Abstract
Bacteria can enter the bloodstream in response to infectious insults. Bacteremia elicits several immune and clinical complications, including thrombocytopenia. A primary cause of thrombocytopenia is shortened survival of platelets. We demonstrate that pathogenic bacteria induce apoptotic events in platelets that include calpain-mediated degradation of Bcl-xL, an essential regulator of platelet survival. Specifically, bloodstream bacterial isolates from patients with sepsis induce lateral condensation of actin, impair mitochondrial membrane potential, and degrade Bcl-xL protein in platelets. Bcl-xL protein degradation is enhanced when platelets are exposed to pathogenic Escherichia coli that produce the pore-forming toxin α-hemolysin, a response that is markedly attenuated when the gene is deleted from E coli. We also found that nonpathogenic E coli gain degrading activity when they are forced to express α-hemolysin. Like α-hemolysin, purified α-toxin readily degrades Bcl-xL protein in platelets, as do clinical Staphylococcus aureus isolates that produce α-toxin. Inhibition of calpain activity, but not the proteasome, rescues Bcl-xL protein degradation in platelets coincubated with pathogenic E coli including α-hemolysin producing strains. This is the first evidence that pathogenic bacteria can trigger activation of the platelet intrinsic apoptosis program and our results suggest a new mechanism by which bacterial pathogens might cause thrombocytopenia in patients with bloodstream infections.
Introduction
Bacteremia is a leading cause of morbidity and mortality in the United States and worldwide.1,2 Risk factors for bacteremia include indwelling catheters,3 trauma,4 and surgery.5 Bloodstream infections also are found frequently in patients with malignancies,6 endocarditis,7 and urinary tract infections.8 In severe cases, bacteremia elicits a vigorous immune response that results in sepsis and septic shock.9
Bloodstream infections often are accompanied by thrombocytopenia.10 Adverse clinical outcomes commonly are observed in thrombocytopenic patients with documented bacteremia, and the severity of thrombocytopenia is associated with an increase in the rate of mortality of patients in the intensive care unit.10,11 It generally is presumed that the infectious milieu induces thrombocytopenia by activating platelets that subsequently deposit in microvascular thrombi or get cleared from the circulation.12 Indeed, bacteria or bacterial products can induce platelet activation by directly or indirectly binding to surface receptors such as integrin αIIbβ3, GPIb, and TLRs.13 However, the precise mechanisms that underpin bacteremia-induced thrombocytopenia remain poorly defined.
In addition to the biochemical pathways that regulate classic platelet functional responses such as aggregation and adhesion, it has been demonstrated recently that megakaryocytes and platelets possess an intrinsic apoptosis program.14,15 Key components of the platelet apoptotic pathway include the prosurvival protein Bcl-xL and its prodeath counterparts Bak and Bax. Genetic ablation of Bcl-xL in mice markedly reduces the life span of circulating platelets,14 and pharmacologic inhibition of Bcl-xL with the BH3-mimetic compounds ABT-737 or ABT-263 triggers platelet apoptosis and thrombocytopenia in mice, dogs, and humans.14,16-21 Deletion of Bak and Bax corrects the defects caused by loss of Bcl-xL, renders platelets refractory to the effects of ABT-737, and results in an almost doubling of steady-state platelet life span in vivo.15,22,23 Thus, Bcl-xL is critical for platelet survival. Nevertheless, it is unclear how this essential factor is regulated, and compelling evidence for time-dependent or signal-dependent Bcl-xL degradation in platelets has yet to emerge.24 Here, we demonstrate that pathogenic bacteria differentially induce calpain-dependent Bcl-xL degradation, providing the first direct evidence that the infectious milieu influences the programmed cell death pathway in human platelets.
Methods
Studies involving platelets and bacteria
All of the studies were conducted in accordance with the Declaration of Helsinki and were approved by the University of Utah Institutional Review Board. As previously described,25 washed platelets were freshly isolated from healthy human subjects who consented to participate in the studies. Contaminating leukocytes were removed from the platelet preparations by CD45-positive selection (Miltenyi Biotec), and the purified platelets were resuspended in M199 culture medium (Lonza).
Platelets were incubated with soluble agonists that included A23187 (EMD Biosciences), platelet-activating factor (PAF; Sigma-Aldrich), thrombin (Sigma-Aldrich), lipopolysaccharide (LPS; Sigma-Aldrich), and established uropathogenic Escherichia coli (see Table 1 and Mulvey et al,26 Wiles et al,27 and Bauer and Welch28 for descriptions of each strain). In select studies, platelets also were incubated with E coli or Staphylococcus aureus bacteria that were isolated from the bloodstream of septic patients (Table 1).
Bacterial strains
| Strain . | Description . | Hemolytic activity* . | Reference . |
|---|---|---|---|
| UTI89 | Cystitis Isolate | ++ | Mulvey et al26 |
| UTI89 ΔhlyA (MM746) | UTI89 hlyA::kan (sw) | − | Wiles et al27 |
| UTI89 Δcnf1 | UTI89 cnf1::clm | ++ | Wiles et al27 |
| WAM582 | K-12 strain DH1 with pSF4000 plasmid | ++ | Bauer and Welch28 |
| WAM783 | K-12 strain DH1 with pSF4000 ΔBamHI plasmid | − | Bauer and Welch28 |
| EC-SP† | Bloodstream isolate | Unknown | This study |
| SA-SP‡ | Bloodstream isolate | ++ | This study |
| Strain . | Description . | Hemolytic activity* . | Reference . |
|---|---|---|---|
| UTI89 | Cystitis Isolate | ++ | Mulvey et al26 |
| UTI89 ΔhlyA (MM746) | UTI89 hlyA::kan (sw) | − | Wiles et al27 |
| UTI89 Δcnf1 | UTI89 cnf1::clm | ++ | Wiles et al27 |
| WAM582 | K-12 strain DH1 with pSF4000 plasmid | ++ | Bauer and Welch28 |
| WAM783 | K-12 strain DH1 with pSF4000 ΔBamHI plasmid | − | Bauer and Welch28 |
| EC-SP† | Bloodstream isolate | Unknown | This study |
| SA-SP‡ | Bloodstream isolate | ++ | This study |
Hemolytic activity was scored as the development of a clear zone around bacterial colonies grown on blood agar plates.
E coli isolated from the bloodstream of a septic patient (EC-SP).
S aureus isolated from the bloodstream of a septic patient (SA-SP).
Similar to recent work,25 a portion of the bacteria were expanded on blood agar plates overnight at 37°C until they reached a stationary growth phase. The bacteria were resuspended in PBS, and their concentration was determined by colorimetry (VITEK Colorimeter; bioMerieux). The bacteria were then resuspended in M199 culture medium.
Bacteria (4.2 × 106/mL) typically were incubated with freshly isolated platelets (1 × 108/mL) resulting in a ratio of 1:24 (bacteria/platelets). Decreasing the ratio of bacteria to platelets did not significantly alter Bcl-xL degradation patterns. In select studies, the platelets were preincubated with MG132 (EMD Biosciences), a cell-permeable inhibitor that primarily targets chymotrypsin-like activity of the proteasome but also inhibits calpains29 or more specific proteasome inhibitors that include lactacystin (EMD Biosciences) or epoxomicin (EMD Biosciences). Platelets also were preincubated with the calpain inhibitor MDL28170 (Tocris Bioscience), calpeptin (EMD Biosciences), E64d (EMD Biosciences), or EGTA (Sigma-Aldrich). At the end of the designated time period, the platelets were prepared for protein analyses as described in the next section.
Stored platelets
Apheresed platelets were obtained from the ARUP Blood Transfusion Services at the University of Utah. The platelets were immediately placed in standardized storage bags and banked under constant agitation in a climate-controlled chamber (Melco Engineering) that was maintained between 20 and 24°C. On select days, samples of the ex vivo–aged platelets were removed to assess Bcl-xL protein expression.
Protein expression analyses
Bcl-xL protein expression was determined by ELISA and Western blot. At the end of each experiment, platelets were centrifuged, and the cell pellets were subsequently lysed in RIPA or Laemmli buffer for ELISA or Western blot analysis, respectively. The Bcl-xL ELISA was from R&D Systems. The antibody used to detect Bcl-xL protein by Western blot was from BD Biosciences. Antibodies against Bax and actin were from Abcam. The 20S proteasome activity assay was from Millipore.
Mitochondrial membrane potential
Platelets were incubated with bacteria for designated times to determine whether morphologic changes suggestive of apoptosis decreased mitochondrial membrane potential (ΔΨm) in platelets. Twenty minutes before the end of each experiment, Mito PT JC-1 (ImmunoChemistry) was added to the cell suspension. Impaired mitochondrial membrane potential (loss of FL2 and gain of FL1 signal) was subsequently assessed by flow cytometry.
Microscopy
Platelets and E coli were cultured in suspension followed by fixation in 4% paraformaldehyde in PBS buffer. Platelets were stained with phalloidin to image polymerized actin, whereas E coli were visualized with topro-3, a specific DNA dye. High-resolution confocal reflection microscopy was performed with an Olympus IX81, FV300, and a FV1000 (Olympus) confocal-scanning microscope equipped with a 60 × /1.42 NA oil objective for viewing platelets. An Olympus FVS-PSU/IX2-UCB camera and scanning unit and Olympus Fluoview FV 300 and FV1000 Version 5.0 image acquisition software were used for recording. The images were further analyzed with the use of Adobe Photoshop CS Version 8.0, Metamorph Version 7.0 software (Molecular Devices), and ImageJ Version 1.47c (National Institutes of Health).
For the ultrastructural analyses, platelets and E coli were cultured in suspension followed by fixation in 2.5% glutaraldehyde in PBS buffer. The samples were subsequently washed and postfixed with 2% osmium tetroxide, rewashed, dehydrated by a graded series of acetone concentrations (50%, 70%, 90%, 100%; 2 × 10 minutes each), and embedded in Epon. Thin sections were counterstained (ie, uranyl acetate and lead citrate), viewed with a JEOL JEM-1011 electron microscope (JEOL), and digital images were captured with a side-mounted Advantage HR CCD camera (Advanced Microscopy Techniques).
Statistics
For relevant studies, we calculated the mean ± SEM and performed ANOVAs to identify differences among multiple experimental groups. If significant differences existed, a Student Newman-Keuls posthoc procedure was used to determine the location of the difference between groups. Statistical significance was set at P < .05.
Results
E coli induces apoptotic-like features in platelets
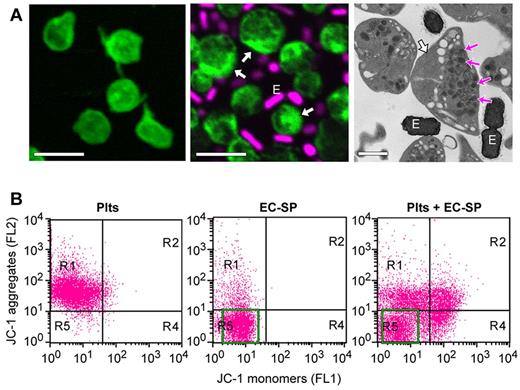
To determine how bacteria regulate platelet function, we isolated E coli from the bloodstream of a patient diagnosed with sepsis. This strain of E coli, referred to as EC-SP (Table 1), was expanded as described in “Methods” and then coincubated with freshly isolated human platelets. As shown in Figure 1A, EC-SP interacts with and forms rosettes around platelets. Infection with EC-SP is accompanied by condensation of actin toward the cell periphery (see white arrows in the middle and right panels of Figure 1A) and parallel movement of granules to the opposite side of platelets. Despite being translocated away from condensed actin, platelet granules did not display features of overt morphologic vacuolation (see magenta arrows in the right panel of Figure 1). Actin condensation also was detected readily in platelets incubated with pore-forming bacteria, but in contrast to EC-SP, these bacterial strains induced morphologic vacuolation of granules rather than peripheral translocation (data not shown and Kraemer et al25 ). Actin condensation or changes in granular translocation or vacuolation were not observed in platelets that were cultured alone (Figure 1A left panel; and data not shown).
E coli induces apoptotic-like features in platelets. (A) Platelets were incubated for 8 hours alone (left) or with E coli (EC-SP), an isolate from the bloodstream of a patient diagnosed with sepsis (middle and right). Left and middle: Confocal microscopy of polymerized actin (phalloidin, green stain) and DNA (topro 3, magenta stain) in platelets and EC-SP (E), respectively. Far right: Transmission electron micrograph of the platelet and EC-SP (E) suspension cultures. The white arrows point to lateral condensation of polymerized actin, whereas the magenta arrows (right) identify granules that have moved to one side of the cell. Scale bars = 2 μm in the left and middle, 1 μm in the right. These micrographs are representative of 3 independent experiments. (B) Mitochondrial membrane potential (ΔΨm) in platelets or EC-SP that were cultured (8 hours) alone (left and middle) or together (right). Bacteria population in middle and right panels is indicated by green box. This flow cytometric experiment is representative of 3 independent studies. The green box identifies the major population of EC-SP.
E coli induces apoptotic-like features in platelets. (A) Platelets were incubated for 8 hours alone (left) or with E coli (EC-SP), an isolate from the bloodstream of a patient diagnosed with sepsis (middle and right). Left and middle: Confocal microscopy of polymerized actin (phalloidin, green stain) and DNA (topro 3, magenta stain) in platelets and EC-SP (E), respectively. Far right: Transmission electron micrograph of the platelet and EC-SP (E) suspension cultures. The white arrows point to lateral condensation of polymerized actin, whereas the magenta arrows (right) identify granules that have moved to one side of the cell. Scale bars = 2 μm in the left and middle, 1 μm in the right. These micrographs are representative of 3 independent experiments. (B) Mitochondrial membrane potential (ΔΨm) in platelets or EC-SP that were cultured (8 hours) alone (left and middle) or together (right). Bacteria population in middle and right panels is indicated by green box. This flow cytometric experiment is representative of 3 independent studies. The green box identifies the major population of EC-SP.
Next, we examined mitochondrial membrane potential (ΔΨm) in platelets using the JC-1 indicator dye. Unlike control platelets (ie, cultured alone), ΔΨm was significantly decreased in platelets that were coincubated with EC-SP (Figure 1B). As shown in Figure 1B, the JC-1 dye was specific for platelets because minimal fluorescence was observed in EC-SP (middle panel as indicated by green box).
E coli degrades Bcl-xL protein in platelets
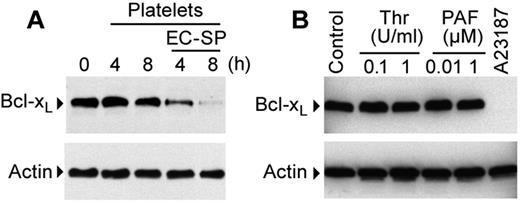
We subsequently determined whether EC-SP altered the expression of Bcl-xL, an essential regulator of platelet survival and apoptosis.14,22 EC-SP induced Bcl-xL degradation over the course of several hours (Figure 2A). In contrast, Bcl-xL protein was unchanged in platelets that were cultured alone (Figure 2A). Similarly, Bcl-xL protein levels did not decrease during a 7-day period in apheresed platelets that were placed in standardized transfusion bags and stored under blood bank conditions (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
EC-SP degrades Bcl-xL protein in platelets. (A) Western blot analysis of Bcl-xL protein expression in platelets incubated with EC-SP (4 or 8 hours). (B) Western blot analysis of platelets stimulated with vehicle (control), thrombin (Thr), PAF, or A23187 (1μM) for 8 hours. The Western blots in panels A and B are representative of 2-4 independent experiments for each experimental group.
EC-SP degrades Bcl-xL protein in platelets. (A) Western blot analysis of Bcl-xL protein expression in platelets incubated with EC-SP (4 or 8 hours). (B) Western blot analysis of platelets stimulated with vehicle (control), thrombin (Thr), PAF, or A23187 (1μM) for 8 hours. The Western blots in panels A and B are representative of 2-4 independent experiments for each experimental group.
Degradation of Bcl-xL was not driven by purified LPS, an endotoxin found in the outer membrane of gram-negative bacteria, in the presence or absence of LPS-binding protein and soluble CD14 (supplemental Figure 2). Similarly, thrombin or PAF did not alter Bcl-xL protein expression (Figure 2B and supplemental Figure 2). However, degradation was induced by A23187 (Figure 2B and supplemental Figure 2), a calcium ionophore that induces a range of responses in platelets that includes triggering of the intrinsic apoptosis pathway.23,30 Unlike EC-SP–mediated degradation that occurred over hours, A23187 reduced the expression of Bcl-xL protein as early as 15 minutes (supplemental Figures 3 and 4A). A23187 induced Bcl-xL degradation occurred in a concentration-dependent manner (supplemental Figure 3). Unlike Bcl-xL, minimal degradation of Bax protein was observed during a period of 1 hour (supplemental Figure 4A).
Bacteria that produce pore-forming toxins invoke Bcl-xL protein degradation
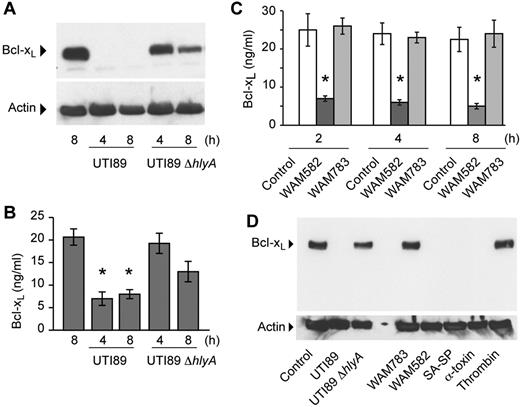
To assess whether bacterial strains differentially influence Bcl-xL degradation, we incubated platelets with the human E coli isolate UTI89. This pathogenic strain expresses filamentous adhesive organelles (type 1 pili) and secretes cytotoxic necrotizing factor 1 and α-hemolysin (HlyA), a pore-forming toxin.27 UTI89 markedly induced degradation of Bcl-xL as assessed by Western blot analysis (Figure 3A) and quantification of the protein by intracellular ELISA (Figure 3B), as did a strain (UTI89Δcnf1) deficient in cytotoxic necrotizing factor 1 (data not shown). Degradation of Bcl-xL, but not Bax, occurred as early as 1 hour (supplemental Figures 4B and 5) and required live bacteria because heat-killed UTI89 did not degrade Bcl-xL protein in platelets (supplemental Figure 6). UTI89 did not dramatically alter the expression of actin up to 8 hours (Figure 3A,D and supplemental Figure 4B).
E coli that express HlyA invoke Bcl-xL protein degradation in platelets. (A) Platelets were incubated with wild-type UTI89 or UTI89 ΔhlyA bacteria for 4 or 8 hours and Bcl-xL and actin protein were assessed by Western blot analysis. (B) Platelets were incubated with wild-type UTI89 or UTI89 ΔhlyA bacteria for 4 or 8 hours and intracellular Bcl-xL protein was quantified by ELISA. The bars in the graph are the mean ± SEM (n = 3) and the single asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with all other conditions. (C) Platelets were incubated with WAM582 or WAM783 for 2, 4, or 8 hours, and Bcl-xL protein was assessed by ELISA. The bars in the graph are the mean ± SEM (n = 3), and the asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels in WAM582 treated platelets compared with all other conditions. (D) Platelets were incubated with wild-type UTI89, UTI89 ΔhlyA, WAM783, WAM582, and S aureus isolated from the bloodstream of a patient diagnosed with sepsis (SA-SP), α-toxin (500 ng/mL), or thrombin (1 U/mL). After 8 hours, protein for Bcl-xL and actin were assessed by Western blot analysis. The Western blots in this figure are representative of 2 independent experiments for each group.
E coli that express HlyA invoke Bcl-xL protein degradation in platelets. (A) Platelets were incubated with wild-type UTI89 or UTI89 ΔhlyA bacteria for 4 or 8 hours and Bcl-xL and actin protein were assessed by Western blot analysis. (B) Platelets were incubated with wild-type UTI89 or UTI89 ΔhlyA bacteria for 4 or 8 hours and intracellular Bcl-xL protein was quantified by ELISA. The bars in the graph are the mean ± SEM (n = 3) and the single asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with all other conditions. (C) Platelets were incubated with WAM582 or WAM783 for 2, 4, or 8 hours, and Bcl-xL protein was assessed by ELISA. The bars in the graph are the mean ± SEM (n = 3), and the asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels in WAM582 treated platelets compared with all other conditions. (D) Platelets were incubated with wild-type UTI89, UTI89 ΔhlyA, WAM783, WAM582, and S aureus isolated from the bloodstream of a patient diagnosed with sepsis (SA-SP), α-toxin (500 ng/mL), or thrombin (1 U/mL). After 8 hours, protein for Bcl-xL and actin were assessed by Western blot analysis. The Western blots in this figure are representative of 2 independent experiments for each group.
Targeted disruption of the hlyA gene in UTI89 (UTI89 ΔhlyA) revealed that HlyA increases the rate and magnitude of Bcl-xL protein degradation in platelets compared with wild-type UTI89 (Figure 3A). We did, however, observe partial degradation over time with the UTI89 ΔhlyA strain, indicating that pathogenic E coli can degrade Bcl-xL protein through HlyA-independent mechanisms.
The pore-forming activity of HlyA requires HlyC to form transmembrane pores.28,31 Incubation of platelets with WAM582, a nonpathogenic K-12 laboratory strain of E coli that encodes for the wild-type hlyA operon, induced Bcl-xL degradation within 2 hours (Figure 3C). In contrast, WAM783, which carries the hlyA operon lacking hlyC, failed to induce Bcl-xL degradation in incubations lasting up to 8 hours (Figure 3C). These results demonstrate that pore formation by HlyA facilitates Bcl-xL degradation. They also indicate that nonpathogenic bacteria have minimal effects on Bcl-xL protein expression in platelets.
Many pathogenic bacteria express pore-forming toxins, including α-toxin, which is produced by S aureus. α-toxin creates permeable pores that are ∼1-4 nm in diameter, similar in size to those formed by HlyA.32 Purified α-toxin, at concentrations that activate platelets,33 induced Bcl-xL degradation (Figure 3D). Consistent with this finding, incubation of platelets with a pathogenic strain of S aureus (SA-SP), which was isolated from the bloodstream of a patient diagnosed with sepsis, also induced Bcl-xL degradation (Figure 3D). These results indicate that gram-negative and gram-positive bacteria, which express pore-forming toxins, induce Bcl-xL degradation.
Calpains regulate bacterial-induced BclxL degradation in platelets
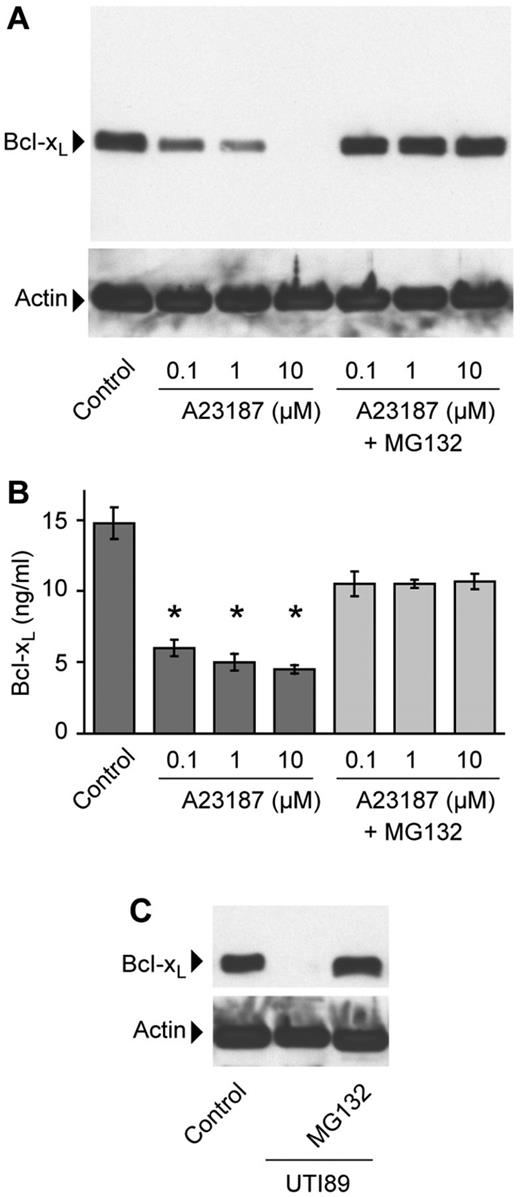
We identified distinct cleavage products by Western blot analysis after platelets were treated with UTI89, suggesting that specific proteases were involved in Bcl-xL degradation (supplemental Figure 7). Therefore, we treated platelets with MG132 and found that it rescued A23187-induced Bcl-xL degradation in platelets (Figure 4A-B). Likewise, MG132 rescued Bcl-xL degradation induced by UTI89 E coli isolates that produce HlyA (Figure 4C and supplemental Figure 7).
Inhibition of calpain activity rescues Bcl-xL degradation in platelets. (A-B) Platelets were stimulated with increasing concentrations of A23187 in the presence or absence of MG132 (10μM). After 1 hour, Bcl-xL and actin protein were assessed by Western blot analysis (A) or intracellular Bcl-xL protein was measured by ELISA (B). The bars in panel B represent the mean ± SEM of 4 independent experiments, and the asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control and MG132-treated platelets. (C) Platelets were pretreated with or without MG132 and then incubated alone (control) or with UTI89 for 2 hours. Bcl-xL and actin protein in this blot and panel A are representative of 3 independent experiments.
Inhibition of calpain activity rescues Bcl-xL degradation in platelets. (A-B) Platelets were stimulated with increasing concentrations of A23187 in the presence or absence of MG132 (10μM). After 1 hour, Bcl-xL and actin protein were assessed by Western blot analysis (A) or intracellular Bcl-xL protein was measured by ELISA (B). The bars in panel B represent the mean ± SEM of 4 independent experiments, and the asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control and MG132-treated platelets. (C) Platelets were pretreated with or without MG132 and then incubated alone (control) or with UTI89 for 2 hours. Bcl-xL and actin protein in this blot and panel A are representative of 3 independent experiments.
To decipher whether the inhibitory properties of MG132 occurred through proteasome- or calpain-dependent pathways, we preincubated platelets with proteasome-specific inhibitors. As shown in Figure 5A and B, all 3 of the calpain inhibitors (ie, MDL28170, calpeptin, or E64d) rescued Bcl-xL from degradation in ionophore-stimulated platelets. In contrast, neither of the proteasome inhibitors prevented the degradation of Bcl-xL even though they neutralized intracellular 20S proteasome activity in platelets (3900 ± 500 relative fluorescence units in vehicle-treated platelets vs 750 ± 150 and 420 ± 70 in lactacystin- and epoxomicin-treated platelets, respectively). Similarly, inhibition of calpain activity, but not proteasome activity, rescued Bcl-xL from degradation in platelets coincubated with UTI89 (Figure 5C-D; also see supplemental Figure 7). We also found that chelation of extracellular calcium with EGTA prevented A23187 or UTI89 from degrading Bcl-xL protein in platelets (Figure 5E).
Inhibition of calpain activity rescues E coli–induced Bcl-xL degradation in platelets. (A-B) Platelets were left alone or pretreated with specific inhibitors (10μM epoxomicin [EPO]; 25μM lactacystin [Lact]; 10μM MG132; 50μM calpeptin [Calp]; 50μM E64d; and 50μM MDL28170 [MDL]) before being stimulated with A23187 (1μM) for 1 hour and intracellular Bcl-xL protein was assessed by Western analysis (A) or quantified by ELISA (B). The bars for panel B show the mean ± SEM of 4 independent experiments, and the asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control. (C-D) Platelets were pretreated with inhibitors (A and B) and then left alone (control) or incubated with wild-type UTI89 for 4 hours. Intracellular Bcl-xL protein was subsequently assessed by Western analysis (C) or quantified by ELISA (D). The bars for panel D depict the mean ± SEM of 4 independent experiments, and the single asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control. (E) Platelets were incubated in the presence or absence of EGTA (5mM) for 15 minutes and then left alone or stimulated with 1μM A23187, UTI89, or UTI89 ΔhlyA for 2 hours and Bcl-xL protein was assessed by Western analysis. This blot is representative of 3 independent experiments. (F) Whole blood was incubated with UTI89 in the presence or absence of calpeptin. After 4 hours, platelets were isolated, and intracellular Bcl-xL protein was measured by ELISA. The bars represent the mean ± SEM of 3 independent experiments, and the single asterisk indicates a significant decrease (P < .05) in Bcl-xL protein levels compared with control.
Inhibition of calpain activity rescues E coli–induced Bcl-xL degradation in platelets. (A-B) Platelets were left alone or pretreated with specific inhibitors (10μM epoxomicin [EPO]; 25μM lactacystin [Lact]; 10μM MG132; 50μM calpeptin [Calp]; 50μM E64d; and 50μM MDL28170 [MDL]) before being stimulated with A23187 (1μM) for 1 hour and intracellular Bcl-xL protein was assessed by Western analysis (A) or quantified by ELISA (B). The bars for panel B show the mean ± SEM of 4 independent experiments, and the asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control. (C-D) Platelets were pretreated with inhibitors (A and B) and then left alone (control) or incubated with wild-type UTI89 for 4 hours. Intracellular Bcl-xL protein was subsequently assessed by Western analysis (C) or quantified by ELISA (D). The bars for panel D depict the mean ± SEM of 4 independent experiments, and the single asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control. (E) Platelets were incubated in the presence or absence of EGTA (5mM) for 15 minutes and then left alone or stimulated with 1μM A23187, UTI89, or UTI89 ΔhlyA for 2 hours and Bcl-xL protein was assessed by Western analysis. This blot is representative of 3 independent experiments. (F) Whole blood was incubated with UTI89 in the presence or absence of calpeptin. After 4 hours, platelets were isolated, and intracellular Bcl-xL protein was measured by ELISA. The bars represent the mean ± SEM of 3 independent experiments, and the single asterisk indicates a significant decrease (P < .05) in Bcl-xL protein levels compared with control.
Finally, we determined whether calpain-dependent pathways regulate Bcl-xL degradation in platelets isolated from bacteria-inoculated whole blood. As shown in Figure 5F, Bcl-xL protein was degraded in platelets isolated from incubations of UTI89 and whole blood. Degradation of Bcl-xL, however, was rescued when whole blood was pretreated with a calpain inhibitor (Figure 5F).
Discussion
In the present study, we showed for the first time that E coli or S aureus isolated from the bloodstream of patients with sepsis induces Bcl-xL protein degradation in platelets. Bcl-xL protein also was degraded rapidly by uropathogenic E coli that produce HlyA. In contrast, nonpathogenic and heat-inactivated bacteria failed to degrade Bcl-xL protein. These results are consistent with previous reports in which the authors demonstrated that individual strains of bacteria differentially modulate platelet adhesion and/or aggregation.13 They also identify a previously unrecognized mechanism by which bacteria can compromise platelet function and survival.
During the last decade in Europe, the number of E coli and S aureus infections in the bloodstream increased by 71% and 34%, respectively.34 As reported here and elsewhere,35 E coli bloodstream isolates induce morphologic alterations and changes in platelet mitochondrial function that are consistent with apoptosis. We also show that bacteria differentially induce degradation of Bcl-xL protein by platelets. This finding provides the first demonstration that platelets degrade Bcl-xL in response to a pathogenic organism. Bcl-xL protein degradation has been observed and linked to apoptosis in nucleated cells. It has been shown that calcium overload or ultraviolet radiation B induces Bcl-xL protein degradation and apoptosis in lung and basal carcinoma cells, respectively.36,37
In addition to E coli and S aureus isolated from the bloodstream of sepsis patients, a strain of HlyA-positive E coli from a patient with a urinary tract infection induced a rapid and intense degradation of Bcl-xL protein in platelets. Targeted disruption of HlyA in this strain significantly decreased Bcl-xL degradation in platelets. This finding suggests that HlyA-positive E coli, which frequently invade the bloodstream and are associated with increased clinical severity compared with toxin-negative strains,27,38 have potent apoptotic effects on platelets. HlyA is a 107-kDa protein that induces hemolysis and apoptosis in erythrocytes by creating pores in membranes.32 HlyA also induces apoptosis in neutrophils, monocytes, and endothelial cells.39-41 In epithelial cells, HlyA can stimulate degradation of multiple host proteins and inactivation of the prosurvival host kinase Akt.27,42
The current studies extend the apoptotic effects of HlyA to platelets and demonstrate a new link to Bcl-xL protein degradation. They also suggest that HlyA triggers degradation of Bcl-xL, in part, by forming pores in platelets. This conclusion is determined on the basis of studies in which authors used HlyA-producing E coli that express or lack hemolysin C. Previous groups have shown that HlyA inserts itself into host membranes, but it is unable to form pores in the absence of hemolysin C.28,31 When hemolysin C was absent, a nonpathogenic HlyA-positive strain of E coli (WAM783) failed to degrade Bcl-xL. In contrast, nonpathogenic E coli that coexpress HlyA and hemolysin C (WAM582) gain robust Bcl-xL degrading capacity.
As it forms pores, HlyA triggers the entry of calcium into cells43,44 that presumably promotes Bcl-xL degradation. Consistent with this notion, the treatment of platelets with the calcium ionophore A23187 induced rapid and sustained Bcl-xL degradation, a response that was blocked by calcium chelation. In previous studies, authors have shown that calcium ionophores induce calpain activation in lung carcinoma cells, and ubiquitous calpains are capable of cleaving Bcl-xL.36 Indeed, inhibition of calpain activity prevented A23187 from degrading Bcl-xL protein in platelets. A similar inhibition was observed in platelets that were coincubated with strains of E coli that express HlyA. Together, these results indicate that pore-forming E coli induce calpain activity in platelets and subsequent Bcl-xL protein degradation. Induction of calpain activity in platelets has also been linked to other markers of platelet apoptosis.45
Like HlyA, bacterial-derived α-toxin forms pores in target cells and elicits hemolysis.46 α-toxin also activates platelets and triggers cytoskeletal reorganization.33,47 In the present study we found that S aureus–derived α-toxin induces Bcl-xL protein degradation. Similarly, a bloodstream isolate of S aureus, which produces α-toxin (see Table 1 and data not shown), induced Bcl-xL protein degradation in platelets. This result indicates that pore-forming exotoxins are capable of inducing Bcl-xL degradation and raises the possibility that other bacterial toxins, such as shiga-toxin, β-hemolysin, and porB, will have a similar effect on platelets.
Although similarities may exist, the mechanisms by which gram-negative and -positive bacteria drive degradation may be distinct because, unlike UTI89, S aureus does not impair mitochondrial membrane potential in platelets under the conditions of our experiments (data not shown). We also found that cleavage of protease-activated receptors by thrombin, which has previously been shown to induce intracellular calcium transients in platelets,48 does not promote Bcl-xL degradation. Likewise, factors present in the septic milieu such as LPS and PAF do not alter Bcl-xL protein expression in platelets. Altogether, these data support the concept that the signaling pathways leading to platelet activation, apoptosis, and degradation are complex and, in many cases, distinct from one another.
It is intriguing to speculate that bacteria accelerate the clearance of platelets and induce thrombocytopenia in infectious diseases through mechanisms that involve Bcl-xL degradation. This scenario is supported by genetic-based studies demonstrating that deficiencies in Bcl-xL reduce the life span of platelets and cause thrombocytopenia in mice.14 Presumably, bacterial-induced degradation of Bcl-xL protein would provide an opportunity for Bak and Bax to become activated and trigger mitochondrial outer membrane permeabilization. The net result would be rapid clearance of compromised platelets and thrombocytopenia.
Platelets have important roles in the pathogenesis of infective endocarditis and sepsis, and thrombocytopenia is a strong predictor of mortality in these syndromes as well as in other infectious diseases.9,12,49 The current data demonstrate that gram-negative and -positive strains of bacteria target degradation of the prosurvival protein Bcl-xL in platelets. These studies provide the first clear evidence that bacteria can activate the intrinsic apoptosis pathway in platelets. Our results underscore the importance of measures that prevent bacterial contamination in stored platelet concentrates. Moreover, they garner momentum for more in-depth investigations of signals that trigger programmed cell death pathways in platelets that may reveal new therapeutic targets for the treatment of sepsis, endocarditis, and other infectious diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Diana Lim for preparing the figures, Alex Greer for editorial assistance, Peter Seizer for thoughtful discussions and contributions, and Estelle Harris for supplying bacterial strains from clinical patients.
The work in this report was supported by grants from the National Institutes of Health (R01s HL66277, HL91754, HL44525, and HL90870; K23 HL92161 and U54HL112311; and AI095647 AI090369, and AI088086), a grant from the ARUP Institute for Clinical and Experimental Pathology, a Hematology Training grant (5T32DK007115-35), the American Heart Association (0625098Y), an operating grant from the Canadian Institutes of Health Research (CIHR, MOP-81208), a Pfizer Grant of the German Cardiac Society, the FöFoLe-program of the University of Munich, the Australian National Health and Medical Research Council, the Sylvia and Charles Viertel Foundation, and an Operational Infrastructure Support Grant from the Victorian State Government of Australia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
National Institutes of Health
Authorship
Contribution: B.F.K., R.A.C., H.S., B.T.K., M.A.M, G.A.Z., and A.S.W. conceived and designed the experiments; B.F.K., R.A.C., Z.G.F., A.V.d.A., K.G., B.K.D., and W.H.A.K. performed experiments; B.F.K., R.A.C., H.S., B.T.K., and A.S.W. analyzed the data; M.T.R., W.H.A.K., M.A.M., R.C.B., G.A.Z., and A.S.W. provided resources for experiments; and B.F.K., R.A.C., H.S., Z.G.F., B.T.K., M.T.R., W.H.A.K., M.A.M, R.C.B., G.A.Z., and A.S.W. wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew S. Weyrich, PhD, Professor of Internal Medicine, Eccles Institute of Human Genetics, Bldg 533, Rm 4220, University of Utah, Salt Lake City, UT 84112; e-mail: andy.weyrich@u2m2.utah.edu.

![Figure 5. Inhibition of calpain activity rescues E coli–induced Bcl-xL degradation in platelets. (A-B) Platelets were left alone or pretreated with specific inhibitors (10μM epoxomicin [EPO]; 25μM lactacystin [Lact]; 10μM MG132; 50μM calpeptin [Calp]; 50μM E64d; and 50μM MDL28170 [MDL]) before being stimulated with A23187 (1μM) for 1 hour and intracellular Bcl-xL protein was assessed by Western analysis (A) or quantified by ELISA (B). The bars for panel B show the mean ± SEM of 4 independent experiments, and the asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control. (C-D) Platelets were pretreated with inhibitors (A and B) and then left alone (control) or incubated with wild-type UTI89 for 4 hours. Intracellular Bcl-xL protein was subsequently assessed by Western analysis (C) or quantified by ELISA (D). The bars for panel D depict the mean ± SEM of 4 independent experiments, and the single asterisk indicates a significant decrease (P < .01) in Bcl-xL protein levels compared with control. (E) Platelets were incubated in the presence or absence of EGTA (5mM) for 15 minutes and then left alone or stimulated with 1μM A23187, UTI89, or UTI89 ΔhlyA for 2 hours and Bcl-xL protein was assessed by Western analysis. This blot is representative of 3 independent experiments. (F) Whole blood was incubated with UTI89 in the presence or absence of calpeptin. After 4 hours, platelets were isolated, and intracellular Bcl-xL protein was measured by ELISA. The bars represent the mean ± SEM of 3 independent experiments, and the single asterisk indicates a significant decrease (P < .05) in Bcl-xL protein levels compared with control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/25/10.1182_blood-2012-04-420661/4/m_zh89991200120005.jpeg?Expires=1765893636&Signature=cQTy9dYElRfsNnfc9twOppD37Oox5X-~0yz8UNbRCIYMqqOa6H-xioIhEvxB-0ySnxN5JwZVcsaNdViA6ctiC3xfgGxly7gpMdCM1k5~6xCOpkbf6HYDnFfcx2gD5xHr4v08AkJM4QjLGjoChQjiUXIC5IT4ngPDtILhpuWfejAp7cPJhFVGvQxslpcvhibJImr7JuDN8cxQ0kKjuDlULSLl1xi3IQfQKa1gJFcKZj1btK5kRLgwGNlqoASNHc-WgPfar3YAUH~-ZiE~yviXpZnj~lNTHwxjW82t3NGF8eQY2B9RgoJIvbf87PMHZdpU4CP3XNKG9dD5UAF8wYOOWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)