Key Points
Genetic lupus risk factors enhance HSC repopulation capacity.
Inflammatory cytokines and HMGB1 in lupus mice modify HSC function.
Abstract
Hematopoietic stem cells (HSCs) are protected in a metabolically dormant state within the bone marrow stem cell niche. Inflammation has been shown to disrupt HSC dormancy and cause multiple functional changes. Here, we investigated whether HSC functions were altered in systemic lupus erythematosus (SLE)-prone mice and whether this contributed to clinical manifestations of SLE. We found that HSCs were significantly expanded in lupus mice. The increase in HSC cellularity was caused by both genetic lupus risk factors and inflammatory cytokines in lupus mice. In addition, the inflammatory conditions of lupus led to HSC mobilization and lineage-biased hematopoiesis. Strikingly, these functionally altered HSCs possessed robust self-renewal capacity and exhibited repopulating advantages over wild-type HSCs. A single-nucleotide polymorphism in the cdkn2c gene encoding p18INK4c within a SLE susceptibility locus was found to account for reduced p18INK4c expression and the increase in HSC self-renewal capacity in lupus mice. Lupus HSCs with enhanced self-renewal capacity and resistance to stress may compete out transplanted healthy HSCs, thereby leading to relapses after HSC transplantation.
Introduction
The hematopoietic system is constantly replenished throughout life from a rare population of pluripotent hematopoietic stem cells (HSCs). Maintaining a lifelong stable pool of functionally competent HSCs is achieved through a delicate balance between quiescence vs proliferation and self-renewal vs differentiation. Under steady state, most HSCs are quiescent and only a small fraction of HSCs are self-renewing or differentiating into rapidly proliferating progenitors that give rise to the entire population of blood cells.1,2 The dormancy of HSCs has been shown to be essential for preserving stemness because excessive proliferation of HSCs compromises their regenerative activity and eventually leads to bone marrow (BM) failure.3 Quiescence and self-renewal of HSCs are tightly regulated by both cell-intrinsic signals and environmental factors derived from the surrounding BM stem cell niche.4,5 However, this niche-signal–mediated enforcement of HSC dormancy can be subverted by a number of stress conditions, such as chemotherapy, radiation, or severe bleeding.6,7 In response to blood cell loss, HSCs grow rapidly and differentiate to restore the injured hematopoietic system. Likewise, transient neutropenia caused by bacterial and viral infections also stimulates HSC expansion.8,9 However, unlike HSCs that generate balanced populations of blood cells after cytoablation, HSCs exposed to microbial infections selectively lose lymphoid potential and produce more myeloid lineage cells.10-14 HSCs in animals with microbial infections are also more inclined to migrate to and accumulate in extramedullary tissues.15,16 Thus, responses and functional changes of HSCs to different types of hematopoietic insults are highly context dependent.
Lymphopenia or leukopenia is also frequently observed in patients with autoimmune diseases, such as systemic lupus erythematosus (SLE).17 The mechanisms underlying these clinical manifestations have not been well studied. Although autoantibodies may cause leukocyte apoptosis,18 it is also possible that leukopenia in SLE patients may reflect an HSC disorder. Many inflammatory cytokines induced by microbial infections are also elevated in the serum of SLE patients. For instance, interferon α, produced by innate immune cells upon pathogen stimulation, is known to be a key cytokine for SLE pathogenesis.19,20 In addition, high serum levels of proinflammatory cytokines interleukin (IL)-6, IL-10, and tumor necrosis factor α (TNFα) are found in patients with SLE and with microbial infections.21-24 Moreover, receptors for these cytokines are expressed by HSCs and signaling through these receptors can promote HSC proliferation and modify HSC functions.25-29 In this regard, it is conceivable that lupus conditions may impair the functional integrity of HSCs as infection does, and the answer to this issue will inform strategies for HSC transplantation based treatment of severe and refractory SLE.
In this study, we investigated HSC function in a lupus mouse model. Our findings show that HSCs in mice with active lupus have undergone similar functional alterations as are seen in chronically infected animals. These include a greatly expanded HSC population in the BM, abnormal accumulation of HSCs in the periphery, and a biased hematopoietic output skewing toward the myeloid than the lymphoid lineage. Interestingly, whereas all these changes of HSC function can be induced by common inflammatory cytokines in both lupus and infected mice, HSCs in lupus mice also exhibit a unique feature. Unlike infected animals in which loss of dormancy compromises regenerative activity of HSCs, lupus mice possess an expanded HSC population imbued with enhanced self-renewal capacity, and a single-nucleotide polymorphism in the SLE susceptibility locus downregulating p18INK4c accounts for this enhanced repopulating ability.
Materials and methods
Mice
C57BL/6 Ly5.2 (CD45.2), B6.SJL Ly5.1 (CD45.1), and BcN/LmoJ triple-congenic (TC) lupus-prone mice (The Jackson Laboratory) are maintained under specific pathogen-free conditions in the animal facility of The Feinstein Institute for Medical Research. All animal experiments were performed in accordance with approved Institutional Animal Care and Use Committee protocols.
Retroviral vector construction and viral infection
p18INK4c complimentary DNA (cDNA) was cloned into the retroviral expression vector MSCV-GFP to generate the murine stem cell virus (MSCV)-p18 construct. Retrovirus was produced by calcium phosphate transfection of 293FT cells (Invitrogen) with MSCV-p18 and a helper plasmid pCL-Eco. Virus was collected 36 hours after transfection and spinoculated onto RetroNectin-coated plates (Takara). BM cells were harvested on day 5 after 5-FU treatment (3 mg/mouse) and cultured for 24 hours in Dulbecco’s modified Eagle medium supplemented with IL-3 (10 ng/mL), IL-6 (10 ng/mL), and stem cell factor (50 ng/mL), followed by transduction in retrovirus binding plates for 48 hours in the presence of cytokines. Retrovirus-transduced BM cells were then transplanted into irradiated recipient mice.
Transplantation
Eight- to 12-week-old B6.SJL recipient mice received a total 9.5 Gy irradiation from an x-ray source (RS-2000 Biological Irradiator) delivered in 2 split doses with a 4-hour interval. For noncompetitive transplantation assays, 2 × 106 BM cells from lupus TC mice or age-matched B6 mice were injected intravenously into the irradiated recipients. For competitive transplantation assays, 106 BM cells from healthy TC mice were mixed with 106 BM cells from B6.SJL mice and cotransferred into irradiated recipients. For serial transplantation assays, 2 × 106 BM cells from either lupus TC mice or B6 controls were injected into the recipients. Four months later, 2 × 106 BM cells collected from the primary recipients were transferred into secondary recipients. Two more rounds of transplantation were carried out at 2- to 3-month intervals.
Flow cytometry
All antibodies were obtained from eBioscience unless otherwise stated. For detecting LSK cells, cells were stained with eFluor450-conjugated antibodies against lineage markers (B220, CD3, CD4, CD8, CD11b, Gr1, and TER119) together with biotinylated anti-CD150, anti–CD48- fluorescein isothiocyanate (FITC), anti–c-Kit-PE-Cy7, and anti-Sca-1-APC. Biotinylated anti-CD150 was revealed by streptavidin-APC-Cy7. To analyze myeloid progenitors, cells were stained with the same antibodies used for detecting LSK cells together with additional anti–IL-7Rα-PE, anti–CD34-Alexa Fluor 700, and anti–FcγRIIb-FITC (BD Biosciences). The lymphoid and myeloid lineage cells in the BM were examined by anti–B220-APC-eFluor 780 and anti–Gr1-PE. Flow cytometry was performed on a BD LSR II (BD Biosciences) and analyzed with FlowJo software (Tree Star).
BrdU incorporation
BrdU (5-bromo-2′-deoxyuridine) was injected intraperitoneally at 1 mg/mouse. BM cells were collected 16 hours later and stained for cell surface markers, followed by intracellular staining with anti-BrdU using the FITC BrdU flow kit (BD Biosciences).
Quantitative real-time RT-PCR
RNA was isolated from total BM cells or LSK cells using the RNeasy Micro Kit (QIAGEN). cDNA was made using an iScript cDNA synthesis kit (Bio-Rad). Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed with a Power SYBR Green PCR kit (Applied Biosystems) on the LightCycler 480 system (Roche). The sequences of the primers used for qRT-PCR are listed in Table 1.
Primer sequences
| Genes . | Forward 5′-3′ (or ID) . | Reverse 5′-3′ . |
|---|---|---|
| RNA pol2a | Mm00839493_m1 | — |
| MX1 | Mm00487796_m1 | — |
| β-actin | AAGGAGATTACTGCTCTGGCTCCTA | ACTCATCGTACTCCTGCTTGCTGAT |
| p18 | AATAGATCTATGGCCGAGCCTTGGGGG | AATGAATTCTCACTGCAGGCTTGTGGCTC |
| p19 | CTGAACCGCTTTGGCAAGAC | ATCATGCACAGGACTAGTACC |
| p21 | CTGTCTTGCACTCTGGTGTCTGAG | TTTTCTCTTGCAGAAGACCAATCTG |
| p27 | TTTAATTGGGTCTCAGGCAAACTCT | CCGTCTGAAACATTTTCTTCTGTTC |
| Gfi-1 | GGGCGGCGGCTCCTACAAAT | TCAAAGCTGCGTTCCTGGGAGT |
| Bmi-1 | TGACTGTGATGCACTTGAGAAAGTT | GTAGGCAATGTCCATTAGCGTGTAG |
| PTEN | CAGACCCGTGGCACTGCTGTTT | ATGAACTTGTCCTCCCGCCGC |
| IL-6 | GTTGCCTTCTTGGGACTGATG | GGGAGTGGTATCCTCTGTGAAGTCT |
| IL-10 | GGACTTTAAGGGTTACTTGGGTTGC | TCTTATTTTCACAGGGGAGAAATCG |
| TNFα | GGGTGATCGGTCCCCAAAGGG | GTGGTTTGCTACGACGTGGGC |
| Genes . | Forward 5′-3′ (or ID) . | Reverse 5′-3′ . |
|---|---|---|
| RNA pol2a | Mm00839493_m1 | — |
| MX1 | Mm00487796_m1 | — |
| β-actin | AAGGAGATTACTGCTCTGGCTCCTA | ACTCATCGTACTCCTGCTTGCTGAT |
| p18 | AATAGATCTATGGCCGAGCCTTGGGGG | AATGAATTCTCACTGCAGGCTTGTGGCTC |
| p19 | CTGAACCGCTTTGGCAAGAC | ATCATGCACAGGACTAGTACC |
| p21 | CTGTCTTGCACTCTGGTGTCTGAG | TTTTCTCTTGCAGAAGACCAATCTG |
| p27 | TTTAATTGGGTCTCAGGCAAACTCT | CCGTCTGAAACATTTTCTTCTGTTC |
| Gfi-1 | GGGCGGCGGCTCCTACAAAT | TCAAAGCTGCGTTCCTGGGAGT |
| Bmi-1 | TGACTGTGATGCACTTGAGAAAGTT | GTAGGCAATGTCCATTAGCGTGTAG |
| PTEN | CAGACCCGTGGCACTGCTGTTT | ATGAACTTGTCCTCCCGCCGC |
| IL-6 | GTTGCCTTCTTGGGACTGATG | GGGAGTGGTATCCTCTGTGAAGTCT |
| IL-10 | GGACTTTAAGGGTTACTTGGGTTGC | TCTTATTTTCACAGGGGAGAAATCG |
| TNFα | GGGTGATCGGTCCCCAAAGGG | GTGGTTTGCTACGACGTGGGC |
RNA pol2a, RNA polymerase IIa.
CFU assay
Splenocytes from TC or B6 mice were seeded in MethoCult M3434 medium (StemCell Technologies) at 2 × 105 cells/plate in 35-mm Petri dishes. Colonies were scored 14 days after culture.
Cytokine and HMGB1 measurement
Cytokine production in TC and B6 mice was examined using a mouse cytokine antibody array (RayBiotech) following the manufacturer’s instruction. Serum levels of IL-6 and IL-10 were further measured by the MSD multispot cytokine assay (Meso Scale Discovery). Briefly, serum samples were added to the plate and incubated at room temperature for 1 hour. After addition of detection antibodies, the plate was read on the SECTOR Imager (Meso Scale Discovery). Serum levels of high-mobility group protein B1 (HMGB1) were determined by an enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (Shino-Test).
Results
Mice with active lupus have an expanded HSC population with enhanced self-renewal capacity
Sle1.2.3 TC mice carry 3 major lupus susceptibility loci on the C57BL/6 (B6) background.30 A total of 98% of female TC mice develop severe systemic autoimmunity and nephritis by 6 to 8 months of age.31 To determine whether a chronic inflammatory condition of lupus affects HSC function, we selected 8- to 13-month-old female TC mice that had proteinuria and >30 μg/mL anti–double-stranded DNA immunoglobulin G as mice with active lupus (referred to hereinafter as lupus TC mice). Age-matched female B6 mice served as controls.
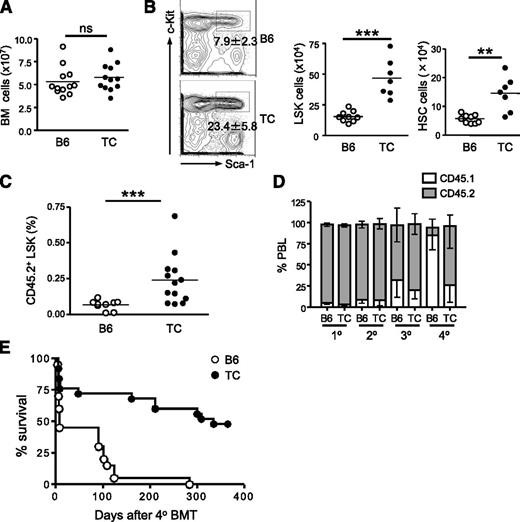
Lupus TC mice had equivalent numbers of total nucleated BM cells compared with B6 mice (Figure 1A). Within the lineage marker-negative population (Lin−), hematopoietic stem and progenitor cells (HSPCs) are enriched in the c-Kit+ Sca-1+ compartment (LSK cells). We noticed that the LSK population was increased 3-fold in the BM of lupus TC mice and the cellularity of long-term HSCs (CD150+CD48− LSK cells) was increased approximately 2.5-fold (Figure 1B). To confirm that the expanded population of phenotypic HSCs in lupus TC mice contained true repopulating HSCs, we carried out a transplantation assay. Equal numbers of BM cells from lupus TC or age-matched B6 mice (CD45.2+) were transferred into irradiated recipient mice (CD45.1+) and donor engraftment was analyzed 4 months later. As shown in Figure 1C, lupus LSK cells repopulated the recipient BM better than B6 LSK cells did. To determine whether enhanced engraftment resulted from a greater self-renewal capacity of HSCs from lupus TC mice, we performed a serial BM transplantation study by infusing the primary irradiated hosts (CD45.1) with the same number of BM cells from either lupus TC or age-matched B6 mice (CD45.2) and then sequentially transplanting BM cells collected from the prior hosts for an additional 3 rounds at an interval of 2 to 3 months. A decline of hematopoietic regeneration by wild-type (WT) donor cells was evident at the third round and became prominent at the fourth round of BM transplantation. In contrast, BM cells from lupus TC mice sustained hematopoiesis after quaternary BM transplantation (Figure 1D). Whereas 48% of recipient mice repopulated by BM cells from lupus TC mice survived at least a year after quaternary BM transplantation, all host mice died within 10 months after receiving B6 BM cells from tertiary hosts, indicating that long-term hematopoietic regeneration capacity is maintained in HSCs from lupus TC mice but exhausted in WT HSCs after multiple rounds of BM transplantation (Figure 1E). Together, these results indicate that lupus TC mice have an expanded HSC population with enhanced self-renewal capacity.
Lupus TC mice possess an expanded population of HSPCs with enhanced self-renewal capacity. (A) The total number of nucleated BM cells from lupus TC and age-matched B6 mice were enumerated. Each symbol shows data from 1 mouse. (B) Lineage-negative BM cells from lupus TC and B6 control mice are displayed by c-Kit and Sca-1 staining in the contour plots. The LSK population in the rectangular gate is shown with percentages ± SD. The absolute number of LSK cells (middle panel) and long-term HSCs (right panel) in the BM from each mouse is shown by each dot. ***P < .001; **P < .01. (C) Irradiated B6.SJL mice were engrafted with 2 × 106 BM cells of lupus TC or B6 mice, and analyzed for donor-derived LSK cells in the BM 4 to 5 months after transplantation. Each dot corresponds to the percentage of BM LSK cells in each mouse. (D) The frequencies of donor-derived peripheral blood leukocytes (PBL) in recipients at each round of serial transplantation are shown by gray bars representing the mean ± SD (n = 10). (E) Survival curve of recipient mice after quaternary transplantation with BM cells of B6 or lupus TC origin (B6, n = 20; TC, n = 25). BMT, bone marrow transplantation; ns, not significant.
Lupus TC mice possess an expanded population of HSPCs with enhanced self-renewal capacity. (A) The total number of nucleated BM cells from lupus TC and age-matched B6 mice were enumerated. Each symbol shows data from 1 mouse. (B) Lineage-negative BM cells from lupus TC and B6 control mice are displayed by c-Kit and Sca-1 staining in the contour plots. The LSK population in the rectangular gate is shown with percentages ± SD. The absolute number of LSK cells (middle panel) and long-term HSCs (right panel) in the BM from each mouse is shown by each dot. ***P < .001; **P < .01. (C) Irradiated B6.SJL mice were engrafted with 2 × 106 BM cells of lupus TC or B6 mice, and analyzed for donor-derived LSK cells in the BM 4 to 5 months after transplantation. Each dot corresponds to the percentage of BM LSK cells in each mouse. (D) The frequencies of donor-derived peripheral blood leukocytes (PBL) in recipients at each round of serial transplantation are shown by gray bars representing the mean ± SD (n = 10). (E) Survival curve of recipient mice after quaternary transplantation with BM cells of B6 or lupus TC origin (B6, n = 20; TC, n = 25). BMT, bone marrow transplantation; ns, not significant.
Enhanced proliferation and repopulation activity of HSCs in TC mice are regulated by a cell-intrinsic mechanism
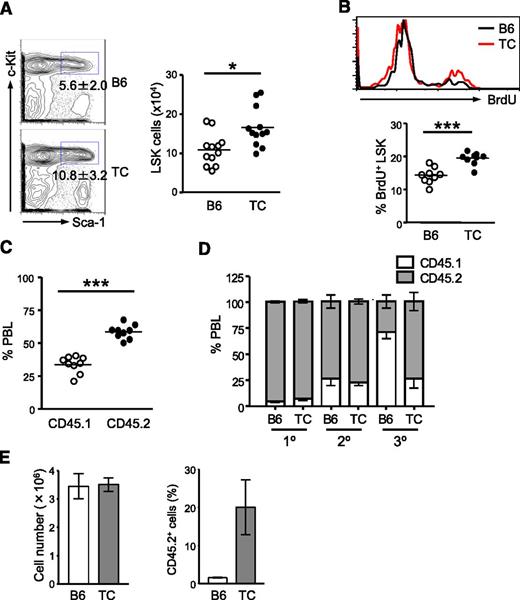
Recipient mice transplanted with lupus TC BM cells did not develop lupus-like symptoms over the 10-month experimental period. Nevertheless, we found that the number of transplanted TC LSK cells was significantly increased in the intervening time in these healthy hosts. This result suggests that a cell-intrinsic mechanism is responsible for the expansion of HSCs from lupus TC mice, even in the absence of inflammatory conditions. It is conceivable that HSC self-renewal is genetically augmented in TC mice. Additionally, the inflammatory microenvironment in the BM of lupus TC mice may also epigenetically modify HSC function. To address whether genetic or epigenetic factors in TC mice cause the observed changes in HSC function, we first examined the HSC compartment in healthy TC mice that were devoid of lupus-associated inflammation. Eight- to 10-week-old TC mice had not developed lupus, because autoantibodies and proteinuria were undetectable in these mice (data not shown). Interestingly, the LSK population in these healthy TC mice was already 1.5-fold larger than that in age-matched female B6 mice (Figure 2A). To address whether hyperproliferation caused the expansion of LSK cells in healthy TC mice, we determined the proliferation rate of LSK cells by a BrdU uptake assay. After 16-hour BrdU labeling, approximately 14% of LSK cells in B6 mice had incorporated BrdU. During the same labeling period, BrdU+ LSK cells in healthy TC mice were increased 1.4-fold (Figure 2B). Moreover, in recipient mice cotransplanted with an equal number of BM cells from healthy TC (CD45.2+) and WT (CD45.1+) mice, TC BM cells exhibited a strong competitive advantage over WT cells in repopulating the hematopoietic system (Figure 2C). We also directly assessed self-renewal capacity of HSCs from healthy TC mice by serial transplantation assays. In the primary recipients, nearly 100% of blood cells were derived from B6 and healthy TC BM donor cells. However, B6 contribution to the hematopoietic system was drastically declined to approximately 25% in the tertiary recipients, whereas TC donor cells sustained 75% blood chimerism after third-round transplantation (Figure 2D). Thus, HSCs with increased long-term repopulating capacity were hyperproliferative and expanded in TC mice prior to lupus onset. To examine whether stemness was better preserved in HSCs from lupus TC mice in a cell-autonomous manner, equal numbers of LSK cells sorted from lupus TC mice and B6 controls were grown in the presence of hematopoietic cytokines. During a 7-day culture, LSK cells from lupus TC and B6 mice exhibited an equivalent rate of expansion. However, ex vivo–expanded cells from TC mice possessed better repopulation ability than those from B6 controls (Figure 2E). Thus, regenerative potential was better maintained in ex vivo–expanded LSK cells from lupus TC mice.
Expansion of HSPCs with higher repopulating capacity occurs before lupus onset. Eight- to 10-week-old B6 and healthy TC mice were used for this study. (A) Percentages of LSK cells in the BM of B6 and TC mice are displayed as mean ± SD in contour plots. Right panel shows the total number of BM LSK cells with each dot symbolizing the value from 1 mouse. (B) B6 and TC mice were labeled with BrdU for 16 hours. The histogram shows a representative profile of BrdU staining of LSK cells. The percentage of BrdU+ cells in the BM LSK population in B6 and TC mice is shown by individual open and closed dots. (C) Competitive transplantation was done by transferring 106 BM cells from TC mice (CD45.2+) together with 106 BM cells from B6.SJL mice (CD45.1+) into irradiated B6.SJL F1 mice (CD45.1+). Hematopoietic repopulation by cotransferred TC and B6.SJL BM cells was analyzed 18 weeks after transplantation by determining the percentages of CD45 isotype expressing PBL. Each dot corresponds to the percentage of donor-derived BM LSK cells in each mouse. (D) The frequencies of donor-derived PBL in recipients at each round of serial transplantation are shown by gray bars representing the mean ± SD (n = 6). (E) An equal number of LSK cells purified from TC or B6 mice were cultured in medium containing 50 ng/mL stem cell factor, 10 ng/mL IL-6 and 10 ng/ml IL-3. Seven days later, cells were harvested and counted. A total of 106 cells derived from TC or B6 LSK cells were transferred into irradiated B6.SJL mice. Reconstitution was examined 10 months later. Left: Bars depict the number of total nucleated cells harvested from the 7-day culture. Right: Bars show the percentage of donor-derived PBL. Data are presented as mean ± SD (n = 4).
Expansion of HSPCs with higher repopulating capacity occurs before lupus onset. Eight- to 10-week-old B6 and healthy TC mice were used for this study. (A) Percentages of LSK cells in the BM of B6 and TC mice are displayed as mean ± SD in contour plots. Right panel shows the total number of BM LSK cells with each dot symbolizing the value from 1 mouse. (B) B6 and TC mice were labeled with BrdU for 16 hours. The histogram shows a representative profile of BrdU staining of LSK cells. The percentage of BrdU+ cells in the BM LSK population in B6 and TC mice is shown by individual open and closed dots. (C) Competitive transplantation was done by transferring 106 BM cells from TC mice (CD45.2+) together with 106 BM cells from B6.SJL mice (CD45.1+) into irradiated B6.SJL F1 mice (CD45.1+). Hematopoietic repopulation by cotransferred TC and B6.SJL BM cells was analyzed 18 weeks after transplantation by determining the percentages of CD45 isotype expressing PBL. Each dot corresponds to the percentage of donor-derived BM LSK cells in each mouse. (D) The frequencies of donor-derived PBL in recipients at each round of serial transplantation are shown by gray bars representing the mean ± SD (n = 6). (E) An equal number of LSK cells purified from TC or B6 mice were cultured in medium containing 50 ng/mL stem cell factor, 10 ng/mL IL-6 and 10 ng/ml IL-3. Seven days later, cells were harvested and counted. A total of 106 cells derived from TC or B6 LSK cells were transferred into irradiated B6.SJL mice. Reconstitution was examined 10 months later. Left: Bars depict the number of total nucleated cells harvested from the 7-day culture. Right: Bars show the percentage of donor-derived PBL. Data are presented as mean ± SD (n = 4).
These results demonstrate that the quantitative and functional enhancement of HSCs in TC mice is mediated by a cell-intrinsic mechanism, and strongly suggest that genetic lesions in addition to the inflammatory conditions of TC mice boost HSC self-renewal.
TC HSC functionality is closely correlated with the altered expression of p21CIP and p18INK4c
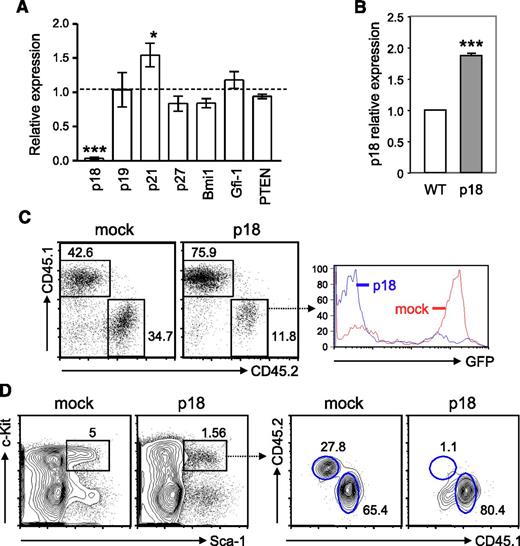
HSC self-renewal is tightly controlled by various signaling and cell-cycle regulators and transcription factors. It has been shown that ablation of PTEN, a negative regulator of the PI3K-Akt pathway, induces short-term expansion of HSCs.32,33 Additionally, cyclin-dependent kinase inhibitors, such as p21CIP1 and p18INK4c, impose a significant impact on HSC quiescence and self-renewal capability.34 Moreover, although the key transcriptional regulation of HSC self-renewal remains elusive, transcription factors Bim-1 and Gfi-1 have been shown to play a critical role in regulation of cell-cycle progression and HSC quiescence.35-37 To identify the cell-intrinsic factors responsible for the increased proliferation and self-renewal of TC HSCs, we decided to analyze the expression of the above molecules known to be involved in HSC proliferation and self-renewal. We found that the expression of PTEN, Bmi-1, and Gfi-1 in TC HSPCs was comparable to that in WT counterparts, indicating that the altered TC HSPC function is unlikely affected by these molecules. In contrast, the expression of p21CIP1 was upregulated by 1.5-fold, whereas the expression of p18INK4c was markedly downregulated in TC HSPCs (Figure 3A). Our findings are in good agreement with the previous report showing that p18INK4c-deficient mice had a significant increase in HSC self-renewal capacity.38 To determine whether p18INK4c negatively regulated HSC repopulation potential, we generated BM chimeras with BM cells that were transduced with either p18INK4c-expressing or an empty retroviral vector (mock infection) and then examined hematopoietic reconstitution 1 month later. We found that p18INK4c-transduced Lin− BM cells expressed approximately 2-fold higher p18INK4c as compared with Lin− BM cells from B6 mice (Figure 3B). Mice receiving p18INK4c-transduced BM cells had a poorer peripheral engraftment than those transplanted with mock-infected cells, and GFP+ cells that overexpressed p18INK4c were underrepresented within the CD45.2+ donor-cell population (Figure 3C). To determine whether the underrepresentation of peripheral p18INK4c-overexpressing cells was due to a growth or survival disadvantage of these cells or caused by impaired HSC reconstitution, we examined the LSK compartment in the BM chimeras. Our data show that the percentage of LSK cells was reduced in recipient mice with p18INK4c virus–infected BM cells compared with the controls. Furthermore, p18INK4c virus–infected BM cells reconstituted only 1.3% of the LSK population whereas mock-infected BM cells contributed ∼30% chimerism (Figure 3D). These findings thus lead us to conclude that overexpression of p18INK4c reduces HSC engraftment and defective expression of p18INK4c in TC mice is at least one of the factors that enhance self-renewal capacity of HSCs.
p18INK4c, which negatively regulates HSC repopulating potential, is undetectable in HSPCs from TC mice. (A) Quantitative RT-PCR analysis was performed with fluorescence-activated cell sorted LSK cells from 2 TC or B6 mice. cDNA input was normalized to the level of β-actin. Relative expression levels of each gene transcript in TC LSK cells relative to those in B6 controls are shown as the mean ± SD of 3 independent experiments. *P < .05; ***P < .001. (B) The relative expression level of p18INK4c in Lin− BM cells from BM chimeras engrafted with MSCV-p18 transduced BM cells (p18) is presented as fold induction compared with that detected in B6 Lin− BM cells (WT). ***P < .001, n = 3. (C) Peripheral engraftment was examined by fluorescence-activated cell sorting analysis with antibodies against CD45.1 and CD45.2. Percentages of donor (CD45.2) and recipient PBL (CD45.1) are indicated in the dot plots. Histograms show GFP expression within the donor population. (D) Left contour plots show the percentage of LSK cells in mice reconstituted with BM cells transduced with control MSCV retroviral vector (mock) or with MSCV-p18 vector (p18). Right contour plots show the chimerisms of the LSK population.
p18INK4c, which negatively regulates HSC repopulating potential, is undetectable in HSPCs from TC mice. (A) Quantitative RT-PCR analysis was performed with fluorescence-activated cell sorted LSK cells from 2 TC or B6 mice. cDNA input was normalized to the level of β-actin. Relative expression levels of each gene transcript in TC LSK cells relative to those in B6 controls are shown as the mean ± SD of 3 independent experiments. *P < .05; ***P < .001. (B) The relative expression level of p18INK4c in Lin− BM cells from BM chimeras engrafted with MSCV-p18 transduced BM cells (p18) is presented as fold induction compared with that detected in B6 Lin− BM cells (WT). ***P < .001, n = 3. (C) Peripheral engraftment was examined by fluorescence-activated cell sorting analysis with antibodies against CD45.1 and CD45.2. Percentages of donor (CD45.2) and recipient PBL (CD45.1) are indicated in the dot plots. Histograms show GFP expression within the donor population. (D) Left contour plots show the percentage of LSK cells in mice reconstituted with BM cells transduced with control MSCV retroviral vector (mock) or with MSCV-p18 vector (p18). Right contour plots show the chimerisms of the LSK population.
Lupus conditions cause HSC mobilization and promote myelopoiesis
Inflammatory conditions caused by viral and bacterial infections have been shown to trigger a transient expansion of HSCs.39,40 These inflammation-injured HSCs egress from the BM to distal tissues, where they proliferate and generate immune effector cells.16 To examine whether chronic inflammatory conditions of lupus mice also mobilize HSPCs into the periphery, we determined the frequency of LSK cells in the spleens of healthy and lupus TC mice as well as age-matched B6 mice. Although very few LSK cells can be found in the spleen of B6 and healthy TC mice, a substantial number of LSK cells accumulated in the spleen of lupus TC mice (Figure 4A). Methylcellulose-based colony-forming unit (CFU) assays also showed that splenocytes from lupus TC mice gave rise to drastically increased CFUs compared with splenocytes from healthy TC and B6 mice, demonstrating that there are many more clonogenic HSPCs in the periphery of lupus TC mice than that in healthy controls (Figure 4B).
Disease conditions of lupus correlate with HSPC mobilization and lineage biased hematopoietic output. (A) Each symbol represents the number of LSK cells in the spleen of each mouse of indicated groups. (B) CFU assays were performed to evaluate relative frequencies of clonogenic HSPCs in total splenocytes of mice for indicated groups. Each symbol represents the value of 1 mouse. (C) Each symbol displays the absolute number of B220+ cells in the BM of mice for indicated groups. (D) Each symbol displays the absolute number of Gr1+ cells in the BM of mice for indicated groups. (E) Dot plots show fluorescence-activated cell sorting analysis of myeloid progenitors. The absolute numbers of GMPs in each mouse of indicated groups are shown by each symbol. *P < .05; ***P < .001. B6-O, old B6 mice at 10 months of age; B6-Y, young B6 mice at 8 to 10 weeks of age; TC-H, healthy TC mice at 8 to 10 weeks of age; TC-L, lupus TC mice at 10 months of age.
Disease conditions of lupus correlate with HSPC mobilization and lineage biased hematopoietic output. (A) Each symbol represents the number of LSK cells in the spleen of each mouse of indicated groups. (B) CFU assays were performed to evaluate relative frequencies of clonogenic HSPCs in total splenocytes of mice for indicated groups. Each symbol represents the value of 1 mouse. (C) Each symbol displays the absolute number of B220+ cells in the BM of mice for indicated groups. (D) Each symbol displays the absolute number of Gr1+ cells in the BM of mice for indicated groups. (E) Dot plots show fluorescence-activated cell sorting analysis of myeloid progenitors. The absolute numbers of GMPs in each mouse of indicated groups are shown by each symbol. *P < .05; ***P < .001. B6-O, old B6 mice at 10 months of age; B6-Y, young B6 mice at 8 to 10 weeks of age; TC-H, healthy TC mice at 8 to 10 weeks of age; TC-L, lupus TC mice at 10 months of age.
Chronic infections cause dormant HSCs to enter the cell cycle and may accelerate HSC senescence, because HSCs that have been exposed to infections and inflammatory signals manifest functional changes characteristic of natural aging HSCs, such as impaired self-renewal capacity and biased hematopoietic output favoring the myeloid over the lymphoid lineage.41-43 The observation that lymphopenia was closely associated with SLE prompted us to assess whether lupus conditions skewed HSCs differentiation potential to generate more myeloid lineage cells at the expense of lymphocytes. We measured the cellularity of the BM for myeloid cells and B cells in lupus and healthy TC mice as well as age-matched B6 mice. We found that the numbers of B-cell precursors and newly generated B cells were significantly decreased in lupus TC mice compared with the numbers in healthy TC and age-matched B6 mice (Figure 4C). Accompanying the B-cell reduction, an increase in myeloid lineage cells was observed in lupus TC mice (Figure 4D). To further address whether the biased lineage output in lupus TC mice was regulated at the stem cell level, we examined the lymphoid and myeloid precursor compartments. The populations of common lymphoid progenitors and common myeloid progenitor were balanced in all groups of TC and B6 mice analyzed (data not shown), but the number of granulocyte/monocyte progenitors (GMPs) was significantly increased in lupus TC mice compared with that in healthy TC and B6 mice (Figure 4E). These data indicate that HSC differentiation potential is not skewed by the inflammatory conditions in lupus mice, and that biased differentiation of the lymphoid and myeloid lineage cells occurs at the late precursor stage through a selective expansion of GMPs and impaired maturation of B-lineage cells from common lymphoid progenitors.
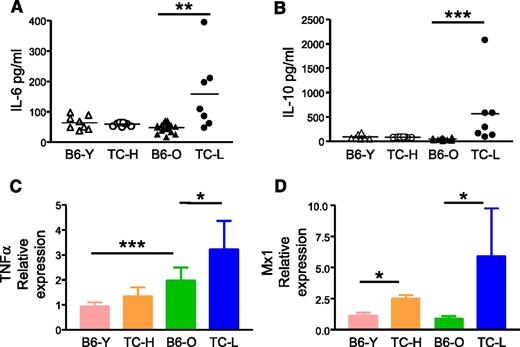
To identify the lupus inflammatory factors that account for the biased lineage output, we performed cytokine array analysis to screen for those that were upregulated in lupus TC mice. Among a total of 96 cytokines and chemokines screened, the serum levels of IL-6, IL-10, TNFα, and type I interferon (IFN) were elevated in lupus TC mice compared with age-matched B6 mice (data not shown). Notably, these cytokines are highly expressed in SLE patients and critical for lupus pathogenesis.22 Thus, the cytokine expression pattern of lupus TC mice closely resembles that of SLE patients. We further quantified by enzyme-linked immunosorbent assay the serum levels of IL-6 and IL-10 in healthy and lupus TC mice and in age-matched B6 mice. Serum levels of IL-6 and IL-10 were normal in healthy TC mice but significantly elevated in lupus TC mice compared with those in 2- or 10-month-old B6 mice (Figures 5A-B). Next, we measured local production of TNFα in the BM of healthy, lupus TC mice and B6 controls by qRT-PCR using total BM cells. As shown in Figure 5C, TNFα expression was already elevated in the BM of TC mice before lupus onset and increased further in mice with active lupus. Likewise, expression of type I IFN was also increased in healthy TC mice and further augmented in lupus TC mice, as revealed by the upregulation of the IFN-induced gene Mx1 in LSK cells (Figure 5D).
Production of proinflammatory cytokines is increased in lupus TC mice. (A) Serum levels of IL-6 were determined. Data were shown by symbols representing the value of each mouse. (B) Serum levels of IL-10 were shown by symbols representing the value of each mouse. (C) Expression levels of TNFα in BM cells were determined by qRT-PCR. cDNA input was normalized to the level of RNA polymerase IIa. Mean values ± SD obtained from at least 4 experiments are shown. (D) Expression levels of Mx1 in LSK cells measured by qRT-PCR are indicated as means ± SD (n = 3). *P < .05; **P < .01; ***P < .001. B6-O, old B6 mice at 10 months of age; B6-Y, young B6 mice at 8 to 10 weeks of age; TC-H, healthy TC mice at 8 to 10 weeks of age; TC-L, lupus TC mice at 10 months of age.
Production of proinflammatory cytokines is increased in lupus TC mice. (A) Serum levels of IL-6 were determined. Data were shown by symbols representing the value of each mouse. (B) Serum levels of IL-10 were shown by symbols representing the value of each mouse. (C) Expression levels of TNFα in BM cells were determined by qRT-PCR. cDNA input was normalized to the level of RNA polymerase IIa. Mean values ± SD obtained from at least 4 experiments are shown. (D) Expression levels of Mx1 in LSK cells measured by qRT-PCR are indicated as means ± SD (n = 3). *P < .05; **P < .01; ***P < .001. B6-O, old B6 mice at 10 months of age; B6-Y, young B6 mice at 8 to 10 weeks of age; TC-H, healthy TC mice at 8 to 10 weeks of age; TC-L, lupus TC mice at 10 months of age.
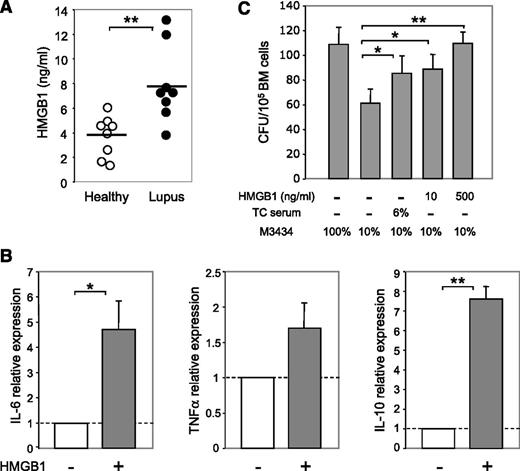
Recently, HMGB1 has emerged as a critical mediator of inflammation in lupus.44 High serum levels of HMGB1 are detected in SLE patients and correlate with disease activity.45 Of particular interest is the finding that HMGB1-containing nucleosomes can stimulate primary human monocyte–derived macrophages to secrete IL-6, IL-10, and TNFα.46 We therefore tested the role of HMGB1 in modulating the BM cytokine milieu. Consistent with human studies, we found a significantly higher level of HMGB1 in the plasma of lupus TC mice than in healthy TC mice (Figure 6A). Culture of BM cells from B6 mice with 500 ng/mL HMGB1 for 24 hours potently induced production of IL-6, IL-10, and TNFα (Figure 6B). In line with these data, we found that serum of lupus TC mice or HMGB1 promoted myeloid development, because addition of serum from lupus TC mice or HMGB1 to the suboptimal methylcellulose-based medium boosted myeloid CFU numbers in an in vitro CFU assay (Figure 6C). Thus, given that addition of HMGB1 is sufficient to induce IL-6, IL-10, and TNFα expression by BM cells, our data suggest that HMGB1 is an important regulator that boosts the production of these inflammatory cytokines in lupus TC mice, which subsequently promotes the outgrowth of myeloid precursors.
Increased levels of HMGB1 in the plasma of lupus TC mice can induce production of inflammatory cytokines by BM cells and promote myeloid development. (A) Levels of HMGB1 detected in the plasma of healthy and lupus TC mice. Each symbol shows data from 1 mouse. (B) B6 BM cells were cultured with or without 500 ng/mL HMGB1 for 24 hours. Relative expression levels of cytokines were quantified by qRT-PCR and are shown as the ratio of each gene transcript in the presence versus absence of HMGB1 after being normalized to the level of β-actin. (C) Hematopoietic cytokines in the methylcellulose-based medium were diluted to establish a suboptimal condition (10% MethoCult M3434) under which the number of CFU reduced to approximately 50% of those formed in the optimal medium (100% MethoCult M3434). Colonies formed under the suboptimal condition with serum from lupus TC mice or with different doses of HMGB1 were counted at day 14 of culture and presented as bars with mean ± SD from 3 independent experiments. *P < .05; **P < .01.
Increased levels of HMGB1 in the plasma of lupus TC mice can induce production of inflammatory cytokines by BM cells and promote myeloid development. (A) Levels of HMGB1 detected in the plasma of healthy and lupus TC mice. Each symbol shows data from 1 mouse. (B) B6 BM cells were cultured with or without 500 ng/mL HMGB1 for 24 hours. Relative expression levels of cytokines were quantified by qRT-PCR and are shown as the ratio of each gene transcript in the presence versus absence of HMGB1 after being normalized to the level of β-actin. (C) Hematopoietic cytokines in the methylcellulose-based medium were diluted to establish a suboptimal condition (10% MethoCult M3434) under which the number of CFU reduced to approximately 50% of those formed in the optimal medium (100% MethoCult M3434). Colonies formed under the suboptimal condition with serum from lupus TC mice or with different doses of HMGB1 were counted at day 14 of culture and presented as bars with mean ± SD from 3 independent experiments. *P < .05; **P < .01.
Discussion
HSCs are the first type of somatic stem cells that have been clinically used to treat a variety of severe hematopoietic disorders, including autoimmune diseases such as SLE. Currently, remission has been achieved in approximately two thirds of the SLE patients receiving HSC transplantation.47 However, HSC transplantation still remains a highly complex and risky procedure. An imperative issue that needs to be resolved is whether and how HSC function is modified by disease conditions. In the present study, we used a lupus mouse model to demonstrate that multiple etiologic factors of lupus impact hematopoiesis through different molecular and cellular mechanisms.
TC mice are particularly well suited for the study of HSC function under the chronic inflammatory conditions of lupus because these mice develop spontaneous autoimmune symptoms that closely resemble human SLE and their B6 genetic background permits testing HSC repopulating capacity using transplantation assays. Studying HSC function in TC mice before and after lupus onset provides a unique system to dissect genetic and inflammatory factors that contribute to HSC functional alterations. Our results showed that HSCs were mobilized into the periphery in lupus but not in healthy TC mice, suggesting that inflammatory factors impair homing and retention of HSCs in the BM. In addition, we showed that inflammatory conditions of lupus suppressed lymphopoiesis and fostered generation of myeloid lineage cells, and such a biased lineage output in lupus mice was not caused by defective HSC differentiation but was rather due to the selective growth of GMPs.
Increases in serum levels of HMGB1, IL-6, IL-10, type I IFN, and TNFα closely correlate with lupus disease activity, because these inflammatory factors directly modulate activation and survival of immune effector cells. The regulatory function of IL-6, IL-10, and TNFα in HSPC function has also been studied in various models of infections. It has been shown that IL-6 and IL-10 promote myeloid development and TNFα reduces lymphopoiesis without affecting granulopoiesis,11,13,25 but the role of HMGB1 in hematopoiesis has not been determined. HMGB1 is a nonhistone chromosomal protein that regulates transcription and DNA repair. It also acts as an alarmin after being secreted by activated cells or released from dead cells.48 Serum levels of HMGB1 are significantly elevated in SLE patients, probably due to increased cell death or impaired clearance of dead cells.49 HMGB1 in SLE patients forms complexes with nucleosomes and serves as a key autoantigen that drives inflammation and tissue damages.46 In line with studies of SLE patients, we show that HMGB1 level is also increased in lupus TC mice. Circulating HMGB1 in lupus animals may change the BM environment to be more proinflammatory, as our data show that HMGB1 induces BM cells to express IL-6, IL-10, and TNFα within 24 hours. Importantly, we find that HMGB1 and serum from lupus TC mice promote myeloid lineage development. Therefore, exposure to HMGB1 and inflammatory cytokines is likely a major cause for the expansion of GMPs while shrinking the pool of B-lineage precursors in lupus TC mice. Further studies are necessary to clarify an obligatory role of HMGB1 in the observed hematopoietic alterations in lupus animals. In addition, our findings also provide a mechanistic insight into the long-recognized association between lymphopenia and active lupus, and should have significant implications for tailoring treatment to increase lymphocyte counts in SLE patients.
HMGB1, IL-6, type I IFN, and TNFα are also typically induced by bacterial and viral infections. It is, therefore, not surprising that HSPCs from lupus mice and from infected mice share most functional changes, such as hyperproliferation, myeloid skewed output, and migration to extramedullary sites. However, lupus HSCs display a remarkable functional feature distinct from HSCs exposed to pathogen-infected microenvironment, namely, their regenerative capacity is enhanced. This finding is unexpected in light of numerous reports showing that increased proliferation of stem cells exhausts their self-renewal capacity. Our further analysis reveals that the enhanced HSC self-renewal in lupus mice is accompanied with abnormal expression of cell-cycle inhibitors p21CIP1 and p18INK4c. Currently, our knowledge about the role of p21CIP1 in HSC function remains limited. An initial study reported that the inactivation of p21CIP1 led to premature HSC exhaustion caused by loss of cell quiescence.3 Later, more experiments on p21CIP1 knockout mice corrected this notion and showed that p21CIP1 was dispensable for the maintenance of HSC quiescence and functionality under steady state.50,51 Indeed, the observation that p21CIP1 expression is upregulated when quiescent long-term HSCs differentiate into more cycling short-term HSPCs also argues against the role of p21CIP1 in enforcing HSC quiescence.52 The duality of p21CIP1 function in cell-cycle regulation has long been noticed. On one hand, p21CIP1 can inhibit CDK4/cyclin D1 activity, consequently blocking G1 to S phase transition. On the other hand, it also acts as a positive regulator for cyclin D1 nuclear accumulation.53,54 The major function of p21CIP1 in HSCs is likely to promote survival of rapidly cycling HSCs under stress conditions and to prevent HSC senescence.55 In this regard, our finding that p21CIP1 expression is upregulated in TC HSPCs supports a role of p21CIP1 in preserving the HSC pools under the inflammatory condition of lupus mice.
p18INK4c is a negative cell-cycle regulator encoded by the cdkn2c gene located within the Sle2 lupus susceptibility locus. A single-nucleotide polymorphism in the cdkn2c promoter region has been recently mapped to associate with severely impaired expression of p18INK4c in TC mice.31 The functional consequence of impaired p18INK4c expression in HSCs has been underscored by a genetic study showing that deletion of cdkn2c results in an increase in HSC self-renewal.38 In line with this finding, TC LSK cells that expressed an undetectable level of p18INK4c also exhibit enhanced long-term repopulation activity. In contrast, overexpression of p18INK4c impairs HSC repopulating capacity. Our data indicate that the point mutation in the cdkn2c promoter in the Sle2 locus is likely the causal lupus genetic factor for the enhanced HSC self-renewal in TC mice.
In summary, the loss of p18INK4c expression and an enhanced level of p21CIP1 in TC mice may protect HSCs from stress-induced depletion. Therefore, WT HSCs, when exposed to inflammatory signals after transplantation into the lupus BM, would be gradually outcompeted by resident lupus HSCs. These results have important implications in HSC transplantation based clinical interventions for refractory SLE. Modification of p18INK4c function in HSCs may increase the efficacy of HSC transplantation for SLE treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Heriberto Borrero for cell sorting, Drs Hua Gu and Darran Cronshaw for critical reading of the manuscript, and all members of Zou laboratory for their technical support.
This work was supported in part by a research grant from the American Autoimmune Related Diseases Association (H.N.) and by a grant from the National Institutes of Health (RO1AI068965) (L.M.).
National Institutes of Health
Authorship
Contribution: H.N., B.D., and Y.-R.Z. designed the experiments; H.N., G.F., Y.T., and L.X. did the experiments and/or analyzed data; J.Y. generated reagents; M.L. and B.D. assisted in lupus studies and manuscript preparation; H.N. and Y.-R.Z. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.X. is Department of Pharmacology and Neuroscience, University of North Texas Health Science Center, 3500 Camp Bowie Blvd, Fort Worth, TX 76107.
Correspondence: Yong-Rui Zou, The Feinstein Institute for Medical Research, North Shore LIJ Health System, 350 Community Dr, Manhasset, NY 11030; e-mail: yzou@nshs.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal