Key Points
This analysis demonstrates the universality of the early response in CML, regardless of the treatment modality used.
Factors correlating with poor cytogenetic responses at 3-mo assessment in a multivariate analysis across all 4 TKIs.
Abstract
Early responses to tyrosine kinase inhibitors (TKIs) in chronic myeloid leukemia (CML)-chronic phase (CP) are associated with improved outcome. We analyzed the impact of such a response on outcomes among patients treated with 4 TKI modalities as frontline therapy in CML-CP. A total of 483 patients who received 400 or 800 mg imatinib, nilotinib, or dasatinib were analyzed. The median follow-up was 72 mo. Landmark analysis at 3 mo by molecular response showed that the cumulative proportions of 3-y event-free survival (EFS) for 3-mo BCR-ABL levels was 95% for those with ≤1%, 98% for >1% to 10%, and 61% for those with >10% (P = .001). The corresponding values by cytogenetic responses were 97% if Ph+ = 0%, 89% if Ph+ = 1% to 35%, and 81% if Ph+ >35% (P = .001). Cytogenetic response at 3 mo significantly discriminated for 3-y overall survival (OS): 98%, 96%, and 92%, respectively (P = .01). In multivariate analysis, young patients, high Sokal index, and treatment with imatinib 400 significantly predicted for poor (>35%) cytogenetic response at 3 mo. Early responses are predictive for EFS and failure-free survival and to a lesser extent OS, regardless of the treatment modality, although therapies other than standard-dose imatinib result in higher rates of deep early responses. This trial was registered at www.clinicaltrials.gov as ID01-151 NCT00038649, ID01-015 NCT00048672, DM00-163 NCT00333840, ID02-534 NCT00050531, 2005-0422 NCT00254423, and 2005-0048 NCT00129740.
Introduction
Long-term follow-up data from a phase III International Randomized Study of Interferon and STI571 trial have shown excellent patient outcomes with imatinib as an initial therapy for patients with chronic myeloid leukemia (CML).1 Imatinib therapy resulted in a complete cytogenetic response (CCyR) rate of 83% and an estimated overall survival (OS) of 93% considering only CML-related deaths. Despite these excellent results, imatinib resistance occurs in many patients,2 frequently through BCR-ABL kinase domain mutations,3 and some patients may be intolerant to imatinib. This led to the search for more effective and better-tolerated second-generation tyrosine kinase inhibitors (TKIs) such as nilotinib4,5 and dasatinib.6
The depth of the response as early as 3 mo from the start of imatinib therapy has been proposed to be correlated with improved long-term outcome for nearly a decade.7-12 Recent analyses have further emphasized the importance of achieving a major cytogenetic response (MCyR) or BCR-ABL/ABL transcripts <10% at 3 mo. Patients who achieve these responses have an improved probability of achieving a favorable progression-free survival and, to some extent, OS.13-16 As the search for improving the long-term outcome of patients with CML evolved from the use of standard-dose imatinib to high-dose imatinib17 to second generation TKIs,18,19 it became evident that deeper responses occurred earlier with the newer modalities compared with what was achieved with standard-dose imatinib. Randomized studies later confirmed the benefit of these treatment options compared with standard-dose imatinib.4-6,20,21 In this study, we report the patterns of early (3 and 6 mo) molecular and cytogenetic responses and their impact on OS, event-free survival (EFS), failure-free survival (FFS), and transformation-free survival (TFS) in patients with CML-chronic phase (CP) receiving different TKI modalities.
Methods
All evaluable patients with newly diagnosed CML-CP treated with TKIs as an initial therapy in consecutive or parallel clinical trials at MD Anderson Cancer Center from May 2000 to January 2012 were included in this analysis. The entry criteria were similar for all trials, and TKI therapy had to be started within 6 mo from the initial diagnosis. CML-CP was defined as the presence in the peripheral blood of <15% blasts, <20% basophils, <30% blasts and promyelocytes, and platelets >100 × 109/L. Patients were treated with protocols approved by the institutional review board and informed consent was obtained in accordance with the Declaration of Helsinki.
The response criteria were as previously described.22 Cytogenetic response was assessed by conventional cytogenetic analysis done in bone marrow cells using the G-banding technique with at least 20 metaphases analyzed. Fluorescent in situ hybridization on peripheral blood was used only when routine cytogenetic analysis was not successful (ie, insufficient metaphases). Cytogenetic response categories included CCyR (0% Ph-positive metaphases), partial cytogenetic response (PCyR) (1% to 35% Ph-positive metaphases), and minor cytogenetic response (>35% Ph-positive metaphases). The molecular response was assessed by polymerase chain reaction (PCR) and expressed as the BCR-ABL/ABL ratio (International Scale). A major molecular response (MMR) was defined as a BCR-ABL/ABL transcript ratio <0.1% and MR4.5 as a ratio of ≤0.0032%. Only patients with typical BCR-ABL transcripts (b2a2, b3a2, or both) were included in the molecular analyses. Patients were followed for cytogenetic analysis every 3 mo for the first year, then every 6 mo for the next 2 to 3 y, and then every 1 to 2 y. Molecular response was generally assessed every 3 mo for the first year, then every 6 mo.
Statistical analysis
Landmark analyses for cytogenetic and molecular responses were performed at 3 and 6 mo (excluding patients with missing values at each time point) (supplemental Table 1). EFS was measured from the start of treatment to the date of any of the following events (as defined in the International Randomized Study of Interferon and STI571 study) while on therapy: loss of complete hematologic remission, loss of MCyR, progression to accelerated or blast phase, or death from any cause while on study. Because of the limitations of this definition, we also measured the FFS that accounts for other events such as failure to achieve response at set times as defined by European Leukemia Net, loss of CCyR, intolerance, or treatment discontinuation for any reason. The OS was measured from the time treatment was started to the date of death from any cause at any time or date of last follow-up. TFS was measured from the start of therapy to the date of transformation to accelerated or blast phases while on therapy or to the date of last follow-up.
Differences between groups were evaluated by the χ2 test and Mann-Whitney U test for categorical and continuous variables, respectively. Survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate analyses were performed to identify potential factors predicting for early cytogenetic responses. Multivariate analysis of response used the logistic regression model, and for time to event outcomes, we used the Cox proportional hazard regression.
Results
Patients
A total of 483 consecutive patients with newly diagnosed CML-CP treated with various TKI modalities were included in this analysis and 477 patients were evaluable (6 patients did not have any response assessment) and constitute the basis for this report. Patients received 400 mg/d imatinib (n = 71), 800 mg/d imatinib (n = 199), 100 mg/d dasatinib (n = 102), or 400 mg nilotinib twice per day (n = 105). Their median age was 48 y (range, 15-86 y), and the overall median follow-up was 72 mo (2-136 mo). The majority of patients (60%) had a low Sokal score. Patient characteristics are shown in supplemental Table 2. Patient characteristics were similar among patients treated with each of the 4 TKI modalities (supplemental Table 3). Follow-up is longer for patients treated with imatinib (imatinib 400, median 123 mo, range 16-136 mo; imatinib 800, median 97 mo, range, 4-129 mo) than with second generation TKIs (nilotinib, median 30 mo, range 3-77 mo; dasatinib, median 36 mo, range 2-73 mo). In total, there were 45 (9%) deaths, 53 events (11%), and 10 transformations (2%).
Evaluable responses to TKI modalities
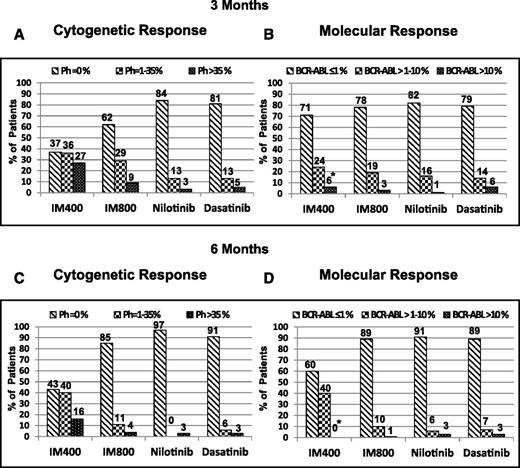
Overall, 421 (88%) patients achieved CCyR, 392 (82%) a MMR, and 329 (69%) a MR4.5. The 3-y estimates for EFS were 93%, with an FFS of 77%. The corresponding figures for TFS and OS were 98% and 97%, respectively. At 3 mo from the start of therapy, 300 (67%) patients achieved a CCyR, 104 (23%) achieved PCyR, and 44 (10%) <MCyR (ie, Ph+ >35%). By PCR, among 379 patients with available information, 300 (79%) patients had achieved at 3 mo BCR-ABL/ABL transcripts ≤1%, 66 (17%) achieved transcripts of >1% to 10%, and 13 (3%) had >10% (Table 1). For the 448 patients with available data, the proportion of patients achieving MCyR (≤35% Ph+) at 3 mo was higher among patients treated with imatinib 800 (91%), nilotinib (97%), or dasatinib (95%) compared with those treated with imatinib 400 (73%). All patients treated with imatinib 400 initiated therapy between May 2000 and June 2001, when molecular analysis was not being routinely done at the earliest time points, making the analysis of molecular response at 3 mo noninformative for this group. Therefore, for the time to event analyses by molecular response categories at 3 and 6 mo, patients treated with imatinib 400 were excluded. Patients treated with the other 3 treatment modalities had similar rates of PCR ≤1% at 3 mo (78%, 82%, and 79%, respectively) (Figure 1A-B). Similar results were obtained at 6 mo from the start of therapy (Figure 1C-D).
Cytogenetic and molecular response categories according to TKI modality at 3 and 6 mo (among evaluable patients for which corresponding values were available)
| 3- and 6-mo response categories . | Response, n (%) . | |||||
|---|---|---|---|---|---|---|
| Imatinib 400 . | Imatinib 800 . | Imatinib (400 and 800) . | Nilotinib . | Dasatinib . | Overall . | |
| 3-mo cytogenetic response (n = 448) | ||||||
| 0 | 25 (37) | 118 (62) | 143 (55) | 79 (84) | 78 (81) | 300 (67) |
| 35 < 0 | 24 (35) | 55 (29) | 79 (30) | 12 (13) | 13 (13) | 104 (23) |
| >35 | 18 (26) | 18 (9) | 36 (14) | 3 (3) | 5 (5) | 44 (10) |
| 3-mo molecular response (n = 379) | ||||||
| ≤1 | 12 (70) | 133 (78) | 145 (77) | 79 (8) | 76 (79) | 300 (79) |
| >1-10 | 4 (23) | 32 (19) | 36 (19) | 16 (16) | 14 (14) | 66 (17) |
| >10 | 1 (6) | 5 (3) | 6 (3) | 1 (1) | 6 (6) | 13 (3) |
| 6-mo cytogenetic response (n = 424) | ||||||
| 0 | 29 (43) | 154 (84) | 183 (73) | 85 (96) | 79 (91) | 347 (81) |
| 35 < 0 | 27 (40) | 21 (11) | 48 (19) | — | 5 (6) | 53 (12) |
| >35 | 11 (16) | 7 (4) | 18 (7) | 3 (3) | 3 (3) | 24 (7) |
| 6-mo molecular response (n = 360) | ||||||
| ≤1 | 3 (60) | 156 (89) | 159 (88) | 81 (91) | 81 (89) | 321 (89) |
| >1-10 | 2 (40) | 17 (9) | 19 (10) | 5 (6) | 7 (8) | 31 (9) |
| >10 | — | 2 (1) | 2 (1) | 3 (3) | 3 (3) | 8 (2) |
| 3- and 6-mo response categories . | Response, n (%) . | |||||
|---|---|---|---|---|---|---|
| Imatinib 400 . | Imatinib 800 . | Imatinib (400 and 800) . | Nilotinib . | Dasatinib . | Overall . | |
| 3-mo cytogenetic response (n = 448) | ||||||
| 0 | 25 (37) | 118 (62) | 143 (55) | 79 (84) | 78 (81) | 300 (67) |
| 35 < 0 | 24 (35) | 55 (29) | 79 (30) | 12 (13) | 13 (13) | 104 (23) |
| >35 | 18 (26) | 18 (9) | 36 (14) | 3 (3) | 5 (5) | 44 (10) |
| 3-mo molecular response (n = 379) | ||||||
| ≤1 | 12 (70) | 133 (78) | 145 (77) | 79 (8) | 76 (79) | 300 (79) |
| >1-10 | 4 (23) | 32 (19) | 36 (19) | 16 (16) | 14 (14) | 66 (17) |
| >10 | 1 (6) | 5 (3) | 6 (3) | 1 (1) | 6 (6) | 13 (3) |
| 6-mo cytogenetic response (n = 424) | ||||||
| 0 | 29 (43) | 154 (84) | 183 (73) | 85 (96) | 79 (91) | 347 (81) |
| 35 < 0 | 27 (40) | 21 (11) | 48 (19) | — | 5 (6) | 53 (12) |
| >35 | 11 (16) | 7 (4) | 18 (7) | 3 (3) | 3 (3) | 24 (7) |
| 6-mo molecular response (n = 360) | ||||||
| ≤1 | 3 (60) | 156 (89) | 159 (88) | 81 (91) | 81 (89) | 321 (89) |
| >1-10 | 2 (40) | 17 (9) | 19 (10) | 5 (6) | 7 (8) | 31 (9) |
| >10 | — | 2 (1) | 2 (1) | 3 (3) | 3 (3) | 8 (2) |
The percentages represent the proportion of patients with respect to the total number of patients in each TKI arm falling into each response category.
Evaluable cytogenetic and molecular responses to different TKI modalities at 3- and 6-mo follow-up (imatinib 400, imatinib 800, nilotinib, and dasatinib). (A) Cytogenetic responses at 3 mo. (B) Molecular responses at 3 mo. * indicates that the majority of patients in the imatinib 400 group did not have molecular response values, as these patients were enrolled in the year 2000-2001, when PCR was not routinely done. (C) Cytogenetic responses at 6 mo. (D) Molecular responses at 6 mo.
Evaluable cytogenetic and molecular responses to different TKI modalities at 3- and 6-mo follow-up (imatinib 400, imatinib 800, nilotinib, and dasatinib). (A) Cytogenetic responses at 3 mo. (B) Molecular responses at 3 mo. * indicates that the majority of patients in the imatinib 400 group did not have molecular response values, as these patients were enrolled in the year 2000-2001, when PCR was not routinely done. (C) Cytogenetic responses at 6 mo. (D) Molecular responses at 6 mo.
Clinical outcomes according to evaluable cytogenetic and molecular responses at 3 and 6 mo
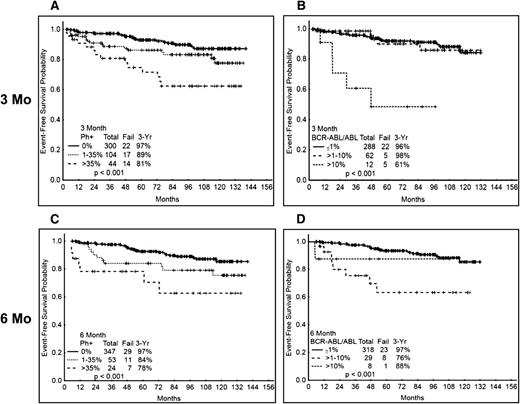
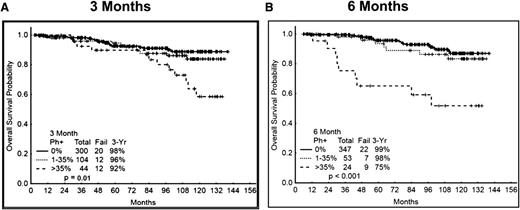
We then analyzed the time to event variables according to the 3- and 6-mo responses (Table 2). Patients with Ph+ >35% at 3 mo had a significantly inferior probability of 3-y EFS (81%) compared with those with CCyR (97%) or PCyR (89%) (95% for all patients with MCyR) (P = .001) (Figure 2A). When analyzed by molecular response at 3 mo, the patients with >10% BCR-ABL also had an inferior 3-y EFS probability compared with those with lower transcript levels (Figure 2B). At 6 mo, the cytogenetic response also correlated with outcome, with 3-y probabilities of EFS of 97% for those with CCyR, 84% for those with PCyR, and 78% for patients without MCyR (Figure 2C). A similar trend was observed analyzing by molecular response at 6 mo (P ≤ .001) (Figure 2D). Importantly, the EFS probability was not significantly different among the 4 TKI modalities in any of the cytogenetic (supplemental Figure 1A-C for EFS at 3 mo) or molecular response categories (excluding imatinib 400) (not shown), whether assessed at 3 or 6 mo.
Landmark analysis at 3 and 6 mo by evaluable cytogenetic and molecular response categories for cumulative probability of 3-y EFS, OS, and FFS in response to each TKI therapy
| Response . | IM400 . | IM800 . | Nilotinib . | Dasatinib . | Overall . |
|---|---|---|---|---|---|
| Cytogenetic | |||||
| 3 mo | |||||
| 0 | 92/100/76 | 97/97/85 | 97/99/87 | 99/98/95 | 97/98/87 |
| 35 < 0 | 88/91/67 | 89/96/67 | 92/100/92 | 91/100/81 | 89/96/70 |
| >35 | 83/83/71 | 83/100/44 | 67/100/33 | 75/100/50 | 81/92/51 |
| 6 mo | |||||
| 0 | 96/100/79 | 97/99/87 | 99/100/92 | 99/99/94 | 97/99/89 |
| 35 < 0 | 80/100/78 | 88/95/52 | NA/NA/NA | 75/100/75 | 84/98/69 |
| >35 | 73/64/45 | 83/86/14 | 67/100/33 | 100/100/50 | 77/75/35 |
| Molecular | |||||
| 3 mo | |||||
| ≤1 | NA/NA | 94/78 | 96/88 | 100/95 | 96/85 |
| >1-10 | NA/NA | 100/69 | 94/75 | 100/82 | 98/73 |
| >10 | NA/NA | 80/60 | 100/100 | 27/50 | 61/61 |
| 6 mo | |||||
| ≤1 | NA/NA | 96/85 | 99/92 | 100/96 | 97/89 |
| >1-10 | NA/NA | 79/47 | 100/100 | 42/60 | 76/56 |
| >10 | NA/NA | 100/50 | 67/33 | 100/50 | 88/49 |
| Response . | IM400 . | IM800 . | Nilotinib . | Dasatinib . | Overall . |
|---|---|---|---|---|---|
| Cytogenetic | |||||
| 3 mo | |||||
| 0 | 92/100/76 | 97/97/85 | 97/99/87 | 99/98/95 | 97/98/87 |
| 35 < 0 | 88/91/67 | 89/96/67 | 92/100/92 | 91/100/81 | 89/96/70 |
| >35 | 83/83/71 | 83/100/44 | 67/100/33 | 75/100/50 | 81/92/51 |
| 6 mo | |||||
| 0 | 96/100/79 | 97/99/87 | 99/100/92 | 99/99/94 | 97/99/89 |
| 35 < 0 | 80/100/78 | 88/95/52 | NA/NA/NA | 75/100/75 | 84/98/69 |
| >35 | 73/64/45 | 83/86/14 | 67/100/33 | 100/100/50 | 77/75/35 |
| Molecular | |||||
| 3 mo | |||||
| ≤1 | NA/NA | 94/78 | 96/88 | 100/95 | 96/85 |
| >1-10 | NA/NA | 100/69 | 94/75 | 100/82 | 98/73 |
| >10 | NA/NA | 80/60 | 100/100 | 27/50 | 61/61 |
| 6 mo | |||||
| ≤1 | NA/NA | 96/85 | 99/92 | 100/96 | 97/89 |
| >1-10 | NA/NA | 79/47 | 100/100 | 42/60 | 76/56 |
| >10 | NA/NA | 100/50 | 67/33 | 100/50 | 88/49 |
Patients with imatinib 400 were excluded from the molecular analyses at 3 and 6 mo due to low percentage of patients with available data.
NA, no data applicable.
EFS according to cytogenetic and molecular response at 3 and 6 mo. (A) EFS by cytogenetic response at 3 mo. (B) EFS by molecular response at 3 mo. (C) EFS by cytogenetic response at 6 mo. (D) EFS by molecular response at 6 mo. Molecular response analysis excludes patients treated with imatinib 400.
EFS according to cytogenetic and molecular response at 3 and 6 mo. (A) EFS by cytogenetic response at 3 mo. (B) EFS by molecular response at 3 mo. (C) EFS by cytogenetic response at 6 mo. (D) EFS by molecular response at 6 mo. Molecular response analysis excludes patients treated with imatinib 400.
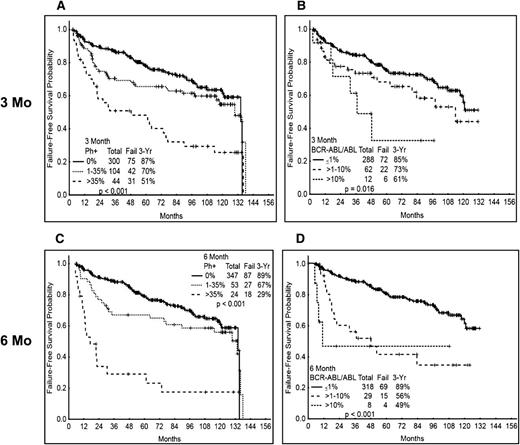
The standard definition of EFS does not consider events that are currently recognized as treatment failure, including failure to achieve established response hallmarks at given times or loss of CCyR (rather than loss of MCyR). We thus analyzed the 3-y FFS probability according to the early cytogenetic and molecular response. Patients with Ph+ >35% at 3 mo have a significantly inferior probability of 3-y FFS (51%) compared with those with CCyR (87%) or PCyR (70%) (82% for all patients with MCyR) (P ≤ .001) (Figure 3A). A similar trend was observed when analyzing the results by molecular response at 3 mo and by cytogenetic or molecular response at 6 mo (Figure 3B-D). As for EFS, FFS probability was not significantly different among the 4 TKI modalities in all 3 cytogenetic categories (supplemental Figure 2A-C for FFS at 3 mo) or molecular response categories (excluding imatinib 400) (not shown) at 3 and 6 mo.
FFS according to cytogenetic and molecular response at 3 and 6 mo. (A) FFS by cytogenetic response at 3 mo. (B) FFS by molecular response at 3 mo. (C) FFS by cytogenetic response at 6 mo. (D) FFS by molecular response at 6 mo. Molecular response analysis excludes patients treated with imatinib 400.
FFS according to cytogenetic and molecular response at 3 and 6 mo. (A) FFS by cytogenetic response at 3 mo. (B) FFS by molecular response at 3 mo. (C) FFS by cytogenetic response at 6 mo. (D) FFS by molecular response at 6 mo. Molecular response analysis excludes patients treated with imatinib 400.
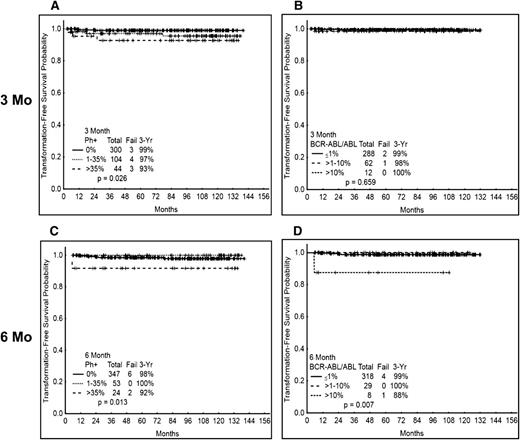
We then analyzed the TFS probability according to the response at 3 and 6 mo. Overall, only 10 (2%) patients transformed to accelerated (n = 2) or blast (n = 8) phase. There was a trend for an inferior probability of 3-y TFS for patients with no MCyR at 3 mo compared with those with at least a PCyR. Still, 93% of patients in the worse response group were projected to remain alive and free from transformation at 3 y. In contrast, the 3-y TFS was projected to be 99% for those with CCyR and 97% with PCyR at 3 mo. No difference was observed according to the 3-mo molecular response (excluding imatinib 400). Of the 10 patients that had transformed after 3 mo, 4 were treated with imatinib 400, 4 with imatinib 800, and 2 with nilotinib. Similar outcomes were observed according to response at 6 mo, with the worse probability of 3-y TFS among patients with poor cytogenetic (92% TFS) or molecular (88%) response (Figure 4A-D). As for other outcomes, the outcome probability was similar for patients with similar response at 3 or 6 mo, regardless of the treatment received (not shown).
TFS according to cytogenetic and molecular response at 3 and 6 mo. (A) TFS by cytogenetic response at 3 mo. (B) TFS by molecular response at 3 mo. (C) TFS by cytogenetic response at 6 mo. (D) TFS by molecular response at 6 mo. Molecular response analysis excludes patients treated with imatinib 400.
TFS according to cytogenetic and molecular response at 3 and 6 mo. (A) TFS by cytogenetic response at 3 mo. (B) TFS by molecular response at 3 mo. (C) TFS by cytogenetic response at 6 mo. (D) TFS by molecular response at 6 mo. Molecular response analysis excludes patients treated with imatinib 400.
Finally, we analyzed OS as a function of response at 3 and 6 mo. There was again a trend for inferior outcome for patients without MCyR at 3 mo, with a projected 3-y OS rate of 92% compared with 96% to 98% for those with MCyR. Interestingly, the difference appears to widen after 5 y, with an estimated 10-y survival of 58% compared with 84% to 89% for those patients with MCyR. However, only 4 of the 12 deaths in this group (Ph+ >35%) were related to CML due to transformation to blast phase. The cause of death for the other patients include other cancers in 2, other comorbidities (advanced parkinsonism) in 1, unknown but in remission in 4, and 1 from complications from stem cell transplant while in complete cytogenetic and molecular remission. All these 12 deaths were among patients who received treatment with imatinib 400. Among the causes of deaths after 5 y (n = 8), 3 were due to blast phase (2 other cancers, 1 comorbidities, 2 unknown). The OS was similar by cytogenetic response measured at 6 mo (Figure 5A-B). Because most of the poor responders were treated with imatinib 400 and, as mentioned earlier, molecular analysis was not routinely done at earlier time points, and in view of the few events, survival by molecular response at 3 and 6 mo was noninformative (not shown). OS was similar by cytogenetic response categories regardless of the TKI modality used (supplemental Figure 3A-C).
OS according to cytogenetic response at 3 and 6 mo. (A) OS by cytogenetic response at 3 mo. (B) OS by cytogenetic response at 6 mo.
OS according to cytogenetic response at 3 and 6 mo. (A) OS by cytogenetic response at 3 mo. (B) OS by cytogenetic response at 6 mo.
Factors predictive of early response
Because response at 3 mo appeared to be predictive of long-term outcome, we then investigated the factors associated with increased probability of having a poor cytogenetic response (ie, Ph+ >35%) at 3 mo. Supplemental Table-5A shows the results of the univariate analysis. In a multivariate analysis (supplemental Table-5B), factors associated with a poor 3-mo cytogenetic response were: age [OR = 0.96, 95% confidence interval (CI) = 0.933-0.984 (P value ≤ 0.01)], Sokal score [OR = 2.88, 95% CI = 1.654-5.018 (P value ≤ 0.001)], and treatment modality. Compared with patients treated with 400 mg imatinib, those treated with 800 mg had an OR = 0.17, 95% CI 0.074-0.39 (P value ≤ 0.001), and the group treated with nilotinib or dasatinib had an OR = 0.06, 95% CI = 0.022-0.175). Compared with patients treated with 400 mg imatinib, patients who received 800 mg imatinib had 83% less risk and those treated with nilotinib or dasatinib had a 96% lower risk of a poor cytogenetic response at 3 mo. Treatment with imatinib 800 was similar to second generation TKIs in predicting deeper early cytogenetic response. Age constituted a protective factor, with an increase of a year of age decreasing the odds of a poor 3-mo cytogenetic response by 4%. Increments of one category of Sokal risk-score increased the odds of a poor 3-mo cytogenetic response by 2.8 times.
Discussion
In recent years, the therapeutic landscape of CML-CP has changed significantly.23 Imatinib first proved to be superior to interferon in achieving MCyR, resulting in an improved long-term outcome, as manifested by better EFS.1,24 More recently, second generation TKIs such as dasatinib and nilotinib have been shown to be superior to imatinib in achieving faster and deeper molecular and cytogenetic responses that result in a decreased probability of transformation to accelerated and blast phase.4,6,18,19,25 With this improvement comes the need for precisely assessing the response kinetics as a predictor for long-term outcomes among the patients with CML-CP on various TKI therapies.11,14,26 It is now nearly universally accepted that patients not achieving certain response hallmarks at given times have a significantly worse long-term outcome. This notion is incorporated in the definitions of optimal response defined by the European Leukemia Net.22 These definitions have been confirmed by independent analyses to predict for the long-term outcome of patients.27,28 However, with the availability of more, and possibly better, treatment options, increased emphasis has been placed on earlier identification of those patients less likely to have a favorable outcome.
Nearly 10 y ago, Wang et al8 first proposed that the transcript level at 3 mo could identify those patients destined to eventually have a poor outcome. Patients with <10% transcripts (compared with baseline, not by International Scale) at 3 mo had a significantly better progression-free survival compared with those with >10% transcripts.8 Several subsequent studies confirmed this notion with further evidence of a lower probability of achieving a MMR10 and greater probability of eventually progressing10,29 for those patients with a higher level of transcripts or higher percentage of Ph+ metaphases at 3 mo, with the worse outcome for those with >10% transcripts (or <1-log reduction) or with no MCyR. Recently, Marin et al13 confirmed that patients who were treated with imatinib therapy with transcript levels >9.8% (International Scale) had a significantly inferior OS (54% at 7 y) compared with those with transcripts <9.8% (93%).13 Several other studies have confirmed this observation among patients treated with imatinib as well as for patients treated with dasatinib as initial therapy.14,15,26,30
Our report is the first to analyze the outcome of patients according to 4 different TKI modalities that have been used as initial therapy for CML in chronic phase in a single institution: standard-dose imatinib, high-dose imatinib, nilotinib and dasatinib. Our analysis shows that nearly one-third of patients treated with 400 mg/d imatinib are “poor responders” (ie, no MCyR) at 3 mo. Notably, this ratio is very consistent among different series.13,14 In contrast, only 10% to 15% of patients treated with second generation TKIs have these poor responses in ours and other series.11,26 Importantly, we find that patients treated with high-dose imatinib (ie, 800 mg/d) have a similar low rate of poor responders at 3 and 6 mo to those treated with second generation TKIs. This finding might be of relevance, as second generation TKIs are beyond the reach of many patients in some parts of the world and generic imatinib is becoming more widely available. However, this finding needs to be confirmed in prospective, randomized, clinical trials. In multivariate analysis, treatment with 400 mg imatinib was associated with an increased probability of having a poor early response. Such analysis also identified high Sokal risk score and younger age as factors associated with higher risk of poor response at 3 mo. The identification of younger age with poor early response may appear counterintuitive at first glance. However, we recently reported that younger patients have a lower response rate and worse long-term outcome than older patients.31 Although these findings require confirmation, a possible explanation for this inferior outcome is that these patients might be more prone to decreased adherence to treatment and this would affect both their short-term response and long-term outcome. Adherence has been identified as an important predictor of outcome in patients with CML treated with TKIs.32,33
We confirm here that patients with early poor response have an inferior probability of EFS. It is clear, however, that the definition of EFS is insufficient to identify features that are currently recognized as treatment failure. These include loss of CCyR (rather than only loss of MCyR) and failure to achieve desired responses at given time points (eg, no MCyR at 12 mo) identified by the European LeukemiaNet as failure to therapy. We thus analyzed also what we termed FFS that includes all reasons leading to treatment discontinuation. Patients with poor response at 3 mo have significantly inferior EFS and FFS compared with those with better responses. However, most of the events that occur in these patients are loss or lack of cytogenetic response, with very few patients transforming to accelerated or blast phase. This is important, because the outcome of patients with different reasons of failure have different expected probabilities of response with subsequent therapy. For example, patients who lose a cytogenetic response with imatinib have an 85% probability of achieving a CCyR,34 whereas patients who transform to accelerated or blast phase have only a 20% to 30% probability of achieving such response; in this setting, responses are rarely durable.35,36 These are important considerations in the current debate on what the best approach for patients with slow response may be. The options to consider are to prevent slow response (by using 800 mg/d imatinib or second generation TKIs as frontline), to make a change for patients with slow response at 3 mo, to wait and make a change at a slightly later time point (eg, at 6 mo), or to change only when failure by current definitions is identified. This question is not addressed in the current study, as no patient changed dose or treatment at 3 or 6 mo for failure to achieve MCyR or transcripts <10%.
We also analyzed the outcome of patients in different response categories according to their initial therapy. Our analysis shows that the response at the early time points defines the long-term outcome, regardless of whether this response is achieved with imatinib (standard or high dose) or second generation TKIs. The difference, however, is that with 400 mg imatinib, there is a considerably higher percentage of patients (nearly one-third) that have a poor response at 3 or 6 mo compared with the other modalities. Interestingly, there appears to be little or no difference between the rates achieved with high-dose imatinib or second generation TKIs. Based on this observation, the evaluation of early response should be considered universal, regardless of the treatment modality the patient is using.
This analysis has some shortcomings. First, it includes results from different studies performed at different times. However, the trials had nearly identical inclusion criteria and overall design, and the overall results have been consistent with what has been reported in other series with similar therapies. Because of the long timespan covered by these trials, some patients did not have molecular analysis done at the earliest time points. In the earlier days of imatinib, it was not routine to perform PCR until patients achieved CCyR.37 In our series, the cohort of patients treated with 400 mg/d imatinib was mostly affected by this lack of early PCR data. Because this is the treatment modality with the greatest risk of poor response at 3 mo, data from molecular analysis do not include patients treated with this option and thus are less informative than the analysis by cytogenetic response assessment that was performed routinely in all the patients. There is, however, good correlation between cytogenetic and molecular analyses.38
In conclusion, our results show that early achievement of cytogenetic and molecular responses results in improved EFS and FFS, but few of the events are transformations to accelerated or blast phases. The identification of features associated with increased risk of a poor response may help identify patients to target in prospective studies to address the optimal management of this patient population.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 and award number P01 CA049639 from the National Cancer Institute.
Authorship
Contribution: P.J., H.K., and J.C. designed the study; M.C.-T., P.J., H.K., and J.C. analyzed results; P.J., M.C.-T., C.G.R., and J.C. wrote the paper; P.J., M.C.-T., S.P., H.K., A.N., and J.C. conducted clinical correlation; H.K., A.Q.-C., S.O., F.R., E.J., S.V., G.B., and J.C. contributed patient samples; J.C., S.O., H.K., and F.R. contributed patients; and all authors reviewed and gave the final approval for the paper.
Conflict-of-interest disclosure: J.C. is a consultant for Pfizer, Ariad, and Teva and received research support from Pfizer, Ariad, Chemgenex, Bristol Myers Squibb (BMS), and Novartis. F.R. received research funding from BMS and honoraria from BMS, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, Professor and Deputy Chairman, Department of Leukemia, Unit 428, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: jcortes@mdanderson.org.