Key Points
Heparin may have beneficial effects on placental health beyond anticoagulation.
Thrombin receptor activation on maternal platelets is implicated in placental developmental failure independent of thrombosis.
Abstract
Low molecular weight heparin (LMWH) is being tested as an experimental drug for improving pregnancy outcome in women with inherited thrombophilia and placenta-mediated pregnancy complications, such as recurrent pregnancy loss. The role of thrombotic processes in these disorders remains unproven, and the issue of antithrombotic prophylaxis is intensely debated. Using a murine model of factor V Leiden–associated placental failure, we show that treatment of the mother with LMWH allows placental development to proceed and affords significant protection from fetal loss. Nonetheless, the therapeutic effect of LMWH is not replicated by anticoagulation; fondaparinux and a direct Xa inhibitor, C921-78, achieve anticoagulation similar to LMWH but produce little or no improvement in pregnancy outcome. Genetic attenuation of maternal platelet aggregation is similarly ineffective. In contrast, even a partial loss of thrombin sensitivity of maternal platelets protects pregnancies. Neonates born from these pregnancies are growth retarded, suggesting that placental function is only partially restored. The placentae are smaller but do not reveal any evidence of thrombosis. Our data demonstrate an anticoagulation-independent role of LMWH in protecting pregnancies and provide evidence against the involvement of thrombotic processes in thrombophilia-associated placental failure. Importantly, thrombin-mediated maternal platelet activation remains central in the mechanism of placental failure.
Introduction
Placental abnormalities have serious consequences for mothers and babies. Consequences include fetal growth restriction, early and late fetal death, and maternal hypertensive disorders, such as preeclampsia. These are disorders of multifactorial origin that affect more than 5% of all pregnancies, and they are a major cause of preterm deliveries, small birth weight, and maternal/neonatal morbidity and mortality.1 Babies born small for gestation age are also at an increased risk of developmental problems and adult onset cardiovascular and metabolic disorders.2
Maternal inherited thrombophilia is a risk factor for placenta-mediated pregnancy complications.3 The extent of the risk varies with ethnicity, the type of pregnancy complication, and the thrombophilia mutation under investigation.4 The mechanism that connects maternal thrombophilia with adverse pregnancy outcomes is unknown and suspected to involve thrombotic occlusion of the uteroplacental circulation. It is hypothesized that the low-pressure placental flow is susceptible to thrombotic complications, much like the maternal venous circulation. Accordingly, interventions aimed at limiting placental thrombosis, such as treatment with low molecular weight heparin (LMWH), are being tested to prevent placental dysfunction and the related sequelae in at-risk pregnancies.5-7 While these studies suggest that LMWH may be beneficial for a subset of women with heritable thrombophilia and recurrent pregnancy loss (RPL), the risk-to-benefit balance of antithrombotic prophylaxis during pregnancy is a subject of intense ongoing debate.8-11 Study limitations and related methodological concerns contribute to the debate, but it is largely due to the uncertain role of thrombotic processes in placental disease12-14 and a lack of established criteria for identifying high-risk pregnancies that may benefit from the use of LMWH. Given the multifactorial and heterogeneous nature of thrombophilia-associated pregnancy disorders, these issues have proven to be complex and difficult to resolve based on epidemiological and clinical data alone.15,16 In the last few years, new nonanticoagulant roles of LMWH have emerged, some of which are directly related to trophoblast function.17 Thus, any beneficial effects of LMWH treatment may not necessarily reflect a causal thrombotic link between thrombophilia mutations and pregnancy loss.
The mouse is a well-studied experimental model system that shares chorioallantoic placentation with humans; this occurs around the end of first trimester in humans and corresponds to 9 days post coitum (dpc) in mice.18 Both species form a hemochorial placenta where maternal cells are eroded and zygote-derived trophoblast cells become directly exposed to maternal blood. At this fetomaternal interface, hemostasis is coordinately regulated by maternal and fetal factors.19 We have previously provided proof-of-concept evidence that fetal prothrombotic gene mutations expressed on trophoblast cells are risk modifiers of adverse pregnancy outcome in women with thrombophilia.20 We showed that embryos homozygous for a hypomorphic Glu387Pro mutation in thrombomodulin (designated as ThbdPro/Pro) die in utero in pregnancies carried by mothers homozygous for the factor V Leiden mutation (designated as FVQ/Q), but not in mothers with normal alleles for factor V (FV+/+). In pregnancies of FVQ/Q mothers, placentae of ThbdPro/Pro embryos are growth retarded, and most do not complete chorioallantoic morphogenesis, resulting in placental insufficiency and fetal death. If the mother is treated with platelet-depleting antibodies, the chorioallantoic placenta is formed and embryonic development proceeds to term. Genetic absence of Par4 in the mother (the embryo is Par4+/−) also results in the birth of normal-appearing and live ThbdPro/Pro pups.20 In the current study, we used 3 experimental approaches to evaluate the role of thrombin and thrombotic processes in this robust model of thrombophilia-associated fetal loss. In the first approach, we treated the mother with LMWH and other pharmacological agents that inhibit thrombin activity or generation to examine their effect on pregnancy outcome. In the second approach, we attenuated thrombin signaling by making the mother deficient in protease-activated receptor 3 (Par3) through genetic breeding experiments. Thrombin triggers activation of murine platelets via 2 receptors, Par3 and Par4. Unlike Par4, thrombin is the only known agonist for Par3. Genetic absence of Par3 in mice increases the threshold of thrombin-mediated platelet activation.21 In the third approach, we used genetic means to inhibit platelet aggregation in the mother by using mutant animals that lack the ability to activate αIIbβ3 integrin by inside-out signaling.22 This experiment is based on the rationale that Par cleavage on platelets potently activates αIIbβ3 integrin by inside-out signaling, leading to platelet aggregation. The 2 genetic approaches allow us to investigate the role of thrombin-mediated platelet activation and aggregation in the mechanism of placental failure and fetal demise.
Materials and methods
Mice
Animal experiments were conducted following standards and procedures approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Thbd Pro (provided by H. Weiler, Blood Center of Wisconsin, Milwaukee, WI), FV Leiden (provided by D. Ginsburg, University of Michigan, Ann Arbor, MI), Par3−/− (purchased from Mutant Mouse Regional Resource Center, University of North Carolina at Chapel Hill, Chapel Hill, NC), Par4−/− (provided by S. Coughlin, University of California, San Francisco, CA), and β3(L746A) mice have been described in Sood et al20 and Petrich et al.22 These were maintained on a C57BL/6 genetic background and bred to each other to generate double and triple mutant strains. Correct combinations were identified based on tail biopsy and polymerase chain reaction–based genotyping.
Analysis of pregnancies
The stage of pregnancies was assessed from days post coitum, assuming midday of plug as 0.5 dpc. Embryos were dissected in phosphate-buffered saline and photographed under a Nikon SMZ1000 Zoom stereomicroscope (Nikon, Melville, NY) equipped with a high-resolution, 5-megapixel digital camera and NIS Elements F 3.0 imaging software. A small sample from each embryo was used for polymerase chain reaction–based genotyping. Nearly all dead embryos were found at an advanced stage of resorption and were not genotyped. Live embryos were identified based on the presence of heartbeats and movement. Using ImageJ software (version 1.42q; National Institutes of Health, Bethesda, MD), the head-to-tail length and lengthwise cross-sectional area was measured from digital images of embryos photographed on their sides. The cross-sectional area of uteroplacental discs photographed with the maternal side facing the camera (supplemental Figure 1) was similarly measured. Formalin-fixed, paraffin-embedded placentae were sectioned and stained with hematoxylin-eosin and independently examined by 2 pathologists. Placental sections were photographed using a Nanozoomer HT slide scanner equipped with the NDP view-imaging software (Hamamatsu, Japan).
Anticoagulation treatments
Anticoagulant drugs were administered through subcutaneous microosmotic pumps (Model 1002, Durect Corp, Cupertino, CA) with a release rate of 0.25 μL/hour. These were filled with 12.5 or 25 μg/μL hirudin (lepirudin, brand name Refludan; Berlex, Wayne, NJ), 100 μg/μL LMWH (enoxaparin, brand name Lovenox; Aventis Pharmaceuticals Inc, Bridgewater, NJ), 12.5 μg/μL fondaparinux (brand name Arixtra; GlaxoSmithKline, Research Triangle Park, NC), or 100 μg/μL C921-78 (Portola Pharmaceuticals, Inc, South San Francisco, CA)23 before implantation. Treatment was initiated at 5.5 dpc and continued until the day of analysis. Anticoagulation and plasma levels of LMWH, fondaparinux, and C921-78 were measured in terms of Xa inhibitory activity with Coatest heparin (Chromogenix, Lexington, MA). Enoxaparin standards were included in these assays to compare all anticoagulants in terms of enoxaparin-equivalent activity. Anticoagulation by hirudin was assessed by partial thromboplastine time (PTT) assays done using an ST4 instrument and STA PTT reagent (Diagnostica Stago, Parsippany, NJ).
Statistical analysis
P < .05 was used to establish significance for all experiments. The χ2 goodness-of-fit (GOF) test was used to determine deviation from expected Mendelian proportions. Exact binomial 95% confidence intervals (CI) were computed where appropriate. The χ2 test of independence was used for comparing 2 treatments or treated vs untreated groups. The exact binomial for the GOF test or the Fisher’s exact test of independence was used in experiments where the expected number in any cell was lower than 5. The Student t test (2 tailed with unequal variance) was used for comparing embryonic and placental sizes.
Results
LMWH rescues pregnancies in a murine model of thrombophilia-associated fetal loss
ThbdPro/Pro embryos conceived from crosses between ThbdPro/Pro males and FVQ/QThbdPro/+ females were growth retarded by 9.5 days dpc,20 and all or most were dead and resorbed by 12.5 dpc (Table 1, row 1; P = .000003, χ2 GOF test; 95% CI of 0% to 15.4%). We treated pregnant FVQ/QThbdPro/+ females with LMWH and examined its ability to rescue ThbdPro/Pro embryos from intrauterine death. Treatment with LMWH resulted in marked anticoagulation in maternal circulation (measured at 0.3 to 0.5 IU antifactor Xa activity per mL plasma) and significantly improved the yield of live ThbdPro/Pro fetuses examined at 16.5 dpc (Table 1, row 2; P = .0006 compared with untreated in row 1, χ2 test of independence; 95% CI of 23.9% to 57.9% with treatment). The ThbdPro/Pro embryos obtained after LMWH treatment appeared morphologically normal (Figure 1A-B). Treatment significantly reduced the abortion rate from 69.9% to 34% (P = .00006, χ2 test of independence; untreated 22 live, 51 aborted vs treated 35 live, 18 aborted), but it did not bring it down to background levels (<5%) observed in control pregnancies. Thus, LMWH treatment significantly improved survival of ThbdPro/Pro embryos and reduced the overall abortion rate in pregnancies of FVQ/Q mothers.
Pregnancy outcome of FVQ/QThbdPro/+ females mated to ThbdPro/Pro males
| Experimental manipulation . | Stage of analysis (dpc) . | Parental genotype . | Genotype of live embryos (number/%) . | Number (%) of aborted embryos . | Number of embryos/ pregnancies analyzed . | ||
|---|---|---|---|---|---|---|---|
| Female . | Male . | FVQ/+ThbdPro/+ . | FVQ/+ThbdPro/Pro . | ||||
| None | 12.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 22 (100%) | 0* | 51 (69.9%) | 73 (8) |
| LMWH | 16.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 21 (60%) | 14 (40%) | 18 (34%) | 53 (7) |
| Lepirudin | 16.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 21 (100%) | 0* | 27 (56.2%) | 48 (6) |
| Fondaparinux | 16.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 25 (96.2%) | 1* (3.8%) | 20 (43.5%) | 46 (6) |
| Xa inhibitor C921-78 | 15.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 36 (90%) | 4* (10%) | 35 (46.7%) | 75 (8) |
| Mother Par3−/− | 15.5 | Par3−/− | ThbdPro/Pro | 54 (65.9%) | 28* (34.1%) | 41 (33.3%) | 123 (13) |
| FVQ/QThbdPro/+ | |||||||
| Mother β3LA/LA | 15.5 | β3LA/LA | ThbdPro/Pro | 27 (93.1%) | 2* (6.9%) | 72 (71.3%) | 101 (12) |
| FVQ/QThbdPro/+ | |||||||
| Mother β3LA/LA | 12.5 | β3LA/LA | ThbdPro/Pro | 9 (100%) | 0* | 9 (50%) | 18 (2) |
| FVQ/QThbdPro/+ | |||||||
| Experimental manipulation . | Stage of analysis (dpc) . | Parental genotype . | Genotype of live embryos (number/%) . | Number (%) of aborted embryos . | Number of embryos/ pregnancies analyzed . | ||
|---|---|---|---|---|---|---|---|
| Female . | Male . | FVQ/+ThbdPro/+ . | FVQ/+ThbdPro/Pro . | ||||
| None | 12.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 22 (100%) | 0* | 51 (69.9%) | 73 (8) |
| LMWH | 16.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 21 (60%) | 14 (40%) | 18 (34%) | 53 (7) |
| Lepirudin | 16.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 21 (100%) | 0* | 27 (56.2%) | 48 (6) |
| Fondaparinux | 16.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 25 (96.2%) | 1* (3.8%) | 20 (43.5%) | 46 (6) |
| Xa inhibitor C921-78 | 15.5 | FVQ/QThbdPro/+ | ThbdPro/Pro | 36 (90%) | 4* (10%) | 35 (46.7%) | 75 (8) |
| Mother Par3−/− | 15.5 | Par3−/− | ThbdPro/Pro | 54 (65.9%) | 28* (34.1%) | 41 (33.3%) | 123 (13) |
| FVQ/QThbdPro/+ | |||||||
| Mother β3LA/LA | 15.5 | β3LA/LA | ThbdPro/Pro | 27 (93.1%) | 2* (6.9%) | 72 (71.3%) | 101 (12) |
| FVQ/QThbdPro/+ | |||||||
| Mother β3LA/LA | 12.5 | β3LA/LA | ThbdPro/Pro | 9 (100%) | 0* | 9 (50%) | 18 (2) |
| FVQ/QThbdPro/+ | |||||||
ThbdPro/Pro embryos die in midgestation in pregnancies carried by FVQ/QThbdPro/+ females (row 1), but survive if the mother is treated with LMWH (row 2) or is deficient in Par3 (row 6). Anticoagulation with lepirudin (row 3), fondaparinux (row 4), or C921-78 (row 5) or genetic attenuation of maternal platelet aggregation (rows 7 and 8) does not result in comparable rescue. Equal proportions (50% each) of the ThbdPro/+ and ThbdPro/Pro embryos are expected from these crosses.
There is a significant difference from expected proportions (P value < .05) based on the χ2 GOF test.
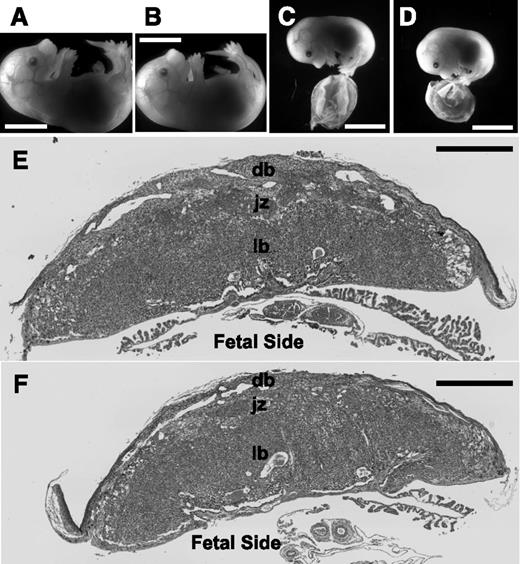
ThbdPro/Pro embryos survive pregnancies of FVQ/QThbdPro/+ females if the mother is treated with LMWH or is deficient in Par3. ThbdPro/Pro (B,D) and littermate ThbdPro/+ (A) and Thbd+/+ (C) embryos from representative pregnancies treated with LMWH (A-B) or with maternal Par3 deficiency (C-D) are shown. These correspond to 16.5 dpc for LMWH treatment and 15.5 dpc for maternal Par3 deficiency. ThbdPro/Pro embryos are growth restricted in pregnancies of Par3−/− FVQ/QThbdPro/+ females (measurements shown in Figure 3A-B). Their placentae tend to be smaller (measurements in Figure 3C) but do not present evidence of overt thrombosis. Hematoxylin-eosin stained histological sections of Thbd+/+ (E) and ThbdPro/Pro placentae (F) corresponding to (C) and (D), respectively, are shown. The plane of sectioning is through the center of the placenta and perpendicular to its flat surface. Regions of the placenta are marked. Scale bars represent 5 mm in (A-D) and 1 mm in (E-F). db, decidua basalis; jz, junctional zone corresponding to basal plate in human placenta; lb, labyrinth or fetal placenta containing trophoblast-covered fetal vessels bathed in maternal blood.
ThbdPro/Pro embryos survive pregnancies of FVQ/QThbdPro/+ females if the mother is treated with LMWH or is deficient in Par3. ThbdPro/Pro (B,D) and littermate ThbdPro/+ (A) and Thbd+/+ (C) embryos from representative pregnancies treated with LMWH (A-B) or with maternal Par3 deficiency (C-D) are shown. These correspond to 16.5 dpc for LMWH treatment and 15.5 dpc for maternal Par3 deficiency. ThbdPro/Pro embryos are growth restricted in pregnancies of Par3−/− FVQ/QThbdPro/+ females (measurements shown in Figure 3A-B). Their placentae tend to be smaller (measurements in Figure 3C) but do not present evidence of overt thrombosis. Hematoxylin-eosin stained histological sections of Thbd+/+ (E) and ThbdPro/Pro placentae (F) corresponding to (C) and (D), respectively, are shown. The plane of sectioning is through the center of the placenta and perpendicular to its flat surface. Regions of the placenta are marked. Scale bars represent 5 mm in (A-D) and 1 mm in (E-F). db, decidua basalis; jz, junctional zone corresponding to basal plate in human placenta; lb, labyrinth or fetal placenta containing trophoblast-covered fetal vessels bathed in maternal blood.
Rescue by LMWH is not replicated by other anticoagulants that inhibit thrombin activity or generation
Because heparins also possess biological effects unrelated to anticoagulation,17 we examined whether the ability of LMWH to rescue pregnancies is mediated through anticoagulation. LMWH inhibits FXa and thrombin, in an antithrombin-dependent manner. We treated pregnant FVQ/QThbdPro/+ females, mated to ThbdPro/Pro males, with a direct thrombin inhibitor, lepirudin. Treatment effectively anticoagulated pregnant females (measured by two- to threefold prolongation in PTT) but did not result in live ThbdPro/Pro embryos (Table 1, row 3; 95% CI of 0% to 16.1%). The abortion rate in lepirudin-treated pregnancies was somewhat reduced (56.2%) but was not significantly different from untreated pregnancies (69.9%; P = .13, χ2 test of independence; untreated 22 live, 51 aborted vs treated 21 live, 27 aborted). Lepirudin may cross the placenta. No obvious bleeding was observed in the embryos with lepirudin treatment. We next used fondaparinux, a synthetic pentasaccharide composed of a minimal antithrombin binding subunit of heparin that retains the ability to inhibit FXa activity. Treatment of pregnancies with fondaparinux resulted in anticoagulation comparable to LMWH (measured at 0.5 to 0.7 IU antifactor Xa activity per mL plasma) but did not result in comparable rescue of ThbdPro/Pro embryos (Table 1, row 4; P = .002 compared with LMWH treated in row 2, χ2 test of independence). Only 1 of 26 live embryos from fondaparinux-treated pregnancies was determined to be ThbdPro/Pro. Thus, despite equivalent anticoagulation, the ability of LMWH to ameliorate fetal loss far exceeded that of fondaparinux.
FXa as part of the prothrombinase complex is 300 000-fold more efficient in thrombin generation than FXa alone.24 However, once incorporated into the prothrombinase complex, FXa is protected from antithrombin-LMWH and antithrombin-fondaparinux–mediated inactivation.25 To overcome this limitation, we treated pregnant females with C921-78, a synthetic peptide and direct Xa inhibitor that efficiently inhibits Xa in solution phase and in membrane-bound prothrombinase complexes.26 Treatment with C921-78 resulted in high, steady-state levels of circulating Xa inhibitory peptide (2.65 ± 0.38 μM/L plasma) and more than 1 IU/mL of plasma Xa inhibitory activity. C921-78–treated pregnancies produced 4 ThbdPro/Pro embryos out of a total of 40 live (Table 1, row 5; P = .0000004, χ2 GOF test; 95% CI of 2.8% to 23.7%). Comparison with pooled historical data from our laboratory (0 ThbdPro/Pro/55 live embryos, 95% CI of 0% to 6.5%) suggests a statistically significant low-level rescue with C921-78 (P = .029, Fisher’s exact test). However, despite more-efficient anticoagulation, C921-78 treatment resulted in significantly fewer live ThbdPro/Pro embryos as compared with treatment with LMWH (Table 1, row 5 compared with row 2; P = .002, χ2 test of independence). Taken together, these data show that placental pathology in the murine model of thrombophilia-associated fetal loss persists even after treatments that significantly reduce thrombin generation or activity. In contrast, LMWH affords rescue in this model.
Anticoagulation protects surviving littermates and reduces overall abortion rate
We noticed an abortion rate of ∼70% in crosses between ThbdPro/Pro males and FVQ/QThbdPro/+ females (Table 1, row 1), higher than accountable from loss of ThbdPro/Pro embryos alone. Although treatment with fondaparinux or C921-78 did not improve specific survival of ThbdPro/Pro embryos, the overall abortion rates were significantly reduced (44% with fondaparinux; P = .004, χ2 test of independence; untreated 22 live, 51 aborted vs treated 26 live, 20 aborted; 47% with C921-78; P = .004, χ2 test of independence; untreated 22 live, 51 aborted vs treated 40 live, 35 aborted). These data suggest that, in addition to ThbdPro/Pro embryos, some of the ThbdPro/+ embryos are also aborted in pregnancies of FVQ/Q mothers, and that the death of ThbdPro/+ embryos is ameliorated with anticoagulation treatment. To compare the survival of ThbdPro/+ with littermate Thbd+/+ embryos, ThbdPro/+ males were mated to FVQ/QThbdPro/+ females and pregnancies were analyzed at 15.5 dpc. No live ThbdPro/Pro embryos were observed in this cross (Table 2, row 1). Although fewer than expected live ThbdPro/+ embryos were observed, this reduction did not reach statistical significance (Table 2, row 1; P = .18, χ2 GOF test, 20 Thbd+/+ and 27 ThbdPro/+). Measurement of embryonic and placental sizes in these pregnancies, however, revealed a significant growth retardation of ThbdPro/+ embryos and placentae, as compared with littermate Thbd+/+ controls (Figure 2 and supplemental Figure 1). Growth retardation was not observed in pregnancies analyzed at 12.5 dpc (ThbdPro/+ males mated to FVQ/QThbdPro/+ females; 9 Thbd+/+, 14 ThbdPro/+, 0 ThbdPro/Pro, 15 aborted not genotyped, 4 pregnancies analyzed; measurement data not shown) or in the reverse genetic crosses where FVQ/QThbdPro/+ males were mated to ThbdPro/+ females (supplemental Figure 2). Embryos of all 3 genotypes, Thbd+/+, ThbdPro/+, and ThbdPro/Pro, were obtained in normal Mendelian proportions from the reverse cross (Table 2, row 2; P = .42, χ2 GOF test). These observations suggest that the growth retardation of ThbdPro/+ embryos, like the fetal demise of ThbdPro/Pro embryos, only occurs in pregnancies of FVQ/Q mothers and is, therefore, specific to the mother-fetus pair. However, because placentae of dead or aborted embryos can activate coagulation in the mother,27 we examined the possibility that dead littermates play a role in growth retardation of ThbdPro/+ placentae in these pregnancies. To address this question, we set up a different genetic cross. FVQ/QThbdPro/+ females were mated to wild-type males, and growth retardation and survival of ThbdPro/+ embryos were assessed in comparison with their Thbd+/+ littermates. No ThbdPro/Pro embryos are expected from this cross. Analysis of these pregnancies revealed normal numbers and sizes of ThbdPro/+ embryos and placentae as compared with littermate Thbd+/+controls (Thbd+/+ males mated to FVQ/QThbdPro/+ females; 35 Thbd+/+, 41 ThbdPro/+, 12 aborted not genotyped; P = .49, χ2 GOF test; supplemental Figure 3). These data demonstrate that ThbdPro/+ embryos are smaller and tend to have reduced survival in pregnancies shared with dead ThbdPro/Pro embryos, but this does not occur in comparable pregnancies that do not include ThbdPro/Pro conceptuses. ThbdPro/+ conceptuses with reduced anticoagulation ability are more susceptible than Thbd+/+ conceptuses to the adverse effects of “toxic factors” produced by their dead littermates. In multifetal human pregnancies, retention of a dead fetus is associated with increased platelet activation, thrombin generation, and a higher risk of disseminated intravascular coagulation in the mother and in morbidity of the surviving fetus.27,28 Our data show that treatments with fondaparinux or C921-78, although not very effective in protecting ThbdPro/Pro fetuses from intrauterine death, do protect ThbdPro/+ fetuses from morbidity associated with sharing the womb with 1 or more dead ThbdPro/Pro fetuses.
Pregnancy outcome of FVQ/QThbdPro/+ females mated to ThbdPro/+ males
| Stage of analysis . | Parental genotype . | Genotype of live embryos (number/%) . | Number (%) of aborted embryos . | Number of embryos/ pregnancies analyzed . | |||
|---|---|---|---|---|---|---|---|
| Female . | Male . | FVQ/+Thbd+/+ . | FVQ/+ThbdPro/+ . | FVQ/+ThbdPro/Pro . | |||
| 15.5 dpc | FVQ/QThbdPro/+ | ThbdPro/+ | 20 (42.6%) | 27 (57.4%) | 0* | 42 (47.2%) | 89 (9) |
| 15.5 dpc | ThbdPro/+ | FVQ/QThbdPro/+ | 26 (29.5%) | 38 (43.2%) | 24 (27.3%) | 4 (4.3%) | 92 (10) |
| 15.5 dpc | Par3−/− | ThbdPro/+ | 14 (26.4%) | 31 (58.5%) | 8 (15.1%) | 24 (31.2%) | 77 (8) |
| FVQ/QThbdPro/+ | |||||||
| Neonates | Par3−/− | ThbdPro/+ | 16 (22.9%) | 38 (54.3%) | 16 (22.9%) | — | 70 (9) |
| FVQ/QThbdPro/+ | |||||||
| Neonates | Par4−/− | ThbdPro/+ | 7 (20%) | 22 (62.9%) | 6 (17.1%) | — | 35 (7) |
| FVQ/QThbdPro/+ | |||||||
| 15.5 dpc | β3LA/LA | ThbdPro/+ | 19 (25%) | 32 (42.1%) | 0* | 25 (32.9%) | 76 (9) |
| FVQ/QThbdPro/+ | |||||||
| Stage of analysis . | Parental genotype . | Genotype of live embryos (number/%) . | Number (%) of aborted embryos . | Number of embryos/ pregnancies analyzed . | |||
|---|---|---|---|---|---|---|---|
| Female . | Male . | FVQ/+Thbd+/+ . | FVQ/+ThbdPro/+ . | FVQ/+ThbdPro/Pro . | |||
| 15.5 dpc | FVQ/QThbdPro/+ | ThbdPro/+ | 20 (42.6%) | 27 (57.4%) | 0* | 42 (47.2%) | 89 (9) |
| 15.5 dpc | ThbdPro/+ | FVQ/QThbdPro/+ | 26 (29.5%) | 38 (43.2%) | 24 (27.3%) | 4 (4.3%) | 92 (10) |
| 15.5 dpc | Par3−/− | ThbdPro/+ | 14 (26.4%) | 31 (58.5%) | 8 (15.1%) | 24 (31.2%) | 77 (8) |
| FVQ/QThbdPro/+ | |||||||
| Neonates | Par3−/− | ThbdPro/+ | 16 (22.9%) | 38 (54.3%) | 16 (22.9%) | — | 70 (9) |
| FVQ/QThbdPro/+ | |||||||
| Neonates | Par4−/− | ThbdPro/+ | 7 (20%) | 22 (62.9%) | 6 (17.1%) | — | 35 (7) |
| FVQ/QThbdPro/+ | |||||||
| 15.5 dpc | β3LA/LA | ThbdPro/+ | 19 (25%) | 32 (42.1%) | 0* | 25 (32.9%) | 76 (9) |
| FVQ/QThbdPro/+ | |||||||
ThbdPro/Pro embryos die in midgestation in pregnancies carried by FVQ/QThbdPro/+ mothers (row 1), but survive in the reverse genetic cross (row 2) in pregnancies carried by FV+/+ mothers. Attenuation of thrombin signaling by genetic absence of Par3 in the mother allows survival of several ThbdPro/Pro embryos (row 3) and neonates (row 4) in these pregnancies, as does the genetic absence of maternal Par4 (row 5). Attenuation of maternal platelet aggregation (row 6) does not result in comparable rescue. Based on Mendelian inheritence, Thbd+/+, ThbdPro/+, and ThbdPro/Pro embryos are expected to be born at 25%, 50%, and 25% proportions, respectively, from these crosses.
There is a significant difference from expected proportions (P value < .05) based on the χ2 GOF test.
ThbdPro/+ embryos and placentae are smaller than littermate Thbd+/+ controls in pregnancies of FVQ/QThbdPro/+ females mated to ThbdPro/+ males. The size distribution of ThbdPro/+ and littermate Thbd+/+ embryos and placentae measured at 15.5 dpc is shown. Lengthwise cross-sectional area (A), length of the embryo (B), and placental cross-sectional area (C) were measured from digital photographs taken at a known magnification and resolution. P values were computed in comparison with Thbd+/+ controls. No live ThbdPro/Pro embryos were obtained from these pregnancies; these die in utero before 12.5 dpc.
ThbdPro/+ embryos and placentae are smaller than littermate Thbd+/+ controls in pregnancies of FVQ/QThbdPro/+ females mated to ThbdPro/+ males. The size distribution of ThbdPro/+ and littermate Thbd+/+ embryos and placentae measured at 15.5 dpc is shown. Lengthwise cross-sectional area (A), length of the embryo (B), and placental cross-sectional area (C) were measured from digital photographs taken at a known magnification and resolution. P values were computed in comparison with Thbd+/+ controls. No live ThbdPro/Pro embryos were obtained from these pregnancies; these die in utero before 12.5 dpc.
Attenuation of thrombin signaling in the mother partially rescues fetal loss
The inability of anticoagulation therapy to protect ThbdPro/Pro embryos was unexpected given the role of platelets and Par4 in their demise. It prompted us to reevaluate the involvement of thrombin in the loss of ThbdPro/Pro embryos. Although generally recognized as a thrombin receptor, Par4 is also activated by other proteases such as neutrophilic granule protease cathepsin G, tissue kallikrein, and trypsin.29,30 Thrombin triggers activation of murine platelets via 2 receptors, Par3 and Par4. In contrast to Par4, thrombin is the only known agonist for Par3. To more rigorously evaluate the role of thrombin in placental pathology, we conducted genetic experiments to determine whether Par3 deficiency in the mother ameliorates placental phenotype and fetal death. ThbdPro/Pro males were mated to Par3−/−FVQ/QThbdPro/+ females, and pregnancies were analyzed at 15.5 dpc. Several live ThbdPro/Pro embryos were obtained from this cross, and rescue was found to be highly significant (Table 1, row 6; P = .0001 compared with untreated in row 1, χ2 test of independence). Consistent with this observation, the genetic absence of Par3 in the mother significantly reduced the abortion rate (33.3% compared with 69.9% in untreated pregnancies; P = .0000007, χ2 test of independence; untreated 22 live, 51 aborted vs treated 82 live, 41 aborted). Rescue of ThbdPro/Pro embryos observed in the absence of maternal Par3 was comparable to LMWH treatment (Table 1, row 6 compared with row 2; P = .55, χ2 test of independence) but was partial as compared with expected Mendelian proportions (Table 1, row 6; P = .004, χ2 GOF test; 95% CI of 24% to 45%). Thus, even a partial loss of thrombin signaling allows placental development to proceed and results in significant rescue. These data provide strong evidence that thrombin-mediated activation of maternal platelets causes placental failure and fetal death in this model.
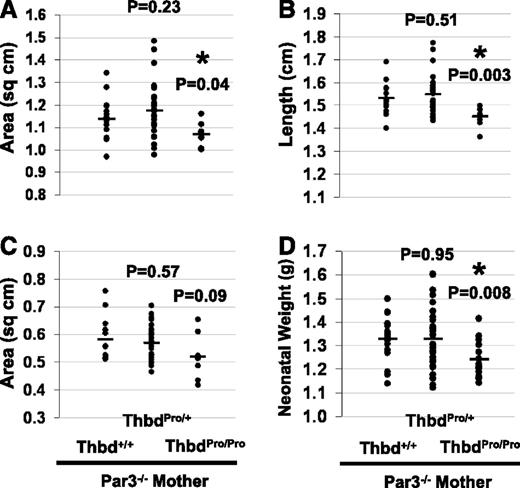
We investigated whether the ThbdPro/Pro embryos that do not die in pregnancies carried by FVQ/Q mothers exhibit normal placental and embryonic growth. For these experiments, Par3−/−FVQ/QThbdPro/+ females were mated to ThbdPro/+ males to generate Thbd+/+ littermate controls. Only 25% of all conceptuses in this cross are expected to be ThbdPro/Pro. Once again, live ThbdPro/Pro embryos were observed in late pregnancies (Table 2, row 3; P = .236, χ2 GOF test). The abortion rate was reduced as compared with the untreated (31.2% in Table 2, row 3 compared with 47.2% in row 1; 53 live and 24 aborted vs untreated 47 live and 42 aborted; P = .035, χ2 test of independence) but remained higher than background (31.2% in Table 2, row 3 compared with 4.3% in row 2; 53 live and 24 aborted vs 88 live and 4 aborted; P = .000003, χ2 test of independence). Although ThbdPro/Pro embryos survived in statistically significant numbers, these were growth restricted as compared with the Thbd+/+ littermates and tended to have smaller placentae (Figure 1C-D; Figure 3A-C). Histological evaluation of ThbdPro/Pro placentae did not reveal any evidence of increased thrombosis (Figure 1E-F). ThbdPro/Pro neonates born from pregnancies of FVQ/QThbdPro/+ mothers lacking Par3 appeared normal but exhibited lower birth weights as compared with their littermates (Table 2, row 4; Figure 3D). These data suggest that although several ThbdPro/Pro embryos survive in pregnancies of FVQ/Q mothers that lack Par3, their placental function is not fully restored, resulting in fetal growth retardation. For comparison, we also weighed neonates from pregnancies of Par4−/−FVQ/QThbdPro/+ females mated to ThbdPro/+ males (Table 2, row 5). These mothers completely lack platelet responsiveness to thrombin. ThbdPro/Pro neonates did not exhibit smaller birth weights (supplemental Figure 4). Our observations underscore the sensitivity of placental growth and development to maternal platelet activation. The mechanisms by which activated platelets alter placental growth are currently unknown.
Par3 deficiency in the mother allows survival of ThbdPro/Proembryos, but these are growth retarded in utero and smaller at birth. Pregnancies of Par3−/−FVQ/QThbdPro/+ females mated to ThbdPro/+ males were analyzed at 15.5 dpc. Lengthwise cross-sectional area (A), length of the embryo (B), and placental cross-sectional area (C) were measured from digital photographs taken at a known magnification and resolution. Neonatal weight was taken within 18 hours of birth (D). P values were computed in comparison with Thbd+/+ controls.
Par3 deficiency in the mother allows survival of ThbdPro/Proembryos, but these are growth retarded in utero and smaller at birth. Pregnancies of Par3−/−FVQ/QThbdPro/+ females mated to ThbdPro/+ males were analyzed at 15.5 dpc. Lengthwise cross-sectional area (A), length of the embryo (B), and placental cross-sectional area (C) were measured from digital photographs taken at a known magnification and resolution. Neonatal weight was taken within 18 hours of birth (D). P values were computed in comparison with Thbd+/+ controls.
Attenuation of platelet aggregation does not rescue fetal loss
Thrombotic clogging of placental vessels and local hypoxia, albeit transient, may explain the failure of ThbdPro/Pro placentae to complete morphogenesis in FVQ/Q mothers. In our previous work, staining for fibrin clots did not reveal any evidence of increased thrombosis in ThbdPro/Pro placentae in pregnancies of FVQ/QThbdPro/+ females.20 Par cleavage on platelets potently activates αIIbβ3 integrin by inside-out signaling, leading to platelet aggregation. We examined if platelet aggregation plays a role in fetal loss. The L746A mutation in the β3 subunit (homozygous, β3LA/LA) impairs agonist-induced inside-out activation of αIIbβ3.22 Homozygous animals show attenuated platelet aggregation in vitro and in vessel injury models.22,31 We used the β3(L746A) mutation as a genetic tool to investigate the role of maternal platelet aggregation in placental failure and fetal loss. ThbdPro/Pro males were mated to β3LA/LAFVQ/QThbdPro/+ females, and pregnancies were analyzed at 15.5 dpc. Of the 101 embryos analyzed in a total of 12 pregnancies, only 2 live ThbdPro/Pro embryos were obtained (Table 1, row 7; P = .000003, χ2 GOF test). The survival of ThbdPro/Pro embryos was significantly lower than observed with the absence of maternal Par3 (Table 1, row 7 compared with row 6; P = .005, χ2 test of independence) or with LMWH treatment (Table 1, row 7 compared with row 2; P = .002, χ2 test of independence). The placentae of the 2 surviving ThbdPro/Pro embryos did not show any evidence of overt thrombosis (data not shown). In a different cross, β3LA/LAFVQ/QThbdPro/+ females were mated to ThbdPro/+ males and analyzed at 15.5 dpc. No ThbdPro/Pro embryos were obtained from this cross (Table 2, row 6; P = .000003, χ2 GOF test). Analysis of pregnancies at 12.5 dpc also did not reveal any live ThbdPro/Pro embryos (Table 1, row 8; P = .004, exact binomial test for GOF; 95% CI of 0.4% to 12.1% from pooled data from all 3 crosses). Thus, attenuation of maternal platelet aggregation due to the β3(L746A) mutation produced little improvement in the yield of live ThbdPro/Pro embryos.
Discussion
Antithrombotic prophylaxis of pregnant women with inherited thrombophilia and RPL is an intensely debated issue in obstetric medicine.8-11 LMWH is suspected to be beneficial in a subgroup of high-risk pregnancies, but the multifactorial nature of RPL and the inherent heterogeneity in study populations precludes easy identification of this subgroup. It has also been questioned if thrombophilia mutations disrupt placental function via thrombotic processes. Evaluation of placental pathologies has not revealed a clear correlation between maternal inherited thrombophilia and increased thrombotic lesions in the placenta.12-14 Meanwhile, animal models have identified a critical role of coagulation components in the development and function of extraembryonic tissues. Complete absence of Thbd or the endothelial protein C receptor (EPCR) results in embryonic lethality in mice, secondary to placental defects. Treatment with heparin extends survival of a small fraction of EPCR−/− embryos,32 and it does not improve survival of Thbd−/− embryos.33 These observations are often quoted as evidence against the role of thrombotic processes in thrombophilia-associated placental disorder.10 Because these are gene knockout models, the inability of anticoagulation to rescue these pregnancies may reflect other essential functions of the missing gene products. In contrast to the complete absence of Thbd and EPCR, reduced expression or compromised function of these components allows placental and embryonic development to proceed and results in viable adult animals.34,35 Placental failure and embryonic death is once again observed when reduced thrombomodulin function or reduced EPCR expression in the fetus is combined with the prothrombotic factor V Leiden mutation in the mother.20 These studies provided us with an animal model of high-risk pregnancy where a combination of maternal and fetal inherited thrombophilia causes placental developmental failure. We asked if placental failure and fetal loss can be counteracted with antithrombotic therapy.
We demonstrated, first, that LMWH unequivocally protects pregnancies in the murine model, lending support to its reported efficacy in improving pregnancy outcome in thrombophilic women.5,6 Protection was observed both in terms of rescue of ThbdPro/Pro embryos from intrauterine death in pregnancies of FVQ/Q mothers and in a significantly improved overall abortion rate. Nonetheless, our data show that the observed therapeutic effect of LMWH is not replicated by anticoagulation alone. Factor Xa and thrombin inhibition are the 2 most well-known anticoagulant functions of LMWH. We used fondaparinux to address whether the Xa-inhibitory activity of LMWH is sufficient to rescue pregnancies. Treatment with fondaparinux resulted in anticoagulation comparable to LMWH, but it did not protect ThbdPro/Pro embryos from in utero death. Inhibition of thrombin activity using the direct thrombin inhibitor, lepirudin, was similarly effective in anticoagulation but did not ameliorate pregnancy loss. Using the direct Xa inhibitor C921-78 resulted in anticoagulation much higher than achieved with LMWH but led to a significantly lower rescue of ThbdPro/Pro embryos. These observations are striking in the extent of rescue observed with LMWH, all or most of which could not be reproduced with thrombin or FXa inhibition alone. The molecular basis of LMWH’s ability to protect pregnancies remains to be determined and is a subject of our ongoing research. LMWH rescues pregnancies in a murine model of antiphospholipid antibody–induced fetal loss by suppressing complement activity.36 In preliminary studies, we treated pregnant factor V Leiden females with complement-inhibitory anti-C5 monoclonal antibodies. This treatment has been previously shown to prevent antiphospholipid antibody–induced fetal loss in mice.37 Anti-C5 monoclonal antibody treatment was ineffective in preventing fetal loss of ThbdPro/Pro embryos (Sood et al, unpublished observations), indicating that the pathological mechanism of fetal loss in our model of inherited thrombophilia is distinct from the complement-driven mechanism operating in the murine model of antiphospholipid antibody–induced fetal loss, where C5 activation is a critical effecter. Other candidate mechanisms include inhibition of p-selectin–mediated platelet tethering to trophoblast cells or to immune cells38,39 and possible direct actions of LMWH on trophoblast cells.17,40,41
The failure to suppress placental pathology with lepirudin, fondaparinux, or C921-78, despite demonstrated efficacy of anticoagulation, was puzzling. Because Par4 can also be activated by agonists other than thrombin,29,30 we asked whether the genetic absence of Par3, a second thrombin receptor on mouse platelets, ameliorates fetal loss. Par3 and Par4 on mouse platelets are analogous to PAR1 and PAR4 on human platelets in their threshold for activation by thrombin. Par3 promotes cleavage and activation of Par4 at low concentrations, such that the 50% effective concentration (EC50) of platelet activation by thrombin increases from 0.10 ± 0.08 nM in wild-type platelets to 1.5 ± 0.7 nM in Par3−/− platelets.21 We demonstrated, second, that the genetic absence of Par3 in the mother allows placental development to proceed and significantly improves the survival of ThbdPro/Pro fetuses. The observation that ThbdPro/Pro embryos in pregnancies of FVQ/Q mothers survive in each case, in the absence of platelets or in the absence of Par4 or Par3 in the mother, provides a strong argument that placental developmental failure in this model is driven by activation of maternal platelets. Thrombin activates both receptors and is the most likely mediator of platelet activation in this scenario. Our observations demonstrate that even a slight elevation in EC50 of maternal platelet activation by thrombin affords significant rescue. Thus, placental development is highly vulnerable to slight increases in thrombin generation and platelet activation. ThbdPro/Pro embryos that escape arrest of placental morphogenesis in the absence of maternal Par3 are not completely normal. These are growth restricted at late gestation and at birth. A similar reduction in birth weight was not detected in ThbdPro/Pro neonates that survive in the absence of maternal Par4. Although our data do not formally prove that the Par3- and Par4-dependent effects are mediated via activation of these receptors on platelets, the data do associate residual reactivity of maternal platelets to thrombin with suboptimal placental function in a prothrombotic setting. These data, once again, demonstrate that thrombophilia-associated placental dysfunction correlates with developmental or structural abnormalities rather than thrombotic clogging.
If thrombin is the critical mediator of placental dysfunction, why is anticoagulation not effective? This likely reflects the low EC50 of thrombin concentration required for platelet activation. While hirudin, fondaparinux, and direct thrombin inhibitors are increasingly efficient in delaying the onset of the propagation phase and reducing the total amount of thrombin, a low level of thrombin generation and platelet activation is likely to continue in their presence. Notably, C921-78 effectively inhibits fibrin-rich venous clots in a rabbit deep vein thrombosis model, but it severely attenuates and does not completely inhibit platelet-rich arterial clots in an arteriovenous shunt model, even at the highest doses examined.23 Similarly, C921-78 alone significantly delays thrombus formation upon ferric chloride–induced injury of mouse mesentric arteries, but a combination of C921-78 and reduced adenosine 5′-diphosphate receptor expression is required to completely abolish platelet aggregation and thrombus formation.42 Observations such as these have suggested a more crucial role of platelets in arterial thrombosis. The uteroplacental circulation does not conform to traditional definitions of arterial or venous blood flow, and the relative contribution of platelets in causing placental pathology is unclear. Our data suggest that a low level of thrombin generation and platelet activation, rather than accumulation of thrombin, causes the primary pathology in our model. It also excludes the possibility that the contribution of activated platelets in this pathology primarily resides in their ability to enhance thrombin generation, also referred to as their procoagulant function.
We investigated the contribution of platelet aggregation to disease pathology. The αIIbβ3 complex plays a central role in the firm adhesion of platelets to the vessel wall and in platelet aggregation. We used β3(L746A)-mutant mice that maintain αIIbβ3-mediated platelet adhesion but are attenuated in their ability to activate the integrin complex by inside-out signaling in response to thrombin and other agonists. These mice show impaired platelet aggregation in vitro and diminished thrombus growth in laser injury models.22,31 We demonstrated, third, that FVQ/Q mothers homozygous for the L746A mutation continue to abort ThbdPro/Pro embryos, despite attenuated platelet aggregation. Taken together, our genetic and anticoagulation studies suggest that PAR-mediated platelet activation at the fetomaternal interface precipitates placental developmental failure, without the need for thrombotic clogging of uteroplacental vessels. Placental failure appears to involve an activation-dependent, but aggregation-independent, function of platelets. Platelet activation triggers release of stored components from secretory vesicles (α-granules, dense granules, and lysosomes) that range from small molecules and ions to enzymes and growth factors. The repertoire of the cargo stored in platelets includes potently anti-angiogenic platelet factor 4, endostatin, and thrombospondin-1.43-45 The ability of activated platelets to degranulate and release factors that may affect placental development and the ability of platelets to recruit immune cells are candidate mechanisms of placental failure.
Our observations may have relevance for human multifetal pregnancies affected by the death of 1 or more fetuses. Intrauterine fetal death of 1 fetus in a multiple gestation is a rare condition that complicates approximately 0.5% to 6.8% of twin pregnancies and 4.3% to 17% of triplet pregnancies. With greater reliance on assisted reproductive techniques, twins and higher-order multiples are becoming increasingly common. In these pregnancies, retention of a dead fetus is associated with increased coagulation activation in maternal circulation and morbidity for the surviving fetuses.27,28 Our data show that ThbdPro/+ embryos and placentae with reduced anticoagulation ability are more susceptible to the hostile intrauterine environment associated with fetal death in a multifetal pregnancy than their Thbd+/+ littermates. Antithrombotic therapy with fondaparinux and direct Xa inhibitors is beneficial in this scenario.
In summary, we have presented data evaluating the role of thrombin and thrombotic processes in placental developmental failure observed in a murine high-risk pregnancy model, generated by combining maternal and fetal prothrombotic mutations in genes expressed at the fetomaternal interface. Given the multifactorial nature of thrombophilia-associated RPL and the inherent deficiencies of animal models of human disease, the exact implication of our observations for human pregnancies is unclear. Our experiments should, therefore, only be interpreted as proof-of-concept studies. Our data support the clinical observation that LMWH might, indeed, be beneficial for a subset of high-risk pregnancies with maternal thrombophilia and a history of RPL. It further supports the stimulating possibility that LMWH may have beneficial effects beyond anticoagulation and prompts an investigation into the mechanism by which LMWH improves placental health. Two pilot studies have reported a beneficial effect of LMWH in pregnancies of women with a history of placenta-mediated complications without thrombophilia,46,47 but no benefit was observed in 3 other studies.48-50 We report that blocking early steps of PAR-mediated platelet activation is beneficial in preventing fetal loss in mice. Pharmacologically blocking thrombin receptor activation on human platelets may be potentially beneficial for a subset of women with thrombophilia and RPL. Our data, however, caution that certain treatments may improve intrauterine survival but continue to be associated with suboptimal growth and development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank Sara Szabo for examination of placental slides and expert pathology opinion; the CRI Histology, Imaging and Pediatric Biobank and Analytical Tissue Core for expert services; Ann Diamond, Andrew Webb, Micah Tesdall, and Eric Jukkala for administrative support; Mark Ginsberg from University of California–San Diego for β3 (L746A) mutant mice; and Hartmut Weiler from the Blood Center of Wisconsin for critical reading of the manuscript and for suggestions.
This work was supported by the Basil O’Connor Starter Scholar Award (5-FY09-121, March of Dimes Foundation); National Scientist Development Grant (0930200N, American Heart Association); and a CRI Histology and Imaging Core grant to R.S.
Authorship
Contribution: J.A., M.S.W., M.B., and V.A. performed experiments; R.G.H. helped with statistical analysis; B.G.P., U.S., and P.E.N. provided critical reagents and reviewed the manuscript; and R.S. designed and performed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: U.S. is an employee of Portola Pharmaceutical Inc. The remaining authors declare no conflicting financial interests.
Correspondence: Rashmi Sood, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: rsood@mcw.edu.