Key Points
B-cell lymphomas with surface nucleolin-Fas complexes are resistant to Fas-mediated apoptosis through decreased ligand binding.
Expression of nucleolin protects mice from a lethal agonistic Fas challenge, whereas a non-Fas binding nucleolin mutant does not.
Abstract
Resistance to Fas-mediated apoptosis is associated with poor cancer outcomes and chemoresistance. To elucidate potential mechanisms of defective Fas signaling, we screened primary lymphoma cell extracts for Fas-associated proteins that would have the potential to regulate Fas signaling. An activation-resistant Fas complex selectively included nucleolin. We confirmed the presence of nucleolin-Fas complexes in B-cell lymphoma cells and primary tissues, and the absence of such complexes in B-lymphocytes from healthy donors. RNA-binding domain 4 and the glycine/arginine-rich domain of nucleolin were essential for its association with Fas. Nucleolin colocalized with Fas on the surface of B-cell lymphoma cells. Nucleolin knockdown sensitized BJAB cells to Fas ligand (FasL)-induced and Fas agonistic antibody-induced apoptosis through enhanced binding, suggesting that nucleolin blocks the FasL–Fas interaction. Mice transfected with nucleolin were protected from the lethal effects of agonistic anti-mouse Fas antibody (Jo2) and had lower rates of hepatocyte apoptosis, compared with vector and a non-Fas-binding mutant of nucleolin. Our results show that cell surface nucleolin binds Fas, inhibits ligand binding, and thus prevents induction of Fas-mediated apoptosis in B-cell lymphomas and may serve as a new therapeutic target.
Introduction
Survival of individuals with non-Hodgkin’s lymphoma (NHL) has improved with recent advancements in chemotherapy regimens, which now include targeted therapies. Despite these advancements, NHL demonstrates frequent relapses and a high mortality rate (30%).1 The principal source of NHL relapse is the survival and expansion of cells resistant to chemotherapy. Stimulation of Fas, a member of the tumor necrosis factor superfamily of apoptosis receptors, by Fas ligand (FasL)-bearing cells or from within damaged cells is an important mechanism of cell elimination, particularly in the lymphoid system.2,3 Genetic models featuring Fas-disabling mutations develop autoreactive lymphocytes, arising from ineffective negative selection that results in autoimmune disorders and lymphoma.4,5 Moreover, cells lacking Fas or Fas-defective cells are resistant to customary doses of chemotherapy and radiation.6-9 Further investigations determined that Fas is a key component of responses to radiation and chemotherapy regimens,6 as several forms of chemotherapy, including genotoxic chemotherapy, induce higher expression levels of Fas and/or FasL in order to effectively eliminate tumor cells.10,11
However, Fas-resistant NHL cells often express normal levels of wild-type Fas and FasL while remaining resistant to Fas activation. The lack of correlation between Fas levels and sensitivity to Fas-mediated apoptosis in lymphoid cancer cells indicates additional modulation of the apoptosis pathway. Investigations into the defects of Fas-mediated apoptosis have shown multiple layers of control over Fas signaling. The signaling is initiated by binding of trimeric FasL complexes to a Fas receptor, which recruits the adaptor molecule FADD and subsequently procaspase-8 through the homologous death domain and death effector domain, respectively, to form the death-inducing signaling complex.3,12 Formation of this complex promotes cleavage and activation of the initiator caspase-8, resulting in activation of an intricate caspase cascade and cell death.13,14 Each of these signaling stages is subjected to different inhibitory mechanisms aimed at preventing Fas-mediated apoptosis.3 In most cases of NHL, the main cause for disabled Fas signaling is unknown, and restoring Fas apoptotic signaling in NHL would have an enormous impact on cancer therapy.3,6,8,15
Our previous research has revealed that Fas signaling can be regulated at the cell membrane. The human herpesvirus-8 K1 oncoprotein binds to the Fas receptor and disables Fas signaling by preventing binding of FasL.16,17 As viral proteins often mimic the functions of cellular proteins, we sought cellular proteins with a similar capacity to form inhibitory complexes with Fas.16,17 Through a screening process, we identified nucleolin associated with activation-resistant Fas.
Nucleolin is a multifunctional nucleolar phosphoprotein that was first identified in ribosomal RNA processing, and more recently is recognized as having pro-survival functions. Nucleolin levels are frequently upregulated in cancer and cancer-associated endothelial cells.18,19 The localization of nucleolin is altered in highly proliferating cells, where it translocates into the cytoplasm and onto the plasma membrane.18,20,21 Nucleolin is highly expressed on the surface of multiple types of cancer cells, where it serves as a receptor and transport protein.22,23
Numerous pro-survival functions attributed to nucleolin are associated with its selective extranuclear localization. Cytoplasmic nucleolin plays a role in stabilizing Bcl-2, Bcl-xl, and IL-2 mRNAs,24,25 and plasma membrane-associated nucleolin has been identified as a receptor for hepatocyte growth factor and P-selectin.23,26 Nucleolin is also involved in regulating multiple apoptosis-related molecules.27,28 These functions implicate extranuclear nucleolin as a contributor to the survival and anti-apoptotic pathways of cancer cells. Based on the role of nucleolin in the survival of cancer cells, its selective surface expression, and our identification of nucleolin as a Fas-binding partner, we investigated the effect of nucleolin on Fas-mediated apoptosis in NHL.
Methods
Cells
Raji, Jurkat, and BC-3 cell lines were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Pittsburgh, PA); BJAB, Daudi, U937, and 293T cell lines were obtained from American Type Culture Collection. Cells were maintained in RPMI 1640 medium (HyClone; Thermo Scientific, Logan, UT) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA) in 5% CO2 atmosphere at 37°C. Cell lines were authenticated by short tandem repeat analysis (MD Anderson Cancer Center Characterized Cell Line Core, Houston, TX) and regularly tested for mycoplasma (Lonza, Rockland, ME).
Peripheral blood B cells were isolated from the blood of healthy donors obtained from Gulf Coast Blood Center (Houston, TX). Isolation was achieved with CD19-positive magnetic beads (Invitrogen, Oslo, Norway). The isolated B cells were released with the competitive DETACHaBEAD CD19 (Invitrogen).
In accordance with the Declaration of Helsinki, tumor cells were collected with the written consent of patients at The University of Texas MD Anderson Cancer Center under research protocols LAB08-0190, 2008-0075, and 2005-0656. Tumor cell-enriched buffy coats were isolated by Histopaque-1077 (Sigma Aldrich, Ayrshire, United Kingdom) gradient centrifugation.
Purification and identification of Fas-associated proteins
BJAB cells and NHL primary cells were screened for potential binding partners of activation-resistant Fas as previously described29 (and see the Blood website for data supplements).
IB and coIP
For coimmunoprecipitation (coIP), cells, homogenized livers, or primary lymphoma tissues were analyzed as previously described16,29 and as detailed in the data supplements. Supernatants were incubated with 1 to 2 µg of the anti-Fas antibody (B-10) (Santa Cruz Biotechnologies, Santa Cruz, CA) or mouse IgG (Invitrogen, Carlsbad, CA) for 1 hour at 4°C. When primary antibody agarose conjugates were not available, protein A/G sepharose (Pierce Biotechnology, Rockford, IL) or strepavidin agarose (Thermo Scientific) were added. For recombinant assays, 1 µg of chimeric Fc:Fas (BD Biosciences, San Jose, CA) and recombinant nucleolin-GST (Abnova, Heidelberg, Germany) were incubated in 300 µL of RIPA buffer (Cell Signaling, Danvers, MA) for 1.5 hours at 4°C, followed by incubation with protein A agarose for 1 hour at 4°C.
Immunoblotting (IB) was performed according to standard protocols as previously described.16,29 Proteins were analyzed by IB with 1:1 000 dilution of primary antibodies in 5% blotting-grade blocker (BioRad, Hercules, CA) in phosphate-buffered saline with Tween 20: anti-Fas (B-10)-horseradish peroxidase (HRP), anti-Fas (N-18), anti-nucleolin MS3-HRP, anti-histone-3, anti-GST (Santa Cruz Biotechnologies), anti-Flag (M2)-HRP, anti-β-actin-HRP (Sigma Aldrich), anti-poly ADP ribose polymerase (PARP), anti-cleaved capase-3, anti-caspase-8, anti-Bcl-2 (Cell Signaling), and anti-DDK (Origene, Rockville, MD), followed by the corresponding HRP-conjugated secondary antibody when an unconjugated primary antibody was used. Visualization was achieved by Supersignal West Pico chemiluminescent substrate (Thermo Scientific). Intensity of bands was compared by densitometry using ImageJ software (NIH).
Confocal imaging
BJABs, a primary NHL, and peripheral blood mononuclear cells (PBMCs) were stained for surface proteins after washing in phosphate-buffered saline (PBS) and blocking with 1% bovine serum albumin (BSA) and 10% goat serum in PBS. Cells were then stained stepwise with the following antibodies in 1% BSA/PBS: anti-Fas (1 µg; Abcam, Cambridge, MA) and secondary alexaflour-647 (1:500; Invitrogen); anti-nucleolin MS-3 (1 µg; Santa Cruz Biotechnologies); and secondary alexaflour-488 (1:500; Invitrogen) for 1.5 hours on ice. Cells were washed twice in 1% BSA/PBS and incubated with wheat germ agglutinin alexafluor-555 (Invitrogen) for 5 minutes. Cells were washed in PBS with 0.1% Tween-20 (Fisher, Fairlawn, NJ) and mounted onto slides using cytospin and ProLong Gold antifade reagent with 4′,6 diamidino-2-phenylindole dihydrochloride stain (DAPI) (Invitrogen). Images were acquired using a Nikon A1R confocal laser microscope system (Nikon Instruments, Melville, NY).
Plasmids and transfections
Plasmids encoding C-terminal myc- and FLAG (DDK)-tagged nucleolin, pCMV6-Nucleolin (TrueORF complementary DNA clones), and the vector control pCMV-ENTRY (Origene), were used for transfections. Partial nucleolin mutants and corresponding DNA primers (Sigma Aldrich) (supplemental Figure 1A) were designed according to the manufacturer’s protocol for Quick Change II XL site-directed mutagenesis (Stratagene, Cedar Creek, TX) using pCMV6-Nucleolin as a template.
Nucleolin binds activation-resistant Fas and shows altered expression in B-cell lymphomas. (A) Silver-stained gel separating primary NHL and BJAB samples subjected to Fas activation and IP with agonistic antibody CH-11 (BJAB and primary NHL CH-11). The remaining lysates were subjected to a second Fas IP (B-10) of any remaining activation-resistant Fas (BJAB and primary NHL B-10). Specific activation-resistant Fas bands were excised, digested with trypsin, and analyzed by nanoflow-LC-MS/MS fragmentation and collision-induced dissociation spectra profiling. A 100 kDa band of interest (asterisk) reveals the protein that is the focus of the current study. The protein sequence of nucleolin is shown with peptides (red text) from the 100 kDa band identified by nanoflow-LC-MS/MS spectra. Peptides map to the RNA binding domains in the C-terminal region of nucleolin. (B) Whole-cell extracts from BJAB, Raji, Daudi, BC-3, and a histocytic lymphoma (U937) cell line and two B-lymphocyte isolations from healthy donors were subjected to IP with B-10 anti-Fas agarose, which recognizes an epitope within the cytoplasmic region of Fas, and analyzed by IB for the presence of nucleolin with MS-3 anti-nucleolin antibody. Representative data from 3 different experiments are shown. Shown is an analysis of 2 primary lymphoma samples (diffuse large B-cell lymphoma [DLBCL] and mantle cell lymphoma [MCL]) demonstrating nucleolin-Fas complex from a screen of 4 DLBCL and 6 MCL primary samples. Extracts were immunoprecipitated with B-10 and analyzed by IB for the presence of nucleolin in precipitated complexes. BJAB cells and isotype IP were used as positive and negative controls, respectively. β-actin was used as a loading control. (C) Whole-cell lysates of 5 lymphomas cell lines (BJAB, Raji, Daudi, BC-3, and U937) and healthy donor B-lymphocyte populations were analyzed by IB for nucleolin expression. β-actin was used as a loading control (bottom panel). Whole-cell lysates of 4 primary hematologic cancer tissues multiple myeloma (MM), MCL, chronic lymphocytic leukemia (CLL) and DLBCL were subjected to IB analysis of nucleolin. β-actin was used as a loading control. Ratio of nucleolin to β-actin levels determined by densitometry is shown below each sample lane. Representative data from 3 different experiments are shown.
Nucleolin binds activation-resistant Fas and shows altered expression in B-cell lymphomas. (A) Silver-stained gel separating primary NHL and BJAB samples subjected to Fas activation and IP with agonistic antibody CH-11 (BJAB and primary NHL CH-11). The remaining lysates were subjected to a second Fas IP (B-10) of any remaining activation-resistant Fas (BJAB and primary NHL B-10). Specific activation-resistant Fas bands were excised, digested with trypsin, and analyzed by nanoflow-LC-MS/MS fragmentation and collision-induced dissociation spectra profiling. A 100 kDa band of interest (asterisk) reveals the protein that is the focus of the current study. The protein sequence of nucleolin is shown with peptides (red text) from the 100 kDa band identified by nanoflow-LC-MS/MS spectra. Peptides map to the RNA binding domains in the C-terminal region of nucleolin. (B) Whole-cell extracts from BJAB, Raji, Daudi, BC-3, and a histocytic lymphoma (U937) cell line and two B-lymphocyte isolations from healthy donors were subjected to IP with B-10 anti-Fas agarose, which recognizes an epitope within the cytoplasmic region of Fas, and analyzed by IB for the presence of nucleolin with MS-3 anti-nucleolin antibody. Representative data from 3 different experiments are shown. Shown is an analysis of 2 primary lymphoma samples (diffuse large B-cell lymphoma [DLBCL] and mantle cell lymphoma [MCL]) demonstrating nucleolin-Fas complex from a screen of 4 DLBCL and 6 MCL primary samples. Extracts were immunoprecipitated with B-10 and analyzed by IB for the presence of nucleolin in precipitated complexes. BJAB cells and isotype IP were used as positive and negative controls, respectively. β-actin was used as a loading control. (C) Whole-cell lysates of 5 lymphomas cell lines (BJAB, Raji, Daudi, BC-3, and U937) and healthy donor B-lymphocyte populations were analyzed by IB for nucleolin expression. β-actin was used as a loading control (bottom panel). Whole-cell lysates of 4 primary hematologic cancer tissues multiple myeloma (MM), MCL, chronic lymphocytic leukemia (CLL) and DLBCL were subjected to IB analysis of nucleolin. β-actin was used as a loading control. Ratio of nucleolin to β-actin levels determined by densitometry is shown below each sample lane. Representative data from 3 different experiments are shown.
To produce the nucleolin partial knockdown (pKO) BJAB cells, lentiviral pGIPZ plasmids (1.2 μg) (Open Biosystems – Thermo Scientific) encoding green fluorescent protein and one of the following short hairpin RNA expressed as microRNA-30 transcripts (miR30): nonsilencing, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), or nucleolin, were transfected into cells using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s recommendations. Transfected cells were selected with 1 µg/mL of puromycin (Sigma Aldrich) for 2 weeks. Cells were subsequently sorted for green fluorescent protein expression using an Aria flow-activated cell sorter (BD Biosciences). Single-cell clones were developed by a standard dilution method.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was extracted by column centrifugation according to the manufacturer’s instructions (Qiagen Sciences, Germantown, MD). First-strand complementary DNA was synthesized using a Superscript II reverse transcriptase kit (Invitrogen) according to the manufacturer’s protocol. Samples were analyzed on 96-well microtiter plates using the StepOnePlus Real-Time PCR System (Applied Biosystems, Singapore) with nucleolin and GAPDH TaqMan probes (Applied Biosystems) after 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Step-One software version 2.1 was used to analyze the real-time polymerase chain reaction data.
Surface biotinylation
Cells were washed twice with ice-cold PBS with Ca2+ and Mg2+ (MediaTech, Manassas, VA) and incubated with 1 mg/mL of EZlink sulfo-NHS-biotin (Thermo Scientific) using standard current protocols. Cells were quenched in ice-cold serum-free medium, washed twice, lysed, and subjected to immunoprecipitation (IP).
Apoptosis induction and flow cytometry analysis
Cells were incubated with the indicated doses of CH-11 (Millipore, Temecula, CA) in 1 mL of serum-free RPMI 1640 and were rotated for 1 hour at room temperature in the dark. Cells were collected by centrifugation, resuspended in fresh FBS-supplemented medium, and incubated for 18 hours at 37°C. Alternatively, cell death was induced by incubation with the indicated dose of FasL (Enzo Life Sciences, Farmingdale, NY) in FBS-supplemented medium for 18 hours at 37°C. Apoptosis analysis by flow cytometry was performed using PE-Annexin V/7-AAD apoptosis kit (BD Bioscience).16,29,30
For detection of surface proteins and agonist binding, cells were washed with 1% FBS/PBS and subjected to 0.25 µg/mL of mouse IgG blocking reagent (Invitrogen). Cells were incubated with either 7 µg of CH-11(Millipore) or 3 µg of Super FasL (Enzo Life Sciences) at 4°C for 20 minutes in the dark. Subsequent incubation with Allophycocyanin rat anti-mouse IgM (BD Biosciences) and Phycolink anti-FLAG-RPE (Prozyme Hayward, CA) were used for detection, respectively. Flow cytometry was performed on an LSR Fortessa flow cytometer with Diva software (BD Bioscience). Data analysis was performed with FlowJo software (Tree Star Inc, Ashland, OR).
In vivo experiments and immunostaining
The hydrodynamic transfection and Fas agonist lethal challenge were performed as previously described and in the data supplements.16,17,29,31 C57BL/6 mice (6- to 8-weeks-old) (National Cancer Institute, Bethesda, MD) or (8-weeks-old) (Harlan Laboratories, Indianapolis, Indiana) were transfected with plasmids: pnuc-myc, pNK-FLAG pSG5, pSG5, Nucleolin-pCMVENTRY, NR123-pCMVENTRY, or pCMVENTRY. Mice were monitored for 8 hours post-challenge and scored for survival. Livers of mice were harvested for fixation and immunostaining. All procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at MD Anderson Cancer Center.
Formalin-fixed, paraffin-embedded liver tissue sections on microscope slides were stained as previously described16,29 using anti-FLAG M2 (Sigma Aldrich), anti-cleaved PARP, and anti-cleaved caspase-3 antibodies (Cell Signaling). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining was performed using a DeadEnd Fluorometric TUNEL kit (Promega, Madison, WI) by following the manufacturer’s protocol. Stained slides were fixed in ProLong Gold antifade reagent with DAPI (Invitrogen). Staining procedures were performed by Vel-Lab Research (Missouri City, TX).
Statistical analysis
Experimental data are reported as means ± SEM from 3 independent experiments, unless otherwise indicated. Differences between groups were calculated using the two-tailed Student t test (GraphPad Prism, La Jolla, CA). A P value less than .05 was considered statistically significant.
Results
Identification of nucleolin in Fas-resistant complexes
To identify potential binding partners of Fas, contributing to the inhibition of Fas signaling, we analyzed activation-resistant Fas complexes. We subjected the Burkitt’s lymphoma line BJAB, which expresses Fas, but shows low response to Fas activation, and primary NHL cells to Fas activation and immunodepletion by agonistic Fas antibody. The remaining supernatants, harboring any inactive/activation-resistant/inaccessible Fas, were subjected to a second Fas IP. The precipitated proteins were separated and visualized by silver stain. A protein band found selectively in the activation-resistant lane was excised and analyzed by nanoflow-Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (LC-MS/MS).29 The fragmentation spectra results were searched against the National Center for Biotechnology Information nonredundant protein database with Mascot. Six of the peptide spectra found in the 100-kDa-excised band (Figure 1A, asterisk) matched nucleolin (Figure 1A and supplemental Figure 2), suggesting that nucleolin is a part of an activation-resistant Fas complex.
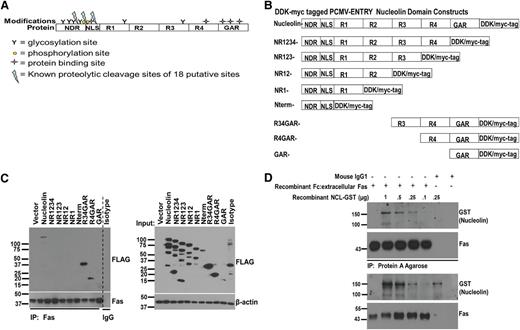
R4 and GAR domains of nucleolin are necessary for its interaction with Fas. (A) Schematic of the nucleolin domains and its known modifications: N-terminal domain region (NDR), nuclear localization signal (NLS), RNA binding domains 1-4 (R1-4), and glycine/arginine-rich domain (GAR). Glycosylation sites are represented by Y, phosphorylation sites by yellow circles, protein-binding sites by stars, and 3 defined proteolytic cleavage sites (of a potential 18 putative sites) by a blue lightning bolt. (B) Domain deletions were created by using the Stratagene Quick Change II XL mutagenesis kit using C-terminal DDK/myc-tagged PCMV-ENTRY construct of full-length nucleolin (Origene) as a template. (C) 293T HEK cells were transfected with the indicated nucleolin domain deletion mutants and lysed for IP/IB analysis. Whole-cell lysates were subjected to Fas IP with agarose conjugated with B-10 anti-Fas antibody. Proteins were separated and immunoblotted for detection of coprecipitated domain mutants with an anti-DDK-HRP antibody. A mixture of all domain mutants was precipitated with mouse IgG and protein G agarose as a negative control. Whole-cell lysate samples prior to IP were immunoblotted with anti-DDK-HRP to reveal expression levels of the transfected constructs. Representative data from 3 different experiments are shown. (D) A chimeric Fc:Fas (Fc:extracellular domain of Fas) was incubated with varying concentrations of recombinant nucleolin-GST for 1.5 hour at 4°C. Fc:Fas was immunoprecipitated with protein A overnight and precipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. IB revealed nucleolin-GST present in Fas-precipitated complexes in a dose-dependent manner. Mouse IgG-1 was used as a control for Fc fragment binding.
R4 and GAR domains of nucleolin are necessary for its interaction with Fas. (A) Schematic of the nucleolin domains and its known modifications: N-terminal domain region (NDR), nuclear localization signal (NLS), RNA binding domains 1-4 (R1-4), and glycine/arginine-rich domain (GAR). Glycosylation sites are represented by Y, phosphorylation sites by yellow circles, protein-binding sites by stars, and 3 defined proteolytic cleavage sites (of a potential 18 putative sites) by a blue lightning bolt. (B) Domain deletions were created by using the Stratagene Quick Change II XL mutagenesis kit using C-terminal DDK/myc-tagged PCMV-ENTRY construct of full-length nucleolin (Origene) as a template. (C) 293T HEK cells were transfected with the indicated nucleolin domain deletion mutants and lysed for IP/IB analysis. Whole-cell lysates were subjected to Fas IP with agarose conjugated with B-10 anti-Fas antibody. Proteins were separated and immunoblotted for detection of coprecipitated domain mutants with an anti-DDK-HRP antibody. A mixture of all domain mutants was precipitated with mouse IgG and protein G agarose as a negative control. Whole-cell lysate samples prior to IP were immunoblotted with anti-DDK-HRP to reveal expression levels of the transfected constructs. Representative data from 3 different experiments are shown. (D) A chimeric Fc:Fas (Fc:extracellular domain of Fas) was incubated with varying concentrations of recombinant nucleolin-GST for 1.5 hour at 4°C. Fc:Fas was immunoprecipitated with protein A overnight and precipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. IB revealed nucleolin-GST present in Fas-precipitated complexes in a dose-dependent manner. Mouse IgG-1 was used as a control for Fc fragment binding.
Nucleolin forms complexes with Fas in B-cell lymphomas
To confirm the formation of nucleolin-Fas complexes in B-cell lymphomas, we subjected lymphoma cell lines, a monocytic-like histocytic lymphoma line, and 2 healthy B-lymphocyte populations (CD19-positive cells isolated from healthy donors) to Fas IP and detection of nucleolin (Figure 1B). The nucleolin-Fas complex was detected in all B-cell lymphoma cell lines, but was absent in the histocytic lymphoma and B-lymphocytes. A repeated IP with increased numbers of healthy B-lymphocytes, to increase Fas IP, confirmed a lack of nucleolin-Fas association in healthy B-lymphocytes (data not shown).
We performed Fas-nucleolin coIP analysis on 9 primary samples to test for the presence of the complex. Two primary lymphoma samples, a diffuse large B-cell lymphoma, and a mantle cell lymphoma yielded nucleolin-Fas complex (Figure 1B). The presence of nucleolin-Fas complexes in some patient lymphoma tissues, at this level of detection, indicates that Fas-nucleolin complex may be clinically relevant.
Nucleolin expression is elevated in B-cell lymphoma tissues
To determine whether the Fas-nucleolin association correlates with an altered nucleolin expression pattern, we quantified the nucleolin levels in B-cell lymphoma cell lines and primary human hematologic cancers, including a chronic lymphocytic leukemia, mantle cell lymphoma, multiple myeloma, and diffuse large B-cell lymphoma, and compared them to healthy B-lymphocytes (Figure 1C). All the primary lymphoma cells and cancer cell lines tested expressed higher levels of nucleolin than healthy B-lymphocytes.
The RNA-binding domain 4 and GAR of nucleolin are required for Fas binding
Nucleolin consists of 6 domains (Figure 2A). To identify the domain responsible for interaction with Fas, we created various single domain and multidomain deletion constructs of nucleolin (Figure 2B). Tagged mutants transiently expressed in 293T cells and subjected to coIP with Fas (Figure 2C) revealed that the interaction of nucleolin with Fas was ablated with the deletion of the glycine/arginine-rich (GAR) domain (lack of detection in the NR1234 lane). The shortest mutant protein capable of binding to Fas consisted of R4 and GAR domains.
To determine whether nucleolin interacts with Fas directly, we incubated increasing concentrations of recombinant nucleolin with recombinant extracellular Fas fused to a chimeric Fc fragment. Precipitation of the Fc fragment revealed a dose-dependent binding of nucleolin (Figure 2D), confirming a direct interaction between nucleolin and the extracellular domain of Fas.
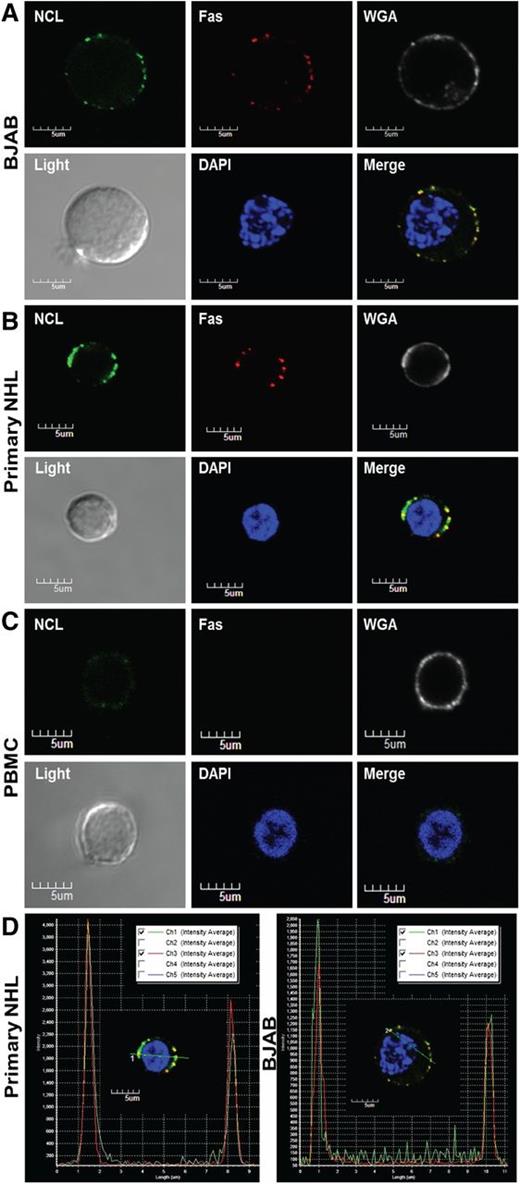
The nucleolin-Fas complex exists selectively on the surface of B-cell lymphomas
To assess the localization of the nucleolin-Fas complex, we analyzed BJAB cells, primary NHL cells, and PBMCs by confocal fluorescence microscopy. Fas and nucleolin colocalized on the surface of BJAB and the primary NHL cells (Figure 3A-D), whereas intracellular staining revealed no complex inside the cells (supplemental Figure 3A-C). PBMCs showed no detectable Fas-nucleolin complex formation (Figure 3C), suggesting that the nucleolin-Fas complex exists selectively on the surface of B-cell lymphomas.
Nucleolin associates with Fas on the surface of primary B-cell lymphoma cells. Localization of nucleolin (NCL)-Fas complexes in B-cell lymphomas. BJAB cells (A) a primary NHL (B) and PBMCs (C) were incubated with an anti-NCL antibody (MS-3), anti-Fas antibody (Abcam), and respective secondary antibodies stepwise at 4°C. Subsequent incubation with wheat germ agglutinin alexa 555 was followed by mounting with prolonged gold anti-fade reagent containing 4,6 diamidino-2-phenylindole (DAPI) and examination by confocal microscopy. The images were captured by the Nikon A1R confocal laser microscope system (Nikon Instruments). All images were acquired at similar voltages for Channel 1 (488 nm) 605-620 V and Channel 3 (647 nm) 510-517 V. An aberration corrected objective (Paplon 1:40) and Nomarski prism for Brightfield DIC image was used for acquiring images. Original image size, 1024 × 1024 with clip size of 302 × 280. PowerPoint was used for further image processing and all panels were adjusted for brightness at correction 44. Top panels (left to right): NCL staining on the surface of BJAB cells (green). Fas staining on the surface of BJAB cells (red). Wheat germ agglutinin revealing surface sialic acid-modified proteins (white) was used as a control for cell membrane localization. Bottom panels (left to right): brightfield image revealing whole cell structure. DAPI nuclear stain (blue). Merged/overlaid images of NCL, Fas, and DAPI; note an almost a complete colocalization of Fas and NCL throughout the surface of BJAB cells (A) and the primary NHL (B) (yellow) and no colocalization on the surface of a healthy lymphocyte, largely because of a lack of surface Fas and low levels of NCL (C). For colocalization staining, we selected a healthy PBMC that had slight positive NCL surface staining as 2 of 15 B-cells scanned by flow cytometry for surface NCL revealed a small shift in staining intensity (MFI) (data not shown) (D) Intensity profile and Pearson’s coefficient analysis for colocalization, revealing positive colocalized staining of Fas and NCL in primary NHL and BJAB cells (merge image from A-B).
Nucleolin associates with Fas on the surface of primary B-cell lymphoma cells. Localization of nucleolin (NCL)-Fas complexes in B-cell lymphomas. BJAB cells (A) a primary NHL (B) and PBMCs (C) were incubated with an anti-NCL antibody (MS-3), anti-Fas antibody (Abcam), and respective secondary antibodies stepwise at 4°C. Subsequent incubation with wheat germ agglutinin alexa 555 was followed by mounting with prolonged gold anti-fade reagent containing 4,6 diamidino-2-phenylindole (DAPI) and examination by confocal microscopy. The images were captured by the Nikon A1R confocal laser microscope system (Nikon Instruments). All images were acquired at similar voltages for Channel 1 (488 nm) 605-620 V and Channel 3 (647 nm) 510-517 V. An aberration corrected objective (Paplon 1:40) and Nomarski prism for Brightfield DIC image was used for acquiring images. Original image size, 1024 × 1024 with clip size of 302 × 280. PowerPoint was used for further image processing and all panels were adjusted for brightness at correction 44. Top panels (left to right): NCL staining on the surface of BJAB cells (green). Fas staining on the surface of BJAB cells (red). Wheat germ agglutinin revealing surface sialic acid-modified proteins (white) was used as a control for cell membrane localization. Bottom panels (left to right): brightfield image revealing whole cell structure. DAPI nuclear stain (blue). Merged/overlaid images of NCL, Fas, and DAPI; note an almost a complete colocalization of Fas and NCL throughout the surface of BJAB cells (A) and the primary NHL (B) (yellow) and no colocalization on the surface of a healthy lymphocyte, largely because of a lack of surface Fas and low levels of NCL (C). For colocalization staining, we selected a healthy PBMC that had slight positive NCL surface staining as 2 of 15 B-cells scanned by flow cytometry for surface NCL revealed a small shift in staining intensity (MFI) (data not shown) (D) Intensity profile and Pearson’s coefficient analysis for colocalization, revealing positive colocalized staining of Fas and NCL in primary NHL and BJAB cells (merge image from A-B).
Ablation of the nucleolin-Fas complex by nucleolin suppression enhances Fas sensitivity and agonist binding
We used a short hairpin RNA miR30 construct to stably reduce the level of nucleolin in BJAB cells, which were selected for these experiments because they are relatively resistant to Fas activation and they lack expression of Bcl-2/Bcl-xl (bcl-2 and bcl-xl messenger RNAs are stabilized by nucleolin and thus could interfere with our studies of apoptosis). We created a pooled pKO BJAB cell line 906P1 and 4 single-cell clones: 906S1, 906S2, 906S4, and 906S5. IB and mRNA analysis revealed at least 50% suppression of nucleolin in all clones (Figure 4A-C). Biotinylation of surface proteins revealed the absence of surface nucleolin in all pKO clones (Figure 4D). Further analysis confirmed ablation of the nucleolin-Fas complex formation in the nucleolin pKOs (Figure 4E).
Downregulation of nucleolin removes surface nucleolin and eradicates the nucleolin-Fas complex. A nucleolin-specific (906), nontargeting (nonsilencing [NS]) control, and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) targeting short hairpin RNA miR30 constructs were used for transfection of BJAB cells to create a pooled NS control, GAPDH control, and nucleolin pKO (906P1) cell line. Four single-cell clones (906S1, 906S2, 906S4, 906S5) were derived from the original pooled cell line 906P1. (A) Whole-cell lysates were analyzed by IB for nucleolin and Fas protein expression. β-actin was used as a loading control. (B) Densitometry analysis revealed a minimum of 50% knockdown of nucleolin in the pKO cells compared with parental BJAB cells, nonsilencing controls, and GAPDH controls (906P1: P < .026; 906S1: P < .035; 906S2: P < .027; 906S4: P < .013; 906S5: P < .0114). Mean value and standard error of the mean of 3 independent experiments are shown. (C) Nucleolin pKO cells, parental BJABs, and nonsilencing controls were analyzed for nucleolin mRNA levels and normalized to GAPDH (906P1: P < .031, 906S1: P < .095, 906S2: P < .038, 906S4: P < .026, 906S5: P < .038). (D) Surface levels of nucleolin and Fas were analyzed in the parental BJAB, NS control, and nucleolin pKOs by biotinylation followed by strepavidin agarose IP. Histone 3 IB was used as a control for purity of the surface fraction. BJAB whole-cell extracts were used as a positive control for antibody specificity. Input levels of nucleolin and β-actin were used as a loading control. Representative data from 3 different experiments are shown. (E) Association of nucleolin and Fas was analyzed in parental BJAB cells, a NS control, a GAPDH control, and pKOs of nucleolin 906P1, 906S1, 906S2, 906S4, and 906S5 by IP with Fas (B-10) agarose beads. Nucleolin was detected by IB. Mouse isotype-matched IgG and protein G agarose were used as a negative control for nonspecific binding. BJAB whole-cell extracts were used as a positive protein control. Representative data from 3 different experiments are shown.
Downregulation of nucleolin removes surface nucleolin and eradicates the nucleolin-Fas complex. A nucleolin-specific (906), nontargeting (nonsilencing [NS]) control, and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) targeting short hairpin RNA miR30 constructs were used for transfection of BJAB cells to create a pooled NS control, GAPDH control, and nucleolin pKO (906P1) cell line. Four single-cell clones (906S1, 906S2, 906S4, 906S5) were derived from the original pooled cell line 906P1. (A) Whole-cell lysates were analyzed by IB for nucleolin and Fas protein expression. β-actin was used as a loading control. (B) Densitometry analysis revealed a minimum of 50% knockdown of nucleolin in the pKO cells compared with parental BJAB cells, nonsilencing controls, and GAPDH controls (906P1: P < .026; 906S1: P < .035; 906S2: P < .027; 906S4: P < .013; 906S5: P < .0114). Mean value and standard error of the mean of 3 independent experiments are shown. (C) Nucleolin pKO cells, parental BJABs, and nonsilencing controls were analyzed for nucleolin mRNA levels and normalized to GAPDH (906P1: P < .031, 906S1: P < .095, 906S2: P < .038, 906S4: P < .026, 906S5: P < .038). (D) Surface levels of nucleolin and Fas were analyzed in the parental BJAB, NS control, and nucleolin pKOs by biotinylation followed by strepavidin agarose IP. Histone 3 IB was used as a control for purity of the surface fraction. BJAB whole-cell extracts were used as a positive control for antibody specificity. Input levels of nucleolin and β-actin were used as a loading control. Representative data from 3 different experiments are shown. (E) Association of nucleolin and Fas was analyzed in parental BJAB cells, a NS control, a GAPDH control, and pKOs of nucleolin 906P1, 906S1, 906S2, 906S4, and 906S5 by IP with Fas (B-10) agarose beads. Nucleolin was detected by IB. Mouse isotype-matched IgG and protein G agarose were used as a negative control for nonspecific binding. BJAB whole-cell extracts were used as a positive protein control. Representative data from 3 different experiments are shown.
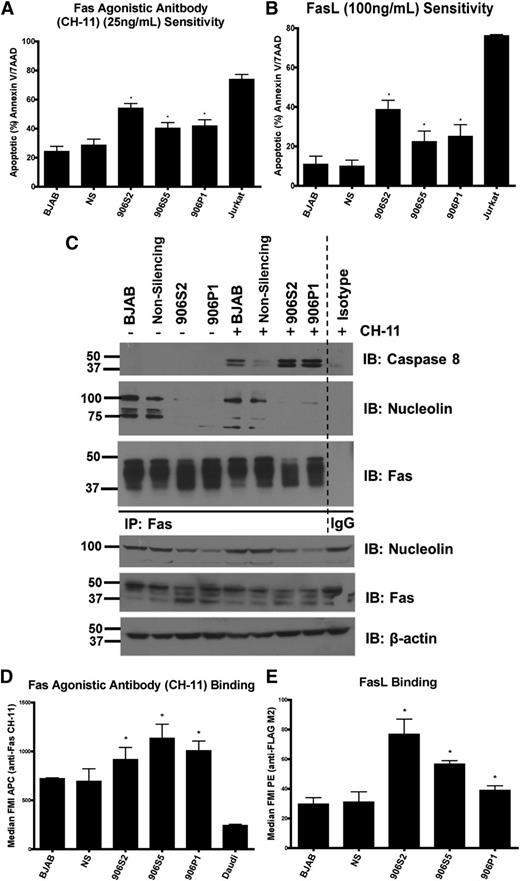
To assess whether the pKO of nucleolin affects Fas signaling, we incubated cells with FasL or Fas-agonistic antibody (Figure 5A-B). Cell death was significantly increased in nucleolin pKOs after either stimulation. Detailed analysis of the Fas signaling cascade in the pKO cells by IB and Fas coIP revealed increased death-inducing signaling complex formation and caspase-8 cleavage (Figure 5C and supplemental Figure 4B-C, respectively), confirming increased initiation of Fas signaling in pKOs. To determine whether the sensitivity to Fas-mediated apoptosis was specific, we challenged pKO and control cells (parental BJABs and a nonsilencing control) with the closely related tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). There was neither significant change in sensitivity to TRAIL (supplemental Figure 4D) nor physical interaction between nucleolin and TRAIL-R1 (supplemental Figure 4E), indicating a Fas-specific effect of nucleolin.
Loss of surface nucleolin and nucleolin-Fas complex sensitizes B-cell lymphomas to Fas-mediated apoptosis. (A) Indicated cells were challenged with the agonistic Fas antibody CH-11 (25 ng/mL) overnight and analyzed for apoptosis levels by Annexin V/7AAD staining and flow cytometry. The nucleolin pKO cells 906S2, 906S5, and 906P1 showed significant increases in sensitivity to agonistic antibody (906S2: P < .001, 906S5: P < .001, 906P1: P < .02). A Fas-sensitive T-cell line, Jurkat, was used as a positive control for Fas activation. Mean value and standard error of the mean (SEM) of 3 or more independent experiments each with 3 replicates are shown. (B) Cells were challenged with FasL (100 ng/mL) overnight and analyzed for apoptosis levels as in (A). The nucleolin pKO cells showed significant increases in FasL sensitivity compared with the nonsilencing control (906S2: P < .001, 906S5: P < .02, 906P1: P < .01). Mean value and SEM of 3 independent experiments each with 3 replicates are shown. (C) Parental BJAB, nonsilencing control, 906P1, and 906S2 cells were subjected to IP of Fas pre-challenge and 1 hour post-challenge with agonistic antibody CH-11 (25 ng/mL). The immunoprecipitated proteins were separated and Fas, caspase-8, and nucleolin were visualized by IB. Note a lack of nucleolin binding to Fas in 906P1 and 906S2 cells. IB of Fas was used as an IP control. Expression levels of nucleolin, Fas, and β-actin in whole-cell extracts as determined by IB analysis as input and loading controls. Representative data from 3 different experiments are shown. (D) Indicated cell lines were incubated with CH-11 (IgM subclass) for 20 minutes and the amount of bound antibody was analyzed by flow cytometry by measuring an anti-IgM–Allophycocyanin secondary antibody signal. The nucleolin pKO cells showed a significant increase in CH-11 signal (906S2: P < .001, 906S5: P < .03, 906P1: P < .04). Mean value and SEM of 3 independent experiments each with 3 replicates are shown. (E) Cells were incubated with FLAG-tagged FasL for 20 minutes and analyzed for the presence of ligand with an anti-FLAG-phycoerythrin secondary antibody by flow cytometry. The nucleolin pKO cells showed significantly increased FasL binding (906S2: P < .01, 906S5: P < .01, 906P1: P < .03). Mean value and SEM of 3 independent experiments each with 3 replicates are shown.
Loss of surface nucleolin and nucleolin-Fas complex sensitizes B-cell lymphomas to Fas-mediated apoptosis. (A) Indicated cells were challenged with the agonistic Fas antibody CH-11 (25 ng/mL) overnight and analyzed for apoptosis levels by Annexin V/7AAD staining and flow cytometry. The nucleolin pKO cells 906S2, 906S5, and 906P1 showed significant increases in sensitivity to agonistic antibody (906S2: P < .001, 906S5: P < .001, 906P1: P < .02). A Fas-sensitive T-cell line, Jurkat, was used as a positive control for Fas activation. Mean value and standard error of the mean (SEM) of 3 or more independent experiments each with 3 replicates are shown. (B) Cells were challenged with FasL (100 ng/mL) overnight and analyzed for apoptosis levels as in (A). The nucleolin pKO cells showed significant increases in FasL sensitivity compared with the nonsilencing control (906S2: P < .001, 906S5: P < .02, 906P1: P < .01). Mean value and SEM of 3 independent experiments each with 3 replicates are shown. (C) Parental BJAB, nonsilencing control, 906P1, and 906S2 cells were subjected to IP of Fas pre-challenge and 1 hour post-challenge with agonistic antibody CH-11 (25 ng/mL). The immunoprecipitated proteins were separated and Fas, caspase-8, and nucleolin were visualized by IB. Note a lack of nucleolin binding to Fas in 906P1 and 906S2 cells. IB of Fas was used as an IP control. Expression levels of nucleolin, Fas, and β-actin in whole-cell extracts as determined by IB analysis as input and loading controls. Representative data from 3 different experiments are shown. (D) Indicated cell lines were incubated with CH-11 (IgM subclass) for 20 minutes and the amount of bound antibody was analyzed by flow cytometry by measuring an anti-IgM–Allophycocyanin secondary antibody signal. The nucleolin pKO cells showed a significant increase in CH-11 signal (906S2: P < .001, 906S5: P < .03, 906P1: P < .04). Mean value and SEM of 3 independent experiments each with 3 replicates are shown. (E) Cells were incubated with FLAG-tagged FasL for 20 minutes and analyzed for the presence of ligand with an anti-FLAG-phycoerythrin secondary antibody by flow cytometry. The nucleolin pKO cells showed significantly increased FasL binding (906S2: P < .01, 906S5: P < .01, 906P1: P < .03). Mean value and SEM of 3 independent experiments each with 3 replicates are shown.
To determine whether the observed sensitization is due to an increased receptor–agonist interaction, we used flow cytometry to evaluate the ability of the nucleolin pKOs to bind Fas agonists (CH-11 and FasL). The nucleolin pKOs bound significantly higher levels of both agonists than did controls (Figure 5D-E), confirming nucleolin-Fas complexes affect Fas receptor-ligand interaction.
To exclude the possibility that increased agonist binding results from increased surface Fas, we analyzed the levels of surface Fas in pKOs relative to controls and surface Fas-negative Daudi cells. No significant increase or decrease in surface Fas levels was observed (supplemental Figure 4A). IP and biotinylation revealed a decreased high-molecular-weight Fas in nucleolin pKO cells, whereas the total levels of high-molecular-weight Fas remained unchanged (Figure 4D-E). These high-molecular-weight bands represent sialylated and glycosylated Fas (supplemental Figure 5) as previously reported.32,33 Fas glycosylation does not alter apoptosis sensitivity,31,32 thus the observed changes do not interfere with our functional studies.
Nucleolin overexpression and nucleolin-Fas complexes protect mice from a lethal Fas-agonist challenge
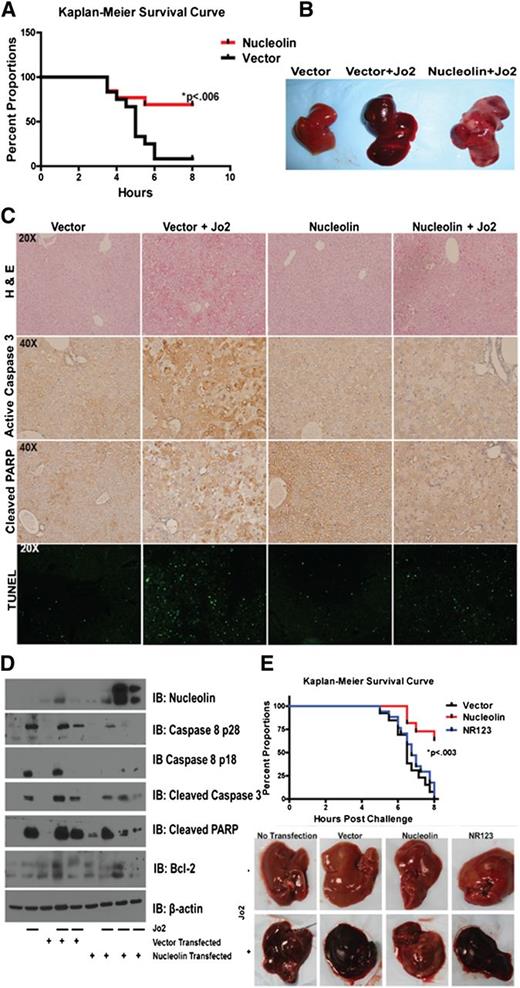
To further confirm the ability of nucleolin to block Fas signaling, we used an in vivo liver nucleolin overexpression model followed by Fas-mediated apoptosis induction. We transfected mice with plasmid expressing full-length nucleolin or empty vector,31,34 and we evaluated their ability to protect the mice from a lethal challenge with Fas agonistic antibody (Jo2) (supplemental Figure 6). Eight hours after the challenge, nucleolin-transfected mice showed a significantly increased survival (P < .006) (Figure 6A). Gross examination of the livers revealed extensive hemorrhaging in vector-transfected livers, which was confined to limited areas in nucleolin-transfected livers (Figure 6B). Hematoxylin and eosin staining confirmed massive hemorrhaging, extravasations, and dead cells in livers transfected with vector alone, whereas these effects were less dramatic in nucleolin-expressing livers (Figure 6C, upper panel). Apoptosis analysis by TUNEL, cleaved caspase-3, and cleaved PARP staining revealed increased numbers of apoptotic cells in the vector-, as compared with the nucleolin-transfected livers (Figure 6C, middle and lower panels). IB of liver tissues (Figure 6D) further confirmed decreased cleavage of PARP, caspase-3, and caspase-8 in nucleolin-transfected livers. These results show that nucleolin overexpression confers resistance to Fas-mediated apoptosis.
Overexpression of nucleolin protects mice from a lethal Fas activation through nucleolin-Fas complex. (A) Mice were hydrodynamically transfected with a vector control or a plasmid expressing DDK-tagged full-length nucleolin. The mice were challenged 24 hours later with a lethal dose of Jo2 agonistic anti-Fas antibody (2 µg/g weight) and monitored for survival for up to 8 hours post-challenge. The survival rate of nucleolin-transfected mice was significantly higher than mice transfected with vector alone (P < .006; log rank Mantel-Cox test). Representative data from 3 different experiments are shown. (B) Gross examination of vector and nontransfected livers challenged with Jo2 revealed massive hemorrhaging as indicated by darkening and swelling. Nucleolin-expressing livers challenged with Jo2 showed decreased hemorrhaging. (C) Livers were harvested, resected, and stained with hematoxylin and eosin (upper panel), or were analyzed using cleaved caspase-3 antibody, cleaved PARP antibody, or TUNEL assay to evaluate apoptosis. The images were captured by the Olympus BX41 (Olympus) UPlan FL N 40×/0.75 objective. Images were acquired with DP Controller (Olympus) with a −2 exposure adjustment for TUNEL staining with a fluorescein isothiocyanate filter (Olympus). Adobe Photoshop PS2 was used for further image enhancement of green fluorescent protein with a +30 brightness for all 4 panels equally. (D) Homogenized vector-tramsfected, nucleolin-transfected, a nontransfected Jo2-challenged and Jo2-unchallenged liver samples were subjected to lysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and IB analysis of nucleolin, caspase-8, caspase-3, PARP, Bcl-2, and β-actin. (E) Mice were transfected with vector control, DDK-tagged full-length nucleolin, or DDK-tagged mutant lacking the Fas-nucleolin binding domain NR123 plasmids. Mice were challenged with a lethal dose of Jo2-agonistic anti-Fas antibody (.5 µg/g weight) and monitored for survival for up to 8 hours post-challenge. On gross examination, nontransfected, vector-transfected, and NR123-transfected livers challenged with Jo2 exhibited massive hemorrhaging as shown by darkening and swelling. Nucleolin livers challenged with Jo2 showed decreased hemorrhaging as shown by light spotting. We confirmed expression of nucleolin and NR123 by IB (data not shown). The survival rate was significantly higher for nucleolin-transfected mice than for mice transfected with vector alone or nonbinding mutant NR123 (P < .003; log rank Mantel-Cox test). Combined data from 2 independent experiments are shown.
Overexpression of nucleolin protects mice from a lethal Fas activation through nucleolin-Fas complex. (A) Mice were hydrodynamically transfected with a vector control or a plasmid expressing DDK-tagged full-length nucleolin. The mice were challenged 24 hours later with a lethal dose of Jo2 agonistic anti-Fas antibody (2 µg/g weight) and monitored for survival for up to 8 hours post-challenge. The survival rate of nucleolin-transfected mice was significantly higher than mice transfected with vector alone (P < .006; log rank Mantel-Cox test). Representative data from 3 different experiments are shown. (B) Gross examination of vector and nontransfected livers challenged with Jo2 revealed massive hemorrhaging as indicated by darkening and swelling. Nucleolin-expressing livers challenged with Jo2 showed decreased hemorrhaging. (C) Livers were harvested, resected, and stained with hematoxylin and eosin (upper panel), or were analyzed using cleaved caspase-3 antibody, cleaved PARP antibody, or TUNEL assay to evaluate apoptosis. The images were captured by the Olympus BX41 (Olympus) UPlan FL N 40×/0.75 objective. Images were acquired with DP Controller (Olympus) with a −2 exposure adjustment for TUNEL staining with a fluorescein isothiocyanate filter (Olympus). Adobe Photoshop PS2 was used for further image enhancement of green fluorescent protein with a +30 brightness for all 4 panels equally. (D) Homogenized vector-tramsfected, nucleolin-transfected, a nontransfected Jo2-challenged and Jo2-unchallenged liver samples were subjected to lysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and IB analysis of nucleolin, caspase-8, caspase-3, PARP, Bcl-2, and β-actin. (E) Mice were transfected with vector control, DDK-tagged full-length nucleolin, or DDK-tagged mutant lacking the Fas-nucleolin binding domain NR123 plasmids. Mice were challenged with a lethal dose of Jo2-agonistic anti-Fas antibody (.5 µg/g weight) and monitored for survival for up to 8 hours post-challenge. On gross examination, nontransfected, vector-transfected, and NR123-transfected livers challenged with Jo2 exhibited massive hemorrhaging as shown by darkening and swelling. Nucleolin livers challenged with Jo2 showed decreased hemorrhaging as shown by light spotting. We confirmed expression of nucleolin and NR123 by IB (data not shown). The survival rate was significantly higher for nucleolin-transfected mice than for mice transfected with vector alone or nonbinding mutant NR123 (P < .003; log rank Mantel-Cox test). Combined data from 2 independent experiments are shown.
Our tissue culture results indicated the need for nucleolin-Fas interaction to block Fas. To confirm this requirement, we transfected mice with plasmids expressing nucleolin, non-Fas-binding nucleolin construct NR123, or empty vector to evaluate their ability to protect the mice from a lethal challenge with Jo2. Eight hours after the challenge, none of the 17 NR123 mice, and only 1 of 13 vector-transfected mice survived, whereas 7 of the 11 nucleolin-transfected mice survived (P < .003) (Figure 6E). We confirmed expression of nucleolin and NR123 by IB of liver tissue (data not shown). These results support our finding that the nucleolin-Fas interaction is important for nucleolin-mediated inhibition of Fas signaling.
Discussion
In this study, we identified nucleolin as a novel modulator of Fas signaling associated with Fas-resistance in B-cell lymphomas. Primary B-cell lymphoma cells express high levels of nucleolin relative to healthy B-lymphocytes. Nucleolin associated with Fas in B-cell lymphoma tissue and cell lines via its R4 and GAR domains. By knocking down nucleolin and subsequent elimination of nucleolin-Fas complexes, we enhanced FasL binding and apoptosis. Conversely, the overexpression of nucleolin in mice effectively blocked Fas signaling, and prevented the lethal effects of Fas activation. Nucleolin-Fas complex was essential to protect mice against Fas-mediated apoptosis, as comparable expression levels of a construct lacking the Fas-binding R4 and GAR domains failed to elicit protection.
Recent studies have found that nucleolin is overexpressed and localized to the cell surface in multiple cancers and cancer-associated endothelial cells, which is commonly associated with a poor prognosis.19,21 Currently, all known functions of cell-surface nucleolin suggest it serves as a docking site for survival molecules, such as P-selectin, hepatocyte growth factor, and for viruses gaining entry into proliferating cells.22,23,26,35,36 It remains to be elucidated how nucleolin adheres to the cell surface and how it is positioned on the plasma membrane. Such knowledge will stimulate further studies of the surface function of nucleolin in B-cell lymphomas. Here we extended observations of overexpressed and surface-localized nucleolin to B-cell lymphomas, which have not been previously characterized. Our results reveal that nucleolin binds Fas and by inducing Fas resistance promotes survival of lymphoma cells, a novel function of surface nucleolin.
It is clear that chemoresistance in cancer has multifactorial origins that converge on Fas signaling.6,8,9,37,38 Multiple chemotherapies, including the lymphoma therapies doxorubicin, methotrexate, mitoxantrone, bleomycin, and rituximab, have been shown to upregulate Fas and/or FasL to achieve high response rates.6,8,15,38-41 Fas signaling in cancer is infrequently blocked through acquired Fas mutations. More often, cancer cells downregulate key players in the Fas-signaling cascade or upregulate apoptosis inhibitors, such as PML-RARα, cFLIP, IAPs, and Bcl-2 family members.3,29,42 In lymphomas, wild-type Fas is commonly expressed and Fas resistance cannot always be explained.43 Recent reports have revealed that cell surface receptors HGFR/c-MET, human herpesvirus-8 K1, and CD44v6/v9, which have their own signaling functions, can also bind and block Fas.16,17,44,45 We provide evidence that nucleolin, similar to these membrane receptors, negatively regulates Fas-mediated apoptosis by inhibiting the Fas-FasL interaction through binding cell surface Fas. We speculate that nucleolin and these previously identified receptors can use a shared mechanism: conceivably they lock Fas in an inactive conformation preventing FasL recognition or prevent conformational changes required for FasL binding. Interestingly, our recent work showed that a peptide derived from the Fas-binding domain of K1 modulates Fas signaling, suggesting these receptor-Fas interactions are accessible and amenable to manipulation.46 Although we did not determine the exact mechanism of nucleolin interference with ligand binding, this deserves further investigation, as a more detailed description of how these Fas-binding proteins prevent Fas activation may result in improving the effectiveness of the numerous Fas-dependent chemotherapies.
Nucleolin is an attractive target for cancer therapy, as it appears to have low off-target effects primarily because of its restrictive expression on the cell surface of cancer cells and cancer-associated endothelial cells. Nucleolin targeting substrates in preclinical trials include: F3 peptide, AS1411 aptamer, and LNA aptamers linked to various drug carriers for delivery of microRNAs, radionuclides, and doxorubicin.47-52 In addition, anti-nucleolin antibodies in pre-clinical models induce downregulation of bcl-2 mRNA levels and subsequent apoptosis.53 However, nucleolin antibodies are present in systemic lupus erythematosus and allograft failure, suggesting potential unwanted effects that could be mediated by anti-nucleolin antibodies.54,55
Some agents have advanced to testing in clinical trials. Nucleolin-targeting Nucant pseudopeptides (Immupharma)56 are currently under evaluation in a phase II clinical trial. A phase II clinical trial of AS1411 G-rich oligonucleotide (Antisoma), which destabilizes bcl-2 and induces apoptosis, has shown a 3% partial response rate and 60% stable disease rate in relapsed renal cell carcinoma.56-58 AS1411 has also shown a 15% response rate in the treatment of relapsed acute myeloid leukemia.59
By defining a novel role for nucleolin as an anti-apoptotic surface protein, we further underscore the potential of these and other nucleolin-targeting therapeutics. Furthermore, our results may explain the variable outcome of Nucant pseudopeptide treatments showing cell death in lymphomas and leukemias while response was restricted to only growth inhibition in other malignant cell types.56,60,61 The apoptotic responses may be associated with the inhibitory effect of nucleolin on Fas-mediated apoptosis that has a more prominent role in lymphoid cancers and warrants further investigation.
We conclude that nucleolin is a novel binding modulator of the Fas death receptor in human lymphomas that effectively blocks Fas signaling. Given the common surface expression of nucleolin and the often impaired Fas signaling in chemoresistant cancers, we anticipate that nucleolin contributes to chemoresistance and is a promising target for new therapeutic interventions. Therefore, we look forward to clinical studies, which should determine whether nucleolin-targeting therapeutics can restore Fas-signaling and chemotherapy responses in cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Cancer Institute of the National Institutes of Health (CA 1206173, CA153170, and CA158692), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091490), the Leukemia & Lymphoma Society (R6187-09), The University of Texas MD Anderson Cancer Center, the American Cancer Society (MSRG-10-052-01-LIB), the Richard Spencer Lewis Memorial Foundation, and patients’ families. J.F.W. is a recipient of the 2009-2011 National Institutes of Health T32 Training Fellowship for the Center for Clinical and Translational Sciences. J.F.W. is a doctoral candidate at the University of Texas Graduate School of Biomedical Sciences and this work is a partial fulfillment of the requirement for the PhD degree. The MD Anderson Flow Cytometry and Cellular Imaging Facility is funded by a Cancer Center Support Grant from the National Cancer Institute (P30C16672).
Authorship
Contribution: J.F.W. designed research studies, analyzed data, and wrote the manuscript; J.F.W., Z.B., R.M., H.Z., F.K.B., R.-H.T., H.M., and X.A. performed experiments; Z.B., A.L.S., and F.S. contributed to the writing of the manuscript; and F.S. contributed to research design.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence to: Felipe Samaniego, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 7455 Fannin St, Houston, TX 77054; e-mail: fsamaniego@mdanderson.org.

![Figure 1. Nucleolin binds activation-resistant Fas and shows altered expression in B-cell lymphomas. (A) Silver-stained gel separating primary NHL and BJAB samples subjected to Fas activation and IP with agonistic antibody CH-11 (BJAB and primary NHL CH-11). The remaining lysates were subjected to a second Fas IP (B-10) of any remaining activation-resistant Fas (BJAB and primary NHL B-10). Specific activation-resistant Fas bands were excised, digested with trypsin, and analyzed by nanoflow-LC-MS/MS fragmentation and collision-induced dissociation spectra profiling. A 100 kDa band of interest (asterisk) reveals the protein that is the focus of the current study. The protein sequence of nucleolin is shown with peptides (red text) from the 100 kDa band identified by nanoflow-LC-MS/MS spectra. Peptides map to the RNA binding domains in the C-terminal region of nucleolin. (B) Whole-cell extracts from BJAB, Raji, Daudi, BC-3, and a histocytic lymphoma (U937) cell line and two B-lymphocyte isolations from healthy donors were subjected to IP with B-10 anti-Fas agarose, which recognizes an epitope within the cytoplasmic region of Fas, and analyzed by IB for the presence of nucleolin with MS-3 anti-nucleolin antibody. Representative data from 3 different experiments are shown. Shown is an analysis of 2 primary lymphoma samples (diffuse large B-cell lymphoma [DLBCL] and mantle cell lymphoma [MCL]) demonstrating nucleolin-Fas complex from a screen of 4 DLBCL and 6 MCL primary samples. Extracts were immunoprecipitated with B-10 and analyzed by IB for the presence of nucleolin in precipitated complexes. BJAB cells and isotype IP were used as positive and negative controls, respectively. β-actin was used as a loading control. (C) Whole-cell lysates of 5 lymphomas cell lines (BJAB, Raji, Daudi, BC-3, and U937) and healthy donor B-lymphocyte populations were analyzed by IB for nucleolin expression. β-actin was used as a loading control (bottom panel). Whole-cell lysates of 4 primary hematologic cancer tissues multiple myeloma (MM), MCL, chronic lymphocytic leukemia (CLL) and DLBCL were subjected to IB analysis of nucleolin. β-actin was used as a loading control. Ratio of nucleolin to β-actin levels determined by densitometry is shown below each sample lane. Representative data from 3 different experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/23/10.1182_blood-2012-12-471094/4/m_4729f1.jpeg?Expires=1769233875&Signature=dGvc1m1FyIO0Tx51pgN7xfgg-2hMmChcY8CW-WCMpw5-WjEGUk3Z27K3mM93dVC4IblZLObqW0BDJNTIpbFCZjD6aXUulPzg03uSMx31NahQO2y50R-D4VoeFqPg5FDyN-sFgKu8HOisbhyuy~4AU6fXBB7~FxCtB6JZ~64l6IETlQ53LeUglUSKDFk6SwSNl9uOcXmFbtgu3tnUjqUqw8bd7XbaFEMKHvJasTdq8V~ON~x887jV7eLH3FFIFL5LWysdqMKCnBYZ9axkleI82eEqH0Uvg6iTpzZLRHdSuj~JDBhDDrEZA~xRLpkxdQTvczccxPKME6y9~5Mi-YAcuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Downregulation of nucleolin removes surface nucleolin and eradicates the nucleolin-Fas complex. A nucleolin-specific (906), nontargeting (nonsilencing [NS]) control, and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) targeting short hairpin RNA miR30 constructs were used for transfection of BJAB cells to create a pooled NS control, GAPDH control, and nucleolin pKO (906P1) cell line. Four single-cell clones (906S1, 906S2, 906S4, 906S5) were derived from the original pooled cell line 906P1. (A) Whole-cell lysates were analyzed by IB for nucleolin and Fas protein expression. β-actin was used as a loading control. (B) Densitometry analysis revealed a minimum of 50% knockdown of nucleolin in the pKO cells compared with parental BJAB cells, nonsilencing controls, and GAPDH controls (906P1: P < .026; 906S1: P < .035; 906S2: P < .027; 906S4: P < .013; 906S5: P < .0114). Mean value and standard error of the mean of 3 independent experiments are shown. (C) Nucleolin pKO cells, parental BJABs, and nonsilencing controls were analyzed for nucleolin mRNA levels and normalized to GAPDH (906P1: P < .031, 906S1: P < .095, 906S2: P < .038, 906S4: P < .026, 906S5: P < .038). (D) Surface levels of nucleolin and Fas were analyzed in the parental BJAB, NS control, and nucleolin pKOs by biotinylation followed by strepavidin agarose IP. Histone 3 IB was used as a control for purity of the surface fraction. BJAB whole-cell extracts were used as a positive control for antibody specificity. Input levels of nucleolin and β-actin were used as a loading control. Representative data from 3 different experiments are shown. (E) Association of nucleolin and Fas was analyzed in parental BJAB cells, a NS control, a GAPDH control, and pKOs of nucleolin 906P1, 906S1, 906S2, 906S4, and 906S5 by IP with Fas (B-10) agarose beads. Nucleolin was detected by IB. Mouse isotype-matched IgG and protein G agarose were used as a negative control for nonspecific binding. BJAB whole-cell extracts were used as a positive protein control. Representative data from 3 different experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/23/10.1182_blood-2012-12-471094/4/m_4729f4.jpeg?Expires=1769233875&Signature=ryyuPWikeUutLenhUGZTVVd4-yjRbCYKtwzLY11H6JEj1esksFP60O4TshgAVR~fieMYPn6QzFJ4wbXphBVATc3KFdw95F73tT~S0WeLOZikR5FuU5C~srIzN~K4yCh4BoCHRX9bC6DcLnn318kGDb1tGIAFw9mGicoJrZre4IPgpPmDhP1mlZ9V8n-W1WnTuKmX7e7SrKuNdI6sODEz0U0b0r0S4SatIkz5uonXnPS-OAUIncS~5C-CDpt-SbkRXqbM9VRloH6bT3VkG9hcTAnv-991qyTi32GnU89o8zqZi1m8AeST8EK4NlfDJ3mim8~FzOsbjO67e6qNQx1XUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)