Key Points
The receptor tyrosine kinase Axl is important for constitutive FLT3 phosphorylation in FLT3-ITD+ AML
Blocking phosphorylation of Axl suppresses the growth of human FLT3-ITD+ AML in vivo, which makes Axl a potential therapeutic target
Abstract
Approximately 20% to 25% of patients with acute myeloid leukemia (AML) have a constitutively activated FLT3-internal tandem duplication (FLT3-ITD), and these patients exhibit a poor prognosis. Here, we report that Axl, a receptor tyrosine kinase (RTK) overexpressed and constitutively active in human AML, targets the RTK FLT3 in FLT3-ITD+ AML. Abrogation of Axl activation by soluble Axl chimeric protein (Axl-Fc) or small interfering RNA (siRNA) diminishes constitutive FLT3 phosphorylation in FLT3-ITD+ AML. In addition, inhibition of Axl activation by Axl-Fc interferes with the physical interaction between Axl and FLT3. We found that Axl-Fc, a pharmacologic Axl inhibitor, or siRNA targeting Axl inhibits cell growth, induces cell-cycle arrest and apoptosis, and relieves a block in myeloid differentiation of FLT3-ITD+ AML in vitro. Axl-Fc also suppresses the growth of human FLT3-ITD+ AML in vivo. Collectively, our data suggest that Axl contributes to the pathogenesis of FLT3-ITD+ AML through, at least in part, positive regulation of constitutive FLT3 activation. This also suggests that Axl should be pursued as a potential target for the treatment of FLT3-ITD+ AML.
Introduction
Axl is a prototypical member of the receptor tyrosine kinase (RTK) Axl family, which is composed of Axl, Tyro3 (or Sky), and Mer. Two natural and highly homologous ligands for the Axl family are growth-arrest–specific gene 6 (Gas6) and protein S.1 Axl has the highest affinity for Gas6 compared with other members of the Axl family, whereas protein S predominantly binds Tyro3 and Mer.2 The Axl family of RTK has been known to be involved in a variety of biological functions, such as spermatogenesis,3 phagocytic clearance of apoptotic cells,4 inhibition of innate immune responses,5 cell proliferation,6 and survival.1,6 Axl has been reported to be overexpressed or activated in the vast majority of cancers,7-9 including acute myeloid leukemia (AML).10-12 Furthermore, a previous study revealed that high AXL levels associate with worse progression-free and overall survival of AML patients,13 which suggests that Axl may be involved in the pathogenesis of AML. However, the mechanism(s) by which Axl contributes to AML has not been explored.
AML is characterized by abnormal proliferation and survival of myeloid progenitor and precursor cells and a premature block in myeloid differentiation.14 A variety of genetic aberrations are associated with the occurrence of AML.14-16 About 20% to 25% of AML patients harbor internal tandem duplication (ITD) mutations in the RTK Fms-like tyrosine kinase-3 (FLT3), which lead to constitutive phosphorylation of FLT3 and activation of its downstream signaling cascades, resulting ultimately in aberrant proliferation and survival.14,16,17 FLT3-ITD mutations have been consistently found to be an adverse prognostic marker in these AML patients.16 This has led to clinical trials treating FLT3-ITD+ AML patients with small-molecule FLT3-selective tyrosine kinase inhibitors.18 To date, however, FLT3 inhibitors used as a single agent or in combination with chemotherapy for FLT3-ITD+ AML have not demonstrated improved clinical efficacy.18 Because FLT3 can crosstalk with a network of various signaling pathways,14,16,17,19 identifying and analyzing the interplay of constitutively active FLT3 with aberrant signaling pathway(s) may lead to the identification of novel therapeutic target(s) for treatment of AML patients harboring constitutively active FLT3.
In human CD34+ hematopoietic progenitor cells, Axl has been reported to be crucial for optimal signaling and biological activities of c-Kit,20 which belongs to the same type III RTK family as FLT3.16 This prompted us to hypothesize that Axl may also play a role in FLT3 signaling and thus in AML driven at least in part by constitutively activated FLT3 such as that found in FLT3-ITD+ AML. In the present study, we provide evidence that supports a role for Axl in regulating the constitutive activation of FLT3 in FLT3-ITD+ AML.
Materials and methods
Cell culture, primary AML samples, and reagents
The MV4;11 human FLT3-ITD+ AML cell line (ATCC, Manassas, VA) was cultured in RPMI1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum and antibiotics and maintained at 37°C and 5% CO2. Thawed, viably cryopreserved primary leukemic blasts for the in vitro studies were previously obtained from FLT3-ITD+ and FLT3–wild-type (WT) AML patients who provided written consent in accordance with the Declaration of Helsinki and with the approval from The Ohio State University Institutional Review Board. Human Control-Fc (Ctrl-Fc) and Axl-Fc chimeric proteins used in the in vitro studies were purchased from R&D Systems (Minneapolis, MN) and were placed in cultures at a final concentration of 1 μg/mL. All antibodies used for flow cytometric analyses were purchased from BD Biosciences (San Diego, CA), unless otherwise noted. PKC412 and XL-880 were purchased from LC Laboratories (Woburn, MA) and Selleck Chemicals (Houston, TX), respectively.
RT-PCR
Total RNA was isolated from the cells treated as indicated in the figures by using RNeasy RNA isolation kit (QIAGEN, Germany). Complementary DNA was generated using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and was subject to polymerase chain reaction (PCR) with Taq DNA polymerase (Invitrogen). Primer sequences and PCR conditions for Axl,10 Mer,21 Gas6,22 and protein S23 were described previously. The mRNA levels for C/EBPα,24 PU.1,24 elastase 2,25 myeloperoxidase,25 and lysozyme25 were measured by real-time reverse transcription PCR (RT-PCR) using SYBR-Green (Applied Biosystems, Foster City, CA). 18S rRNA was measured and used as a normalization control.
Immunoblotting and immunoprecipitation
To detect phospho-Axl, phospho–extracellular signal-regulated kinase (ERK), phospho-AKT, phospho-C/EBPα, C/EBPα, phospho-FLT3 (Tyr589/591), FLT3, and phospho-STAT5, cells were treated as described in the figure legends, lysed, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by transfer to nitrocellulose membrane. The nitrocellulose membrane was then incubated with primary antibodies against proteins mentioned above (Cell Signaling, Danvers, MA), except anti–phospho-STAT5 (BD Biosciences), followed by incubation with either anti-rabbit or anti-mouse secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology). An enhanced chemiluminescence system (GE Healthcare, UK) was used for detection of proteins. Actin was used as a loading control. For immunoprecipitation, cells were lysed and the resulting supernatants were precleared by incubation with normal rabbit immunoglobulin G (IgG; Santa Cruz Biotechnology) for 1 hour at 4°C. After centrifugation, the supernatants were incubated with anti-Axl or anti-FLT3 rabbit antibody (Cell Signaling) at 4°C overnight, which was further incubated with Protein A-agarose beads (Santa Cruz Biotechnology) for 3 hours at 4°C. After centrifugation and washing at 4°C, protein-bead complexes were boiled and subjected to immunoblot analysis.
Measurement of cell proliferation and apoptosis
Viable cell numbers were counted with the trypan blue exclusion assay (Invitrogen). Detection of apoptosis was performed using the Annexin V : FITC Apoptosis Detection Kit I (BD Biosciences). After incubating the cells with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide (PI) in the binding buffer for 15 minutes at room temperature, cells were analyzed using a flow cytometer (FACSCalibur, BD Biosciences).
Cell-cycle analysis
Cells were washed 3 times with phosphate-buffered saline (PBS), fixed with 70% methanol at −20°C for 24 hours, and incubated with RNase (20 μg/mL) and PI (50 μg/mL) for 30 minutes at room temperature. Cells were then subjected to flow cytometric analyses and the percentage of cells in each cell-cycle stage was calculated using FlowJo software (Version 7.2.5; Tree Star, Ashland, OR).
NBT reduction assay for myeloid differentiation study
Nitroblue tetrazolium (NBT) reduction assay was performed as described previously.26 Cells (105 cells per well) were treated with Ctrl-Fc or Axl-Fc for 4 days and then incubated for 30 minutes with medium containing NBT (1 mg/mL) and phorbol myristate acetate (330 nM). Cells were then centrifuged and solubilized in dimethylsulfoxide and the absorbance at 570 nm was measured.
Axl gene expression knockdown using siRNA
Cells were transfected with Ctrl (Santa Cruz Biotechnology, catalog number sc-108060) or Axl small interfering RNA (siRNA; Sigma, catalog number SIHK1049) using the Amaxa nucleofection technology (Amaxa, Germany) as described previously.27 The Axl siRNA from Sigma is a mixture of 5 different (undisclosed) siRNAs targeting Axl.
Generation of Axl-Fc chimeric protein for in vivo mouse studies
Complementary DNA encompassing the extracellular domain (amino acids 1 to 442) of human Axl, which is the same as we used in in vitro studies, was generated by PCR and cloned into the pCR2.1 TA vector (Invitrogen). After enzymatic digestion with EcoRI (Invitrogen), a 1.3-kb fragment was cloned into the pFUSE mammalian expression vector (InvivoGen, San Diego, CA) containing human immunoglobulin G1 (IgG1) Fc fragment. The parental plasmid vector alone or the plasmid vector containing the extracellular domain of human Axl was transfected into 293T human embryonic kidney cell line using Profection Mammalian Transfection System (Promega, Madison, WI). The resulting fusion protein between the extracellular domain of human Axl and human IgG1 Fc fragment (Axl-Fc) was secreted into culture medium due to the presence of the interleukin-2 signal sequence on the plasmid vector. Supernatants from the cells transfected with the parental pFUSE vector contained only human IgG1 Fc, which was used as a Ctrl-Fc. Collected supernatants were applied to Melon gel chromatography cartridge (Pierce, Rockford, IL) and eluted. Ctrl-Fc or Axl-Fc chimeric proteins were kept frozen at −80°C until use.
Animal models for in vivo studies
All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee and according to procedures of the University Laboratory Animal Resources at The Ohio State University. All animals were accommodated and maintained in a clean, sterile animal care facility.
Subcutaneous xenograft tumor model
Imprinting control region (ICR) severe combined immunodeficiency (SCID) mice were purchased from Taconic Farms (Hudson, NY) and maintained in the animal facility at The Ohio State University Comprehensive Cancer Center. Mice (about 6 weeks old) were injected subcutaneously with the MV4;11 human FLT3-ITD+ AML cell line (5 × 106 cells per mouse), and 10 days later Ctrl-Fc or Axl-Fc (each 200 μg per mouse) was subcutaneously injected 3 times a week. PBS (100 μl per mouse) was also injected as a control. Four weeks later, tumor masses were excised from killed mice and weighed.
Leukemia engraftment model
ICR-SCID mice (about 4 to 6 weeks old; from Taconic Farms) were sublethally exposed to X-ray irradiator (200 cGy) 24 hours prior to intravenous injection of leukemic blasts from a cytogenetically normal (CN), FLT3-ITD+ AML patient (2 × 107 cells per mouse). Four weeks after injection of leukemic cells, mice were treated with Ctrl-Fc or Axl-Fc (each 200 μg per mouse) by intraperitoneal injection 3 times a week for the following weeks. In some experiments, PKC412 (100 mg/kg of mouse) was orally administered into mice as described previously.28 Peripheral blood was obtained from each mouse at 8 weeks after injection of primary leukemic blasts and the numbers of white blood cells (WBCs) were counted with Turk’s solution (Carolina Biological Supply, Burlington, NC). For flow cytometry, red blood cells in peripheral blood from each mouse were removed by using Red Blood Cells lysis buffer (StemCell Technologies, Canada), and the prepared cell suspensions were stained with anti–human CD45 antibody and analyzed with a flow cytometer (FACSCalibur, BD Biosciences). Mice were killed when the animals became moribund. Bone marrow cell suspensions were obtained from each mouse as previously reported29 and analyzed by flow cytometry to detect the leukemic cells using an anti-human CD45 antibody.
Statistical analyses
Data from in vitro and in vivo animal studies were analyzed using the Student t test. Estimated probabilities of survival were calculated using the Kaplan-Meier method, and the log-rank test evaluated differences between survival distributions in the in vivo mouse study.
Results
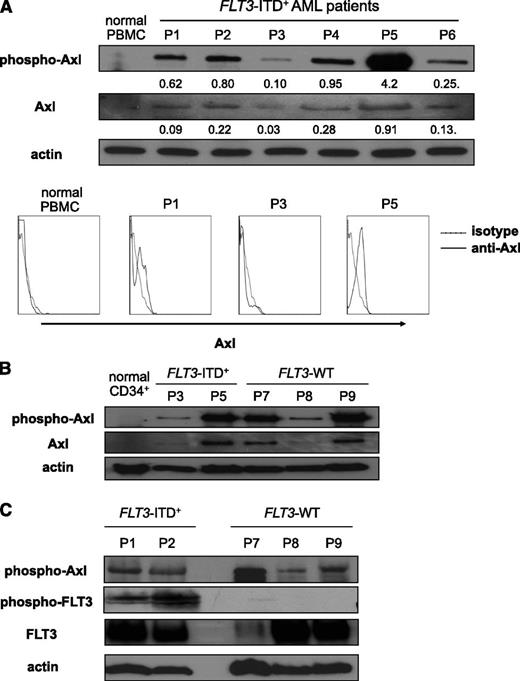
To investigate whether Axl is involved in the pathogenesis of AML, we first examined the status of Axl activation. Axl was constitutively phosphorylated to a variable degree in both the MV4;11 human FLT3-ITD+ AML cell line (supplemental Figure 1B) and in primary patient AML blasts taken prior to treatment in 6 patients with FLT3-ITD+ AML and 3 patients with FLT3-WT AML (Figure 1A-B; information about patients used in this study is described in supplemental Table 1). Axl was not constitutively phosphorylated in peripheral blood mononuclear cells (Figure 1A) or in CD34+ hematopoietic progenitor cells (Figure 1B) from normal donors. We observed an association between the level of phosphorylated Axl and that of total Axl protein in AML patients (Figure 1A). However, we did not find such an association among the level of phosphorylated Axl, the expression of total Axl, and the mutational status of FLT3 (Figure 1B). That is, Axl was present and found to be constitutively phosphorylated in both FLT3-ITD+ cases of AML that displayed phosphorylated FLT3 as well as in FLT3-WT AML patient samples without constitutively active FLT3 (Figure 1C). This suggests that phosphorylation of Axl is independent of FLT3 activation and could be important in the pathogenesis of FLT3-ITD+ and FLT3-WT AML.
Axl is constitutively active in primary human AML blasts. (A) AML blasts from six FLT3-ITD+ patients (P1 to P6) were subjected to immunoblot to detect phospho-Axl and Axl (top). Actin was used as a loading control. Peripheral blood mononuclear cells were obtained from a normal, healthy donor by Ficoll gradient centrifugation and were used as a negative control. Numbers under each row indicate the ratio of the intensity of each band (phospho-Axl or Axl) relative to that of the actin band. Cells from panel A were analyzed for surface expression of Axl by flow cytometry using an antibody against Axl (bottom). A nonreactive isotype antibody was used as a negative control. (B) AML blasts from 2 patients possessing FLT3-ITD mutations (P3 and P5) and from 3 patients harboring FLT3-WT (P7 to P9) were subjected to immunoblot to detect phospho-Axl and Axl. CD34+ cells from a normal, healthy donor were used as a negative control. (C) AML blasts from 2 patients possessing FLT3-ITD mutations (P1 and P2) and from 3 patients harboring FLT3-WT (P7 to P9) were subjected to immunoblot to detect phospho-Axl, phospho-FLT3, and FLT3. PBMC, peripheral blood mononuclear cell.
Axl is constitutively active in primary human AML blasts. (A) AML blasts from six FLT3-ITD+ patients (P1 to P6) were subjected to immunoblot to detect phospho-Axl and Axl (top). Actin was used as a loading control. Peripheral blood mononuclear cells were obtained from a normal, healthy donor by Ficoll gradient centrifugation and were used as a negative control. Numbers under each row indicate the ratio of the intensity of each band (phospho-Axl or Axl) relative to that of the actin band. Cells from panel A were analyzed for surface expression of Axl by flow cytometry using an antibody against Axl (bottom). A nonreactive isotype antibody was used as a negative control. (B) AML blasts from 2 patients possessing FLT3-ITD mutations (P3 and P5) and from 3 patients harboring FLT3-WT (P7 to P9) were subjected to immunoblot to detect phospho-Axl and Axl. CD34+ cells from a normal, healthy donor were used as a negative control. (C) AML blasts from 2 patients possessing FLT3-ITD mutations (P1 and P2) and from 3 patients harboring FLT3-WT (P7 to P9) were subjected to immunoblot to detect phospho-Axl, phospho-FLT3, and FLT3. PBMC, peripheral blood mononuclear cell.
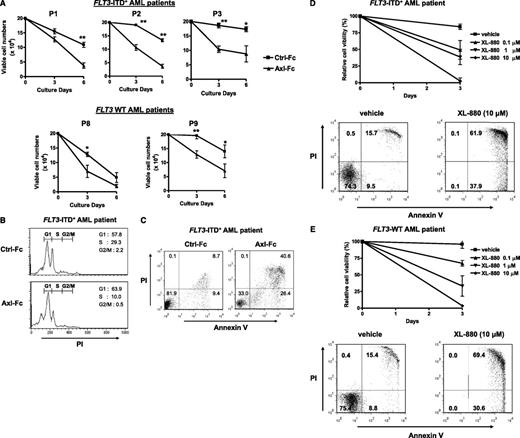
We next investigated whether Axl has a role in the proliferation and survival of AML cells. To study this, we used a soluble fusion protein (termed Axl-Fc) that consists of the extracellular domain of the Axl protein and the Fc portion of human IgG1. As a soluble receptor in excess, Axl-Fc binds its ligands, Gas6 and protein S, and prevents them from interacting with Axl on a cell surface, thereby inhibiting Axl phosphorylation and its downstream signaling cascade,2 such as ERK, STAT5, and AKT (supplemental Figure 1B). Axl-Fc could significantly attenuate the growth of FLT3-ITD+ AML cells in Axl-Fc–treated primary FLT3-ITD+ AML patient blasts (Figure 2A top) and in the Axl-Fc–treated MV4;11 human FLT3-ITD+ AML cell line (supplemental Figure 2A) when compared with Ctrl-Fc–treated cells. Inhibition of the growth of FLT3-ITD+ AML blasts by Axl-Fc was dose dependent (supplemental Figure 2D). XL-880,30 a small-molecule inhibitor of Axl, could also significantly inhibit the growth of FLT3-ITD+ AML cells in a dose-dependent manner (Figure 2D top). This inhibition of FLT3-ITD+ AML cell growth appears to result from both cell-cycle arrest, with a significant decrease in S phase (Figure 2B and supplemental Figure 2B), as well as from an increase in apoptosis (Figure 2C-D and supplemental Figure 2C). Furthermore, Axl-Fc (Figure 2A bottom) and XL-880 (Figure 2E top) were also able to diminish the growth of FLT3-WT AML cells, and XL-880 strongly induced apoptosis (Figure 2E bottom). These results suggest that activated Axl promotes growth and survival of both FLT3-ITD+ and FLT3-WT AML cells, which is consistent with the fact that Axl is constitutively active in both FLT3-ITD+ and FLT3-WT AML cells (Figure 1A-C).
Blocking Axl activation leads to inhibition of cell growth in AML cells. (A) Primary blasts from 3 FLT3-ITD+ AML patients (top, P1 to P3) and those from 2 FLT3-WT AML patients (bottom, P8 and P9) were treated with Ctrl-Fc or Axl-Fc (triplicates for each patient) for the indicated times. Cell numbers were counted using trypan blue. *P < .05, **P < .01. (B) Primary FLT3-ITD+ AML patient blasts were treated with Ctrl-Fc or Axl-Fc for 2 days and the cell cycle was analyzed by flow cytometry after PI staining of DNA of the cells treated as above. Numbers indicate the percentage of each stage (G1, S, and G2/M) in the cell cycle. This is the representative of 3 separate experiments. (C) Blasts from a FLT3-ITD+ AML patient were treated with Ctrl-Fc or Axl-Fc for 3 days, stained with annexin V-FITC and PI, and analyzed by flow cytometry. This is the representative of 3 separate experiments. (D-E) Primary FLT3-ITD+ (panel D top) and FLT3-WT (panel E top) AML patient blasts were treated with vehicle (dimethylsulfoxide) or different concentrations of the pharmacologic Axl inhibitor XL-880 (0.1, 1, or 10 μM) for 3 days (triplicates) and the cell numbers were counted. The cell numbers at the beginning of treatment was set arbitrarily as 100%. Primary blasts from FLT3-ITD+ (panel D bottom) or FLT3-WT (panel E bottom) AML patients were treated with vehicle or XL-880 (10 μM) for 3 days, stained with annexin V-FITC and PI, and analyzed by flow cytometry.
Blocking Axl activation leads to inhibition of cell growth in AML cells. (A) Primary blasts from 3 FLT3-ITD+ AML patients (top, P1 to P3) and those from 2 FLT3-WT AML patients (bottom, P8 and P9) were treated with Ctrl-Fc or Axl-Fc (triplicates for each patient) for the indicated times. Cell numbers were counted using trypan blue. *P < .05, **P < .01. (B) Primary FLT3-ITD+ AML patient blasts were treated with Ctrl-Fc or Axl-Fc for 2 days and the cell cycle was analyzed by flow cytometry after PI staining of DNA of the cells treated as above. Numbers indicate the percentage of each stage (G1, S, and G2/M) in the cell cycle. This is the representative of 3 separate experiments. (C) Blasts from a FLT3-ITD+ AML patient were treated with Ctrl-Fc or Axl-Fc for 3 days, stained with annexin V-FITC and PI, and analyzed by flow cytometry. This is the representative of 3 separate experiments. (D-E) Primary FLT3-ITD+ (panel D top) and FLT3-WT (panel E top) AML patient blasts were treated with vehicle (dimethylsulfoxide) or different concentrations of the pharmacologic Axl inhibitor XL-880 (0.1, 1, or 10 μM) for 3 days (triplicates) and the cell numbers were counted. The cell numbers at the beginning of treatment was set arbitrarily as 100%. Primary blasts from FLT3-ITD+ (panel D bottom) or FLT3-WT (panel E bottom) AML patients were treated with vehicle or XL-880 (10 μM) for 3 days, stained with annexin V-FITC and PI, and analyzed by flow cytometry.
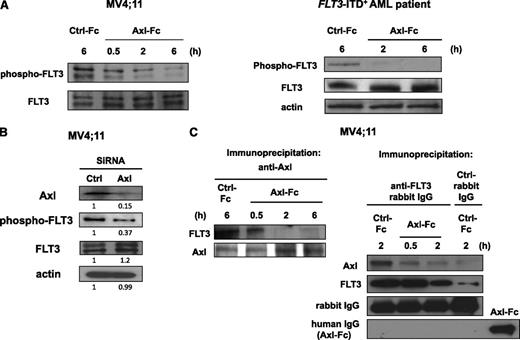
We first looked to our earlier study in an attempt to identify the mechanism(s) by which Axl contributes to the pathogenesis of AML. We reported that Axl is necessary for maintaining activation of c-Kit,20 which belongs to the same type III RTK family as FLT3.31 Indeed, FLT3 inhibitors used in clinical trials have also been shown to inhibit c-Kit activity.18 We thus hypothesized that FLT3 could be a potential target of Axl, at least in cases of FLT3-ITD+ AML. As shown in Figure 3A, abrogation of Axl activation by Axl-Fc resulted in diminished FLT3 phosphorylation in the MV4;11 human FLT3-ITD+ AML cell line (left) as well as in primary FLT3-ITD+ AML blasts (right). To confirm this, we transfected the MV4;11 human FLT3-ITD+ AML cell line with siRNA for silencing Axl expression (Figure 3B). Silencing Axl expression led to significantly and proportionately diminished phosphorylation of FLT3 but did not decrease the expression level of total FLT3 protein, which is consistent with the data shown in Figure 3A using Axl-Fc. The addition of an excess of Axl-Fc to the cultures of MV4;11 cells transfected with Axl siRNA led to a further decrease in phosphorylation of mutant FLT3 (data not shown). These results suggest that Axl is important for constitutive activation of mutant FLT3 in FLT3-ITD+ AML cells, and may explain, at least in part, the mechanism of how Axl plays a role in FLT3-ITD+ AML.
Inhibition of Axl activation diminishes FLT3 phosphorylation in FLT3-ITD+ AML cells. (A) MV4;11 human FLT3-ITD+ AML cell line (left) or primary FLT3-ITD+ AML blasts (right) were treated with Ctrl-Fc or Axl-Fc for the indicated times and subject to immunoblot to detect phospho-FLT3 and FLT3. Cells were treated with Ctrl-Fc for 6 hours. (B) MV4;11 human FLT3-ITD+ AML cell line was transfected with Ctrl or Axl siRNA and were then harvested 16 hours after transfection and subjected to immunoblot. A densitometry measurement is shown below each band. Actin was used as a loading control. (C) After the MV4;11 human FLT3-ITD+ AML cell line was treated with Ctrl-Fc or Axl-Fc for the indicated times, cells were then lysed and coimmunoprecipitated using anti-Axl (left) or anti-FLT3 (right) rabbit IgG. Immunoblots were performed to detect FLT3, Axl, rabbit IgG, and human IgG. Recombinant Axl-Fc protein was used as a positive control for human IgG.
Inhibition of Axl activation diminishes FLT3 phosphorylation in FLT3-ITD+ AML cells. (A) MV4;11 human FLT3-ITD+ AML cell line (left) or primary FLT3-ITD+ AML blasts (right) were treated with Ctrl-Fc or Axl-Fc for the indicated times and subject to immunoblot to detect phospho-FLT3 and FLT3. Cells were treated with Ctrl-Fc for 6 hours. (B) MV4;11 human FLT3-ITD+ AML cell line was transfected with Ctrl or Axl siRNA and were then harvested 16 hours after transfection and subjected to immunoblot. A densitometry measurement is shown below each band. Actin was used as a loading control. (C) After the MV4;11 human FLT3-ITD+ AML cell line was treated with Ctrl-Fc or Axl-Fc for the indicated times, cells were then lysed and coimmunoprecipitated using anti-Axl (left) or anti-FLT3 (right) rabbit IgG. Immunoblots were performed to detect FLT3, Axl, rabbit IgG, and human IgG. Recombinant Axl-Fc protein was used as a positive control for human IgG.
Next, we investigated whether Axl physically interacts with FLT3 in FLT3-ITD+ AML. In the MV4;11 human FLT3-ITD+ AML cell line, anti-Axl antibody could coimmunoprecipitate FLT3 (Figure 3C left), and immunoprecipitation using an anti-FLT3 antibody was also able to pull down Axl (Figure 3C right). This indicates that Axl and FLT3 bind with each other constitutively in FLT3-ITD+ AML. When the cells were treated with Axl-Fc, the physical interaction between Axl and FLT3 was disrupted (Figure 3C), suggesting not only that Axl is a positive regulator of FLT3 phosphorylation but also that this regulation likely occurs through physical interaction between activated Axl and FLT3. To test the possibility that Axl-Fc was inhibiting the interaction between Axl and FLT3 by nonspecific binding to FLT3, anti–human IgG antibody was used to probe coimmunoprecipitated proteins pulled down by anti-FLT3 rabbit antibody. As seen in the second last row of Figure 3C (right), rabbit IgG was easily detected, but in the bottom row of the same figure human IgG, which is a part of Axl-Fc, was not detected. This indicates that Axl-Fc does not nonspecifically bind to FLT3 to interfere with the interaction between Axl and FLT3.
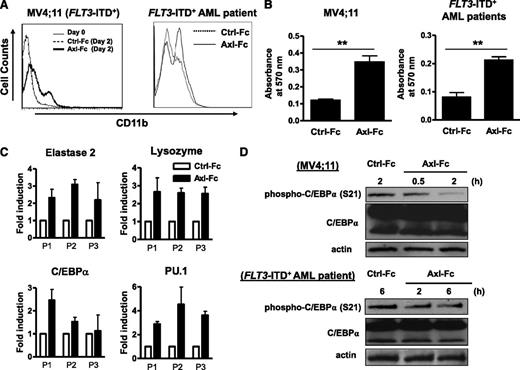
The FLT3-ITD has previously been shown to contribute to an arrest in differentiation along the myeloid lineage, which is a characteristic hallmark of AML.32 Because a block of Axl phosphorylation by Axl-Fc results in downregulation of FLT3-ITD phosphorylation (Figure 3), we examined if treatment with Axl-Fc reversed the arrest of myeloid differentiation. Indeed, primary blasts from FLT3-ITD+ AML patients treated with Axl-Fc in vitro exhibited (1) an increased surface density expression of CD11b, a myeloid lineage marker (Figure 4A); (2) an increase in the number of cells capable of reducing NBT, which is a functional assay of myeloid differentiation measuring superoxide production (Figure 4B); (3) morphologic changes typical of being relieved from myeloid differentiation block (supplemental Figure 3B); and (4) significantly increased expression of genes known to be relevant to myeloid differentiation, such as myeloperoxidase, elastase 2, lysozyme, C/EBPα, and PU.1, in primary human FLT3-ITD+ AML blasts (Figure 4C) and in the MV4;11 human FLT3-ITD+ AML cell line (supplemental Figure 3A,C). Previous studies have shown that the block in myeloid differentiation that occurs in FLT3-ITD+ AML results from suppression of C/EBPα.24,25 In MV4;11 cells as well as in FLT3-ITD+ AML primary patient blasts, Axl-Fc could diminish C/EBPα Ser21 phosphorylation, a posttranslational modification mediated by ERK1/2 that impairs the ability of C/EBPα to induce myeloid differentiation33 (Figure 4D). Collectively, these data indicate that phosphorylation of Axl contributes to the block in myeloid differentiation in FLT3-ITD+ AML.
Inhibition of Axl activation relieves a block in myeloid differentiation. (A) MV4;11 human FLT3-ITD+ AML cell line was treated with Ctrl-Fc or Axl-Fc for 2 days and stained with anti-CD11b antibody conjugated with phycoerythrin (left). Separately, the cells treated with media alone were also stained with the antibody (day 0). The histogram lines for day 0 and Ctrl-Fc (day 2) overlapped indistinguishably. Primary FLT3-ITD+ AML patient blasts were treated with Ctrl-Fc or Axl-Fc for 2 days and stained with anti-CD11b antibody conjugated with phycoerythrin (right). Each histogram is the representative of 2 separate experiments. (B) MV4;11 human FLT3-ITD+ AML cell line (left) or primary FLT3-ITD+ AML patient blasts (right) were treated with Ctrl-Fc or Axl-Fc for 4 days and then NBT reduction was assessed by measuring the absorbance at 570 nm. The graph shows mean + SEM for 3 separate experiments. **P < .01. (C) Primary FLT3-ITD+ AML blasts from 3 patients (P1 to P3) were treated with Ctrl-Fc (empty bar) or Axl-Fc (filled bar) for 2 days and the mRNA of the indicated gene was quantified by real-time RT-PCR. Data shown is the fold induction of each gene expression by Axl-Fc when compared with that by Ctrl-Fc (arbitrarily set at 1.0). (D) MV4;11 human FLT3-ITD+ AML cell line (top) and primary FLT3-ITD+ AML patient blasts (bottom) were treated with Ctrl-Fc or Axl-Fc for the indicated times and phospho-C/EBPα (Ser21) and C/EBPα were detected by immunoblot. Actin was used as a loading control. This is the representative of 2 separate experiments.
Inhibition of Axl activation relieves a block in myeloid differentiation. (A) MV4;11 human FLT3-ITD+ AML cell line was treated with Ctrl-Fc or Axl-Fc for 2 days and stained with anti-CD11b antibody conjugated with phycoerythrin (left). Separately, the cells treated with media alone were also stained with the antibody (day 0). The histogram lines for day 0 and Ctrl-Fc (day 2) overlapped indistinguishably. Primary FLT3-ITD+ AML patient blasts were treated with Ctrl-Fc or Axl-Fc for 2 days and stained with anti-CD11b antibody conjugated with phycoerythrin (right). Each histogram is the representative of 2 separate experiments. (B) MV4;11 human FLT3-ITD+ AML cell line (left) or primary FLT3-ITD+ AML patient blasts (right) were treated with Ctrl-Fc or Axl-Fc for 4 days and then NBT reduction was assessed by measuring the absorbance at 570 nm. The graph shows mean + SEM for 3 separate experiments. **P < .01. (C) Primary FLT3-ITD+ AML blasts from 3 patients (P1 to P3) were treated with Ctrl-Fc (empty bar) or Axl-Fc (filled bar) for 2 days and the mRNA of the indicated gene was quantified by real-time RT-PCR. Data shown is the fold induction of each gene expression by Axl-Fc when compared with that by Ctrl-Fc (arbitrarily set at 1.0). (D) MV4;11 human FLT3-ITD+ AML cell line (top) and primary FLT3-ITD+ AML patient blasts (bottom) were treated with Ctrl-Fc or Axl-Fc for the indicated times and phospho-C/EBPα (Ser21) and C/EBPα were detected by immunoblot. Actin was used as a loading control. This is the representative of 2 separate experiments.
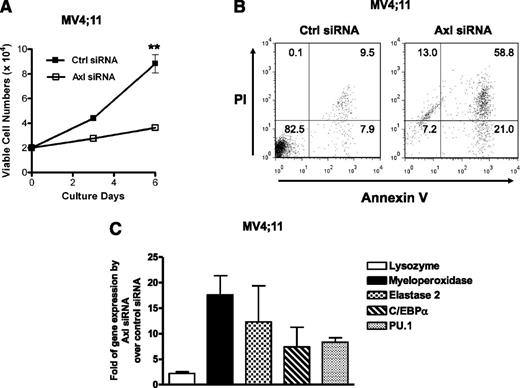
To confirm the results above showing that Axl plays an important role in proliferation, survival, and differentiation of FLT3-ITD+ AML cells, we transfected the MV4;11 human FLT3-ITD+ AML cell line with siRNA against Axl. Whereas MV4;11 cells transfected with Ctrl siRNA showed a robust proliferation, MV4;11 cells transfected with siRNA targeting Axl grew only modestly (Figure 5A). In addition, knockdown of Axl expression by siRNA induced apoptosis (Figure 5B) and increased the expression levels of the genes noted above to be augmented upon myeloid differentiation (Figure 5C). Collectively, these data confirm the results described above using Axl-Fc (Figures 3 and 4) and thus provide further support that activated Axl likely has a role in promoting proliferation, survival, and a block in differentiation of FLT3-ITD+ AML cells.
Knockdown of Axl gene expression by siRNA. (A) After transfecting Ctrl or Axl siRNA, cells from the MV4;11 human FLT3-ITD+ AML cell line were counted in trypan blue at the indicated times. This is the representative of 2 separate experiments and data are the mean ± SD (triplicate). **P < .01. (B) At 3 days after transfection with Ctrl or Axl siRNA, cells from the MV4;11 human FLT3-ITD+ AML cell line were stained with annexin V-FITC and PI for detection of apoptosis. Numbers indicate the percentage of each quadrant. This is the representative of 2 separate experiments. (C) Four days after transfection of Ctrl or Axl siRNA, cells from the MV4;11 human FLT3-ITD+ AML cell line were harvested and measurement of mRNA expression level of each gene was performed by quantitative real-time RT-PCR. The graph shows fold induction of gene expression by Axl siRNA over Ctrl siRNA with mean ± SEM (n = 3).
Knockdown of Axl gene expression by siRNA. (A) After transfecting Ctrl or Axl siRNA, cells from the MV4;11 human FLT3-ITD+ AML cell line were counted in trypan blue at the indicated times. This is the representative of 2 separate experiments and data are the mean ± SD (triplicate). **P < .01. (B) At 3 days after transfection with Ctrl or Axl siRNA, cells from the MV4;11 human FLT3-ITD+ AML cell line were stained with annexin V-FITC and PI for detection of apoptosis. Numbers indicate the percentage of each quadrant. This is the representative of 2 separate experiments. (C) Four days after transfection of Ctrl or Axl siRNA, cells from the MV4;11 human FLT3-ITD+ AML cell line were harvested and measurement of mRNA expression level of each gene was performed by quantitative real-time RT-PCR. The graph shows fold induction of gene expression by Axl siRNA over Ctrl siRNA with mean ± SEM (n = 3).
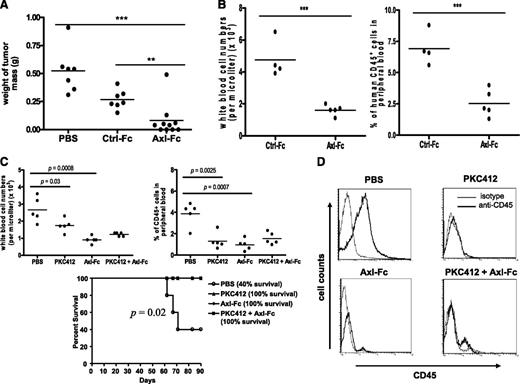
To further explore the role of Axl in FLT3-ITD+ AML in vivo, we first employed a subcutaneous xenograft model. Axl-Fc administered 3 times per week for 4 weeks significantly suppressed growth of the MV4;11 human FLT3-ITD+ AML cell line compared with PBS-treated mice (0.52 ± 0.1 g for PBS-treated mice [n = 7] vs 0.08 ± 0.07 g for Axl-Fc [n = 10], P < .001) (Figure 6A). Ctrl-Fc also inhibited tumor growth as previously reported,34 albeit significantly less than Axl-Fc (0.26 ± 0.04 g for Ctrl-Fc [n = 7] vs 0.08 ± 0.07 g for Axl-Fc [n = 10], P < .01) (Figure 6A).
Blocking Axl activation inhibits FLT3-ITD+ AML in vivo. (A) SCID mice were injected subcutaneously with cells from the MV4;11 human FLT3-ITD+ AML cell line (5 × 106 cells per mouse), and 10 days later treatment of the mice with PBS (n = 7), Ctrl-Fc (n = 7), or Axl-Fc (n = 10) was initiated as described in “Materials and methods,” followed by sacrifice and tumor measurement. Each filled circle indicates the weight of tumor mass from each mouse and the line shows the mean tumor weight of each experimental group. **P < .01, ***P < .001. (B) Irradiated SCID mice received an intravenous injection of primary human FLT3-ITD+ AML blasts (2 × 107 cells per mouse). Four weeks later, thrice-weekly intraperitoneal administration of Ctrl-Fc (n = 4) or Axl-Fc (n = 5) was started. Eight weeks after injection of AML blasts, WBCs (left) and the percentage of human CD45+ cells (right) in peripheral blood were quantified. Each filled circle represents the data from each mouse and the line shows the mean of each experimental group. ***P < .001. (C) Irradiated SCID mice were intravenously injected with primary FLT3-ITD+ AML blasts from a patient (2 × 107 cells per mouse; different from the patient sample used in panel B). Four weeks later, thrice-weekly administration of PBS (n = 5), PKC412 (n = 5), Axl-Fc (n = 5), or PKC412 plus Axl-Fc (n = 5) was started. Whereas PBS and Axl-Fc were administered with intraperitoneal injection, PKC412 was administered orally. Eight weeks after injection of AML blasts, the number of WBCs (top left) and the percentage of human CD45+ cells (top right) in peripheral blood were quantified. Each filled circle represents the data from each mouse and the line shows the mean of each experimental group. Kaplan-Meier plot of survival (bottom). Statistical significance was assessed using log-rank test (P = .02). Mice were sacrificed when they became moribund. The study ended at 90 days after the injection of primary patient FLT3-ITD+ AML cells into SCID mice. (D) At 90 days, all the mice still alive were killed and bone marrow was collected from each mouse and analyzed with fluorescence activated cell sorting to detect human CD45+ cells. Thin and thick lines indicate staining with isotype control antibody and anti–human CD45 antibody, respectively. The histograms shown are the representative of each experimental group.
Blocking Axl activation inhibits FLT3-ITD+ AML in vivo. (A) SCID mice were injected subcutaneously with cells from the MV4;11 human FLT3-ITD+ AML cell line (5 × 106 cells per mouse), and 10 days later treatment of the mice with PBS (n = 7), Ctrl-Fc (n = 7), or Axl-Fc (n = 10) was initiated as described in “Materials and methods,” followed by sacrifice and tumor measurement. Each filled circle indicates the weight of tumor mass from each mouse and the line shows the mean tumor weight of each experimental group. **P < .01, ***P < .001. (B) Irradiated SCID mice received an intravenous injection of primary human FLT3-ITD+ AML blasts (2 × 107 cells per mouse). Four weeks later, thrice-weekly intraperitoneal administration of Ctrl-Fc (n = 4) or Axl-Fc (n = 5) was started. Eight weeks after injection of AML blasts, WBCs (left) and the percentage of human CD45+ cells (right) in peripheral blood were quantified. Each filled circle represents the data from each mouse and the line shows the mean of each experimental group. ***P < .001. (C) Irradiated SCID mice were intravenously injected with primary FLT3-ITD+ AML blasts from a patient (2 × 107 cells per mouse; different from the patient sample used in panel B). Four weeks later, thrice-weekly administration of PBS (n = 5), PKC412 (n = 5), Axl-Fc (n = 5), or PKC412 plus Axl-Fc (n = 5) was started. Whereas PBS and Axl-Fc were administered with intraperitoneal injection, PKC412 was administered orally. Eight weeks after injection of AML blasts, the number of WBCs (top left) and the percentage of human CD45+ cells (top right) in peripheral blood were quantified. Each filled circle represents the data from each mouse and the line shows the mean of each experimental group. Kaplan-Meier plot of survival (bottom). Statistical significance was assessed using log-rank test (P = .02). Mice were sacrificed when they became moribund. The study ended at 90 days after the injection of primary patient FLT3-ITD+ AML cells into SCID mice. (D) At 90 days, all the mice still alive were killed and bone marrow was collected from each mouse and analyzed with fluorescence activated cell sorting to detect human CD45+ cells. Thin and thick lines indicate staining with isotype control antibody and anti–human CD45 antibody, respectively. The histograms shown are the representative of each experimental group.
The in vivo efficacy of Axl-Fc against primary human FLT3-ITD+ AML was examined using a model whereby primary FLT3-ITD+ AML blasts from patients are engrafted into a SCID mouse.35 When compared with Ctrl-Fc, Axl-Fc significantly reduced not only the number of circulating WBCs (4.75 + 0.59 × 106 cells/mL for Ctrl-Fc [n = 4] vs 1.6 + 0.16 × 106/mL for Axl-Fc [n = 5], P < .001), but also the percentage of human CD45+ cells in peripheral blood, which is an indicator of leukemic engraftment (6.9% ± 0.67% human CD45+ cells for Ctrl-Fc vs 2.5% ± 0.59% for Axl-Fc, P < .001, Figure 6B). This indicates that, consistent with the subcutaneous xenograft study with the MV4;11 human FLT3-ITD+ AML cell line presented above, blocking Axl activation by Axl-Fc can suppress primary human FLT3-ITD+ AML in vivo. Moreover, under the dosing and scheduling conditions used, Axl-Fc was as effective against FLT3-ITD+ AML in vivo as the FLT3-selective tyrosine kinase inhibitor PKC412 (Figure 6C-D). Combination of Axl-Fc plus PKC412 was equally effective as either agent alone in reducing total WBC counts and the percentage of human CD45+ cells in blood (Figure 6C top) or in bone marrow (Figure 6D), or in improving survival (bottom, Figure 6C). The absence of an additive effect could be attributed to Axl being upstream of FLT3 as evidenced by the fact that inhibition of Axl activation by Axl-Fc abrogates FLT3 phosphorylation in FLT3-ITD+ AML cells (Figure 2).
Discussion
Our study showed that Axl is constitutively active in FLT3-ITD+ AML as well as in FLT3-WT AML, suggesting that Axl may play a role in the pathogenesis of both types of AML. Here, we have assessed the molecular mechanism responsible for the regulation of constitutive FLT3-ITD phosphorylation in FLT3-ITD+ AML. Using the soluble receptor Axl-Fc, a pharmacologic Axl inhibitor, or siRNA targeting Axl, we have demonstrated that activated Axl contributes to the constitutive activation of the FLT3-ITD, the growth and survival of FLT3-ITD+ AML cells, and the arrest of their differentiation. Axl thus appears be involved in the pathogenesis and/or progression of FLT3-ITD+ AML by at least partially regulating constitutive activation of FLT3-ITD. Our data are consistent with the previous studies showing that, in FLT3-ITD+ AML cells, FLT3-specific inhibitors were able to inhibit proliferation, induce cell-cycle arrest, augment apoptosis, and relieve a myeloid differentiation block.24,25
We also note that Axl likely has a role in FLT3-WT AML where FLT3 is not phosphorylated (Figure 1C). The fact that activated Axl does not have a role in phosphorylation of FLT3 in FLT3-WT AML makes it unlikely that activated Axl is serving as a primary kinase of FLT3 in FLT3-ITD+ AML. Rather, it could be the case that activated Axl interacts physically with FLT3-ITD following the latter’s autophosphorylation (Figure 3). The fact that Axl-Fc can inhibit FLT3-ITD phosphorylation and disrupt the interaction between FLT3-ITD and Axl suggests that the interaction between active Axl and phosphorylated FLT3-ITD may be important for the maintenance of constitutive FLT3-ITD phosphorylation. This notion is indirectly supported by the data showing that the level of phospho-FLT3 corresponds to that of phospho-Axl in FLT3-ITD+ AML cells. When the level of phospho-Axl was similar between two different FLT3-ITD+ AML patient samples (Figure 1C P1 and P2), we observed similar levels of phospho-FLT3. However, when the level of phospho-Axl was higher, that of phospho-FLT3 was also higher (supplemental Figure S1C P3 and P5).
Because the FLT3-WT AML cells that we tested in this study did not possess phosphorylated FLT3, the interaction between Axl and nonactivated FLT3 may not occur. In fact, we have data showing that FL stimulation induces physical interaction between Axl and WT FLT3 protein in CD34+ hematopoietic progenitor cells (I.K. Park and M.A. Caligiuri, unpublished data), indicating that activation of FLT3 may be crucial for the interaction with Axl. Zheng et al36 have shown that WT FLT3, like its mutated form, is often constitutively activated (8 of 12 primary AML samples and 4 of 13 leukemia cell lines in their study) due to autocrine activation by overexpressed FLT3 ligand (FL). This raises the possibility that Axl might bind to phosphorylated FLT3 and contribute to the maintenance of its activated state in some FLT3-WT AML cases.
As noted above, we found Axl to be activated in FLT3-WT AML cells without constitutively active FLT3, and Axl-Fc and the pharmacologic Axl inhibitor XL-880 were each able to inhibit cell growth and induce apoptosis of FLT3-WT AML cells (Figure 2). This suggests that the antileukemic effects of Axl blockade are not exclusive to FLT3-ITD+ AML and that Axl may contribute to leukemogenesis in this molecularly heterogeneous group of AML cases via other pathway(s). Although it remains to be investigated as to how Axl may be involved in such cases, previous studies may provide some insights. Ghosh and et al37 demonstrated that Axl is constitutively active in B-cell chronic lymphocytic leukemia (CLL) and acts as a docking site of nonreceptor kinases, such as Syk and phosphoinositide-3 kinase. They also showed that a specific inhibitor of Axl, R428, could induce apoptosis of B-cell CLL. In addition, the study by Hahn and colleagues38 revealed that Syk is constitutively active in FLT3-WT AML blasts and that a pharmacologic inhibitor of Syk could reduce leukemia burden in vivo. Taken together, these previous data suggest the possibility that Axl might be involved in the pathogenesis of FLT3-WT AML through its regulation of Syk and/or possibly other signaling pathways.
We have shown that blocking the activation of Axl with an excess of soluble Axl-Fc reduced FLT3-ITD+ leukemia burden in vivo, thereby providing the rationale that Axl may have potential as a therapeutic target in FLT3-ITD+ AML. Indeed, small-molecule Axl inhibitors are already in preclinical and clinical development as targeted therapeutics against multiple types of tumors. Foretinib (GSK1363089), an orally available multikinase inhibitor of Axl, c-Met, and vascular endothelial growth factor receptor, has been tested against solid tumors in a phase 1 clinical trial39 and has also been shown to be effective in a mouse lung cancer model.40 Another Axl inhibitor, R428, blocked tumor spread and prolonged survival in mouse models of metastatic breast cancer41 and could induce apoptosis of B-cell CLL in vitro.37 Our data suggest that it would be worthwhile to test whether these Axl inhibitors, alone or in combination with other molecular-targeting compounds or chemotherapy, can improve the outcome of FLT3-ITD+ AML patients.
In summary, our study introduces Axl as a regulator of constitutive FLT3 phosphorylation in FLT3-ITD+ AML and as a potential therapeutic target for treating FLT3-ITD+ AML patients. Its ability to target aberrant proliferation, survival, and a blockade in myeloid differentiation may make inhibitors of Axl useful alone or in combination with chemotherapy for FLT3-ITD+ AML. Future studies may expand its role in a broader spectrum of AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Sabrina Garman for assistance with the animal studies and The Ohio State University Comprehensive Cancer Center Leukemia Tissue Bank Shared Resource.
This work was supported by a grant from the National Cancer Institute (CA16058 and CA89341) (M.A.C.) and a P50 SPORE grant (CA140158) (G.M. and M.A.C.). J.C. was supported by a National Cancer Institute T32 training grant (CA09338).
Authorship
Contribution: I.-K.P. designed and performed research, analyzed and interpreted data, and wrote the paper; A.M. and J.C. designed and performed in vivo mouse experiments; S.P.W. and G.M. analyzed and interpreted data; and M.A.C. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Caligiuri, The Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute, The Ohio State University, 300 W 10th Ave, Columbus, OH 43210; e-mail: michael.caligiuri@osumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal