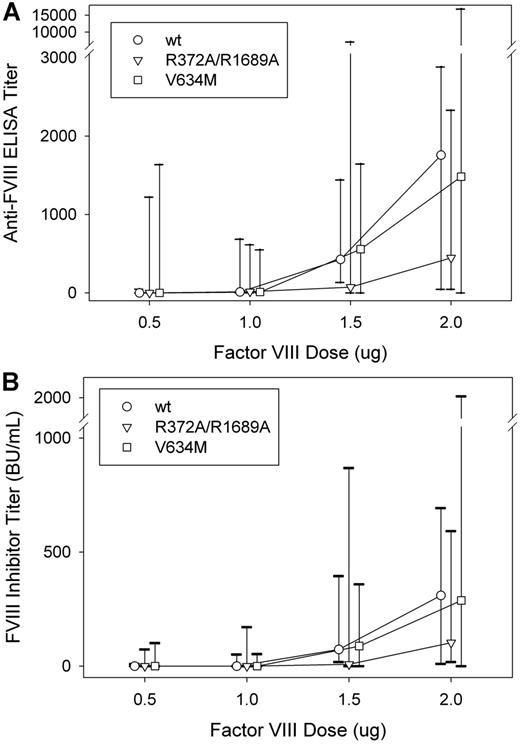
Dose-dependent immunogenicity of wt fVIII, R372A/R1689A fVIII, and V634M fVIII in fVIII−/− mice
To investigate the role of fVIII function and dose in the immunogenicity of fVIII further, we compared the dose-dependent immunogenicity of wt fVIII with R372A/R1689A fVIII and V634M fVIII. Immunogenicity was determined after 4 weekly injections of 0.5, 1.0, 1.5, or 2.0 μg fVIII, followed by an additional injection at twice the nominal dose (Figure 3; Table 2). A dose-dependent increase was observed in total anti-fVIII antibodies measured by ELISA and in the fVIII inhibitor titer for all 3 constructs. The median ELISA titer at 2.0 μg was 1760 for wt fVIII, 450 for R372A/R1689A fVIII, and 1480 for V634M fVIII. The anti-fVIII ELISA titers and fVIII inhibitor titers at each dose were not significantly different between either of the 2 inactive fVIII molecules and wt fVIII (P > .13 and P > .31, respectively, Mann-Whitney test). However, a trend was observed toward significance in the decreased immunogenicity observed in the 2.0-μg subgroup, comparing wt fVIII and R372A/R1689A fVIII (P = .14 for both anti-fVIII ELISA titers and fVIII inhibitors titers, Mann-Whitney test). In addition, no difference was observed in the isotype distribution of IgG1, IgG2a, IgG2b, and IgG3 antibodies in plasmas of mice from the 2.0-μg dosing groups (data not shown). The observation that the inactive R372A/R1689A and V634M fVIII molecules are immunogenic in fVIII−/− mice indicated that fVIII structure not function was the primary driver of immunogenicity. The marginal decreased immunogenicity of R372A/R1689A fVIII compared with wt fVIII suggested that VWF may be partially protective.
Dose-dependent immunogenicity of wt fVIII, R372A/R1689A fVIII, and V634M fVIII in fVIII−/− mice. Cohorts (n = 9-10) of fVIII−/− were injected with 4 weekly doses of fVIII construct (0.5, 1.0, 1.5, or 2.0 μg) weekly, followed by a single boost dose at twice the weekly dose. One week after the last dose plasma was collected for both (A) anti-fVIII ELISA titers and (B) fVIII inhibitor titers. Graphs show medians and interquartile ranges.
Dose-dependent immunogenicity of wt fVIII, R372A/R1689A fVIII, and V634M fVIII in fVIII−/− mice. Cohorts (n = 9-10) of fVIII−/− were injected with 4 weekly doses of fVIII construct (0.5, 1.0, 1.5, or 2.0 μg) weekly, followed by a single boost dose at twice the weekly dose. One week after the last dose plasma was collected for both (A) anti-fVIII ELISA titers and (B) fVIII inhibitor titers. Graphs show medians and interquartile ranges.
Immunogenicity of wt fVIII, R372A/R1689A fVIII, and V634M fVIII in fVIII−/−/VWF−/− mice
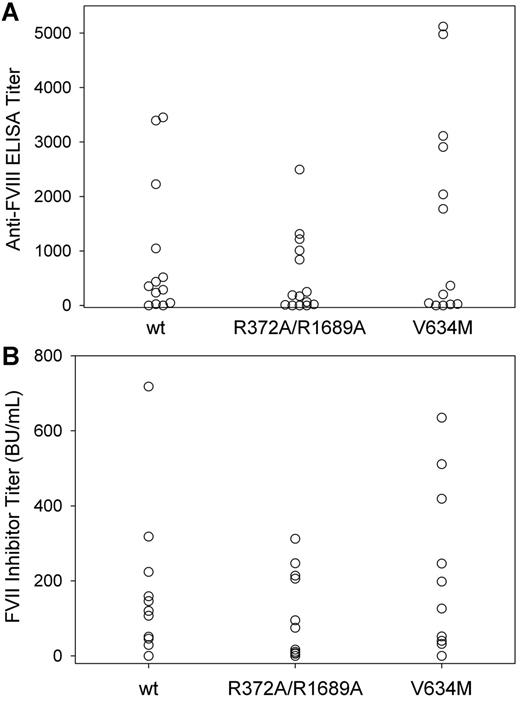
To investigate further the role of VWF in the immunogenicity of fVIII, we compared the immunogenicity of wt fVIII, R372A/R1689A fVIII, and V634M fVIII in fVIII−/−/VWF−/− mice. In initial experiments, using the low-dose wt fVIII regimen described above, none of 4 fVIII−/−/VWF−/− mice treated with wt fVIII and none of 3 fVIII−/−/VWF−/− mice treated with R372A/R1689A fVIII produced anti-fVIII ELISA titers > 40. The lack of an immune response in fVIII−/−/VWF−/− mice contrasted with the immune response to wt fVIII and R372A/R1689A fVIII observed in fVIII−/− mice (Figure 2A). Therefore, a higher dose regimen that compared wt fVIII, R372A/R1689A fVIII, and V634M was used, consisting of 6 weekly injections of 0.6 μg, followed by 2 additional injections of 1.5 μg. With this regimen, most of the fVIII−/−/VWF−/− mice in all 3 cohorts had detectable anti-fVIII antibodies (Figure 4A) and fVIII inhibitors (Figure 4B). Eighty-five percent of the mice had positive ELISA titers in the wt fVIII cohort compared with 79% for R372A/R1689A fVIII and 85% for V634M fVIII (Figure 4A). The median ELISA titers were similar for each group at 350 for wt fVIII, 180 for R372A/R1689A fVIII, and 360 for V634M fVIII. The inhibitor titers also were similar for each group with a median inhibitor titer of 110 BU/mL for wt fVIII, 46 BU/mL for R372A/R1689A fVIII, and 200 BU/mL for V634M fVIII (Figure 4B). The differences in anti-fVIII antibody and fVIII inhibitor titers between wt fVIII and R372A/R1689A fVIII and V634M fVIII were not statistically significant. This result was consistent with the conclusion that fVIII structure and not function is the primary determinant of immunogenicity. In addition, the finding that R372A/R1689A fVIII was equally immunogenic to wt fVIII in fVIII−/−/VWF−/− mice indicated that the slightly decreased immunogenicity seen in the fVIII−/− mice was probably because of small protective effect of VWF on presentation of fVIII to the immune system.
The antibody response to wt fVIII, R372A/R1689A fVIII, or V634M fVIII in fVIII−/−/VWF−/− mice. FVIII−/−/VWF−/− mice were injected with 6 weekly doses of wt fVIII (n = 13), R372A/R1689A fVIII (n = 14), or V634M fVIII (n = 13) at 0.6 μg, followed by 2 weekly doses of 1.5 μg. One week after the last dose plasma was collected for both (A) anti-fVIII ELISA titers and (B) fVIII inhibitor titers. The differences in anti-fVIII antibody and fVIII inhibitor titers between wt fVIII and R372A/R1689A fVIII and V634M fVIII were not statistically significant.
The antibody response to wt fVIII, R372A/R1689A fVIII, or V634M fVIII in fVIII−/−/VWF−/− mice. FVIII−/−/VWF−/− mice were injected with 6 weekly doses of wt fVIII (n = 13), R372A/R1689A fVIII (n = 14), or V634M fVIII (n = 13) at 0.6 μg, followed by 2 weekly doses of 1.5 μg. One week after the last dose plasma was collected for both (A) anti-fVIII ELISA titers and (B) fVIII inhibitor titers. The differences in anti-fVIII antibody and fVIII inhibitor titers between wt fVIII and R372A/R1689A fVIII and V634M fVIII were not statistically significant.
Discussion
In this study, we found that R372A/R1689A fVIII was marginally less immunogenic and that V634M fVIII was equally immunogenic as wt BDD fVIII in mouse model systems. V634M fVIII is associated with a severe hemophilia A mutation and has < 1% of the specific procoagulant of wt fVIII (Table 1; supplemental Methods), yet is cleaved normally (Figure 1A) and dissociates from VWF after exposure to thrombin (Figure 1C). The inability of V634M fVIII to function as a procoagulant, while retaining the immunogenic potential of wt fVIII, indicates that the highly immunogenic nature of fVIII is independent of downstream events that lead to thrombin production and the development of an inflammatory milieu.
This conclusion is in sharp contrast to that of Skupsky et al,4 who found that a heat-inactivated fVIII preparation was less immunogenic than wt fVIII and concluded that the immunogenicity of fVIII was primarily linked to its procoagulant function. Although there was no apparent reduction in the T-cell epitopes in the heat-inactivated fVIII preparation, there was a significant denaturation of B-cell epitope structure. These properties of heat-denatured fVIII are consistent with the fact that T-cell epitopes are short, linear peptides that are resistant to heat denaturation, whereas most B-cell epitopes are conformationally dependent on the overall fold of a protein. Protein denaturation and loss of antigenic structure of fVIII could lead to procoagulant function–independent reduction in immunogenicity compared with the native protein for several reasons. Antibodies are produced by plasma cells, which are the progeny of a direct differentiation pathway or from a memory B-cell pool that each start with naive B cells. Activation of naive and memory B cells into their differentiation pathways is initiated by binding of the intact, native antigen to the surface immunoglobulin (sIg) component of the B-cell receptor. Skupsky et al found that heat denaturation of fVIII led to destruction of the B-cell epitopes for mAbs ESH4, 1B5, and 3E6,4 which recognize the phospholipid and VWF-binding region of the fVIII C2 domain,13 mAbs 2-77 and 3G6, which recognize the so-called nonclassic C2 inhibitor epitope,13 and Abs 413, 4A4, and 2-76, which recognize an immunodominant inhibitory A2 epitope.24,25 Thus, heat denaturation of an antigen could lead to a loss of immunogenicity by destruction of the sIg B-cell epitopes that are required for B-cell activation and differentiation.
B-cell differentiation in response to soluble protein antigens requires CD4+ T-cell help, in which peptide antigen-MCH II complex on the B-cell surface binds to the T-cell receptor on antigen-specific T cells. Antigen-specific T cells are produced from naive T cells after engagement of their T-cell receptors by peptide-MCH II complex on APCs, which include DCs (the “professional” APC), macrophages, and B cells along with appropriate costimulation. The mannose receptor (CD206) has been implicated in endocytosis of fVIII by DCs,26 suggesting that intact, nondenatured fVIII may be required for efficient antigen presentation. Binding of fVIII to an additional CD206-independent DC endocytic receptor has been reported.27 This interaction was blocked by mAb KM33, which recognizes phospholipid binding C1 domain residues 2092-2093.28 Skupsky et al found that heat denaturation of fVIII led to destruction of the B-cell epitope for the mAb 2A9, an anti-C1 mAb.4 Thus, native C1 structure, which was destroyed by heat treatment in the study by Skupsky et al,4 may be important for antigen presentation by DCs.
Binding of antigen to high-affinity sIg on B cells leads to antigen presentation at concentrations of free antigen that are significantly lower than those required for presentation by DCs and macrophages, which lack sIg.29 In addition, DCs engulf intact antigen by macropinocytosis, store it in endocytic compartments, and release it in secondary lymphoid organs, where it binds the sIg on B cells.30 These results further suggest that native antigen is necessary for efficient antibody production. Thus, the decrease in immunogenicity of fVIII after heat-denaturation observed by Skupsky et al may have resulted from destruction of intrinsic structural elements in fVIII that are independent of its procoagulant function but are required for its immunogenicity.4 The observation by Skupsky et al that the immune response to fVIII is decreased in fVIII−/− mice received anticoagulation agents of warfarin or hirudin is consistent with the hypothesis that the coagulation process provides an immunogenic milieu.4 However, our results indicate that the immunogenicity of fVIII is predominantly independent of its procoagulant function.
R372A/R1689A fVIII has < 1% of the specific procoagulant activity of wt fVIII but, unlike V634M fVIII, does not dissociate from VWF on exposure to thrombin (Figure 1C). Thus, R372A/R1689A fVIII is a tool to address the question of whether dissociation of activated fVIII from VWF plays a role in the immune response to fVIII. There was a reduction in immunogenicity of R372A/R1689A fVIII compared with wt fVIII in fVIII−/− mice (Figures 2A-B and 3). This decrease in immunogenicity may be a result of inhibition of antigen presentation of fVIII when bound to VWF. Dasgupta et al found that VWF decreases the endocytosis of fVIII by human DCs and antigen presentation to fVIII-specific T cells.31 Consistent with this, they found interaction between fVIII and the CD206 mannose receptor was inhibited by VWF.
The C2 domain and the acidic NH2-terminal region of the light chain of fVIII determine the high-affinity binding of fVIII to VWF.32 The binding of fVIII to phospholipid membranes involves an interaction with the C2 domain that overlaps the VWF binding site. A large component of the immune response to fVIII in humans and fVIII−/− mice typically includes inhibitory antibodies directed to this site.13,20,33,34 Thus, VWF may have the additional anti-immunogenic property of inhibiting recognition of fVIII by B cells that recognize this immunodominant C2 epitope. Although there was a marginal reduction in immunogenicity, all mice treated with the highest dose of R372A/R1689A fVIII developed an immune response. This residual activity could be from the intrinsic dissociation rate of fVIII from VWF in the absence of thrombin or to factors intrinsic to the structure of fVIII that are maintained. Thus, these data suggest that the protective effect of VWF is minor compared with other mechanisms that drive the immunogenicity of fVIII.
Injection of fVIII−/−/VWF−/− mice with wt fVIII or R372A/R1689A fVIII with the use of the dosing schedule for fVIII−/− mice described in Figure 1 failed to produce an immune response, suggesting that the presence of VWF contributes to the strongly immunogenic nature of fVIII. Alternatively, the greater C57BL/6 background in fVIII−/−/VWF−/− mice may contribute to this difference. VWF potentially brings all fVIII constructs to a hemostatic site even if R372A/R1689A fVIII is not released from the VWF. Cleavage of wt fVIII and V634M fVIII by thrombin may produce an additional marginal increase in immunogenicity relative to R372A/R1689A fVIII. In addition, the circulatory lifetime of fVIII in the presence of normal plasma levels of VWF is similar to that of VWF. In contrast, the circulatory lifetime of fVIII in humans markedly decreases in the absence of VWF.35 Likewise, the clearance of fVIII in VWF−/− mice is markedly faster than in mice with normal levels of VWF.36 This result indicates that human fVIII forms a complex with murine VWF after exogenous administration. In addition, these results indicated that the clearance of exogenous human fVIII in humans and mice is largely governed by the clearance of human and murine VWF, respectively. The clearance receptor for VWF has not been identified, although studies in mice indicate that macrophages play a prominent role.37 As with other blood-born antigens, intravenous injection of human fVIII into fVIII−/− mice results in uptake by the spleen, with preferential uptake by marginal zone macrophages.38 Overall, these results indicate that in VWF+/+ mice, human fVIII binds circulating VWF and is cleared by splenic macrophages by an unknown VWF receptor, whereas in VWF−/− mice, fVIII is cleared by a different mechanism. Several candidate VWF-independent clearance receptors for fVIII have been identified, including low-density lipoprotein receptor-related protein, the low-density lipoprotein receptor, heparan-sulfate proteoglycans, and the asialoglycoprotein receptor.39,40 In the absence of VWF, clearance of fVIII by any 1 of these receptors may lead to degradation of fVIII in a process that does not include antigen presentation. Thus, VWF may decrease the uptake of fVIII by APCs, yet paradoxically may be necessary to prevent clearance of fVIII by pathways that do not promote antigen presentation.
Cross-linking of sIg on B cells is the classic mechanism by which signals are produced that lead to B-cell differentiation into memory and antibody-secreting cells.41 Yet, if individual B cells have identical sIg molecules on their surface, it is difficult to see how antigens with nonrepetitive structures, such as fVIII, could lead to sIg cross-linking. VWF is a multimer that contains multiple repetitive binding sites for fVIII,42 which could promote cross-linking of fVIII-specific B-cell receptors leading to anti-fVIII antibody development.
Another possible reason for the striking immunogenicity of fVIII is that intrinsic structural elements, including B-cell or T-cell epitopes, are particularly well recognized by the immune system. FVIII-specific T-cell responses have been readily identified in humans43 and fVIII−/− mice.44 Although it has been difficult to identify T-cell epitopes that are clearly associated with fVIII inhibitor formation, Steinitz et al recently identified 8 dominant T-cell epitopes associated with antibody production in a humanized MHC class II murine hemophilia A model.45 Studies with model small immunogens indicate that nearly the entire surface of a protein is potentially antigenic.46 Consistent with this, analysis of B-cell epitopes in the C2 domain after immunization of fVIII−/− mice with fVIII found a continuous spectrum of overlapping epitopes.13 Nonetheless, immunodominant B-cell epitopes in fVIII appear to exist, including the classic and nonclassic C2 domain epitopes13,20 and an A2 domain epitope bounded by residues 484-508.24 Conceivably, the immune response to fVIII initially may be focused on these or other B-cell epitope or unidentified immunodominant T-cell epitopes. The immune response to fVIII then may expand by epitope spreading to produce the observed polyclonal response.47
In summary, our study that used structurally intact inactive fVIII molecules indicates that a main component of the immune response to fVIII is independent of its procoagulant function. The immune response is both positively and negatively affected by its association with VWF and may involve intrinsic elements of fVIII structure. Further delineation of the features of fVIII that make it immunogenic may lead to better tolerization strategies for patients with hemophilia A.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Denisa Wagner for her generous gift of VWF−/− mice.
This work was supported by the National Institutes of Health grants U54 HL112309 and K08 HL102262 (S.L.M.); grants U54 HL112309, R01 HL082609, and R01 HL040921 (P.L.); and Hemophilia of Georgia Inc (S.L.M. and P.L.).
National Institutes of Health
Authorship
Contribution: S.L.M. designed and performed research, analyzed data, and co-wrote the paper; C.L.C., J.F.H., E.T.P., B.S.D., B.G., and R.T.B. designed and performed research; P.L. designed research, analyzed data, and co-wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pete Lollar, Emory Children's Center, Rm 426D, 2015 Uppergate Dr, Atlanta, GA 30322; e-mail: jlollar@emory.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal